Abstract
Clinically refractory candidiasis remains uncommon. Following the introduction of antiretroviral therapy, the prevalence of resistant oropharyngeal and esophageal candidiasis dramatically decreased in HIV positive individuals. Nevertheless occasional cases of clinical and in vitro resistant mucosal candidiasis due to C. albicans continue to be reported; however the availability of new azoles, e.g., posaconazole and parenteral echinocandins, usually resolves the therapeutic challenge. Unfortunately recent reports of echinocandin resistance in patients with invasive C. glabrata infection serve as a cause of concern. Considerable progress has been made in determining risk factors for and mechanisms of azole resistance in Candida species.
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
1 Introduction
During the last three decades, there was a marked increase in the population of immunocompromised and severely ill individuals at risk of developing opportunistic fungal infections [1]. In particular, the increased use of immunosuppressive agents particularly in organ transplant patients, chemotherapy, and lifesaving medical technology resulted in this increase of both superficial and serious invasive fungal infections [2–4]. The initial increase in fungal infections occurred at a time when there were few available, effective, systemic antifungal agents. Parenteral and systemically active oral azoles only became available in the 1980s. Accompanying the introduction of these newer azoles, an explosion in numbers of patients with AIDS at high risk of developing oropharyngeal and esophageal candidiasis was encountered.
It was during the 1990s that drug resistance became an important problem in virtually all populations of patients at risk, but predominantly in patients with AIDS [5, 6]. Reports of resistance to antifungal drugs have appeared with increased frequency. Confusion abounds as to how common Candida resistance is and whether fungal isolates should routinely be sent for susceptibility testing. Simultaneously, both clinical resistance and the increased incidence of fungal infections drove the development of new generations and classes of antifungal agents. Although extremely rare prior to the 1990s, antifungal drug resistance has now rapidly become a major problem in certain populations. The highest-risk population has been the most vulnerable, viz., patients with HIV infection. Thus, in the decade of the 1990s, up to a third of advanced-stage AIDS patients had drug-resistant strains of Candida albicans isolated from the oral cavities. However, it is no longer HIV-infected patients that demonstrate major clinical problems with antifungal resistance [7]. Nevertheless, occasional cases of clinical and in vitro resistant mucosal candidiasis due to C. albicans continue to be reported; however, the availability of new azoles, e.g., posaconazole and parenteral echinocandins, usually resolves the therapeutic challenge. Unfortunately, highly immunocompromised patients following both bone marrow and solid organ transplants have become a focus of rising antifungal resistance to both azole and echinocandin antifungal drugs. The purpose of this chapter is to review the epidemiology, pathogenesis, risk factors, and treatment of resistant candidiasis. Understanding cellular and molecular mechanisms of antifungal drug resistance and associated risk factors is crucial to developing successful prophylactic and treatment strategies to prevent emergence of resistant fungi and is discussed in the subsequent chapters. Management of refractory fungal disease caused by resistant Candida species will be reviewed together with methods available to prevent further development of antifungal drug resistance in candidiasis.
2 Epidemiology of Candidiasis
Oropharyngeal candidiasis (OPC) is most prevalent in infants, the elderly, and compromised hosts and also associated with serious underlying conditions including diabetes, leukemia, neoplasia, steroid use, antimicrobial therapy, radiation therapy, and chemotherapy. At least a quarter of cancer patients not receiving antifungal prophylaxis develop OPC, whereas other investigators have observed OPC in more than half of all immunocompromised patients. Prolonged neutropenia appears to be the single most important risk factor for both oropharyngeal colonization with a Candida species and subsequent symptomatic disease [8]. Approximately 80–90 % of patients with HIV infection will develop OPC at some stage of the disease [6], and 60 % of untreated patients develop AIDS-related infection within 2 years of appearance of OPC [9]. Candida albicans remains the most common species responsible for OPC [10]. A small unique population at high risk for developing azole antifungal resistance are individuals with immunodeficiency-related chronic mucocutaneous candidiasis [11, 12].
Vulvovaginal candidiasis (VVC) is considered to be the second most common form of vaginitis worldwide affecting millions of immunocompetent women. More than 90 % of infections are caused by Candida albicans [13]. The high prevalence of this infection in otherwise healthy females is responsible for significant morbidity and use of antifungal therapy.
During the 1980s, data from the National Center for Health Statistics (NCHS) reported that bloodstream infections (BSIs) were the 13th leading cause of death in the USA. Candida bloodstream infection has an attributable mortality of approximately 35 % [1]. Fungal infections, particularly due to Candida species, increased dramatically and accounted for 8–15 % of all nosocomial bloodstream infections [14–17]. The National Hospital Discharge Survey (NHDS) reported rates of oropharyngeal and disseminated candidiasis to have increased fourfold and 11-fold, respectively, between 1980 and 1989, a trend that continued over the next two decades [18]. Bloodstream Candida infections previously predominantly seen in cancer patients became common in ICUs and pediatric wards [19]. The SCOPE study reported that for the 3-year period ending in 1998, Candida species remained the fourth most common cause of nosocomial bloodstream infection [16, 20]. Risk factors for the increased incidence of candidemia have been reviewed [21, 22]. Moreover, candidemia has the highest crude mortality (40–50 %) of all nosocomial bloodstream infections [20, 23, 24]. Autopsy studies have also confirmed the increase in the incidence of disseminated candidiasis. Candidemia is associated with prolongation of hospital stay 70 vs. 40 days compared to matched nonfungemic patients as well as considerable increase in costs of therapy [25].
At present, C. albicans accounts for ~40–60 % of all nosocomial invasive Candida infections, reflecting a continued shift toward Candida species other than C. albicans has occurred, and of relevance because of intrinsic or acquired antifungal resistance in several of these species [2, 15, 23, 26–28]. Within the hospital setting, areas with the highest rates of candidemia include intensive care units, surgical units, trauma units, and neonatal ICUs. In fact, 25–50 % of all nosocomial candidemia occurs in critical care units. Neutropenic patients, formerly the highest-risk group, are no longer the most vulnerable subpopulation, likely as a result of the use of fluconazole prophylaxis during neutropenia [29]. In some tertiary care centers, C. albicans is no longer the most frequent bloodstream isolate, having been replaced by C. glabrata, which in turn replaced C. tropicalis as the most prevalent non-albicans species, causing 3–50 % of all candidemias. The increased frequency of C. glabrata in ICUs is also attributed to fluconazole exposure in ICU patients [30, 31]. There is a wide global variation in the predominance of particular species with C. tropicalis common in South America and C. parapsilosis common in Europe [32].
3 Mechanism of Action of Antifungal Drugs [33]
3.1 Polyenes [34]
The most important polyenes include amphotericin B and nystatin. Amphotericin B binds to sterol, the primary fungal cell membrane altering membrane permeability and ultimately cell death. Amphotericin B also causes oxidative damage to fungal cells (Vol. 1, Chapter 26).
3.2 Fluoropyrimidines
Flucytosine, or 5-fluorocytosine (5-FC), is a synthetic fluorinated pyrimidine. It is transported into susceptible fungal cells by the action of an enzyme cytosine permease and then converted by cytosine deaminase to fluorouracil. The latter molecule is incorporated into RNA in place of uracil. In addition, flucytosine blocks thymidylate synthetase, an essential enzyme for DNA synthesis (Vol. 1, Chapter 27).
3.3 Azoles
The azole antifungal agents in clinical use contain either two or three nitrogens in the azole rank and are therefore classified as imidazoles (ketoconazole, miconazole, clotrimazole, econazole, and butoconazole) or triazoles (itraconazole, fluconazole, terconazole). The newer azole agents include voriconazole, posaconazole, ravuconazole, and albaconazole. The azoles inhibit ergosterol synthesis in the fungal cell membrane through their action on the cytochrome P450-dependent enzyme lanosterol 14α-demethylase. Differences among various azoles relate primarily to their pharmacokinetics as well as their affinity for the target enzymes. There are also some differences in antifungal spectrum. Voriconazole and posaconazole have activity against many yeasts and filamentous fungi as well (Vol. 1, Chapter 27).
3.4 Echinocandins
This new class consists of parenteral caspofungin, micafungin, and anidulafungin. These agents inhibit fungal cell wall synthesis of an enzyme 1,3-β-d-glucan synthase, preventing the formation of 1,3-β-d-glucan, an essential component of the fungal cell wall. These agents result in a weakened cell wall resulting in fungal cell lysis and are considered candidacidal [35] (Vol. 1, Chapter 29).
4 Definition of Resistance
4.1 Refractory Candidiasis
This by no means uncommon condition refers to treatment failure of symptomatic patients with antifungal agents. Only one of the many causes of therapeutic failure is due to the presence of in vitro confirmed resistant Candida spp. (Box 66.1) (Fig. 66.1). Treatment failure can also be the result of failure of the antifungal agent to reach the target site of infection in sufficient concentrations due to inadequate dosing, impaired absorption (food, gastric pH), poor compliance, and drug interactions. Other causes of treatment failure include local factors that either interfere with drug action, e.g., purulent material in an undrained abscess, or prevent access to organisms seeking refuge in a biofilm, e.g., prosthesis both intravascular and intra-articular [32]. A profoundly depressed immune system may also be responsible for failure. Both adequate numbers of functioning polymorphonuclear leukocytes and cell-mediated immunity are also essential in eradicating Candida infection. Clinical resistance refers to treatment failure despite microbial susceptibility in vitro.
Box 66.1. Causes of Treatment Failure Resulting in Refractory Candidiasis
-
1.
In vitro antifungal resistance
-
(a)
Primary (intrinsic)
-
(b)
Secondary
-
(a)
-
2.
Failure of drug to reach the site of infection in effective concentration
-
(a)
Poor adherence
-
(b)
Inadequate dosing
-
(c)
Impaired oral absorption
-
(d)
Drug interactions
-
(a)
-
3.
Failure to drain abscess
-
4.
Local protective mechanisms, e.g., biofilm (catheter, prosthetic valve, device, foreign body)
-
5.
Impaired host immune/defense mechanism
-
(a)
PMNs
-
(b)
CMI
-
(a)
*Mechanisms 2–5 result in clinical resistance with failure associated with susceptible microorganisms.
4.2 Primary or Secondary Resistance
An organism that is resistant to a drug prior to exposure is defined as having intrinsic or primary resistance. Examples of primary resistance include C. krusei to fluconazole and C. krusei and C. lusitaniae to flucytosine. Acquired or secondary resistance develops during or after exposure to an antifungal agent, e.g., HIV-infected patients with fluconazole-resistant OPC and esophageal candidiasis due to C. albicans. Cross-resistance refers to multidrug resistance either within the same class or multiple classes. Heteroresistance refers to variable in vitro susceptibility of different colonies of the same isolate obtained from the same agar plate. All forms of in vitro resistance may be temporary, transient, or irreversible.
5 Antifungal Susceptibility Tests
5.1 Methods
Testing methods and breakpoints for antifungal drugs were first suggested by Rex [36–39]. However, considerable change in methods followed to produce standardized, reproducible susceptibility methods for fungi resulting in the Clinical and Laboratory Standards Institute (CLSI) M27-A3 and the EUCAST methodology [40–47]. Accordingly, interpretive breakpoints determined by these methods are available for testing Candida species to fluconazole, itraconazole, voriconazole, flucytosine, amphotericin B, caspofungin, anidulafungin, and micafungin [42, 48–53] (Table 66.1). Recently, new interpretative standards have been introduced which profoundly impact upon determination and definition of susceptible and resistance isolates, potentially causing confusion for uninformed clinicians. Firstly, a new epidemiologic cutoff value (ECV) is now available and represents a more sensitive measure of change in susceptibility breakpoints [17, 43, 44]. The ECV method statistically determines the distribution of MICs within a given microbial species and is defined as the MIC value that excludes non-wild-type strains, specifically an isolate likely to contain a resistant mutation. Reliance upon the ECV results in variable breakpoints for different Candida species and in many cases a severalfold lowering of the susceptibility breakpoint, e.g., the previous C. albicans breakpoint for susceptibility to fluconazole was ≤8 mg/L, but with the new interpretation, this value is reduced to ≤2 mg/L and elevated to ≤16 mg/L for C. glabrata. The ECV method is valuable for detecting emergence of resistance in a Candida species in an institution. Using this method, most breakpoints have declined, and results of M27-A3 (CLSI) and EUCAST match more frequently. Moreover, as breakpoints decrease more isolates are deemed resistant, but no increased risk of treatment failure has been reported. This conclusion applies to both the triazoles and echinocandins with the new CLSI guidelines (Table 66.1). In the final analysis, therapeutic decisions are always individualized based upon the patient’s response to therapy at the time susceptibility results become available.
In general, the susceptibility of the Candida isolate to the currently available antifungal agents is generally predictable if the species of the infecting isolate is known. However, individual isolates may not follow this general pattern [17].
In the past susceptibility testing of Candida isolates, even blood isolates, was not recommended on a routine basis. Testing was recommended only for persistent disease and failure of organism eradication in symptomatic patients with appropriate antifungal therapy. This principle was based upon the cost and lack of testing facilities available, but also driven by the rarity of in vitro resistance. However, recent surveillance suggests the emergence of reduced susceptibility of some Candida species in relation to azoles and echinocandins. Triazole resistance among C. glabrata isolates has increased to an extent that it is difficult to rely upon triazoles for therapy without performing susceptibility testing [32]. Unfortunately more recently, a similar trend has begun to emerge for a smaller proportion of C. glabrata isolates and the echinocandins [1, 13]. Accordingly, susceptibility testing is now required and recommended to guide the management of candidiasis. It is now recommended the laboratories perform routine antifungal susceptibility testing against both the triazole and echinocandins for C. glabrata isolates from blood and sterile sites and for other Candida species that have failed to respond to antifungal therapy. Although controversial, based upon the overall infrequency of antifungal resistance in C. albicans, routine testing for this species is not indicated in the absence of treatment failure. The value of testing for other Candida species is less clear, although occasional resistance among C. tropicalis and C. parapsilosis has been reported in certain hospitals with high use of antifungals. Hence, some authorities recommend triazole susceptibility testing for all bloodstream and clinically relevant Candida isolates, whereas testing for echinocandin susceptibility should be considered in patients who have had prior treatment with an echinocandin.
The objective of susceptibility testing is to differentiate infecting strains that are susceptible and hence likely to respond to a given antifungal drug from those strains resistant and hence more likely to fail therapy. With regard to echinocandins, it is essential that susceptibility tests capture high-MIC strains containing FKS mutations. To date the CLSI has used limited clinical data but also microbiologic data to define clinical breakpoint for all three echinocandins against Candida spp. [54]. Unfortunately, some resistant Candida strains were often misclassified by this breakpoint [55, 56]. As a result, new breakpoints were determined by CLSI that better accounted for FKS mutations [32, 47] (see Table 66.1). EUCAST established Candida species-specific and echinocandin-specific clinical breakpoints (Vol. 2, Chapter 18).
NCCLS M27-A methodology has only a limited ability to measure MICs of Candida isolates to amphotericin B. Rex et al. recommended the use of antibiotic medium 3 broth to measure resistance [48, 57]. In general, current methods are limited to identifying Candida isolates associated with clinical failure, although breakpoint minimal lethal concentrations (MLCs) and MICs of ≥1 μg/mL at 48 h have been recommended to more accurately predict mycologic Candida spp. failure with amphotericin B [58]. In a multicenter study of candidemia in non-neutropenic patients, all blood isolates demonstrated amphotericin B MICs less than 1.0 μg/mL. As with fluconazole, clinical failures (10–15 %) were all associated with in vitro susceptible isolates with low amphotericin B MICs [50].
The Etest is often used as an alternate to broth dilution methodology and certainly is useful in the setting of refractory clinical disease, and there is no other testing method available. The Etest is considered suitable for testing Candida spp. against amphotericin B or flucytosine, but less reliable for azole susceptibility [49, 51–53, 57].
5.2 In Vitro Susceptibility and Resistance of Candida Species (Table 66.2)
5.2.1 Azoles
The triazole, voriconazole, posaconazole, and ravuconazole exhibit greater potency and spectrum than either fluconazole or itraconazole but are still essentially fungistatic. The activity of this broad-spectrum triazole extends to some fluconazole-resistant strains of Candida.
Primary and secondary azole resistance is species dependent and also shows marked geographic variation [59, 62]. There is no clear evidence for a correlation between the agricultural use of azoles and an increase in antimycotic resistance in Candida species. Primary resistance to azoles remains uncommon in candidiasis, with the exception of Candida glabrata and Candida krusei. Most acquired azole resistance emerged in AIDS patients with OPC and EC following prolonged azole therapy in the presence of advanced immunodeficiency. Azole resistance in other settings is uncommon [32, 63].
-
1.
C. albicans. Primary resistance to fluconazole and itraconazole is extremely rare. Moreover, outside the realm of AIDS, acquired or secondary resistance has likewise remained uncommon especially with regard to bloodstream isolates. Each year, thousands of randomly obtained BSIs isolated from all over the world are tested in a single site (SENTRY), and over several years fluconazole-resistant C. albicans remains <5 % and shows no evidence of changing [60, 63, 64]. In contrast, Antoniadou et al. reported that 9 % of bloodstream isolates of C. albicans were resistant to fluconazole (MIC > 64 μg/mL) [65]. Spontaneous fluconazole resistance in the absence of prior azole therapy is rare but has been reported in otherwise healthy adults [66]. Based upon molecular modeling studies, it has been reported that certain mutations in ERG II result in significant levels of resistance to fluconazole and voriconazole but have less effect on the susceptibility of the organisms to itraconazole and posaconazole, possibly due to the more extensive binding of the latter agents to the target enzymes [67].
-
2.
C. tropicalis. Occasional strains of C. tropicalis demonstrate azole resistance although MIC90 values indicate continued susceptibility. This species has a proclivity to produce trailing grown in vitro often misinterpreted as resistance.
-
3.
C. parapsilosis strains are usually highly susceptible to all azoles [67].
-
4.
C. krusei. This species is intrinsically resistant to fluconazole and has higher MICs to itraconazole in the S-DD range. Voriconazole is, however, very active against C. krusei [60, 68]. C. krusei incidence has remained stable over the last decade.
-
5.
C. dubliniensis. This species has been increasingly identified and implicated in OPC in HIV-infected agents and is usually identified as C. albicans. Most C. dubliniensis strains are susceptible to fluconazole although in vitro resistance can be induced. Acquired resistance develops much more rapidly than in C. albicans.
-
6.
C. glabrata. Among pathogenic yeast species, Candida glabrata, which accounts for 5–40 % of all yeast isolates, ranks second in all clinical forms of candidiasis today and in some studies of nosocomial candidemia is more common than C. albicans [32, 69]. This opportunistic pathogen is particularly relevant in immunocompromised patients including those receiving cytotoxic chemotherapy, undergoing transplantation, and infected with HIV. This critical Candida species represents the Achilles heel of the azole class [70, 71]. C. glabrata isolates exhibit bimodal susceptibility to azoles with 10–15 % of bloodstream isolates demonstrating fluconazole resistance (≥64 μg/mL) [60, 64]. Patterns of fluconazole susceptibility vary by geographic area, patient population, risk factors, and azole exposure [72]. In particular, clinical isolates obtained from patients with AIDS and OPC/EC and those with underlying malignancy show reduced susceptibility to fluconazole and itraconazole. Fluconazole resistance is lowest in Asia-Pacific and Latin-American regions (3–4 %) and highest in North America (10–15 %). Both the frequency of C. glabrata occurrence and azole susceptibility are profoundly affected by azole exposure, with 30–40 % of isolates being S-DD. International surveillance reveals that recently submitted bloodstream isolates (2001–2005) of C. parapsilosis and C. tropicalis in contrast to C. albicans did reveal a slight increase in fluconazole resistance. A similar increase in resistance was observed for C. glabrata with sustained high rates of fluconazole resistance (14.3–18.3 %) over this period [63]. In general, whereas most C. glabrata isolates are still susceptible to voriconazole, most fluconazole-resistant C. glabrata isolates are resistant to itraconazole, and half are also resistant to voriconazole and posaconazole [60, 69, 73]. Not surprisingly, several reports of voriconazole-resistant C. glabrata breakthrough fungemia in bone marrow transplant recipients receiving long-term voriconazole prophylaxis have been reported [74].
5.2.2 Flucytosine
Intrinsic resistance among C. albicans has been described in 6.5–33 % of isolates and is invariably associated with serotype B isolates [75]. More recent studies have shown lower resistance frequency possibly due to infrequent use. Pfaller et al. studying 8803 clinical isolates of Candida spp. reported susceptibility as follows: C. albicans (97 %), C. tropicalis (92 %), C. guilliermondii (100 %), C. dubliniensis (100 %), C. parapsilosis (99 %), and C. glabrata (99 %) [64]. The least susceptible species was C. krusei (5 % susceptible, 67 % intermediate, and 28 % resistant). A smaller study reported that 82 % of C. glabrata were susceptible to flucytosine. The pharmacokinetics and in vitro activity of flucytosine make the agent particularly useful for azole-resistant Candida infections in relatively inaccessible sites such as CSF and the genitourinary tract.
Unfortunately, secondary acquired resistance is common (30 %) and acquired rapidly to flucytosine when used as monotherapy. Accordingly, flucytosine is almost always used in combination with other antifungals.
5.2.3 Polyenes
Resistance to amphotericin B may be intrinsic or acquired [76]. C. albicans resistance is extremely rare, although the NCCLS M27-A methodology may be underestimating its occurrence. For amphotericin B, NCCLS methodology generates a narrow MIC range limiting its ability to identify isolates likely to cause therapeutic failure [58]. Moreover, more important than resistance is the phenomenon of reduced susceptibility without frank resistance. Powderly et al. reported reduced amphotericin B sensitivity of blood isolates of C. albicans in neutropenic patients and correlating higher MICs with poor outcome [77]. Fortunately, such strains are rare and secondary resistance is uncommon [78]. Resistance in C. parapsilosis and C. dubliniensis but not C. tropicalis is rare [79]. Although C. glabrata and C. krusei isolates are usually considered susceptible to amphotericin B, they tend to have higher MICs, justifying initial empiric use of amphotericin B at a higher dose of 1.0 mg/kg/day. Sterling reported the emergence of resistance to amphotericin B during therapy for C. glabrata infection in an immunocompetent host [80]. Many but not all C. lusitaniae and some C. guilliermondii isolates demonstrate intrinsic resistance to amphotericin B [81]. Acquisition of secondary polyene resistance in species, in addition to C. albicans, includes C. lusitaniae and C. guilliermondii during amphotericin B therapy especially in myelosuppressed patients [78, 82–84]. Rare cases of fatal septicemia reported of amphotericin B-resistant C. lusitaniae [85]. Resistance to amphotericin B desoxycholate implies that the organism will be resistant to the various lipid formulations of amphotericin B.
5.2.4 Echinocandins
Early reports of clinical and/or in vitro resistance to any of the echinocandin agents were rare. In 2003, the in vitro activities of caspofungin against 3959 isolates of Candida spp. from 95 different medical centers were determined and compared with fluconazole and itraconazole [61]. No resistant strains of C. albicans were detected. Against all Candida species, 96 % of MICs were ≤2 μg/mL. C. albicans, C. dubliniensis, C. tropicalis, and C. glabrata were the most susceptible species, and C. guilliermondii was the least susceptible (MIC90 > 80 μg/mL). C. parapsilosis MIC90 2–4 μg/mL was significantly increased versus C. albicans 0.25 μg/mL [61]. Echinocandins remain very active against azole-resistant isolates of C. albicans and C. glabrata (99 % of MICs were ≤1 μg/mL). There is no evidence of a significant impact of azole resistance mediated by CDR pumps on echinocandin resistance in clinical Candida isolates.
Similarly, large multinational Candida isolate collections have been used to evaluate in vitro resistance to micafungin and anidulafungin, and identical almost universal susceptibility has been reported and once more higher MICs of C. parapsilosis emerged [63]. Interestingly, caspofungin is not fungicidal for isolates of C. parapsilosis or C. guilliermondii [86].
Breakpoints for the echinocandin class of agents were delayed in appearance since in vitro and in vivo analyses were hampered by a dearth of resistant isolates. As a result Kartsonis et al. failed to establish any relationship between baseline caspofungin MICs and clinical outcome with isolates from both mucosal and invasive Candida infections [87]. An echinocandin MIC of ≤2.0 μg/mL, a blood concentration easily achievable in vivo under normal dosing, would encompass 99.7 % of all clinical isolates of Candida species [63].
While clinical failure due to echinocandin-resistant Candida isolates has been rare, acquired in vivo resistance following echinocandin exposure undoubtedly occurs, and resistant isolates have increasingly been reported. All the resistant isolates were shown to have homozygous mutations in the FKS1 gene. Clinical failure with all Candida species has also increasingly been reported [88–90].
Hernandez et al. in 2004 reported a patient with azole-refractory OPC/EC which in spite of initial improvement eventually failed on caspofungin [91]. Initial isolates exhibited low caspofungin MICs, whereas a late isolate had higher MIC. The clinical response was reproduced in a murine model correlating MIC with the clinical response to caspofungin. Similarly, a case of progressive loss of echinocandin activity following prolonged use for treatment of C. albicans esophagitis was reported [92].
Moudgal et al. in 2005 described a patient with aortic valve endocarditis due to C. parapsilosis [93]. After initially responding to combination therapy with caspofungin (MIC 2 μg/mL) and fluconazole, he cleared his fungemia and was discharged on fluconazole only. He returned three months later with recurrent C. parapsilosis, now resistant to both fluconazole and caspofungin (MIC > 16 μg/mL) and also voriconazole and micafungin but not anidulafungin. Similar case reports regarding acquired echinocandin resistance in C. glabrata are reported more than a decade ago predicting a future likelihood of increased resistance in this species (see Chapter. 29, Volume 1) [94].
5.3 Correlation of In Vitro Susceptibility Testing and Clinical Outcome of Treatment with Antifungal Agents
In vitro susceptibility is only one of the many factors that influence the outcome of therapy of fungal infections [37]. A variety of pharmacokinetic and pharmacodynamic drug factors as well as a multitude of host factors (neutropenia, compliance, catheter presence, APACHE scores, abscess drainage) all interact to impact upon clinical outcome(s). Even the definition of clinical outcome is controversial, ranging from clinical improvement to mycologic evaluation (short or long term) on patient survival (days or weeks). Nevertheless, in vitro susceptibility determination may serve as an objective, reproducible measure that can profoundly influence drug selection with physicians recognizing the limitations of in vitro susceptibility testing.
Establishing that an isolate is resistant to an antifungal agent in vitro is an immensely useful step in selecting therapy. Determining that the isolate is susceptible to antifungal agents in no way predicts survival or fungal eradication. Clinicians should recall the old 90-60 rule in which a clinical response of 90 % or more can be expected when an in vitro sensitive strain is treated with an appropriate antibiotic in comparison to a 60 % response when a resistant strain is treated with drugs showing reduced or no activity in vitro.
With regard to candidiasis, in vitro and clinical outcome correlations have mainly been applied to OPC/EC and candidemia, where the 90-60 rule appears to have been met, recognizing this is merely a minimal standard. The most important principle applied is that organisms deemed resistant in vitro are much less likely to respond in vivo. Yet within the candidemia RCTs involving hundreds of patients, almost all patients failing did so with highly susceptible strains. This emphasizes the principle that susceptibility in vitro does not guarantee successful therapy. Most studies evaluating the 90-60 rule have applied to azoles, specifically fluconazole, and the best correlation was in OPC/EC in AIDS patients. The clinical predictability of amphotericin B susceptibility is less well established. Moreover, Sobel et al. found poor correlation between in vitro MICs and response to fluconazole therapy for VVC [95]. Finally, any discussion of clinical correlation must distinguish resistance developing in a given strain of the same species from the problem of acquiring less susceptible strains from the same or different species.
5.4 Indications for Antifungal Susceptibility Testing in Candida Infections
Apart for reasons of periodic epidemiologic surveillance and resistance monitoring, routine susceptibility testing by any of the aforementioned methods is not indicated. Testing is justified for all bloodstream Candida isolates, especially if associated with persistent, breakthrough, and recurrent candidemia and also refractory mucosal candidiasis, anticipated prolonged or critical therapy, e.g., endocarditis, osteomyelitis especially with non-albicans Candida invasive infections. Also, testing is essential with selected non-albicans Candida species, e.g., C. glabrata, initially treated by non-azole regimens anticipating a switch to oral therapy with either fluconazole or voriconazole to complete therapy. Given the increase of parenteral echinocandins as first-line therapy for candidemia only to have the remainder of the therapeutic course completed by oral triazoles, the Infectious Society of America now recommends that all first bloodstream isolates should be tested for antifungal susceptibility [96].
6 Epidemiology and Risk Factors for Resistant Candidiasis
Does azole use select for antifungal drug resistance? In this context, clinical resistance is encountered with (a) the presence of organisms with intrinsic, de novo resistance to antifungals usually seen with non-albicans Candida and rarely C. albicans, (b) alternately, evolution may occur of the initially sensitive strain to an identical strain that has undergone genetic and molecular changes, or (c) there is replacement of the strain with a new resistant strain of the same species or finally replacement with a new strain of a different species.
Evidence links empirical, prophylactic, and therapeutic use of azoles and selection for yeasts other than C. albicans that exhibit decreased susceptibility to azoles, e.g., C. glabrata and C. krusei infections in patients receiving fluconazole prophylaxis [97–99]. Most of the early data came from AIDS patients. The emergence of antifungal-resistant C. albicans fungemia has increasingly been reported in bone marrow transplant recipients being administered with long-term fluconazole prophylaxis [100]. Similarly, isolated reports of fluconazole-resistant fungi in surgical ICUs are emerging [101].
While molecular changes in a single strain invariably reflect a single or more usually multiple genetic mutations, the dynamics of acquisition of a new strain or species is less well understood. New more resistant Candida strains or species may be acquired during hospitalization from medical staff carriers. This process has been well documented with C. albicans and C. parapsilosis, but C. glabrata is rarely identified on the hands of carriers or in hospital environment. It is hypothesized that patients may be colonized in the gastrointestinal tract simultaneously by multiple strains of Candida, including the possibility of multiple species. Routine culture only captures the dominant strain or species. After antifungal drug ingestion or pressure, more susceptible strains are eliminated or so reduced in number so as to allow growth and emergence and recognition of more resistant strains or species that have coexisted long term but were previously not recognized.
6.1 HIV/AIDS
AIDS patients have been the focal point of much of the scientific inquiry into fluconazole resistance. On the one hand, oral and esophageal candidiasis became extremely common as a clinical manifestation of AIDS in the 1980s. The availability of fluconazole as both treatment and subsequently prophylaxis in patients with recurrent disease was an enormous boon to care. Within a few short years, clinical and in vitro fluconazole resistance was widespread and caused major alarm among AIDS practitioners [6, 32, 102]. Several studies, mainly retrospective, identified risk factors for acquisition of fluconazole resistance (Box 66.2). In addition to the status of the immune system (CMI), i.e., CD4 lymphocyte count, most studies concluded that patterns of fluconazole use particularly drug dose were the dominant factors associated with resistance acquisition [103–106]. In the majority of patients, mutation of a previously susceptible strain of C. albicans to a resistant strain is likely to have occurred, together with coinfection with Candida species resistant to fluconazole, e.g., C. glabrata [107].
Box 66.2. Risk Factors for Azole Resistance in Candidiasis
-
1.
HIV/AIDS
-
(a)
Advanced immunosuppression (low CD4 cells)
-
(b)
High viral load
-
(c)
Fluconazole administration
-
Poor compliance
-
Past fluconazole exposure
-
Total dose
-
Intermittent therapy
-
Prophylaxis versus therapeutic
-
Low dose
-
-
-
(a)
-
2.
Hematologic malignancy/BM transplantation
-
(a)
Azole exposure (prophylactic)
-
(a)
-
3.
Prosthetic devices—foreign bodies
-
(a)
Biofilm
-
(a)
In a prospective, randomized, controlled trial conducted by the Mycoses Study Group, episodic treatment versus continuous prophylaxis with fluconazole was studied. The first conclusion was that overall resistance acquisition was uncommon in this HAART-compliant study population. Secondly, the use of episodic compared to continuous fluconazole prophylaxis was not shown to be protective in preventing emergence of resistance [108]. In general, no pattern of fluconazole prescription or ingestion has been consistently identified as contributing to azole resistance selection, although both dosing and duration have been widely implicated in emergence of resistance. Most importantly, it has not been established whether lower doses used for longer periods of time lead to antifungal resistance and whether intermittent therapy, especially using higher doses for shorter periods, prevents resistance [109]. In contrast to the above, occasionally resistant species were isolated in patients with HIV infection and no prior exposure to fluconazole [110].
It is noteworthy that in the last decade, because of the availability of potent and better tolerated ART, the occurrence of fluconazole-resistant OPC and Candida esophagitis has become infrequent.
6.2 Hematologic Malignancies and Transplant Patients
This growing population is the second focus of resistant candidiasis. Empiric systemic antifungals are widely used as empiric therapy for antibiotic-resistant fever in addition to azole prophylaxis both in neutropenic patients (usually fairly short term) and non-neutropenic high-risk posttransplant patients (often long term). Once more, azole exposure both oral and systemic is recognized as (a) infrequent cause of azole-resistant C. albicans and (b) a more frequent and important cause of selection of non-albicans Candida species, both colonizing the gastrointestinal tract and as a cause of the ensuing infrequent invasive candidiasis [99, 111]. Primary fluconazole resistance has been reported in patients with severe neutropenia [112, 113]. Candidemia due to C. krusei has been associated with prior exposure to fluconazole [94, 114, 115].
6.3 Prosthetic Devices/In Vivo Biofilm
Evidence has been presented based upon in vitro, animal models and clinical studies that Candida organisms found in biofilm may show significant reduced susceptibility to azole drugs [116]. The implications are self-evident, since infections involving intravascular catheters and prosthetic valves and devices invariably fail intensive antifungal therapy and require surgical removal for cure. Clinical failure may also be due to failure of the antifungal drug to penetrate the biofilm access of yeast cells found within the biofilm [117]. The most important explanation for biofilm-related resistance appears to be the phenotypic and genotypic changes that are reported in biofilm containing yeast cells demonstrating in vitro antifungal resistance when compared to planktonic isotype cells. Nett et al. reported increased β-1,3-glucan content in C. albicans cell walls from biofilm compared to planktonic organisms thought to be responsible for polyene resistance and fluconazole resulting in limited intracellular penetration [118]. Biofilm-associated yeast cells are more susceptible to β-glucan inhibitors, i.e., echinocandins [119].
6.4 Antifungal Drugs
While most of the information available on drug-induced resistance followed the use of fluconazole and ketoconazole, usually as oral agents, little is known about the potential for broader-spectrum (itraconazole, voriconazole, posaconazole, caspofungin, micafungin, anidulafungin) or more active/potent in vitro drugs (voriconazole, posaconazole, echinocandins) or Candidacidal drugs (echinocandin) to select for less susceptible C. albicans or non-albicans Candida isolates.
Invasive infections due to amphotericin B-resistant Candida isolates have infrequently been reported in association with the use of this agent [58, 77, 120]. Many C. lusitaniae and some C. guilliermondii isolates demonstrated primary resistance to amphotericin B, but secondary resistance to amphotericin B appears to be uncommon. Acquired resistance associated with disseminated infections due to C. glabrata, C. krusei, and C. albicans that developed during therapy is described but is uncommon [121]. Resistance appears to be due to alteration or a decrease in the amount of ergosterol in the cell membrane. Yoon demonstrated in vitro reversible switching of C. lusitaniae with acquired amphotericin B resistance [122]. Nystatin-resistant C. rugosa was reported a burn unit following extended use of prophylactic topical nystatin [123].
A growing mass of data indicates that frequent and prolonged exposure to azole may influence the emergence of non-albicans Candida species especially C. glabrata but may also select for acquired resistance in C. albicans strains particularly following prolonged exposure to subinhibitory azole concentrations [32, 100, 111, 115]. However, the overall effect of azoles on Candida species distribution and resistance development is incompletely understood [124, 125]. Blott et al. reported that over an 11-year period in a single institution, the volume of fluconazole consumption did not correlate with Candida sp. distribution [124].
6.5 Candida Vaginitis
In spite of widespread use and abuse of over-the-counter (OTC) imidazole antifungals, little evidence has emerged of azole resistance in C. albicans or selection of non-albicans Candida spp. [126, 127]. However, prolonged use of long-term, low-dose (150 mg/week) fluconazole maintenance prophylaxis, in women with recurrent vulvovaginal candidiasis (RVVC), has recently been reported to contribute to both fluconazole and azole class resistance resulting in refractory vaginitis caused by in vitro resistant C. albicans [128, 129]. Moreover, in a study of HIV-positive women with RVVC receiving fluconazole, some evidence did surface of emergence of C. glabrata as a more frequent pathogen [130, 131].
6.6 Azole Cross-Resistance
Given that the azole class of antifungal agents share a common mechanism of action and in most cases of resistance, development of cross-resistance is common.
When selecting antifungal treatment, it is essential to establish whether the patient has received previous antifungal therapy because patients may harbor Candida species resistant to multiple azole agents [132–134]. Both in vitro and clinical studies have clearly demonstrated high frequency of azole cross-resistance [135]. Several studies indicated cross-resistance to itraconazole, ketoconazole, and other imidazoles in isolates resistant to fluconazole [32, 136]. Most of the strains concerned were fluconazole-resistant isolates of C. albicans obtained from patients with advanced AIDS and refractory OPC [137, 138], but others have reported cross-resistance in virtually all species of Candida exposed to non-fluconazole azoles, e.g., itraconazole and ketoconazole [132, 133, 139, 140]. Moreover, resistance found to first- and second-generation azoles may extend, even in the absence of exposure, to newer triazoles, voriconazole and posaconazole, either as absolute resistance or more frequently as higher MIC values [136, 141–144]. In general, fluconazole-resistant strains had higher MICs to voriconazole and posaconazole. Nevertheless, cross-resistance varies considerably among species; hence, some but not all C. parapsilosis and C. albicans isolates maintain susceptibility to itraconazole, posaconazole, and voriconazole despite fluconazole resistance. Cross-resistance is often more predictable for C. tropicalis isolates, and lack of cross-resistance is seen with C. krusei. The development of resistance to azoles invariably requires more than one mutation; hence, isolates with resistance to both fluconazole and itraconazole exhibit multiple mechanisms or types of resistance and therefore are more likely to demonstrate resistance or reduced susceptibility to newer azole agents. Cross-resistance is a very common if not universal feature in azole-resistant C. glabrata isolates, especially in those that are capable of expressing multiple mechanisms of resistance [145, 146].
Susceptibility testing of 6970 Candida isolates from 200 centers worldwide by Pfaller et al. revealed that C. albicans and C. glabrata strains resistant to both fluconazole and itraconazole were less susceptible to posaconazole, ravuconazole, and voriconazole [60]. Slightly less than 50 % of Candida species isolates resistant to fluconazole maintained susceptibility to newer triazole agents [147]. In a study of azole cross-resistance, fluconazole MICs of ≤32 μg/mL predicted susceptibility, and MICs of ≥64 μg/mL predicted resistance of Candida spp. to voriconazole and posaconazole [147]. Voriconazole was active against C. krusei regardless of azole susceptibility. While much has been written of fluconazole prophylaxis leading to widespread azole resistance, similarly itraconazole prophylaxis was shown to be associated with cross-resistance to fluconazole [133, 148]. While much of the literature on azole cross-resistance has focused on mucosal candidiasis, similarly, large surveillance surveys of Candida spp. causing invasive infection including candidemia have shown evidence of cross-resistance.
6.7 Drug Pharmacokinetics, Pharmacodynamics, and Resistance in Candidiasis
Andes et al. reported the impact of fluconazole dosing regimens and pharmacodynamics on resistance development in C. albicans [149, 150]. Fluconazole regimens that produced prolonged sub-MIC concentrations were associated with resistance development. The emergence of the resistant phenotype was associated with increased expression of CDR1- and CDR2-encoded efflux pumps but not MDR1-encoded pumps or ERG II [149, 150]. In a murine systemic candidiasis model, the more frequently administered dosing regimens prevented the emergence of a resistant cell phenotype.
A correlation between in vitro susceptibility and response to therapy of non-mucosal candidiasis has been demonstrated in some studies [151, 152] but not others. Clancy et al. in 2003 evaluated 32 bloodstream Candida isolates and concluded that geometric mean MIC and fluconazole dose/MIC ratio predicted clinical failure [153]. Inadequate dosing of fluconazole (≤200 mg/day) and ratio <50 correlated with therapeutic failure, but not necessarily with resistance development.
6.8 Echinocandin Resistance
Candida sp. isolates resistant to echinocandins were first reported in 2005 [88], but reports of resistance were rare, at <2–3 % with C. albicans and most Candida species [43, 62, 154, 155]. However with time, reports of clinical failure with isolates demonstrating high MIC were increasingly but still not frequently seen [92, 156–165]. Overall, echinocandin resistance among most Candida species has been largely unchanged in the past few years [32]. However, this does not apply to C. glabrata, where echinocandin resistance is increasing and there is justifiable concern especially since many isolates also demonstrate azole resistance [166–168]. The SENTRY Antimicrobial Surveillance Program revealed that 8.0–9.3 % of blood isolates of C. glabrata from 2006 to 2010 were echinocandin drug resistant [154]. Of concern, Alexander et al. reported an increase in echinocandin-resistant C. glabrata bloodstream isolates, in Duke Hospital from 2–3 % in 2001–2006 to more than 13 % in 2009–2010 [166]. This is not widespread throughout the USA in that one recent study showed 3.1–5.7 % resistance in Candida isolates [62, 168]. Nevertheless, echinocandin resistance was similarly linked to azole resistance in C. glabrata. In this large Pham study, nearly all isolates containing an FKS mutation were resistant to at least one echinocandin, and 36 % were also resistant to fluconazole [168].
6.8.1 Mechanism of Acquired Echinocandin Resistance
Echinocandin resistance results from modification of glucan synthase, which is encoded by genes FKS1 and FKS2. Unlike azole drugs, echinocandins are not substitutes for multidrug transporters [169]. Echinocandin resistance is nevertheless well characterized, conferred by restricted mutations in two highly conserved “hot spot” regions of the FKS genes [167]. The FKS mutations result in amino acid mutations that induce MIC values from 20- to 100-fold and reduced sensitivity of glucan synthase to drug by 50–30,000-fold [170]. These less susceptible fks mutant strains respond poorly to echinocandin drugs in pharmacodynamic models of infection [171, 172] and are associated with reduced clinical response [173, 174]. The FKS resistance mechanisms have been observed in many Candida species [175]. In all Candida species, except C. glabrata, mutations occur within two “hot spot” regions of FKS1 [55] (see Chapter 29, Volume 1). In C. glabrata mutations occur in the homologous hot spot regions of FKS1 and FKS2 [55, 155, 170].
The echinocandin drugs are highly serum protein bound potentially reducing susceptibility testing. Serum is considered to reduce the fungicidal characters of the echinocandins, resulting in fungistatic activity against certain Candida species [175].
Biofilms also play a role in antifungal resistance [176]. Decreasing glucan production, accompanying echinocandin use increases susceptibility of yeast organisms contained within the biofilm to the effects of these drugs [177].
7 Refractory Candidiasis: Clinical Resistance Syndromes and Their Management
7.1 Oropharyngeal and Esophageal Candidiasis
Refractory OPC and EC represent the commonest manifestation of clinical azole resistance and failure that is supported by concomitant in vitro azole resistance. Most patients present with highly symptomatic episodes with oropharyngeal pain and debilitating dysphagia and odynophagia requiring hospitalization. The majority of patients with refractory upper gastrointestinal candidiasis have AIDS and advanced immunodeficiency. In the 1990s, the annual incidence of clinical failure of fluconazole in OPC was approximately 5 % [104–107]. Accordingly, refractory superficial candidiasis peaked and became a major clinical problem during the decades of the 1990s prior to the availability of highly active antiretroviral therapy (HAART) [110, 178, 179]. The majority of these patients have refractory disease caused by C. albicans [180]. Only a minority have non-albicans Candida spp. usually C. glabrata, strains of which are usually resistant in vitro to fluconazole. Resistant strains of C. albicans and C. glabrata are frequently, but not invariably, cross-resistant to itraconazole and ketoconazole [181]. Refractory mucosal candidiasis has also been reportedly associated with C. tropicalis and C. krusei [5]. In the absence of coinfection with non-albicans Candida species, refractory candidiasis is seen with both in vitro resistant and sensitive C. albicans. The reason for treatment failure caused by azole-sensitive C. albicans is usually the result of noncompliance with ART therapy, drug underdosing, or drug interactions. Another major factor is simply advanced immunodeficiency. With refractory esophagitis, it is important to exclude concomitant pathology such as CMV or HSV esophagitis. Other explanations for the in vitro-in vivo discrepancy in compliant patients relate to heteroresistance in individual colonies of Candida, with chance selection of a “susceptible” colony. Most patients with refractory OPC and EC almost always have usual Candida spp. isolates with in vitro resistance.
Finally, some experts have questioned the virulence capacity of non-albicans Candida species to induce OPC and EC, let alone refractory disease [5, 182]. It is true that refractory candidiasis in patients with AIDS, from whom NAC strains are isolated, usually represents mixed infections with coexistent C. albicans; however, resistant disease due to C. glabrata in the absence of C. albicans is now widely accepted.
The availability of HAART was rapidly followed by a marked decline in the frequency of refractory OPC and EC [183]. It was assumed that enhanced mucosal immune function was responsible for this phenomenon. However, this issue is more complex in that refractory disease resolved within days and weeks of initiation of HAART, preceding demonstrable improvement or change in CD4 lymphocyte cell count or any other marker of CMI, suggesting that some other beneficial effects might be responsible [184]. Another observation included the disappearance of azole-resistant strains of C. albicans and C. glabrata with the reappearance of azole-sensitive strains. How was improved mucosal CMI selecting susceptible strains of Candida? Another more recent hypothesis relates to a direct effect of HIV structural components in directly influencing genes carried by Candida responsible for virulence expression including development of azole resistance. Accordingly, HIV gp 160 and gp 41 may influence Candida in vitro, selecting for azole resistance [185]. According to this hypothesis, the mucosal viral load (HIV RNA) would enhance Candida virulence in situ and finally induce or select for azole resistance. Introduction of HAART and rapid decrease in viral load, before immune recovery, would explain early resolution of refractory mucosal candidiasis and reemergence of azole-susceptible strains. Therapeutic protease inhibitors may further reduce Candida virulence by inhibiting fungal secretory aspartyl proteinases [186].
It follows that in the post-HAART era, the frequency of refractory disease as well as in vitro azole resistance declined substantially. The majority of patients with chronic and refractory disease are usually noncompliant AIDS patients infected with susceptible C. albicans. In a study of in vitro susceptibility of oral isolates in the HAART era, Tacconelli showed a reduction in azole resistance from 37 to 7 % [187]. The explanation for the reduced or diminished at-risk population is thought to relate to reduced fluconazole exposure, i.e., fewer low-dose regimens and less continuous long-term therapy; however, this hypothesis is unproven. Barchiesi et al. reported that most patients on HAART are colonized by strains of C. albicans susceptible to fluconazole (93 % sensitive) [188]. Most cases of OPC in the HAART era are caused by fluconazole-sensitive C. albicans.
A high prevalence of non-albicans Candida species (C. albicans 49 %, C. glabrata 24 %) with frequent resistance to fluconazole and itraconazole has also been reported in patients with advanced cancer, especially head and neck malignancy [189, 190]. Another small but critically important patient population includes patients with the various genetic forms of chronic mucocutaneous candidiasis such as autoimmune polyendocrinopathy-candidiasis-ectodermal dystrophy (APECED) patients [191]. Frequent decreased susceptibility of C. albicans to fluconazole is a common complication of prolonged fluconazole use in this population.
Clinical management of refractory OPC requires evaluation and determination of etiological mechanisms responsible for clinical resistance, including CD4 count, compliance with HAART therapy, previous OPC, and exposure to azoles, usually fluconazole [192]. Finally, clinical resistance implies failure to respond despite adequate delivery of a tolerable therapeutic concentration of the drug. Once in vitro resistance is suspected, cultures are obtained and susceptibility determined of the responsible organisms. Most commonly, C. albicans is present, sometimes together with a second species usually C. glabrata. While awaiting microbiology and susceptibility results, treatment is initiated. Therapeutic strategies are listed in Box 66.3. Initial options include progressive increasing doses of oral fluconazole from 100 to 400 mg/day, including fluconazole suspension [193] or swish-and-swallow amphotericin B suspension (100 mg/mL, taken as 1 mL qid) [194]. Although cross-resistance with other triazoles is common, in the event of retained itraconazole sensitivity, itraconazole suspension (10 mg/mL, taken as 10 mL bid) is often effective, although usually on a temporary basis only [195]. However, the most important advance in therapy of fluconazole-refractory OPC is oral posaconazole. Although initially available only as an oral suspension, it is now prescribed as posaconazole tablet 400 mg bid for 14 days. Given its safety profile, posaconazole is used preferentially to oral voriconazole.
Box 66.3. Therapy of Fluconazole-Refractory Oropharyngeal (OPC) and Esophageal Candidiasis (EC)
OPC
-
High doses of fluconazole tablets
-
Fluconazole suspension
-
Itraconazole capsules/suspension
-
Amphotericin B oral suspension
-
IV amphotericin B/lipid formulation
-
Posaconazole oral/IV
-
Voriconazole oral/IV
-
IV echinocandin
-
Immunomodulation
-
G-CSF
-
GM-CSF
-
α-Interferon
-
EC
-
IV echinocandin
-
Fluconazole
-
IV lipid formulation of amphotericin B
-
IV voriconazole*
*If susceptible in vitro
Parenteral antifungals have become the last resort employing intravenous amphotericin B, echinocandin, or voriconazole [196]. All these options may successfully control and eradicate acute symptomatic infection; however, unless immune reconstitution follows, relapse is inevitable. Potentially, the aforementioned parenteral antifungals could be given on an intermittent maintenance basis; however, maintenance suppressive therapy with oral posaconazole 400 mg per day is effective [197].
While HAART therapy offers a definite solution in AIDS patients, the same cannot be said from CMC patients with progressive azole resistance starting with fluconazole and extending sequentially to itraconazole and then voriconazole with either C. albicans or C. glabrata. Intermittent parenteral echinocandins or lipid formulation of amphotericin B will be necessary, although the use of oral posaconazole is preferred [198].
7.2 Refractory Esophageal Candidiasis (Box 66.3)
As for refractory OPC, clinically resistant EC is mainly seen in untreated AIDS patients with advanced immunodeficiency, with a history of sporadic previous treatment with fluconazole. Refractory, especially chronic, EC is associated with a profound impact on general health leading to weight loss, malnutrition, and overall reduced general health status. Oral cultures usually reveal the Candida species responsible for esophageal disease, recognizing that more than one resistant species may coexist. Most cases of fluconazole-resistant EC are similarly resistant to itraconazole [199]. In a minority of patients still capable of swallowing, oral posaconazole is still a therapeutic possibility. If swallowing is not possible, therapeutic options now include amphotericin B deoxycholate or lipid formulations used parenterally in hospitalized patients, and while widely recognized as efficacious, there are little published data documenting efficacy. Cost with the use of lipid formulations and toxicity associated with conventional AmB remain issues. Regardless of which formulation is chosen, low-dose regimens frequently fail in patients with azole-resistant C. albicans and/or C. glabrata. Response to IV therapy is frequently slow, and >0.8 mg/kg AmB or 5 mg/kg of lipid AmB should be used.
Fortunately, the drugs of choice are IV echinocandins. Studies confirm similar efficacy with daily IV caspofungins, anidulafungin, and micafungin. Accordingly, caspofungin was found to have ~70 % efficacy rate in treating patients with fluconazole-refractory EC [6, 198, 200–202]. No cross-resistance exists between azoles and echinocandins. Similar efficacy for EC has been observed with parenteral voriconazole, also achieving ~70 % response rates but with little experience published with fluconazole-resistant species [203]. Table 66.1 shows the impact of fluconazole-resistant C. albicans on susceptibility to voriconazole; hence, higher doses of voriconazole may well be indicated [87, 144]. The recent availability of parenteral posaconazole increases therapeutic options, and oral posaconazole is recommended as de-escalation therapy to complete parenteral echinocandin treatment.
Regardless of the parenteral regimen selected, the dominant issue remains the maintenance antifungal prophylaxis in these severely immunocompromised individuals. It cannot be emphasized sufficiently that the key to preventing further recurrences or inevitable relapses of refractory EC lies with successful initiation of HAART therapy. Noteworthy several studies indicated that relapse rates of EC are higher following initially successful echinocandin treatment [204]. Until HAART therapy reverses susceptibility, maintenance prophylaxis is best afforded with oral posaconazole.
7.3 Refractory Candida Vaginitis (VVC)
Two forms of vulvovaginal candidiasis (VVC) exist. In the first place, an individual episode of symptomatic vaginitis may not respond to conventional topical or oral antifungal therapy. The other form of refractory disease is found in a larger population of women with frequently recurring episodes of relapsing symptomatic vaginitis although each individual episode of VVC responds to conventional therapy (RVVC).
Failure to achieve clinical improvement and symptom resolution, i.e., azole-resistant vaginal C. albicans, is still uncommon but has increasingly been reported in both HIV-positive and HIV-negative women [205]. It is actually remarkable that resistance is not more frequent given the widespread use of low-dosage fluconazole as single-dose therapy or once-weekly maintenance prophylaxis for RVVC. Nevertheless, any patient with acute Candida vaginitis, failing to improve with a standard regimen of oral or topical azoles, with persistent symptoms, positive microscopy, and culture, should be treated with topical vaginal boric acid 600 mg daily for 14 days. At the same time, the C. albicans isolate should be sent for azole susceptibility testing. The same cannot be said for acute C. glabrata vaginitis which responds to azole agents with a 50 % rate only [206]. Acute C. glabrata vaginitis should be treated with topical boric acid 600 mg suppositories daily for 11–21 days with an anticipated clinical and mycological response rate of ~70 % [179]. Higher cure rates (>90 %) can be obtained with topical 17 % flucytosine intravaginal cream, 5 g nightly for 14 days, although the cream must be compounded and is not widely available and hence is expensive [206, 207]. High cure rates also follow daily intravaginal amphotericin B 50 mg suppository for 14 days or in combination with topical flucytosine [208].
Acute vaginitis due to C. krusei, although rare, will not surprisingly fail to respond to oral fluconazole, due to innate or primary resistance [209]. Occasionally, patients may respond to oral itraconazole or topical miconazole or clotrimazole prescribed for 14 days. C. krusei is also resistant to flucytosine, and hence vaginitis due to this species is often extremely difficult to control.
It should be emphasized that refractory acute vaginitis is extremely rare, although busy practitioners might not agree. This is because of incorrect diagnosis on the part of practitioners who treat vaginitis on an empiric basis, invariably failing to measure vaginal pH, perform microscopy, and obtain a vaginal culture. Several studies have confirmed the poor diagnostic acumen of practitioners. Self-diagnosis by women is no better. Other species of Candida can cause vaginitis, but tend to rapidly respond to azole therapy.
Much more common and affecting millions of women, in their childbearing decades worldwide, is recurrent vulvovaginal candidiasis (RVVC) thought to affect 6–8 million women in the USA. Under these circumstances recurring episodes of vaginitis respond appropriately to antifungal therapy regardless of route, only for symptoms and signs to recur within a month or two but rarely monthly [210]. RVVC is mostly caused by azole-sensitive C. albicans (>90 %) and less commonly by C. glabrata (5 %). RVVC is rarely a manifestation of drug resistance but of host factors that predispose to genital tract yeast colonization and host immune response hyperreactivity to Candida antigens [210]. RVVC is best controlled by once-weekly fluconazole maintenance prophylaxis administered for 6 or more months [128], although other forms of suppressive azole therapy are effective but less convenient [211, 212]. Boric acid has also been used effectively [213].
The management of azole-refractory vaginitis due to in vitro confirmed fluconazole-resistant C. albicans is initially managed with daily vaginal boric acid for 2 weeks, while in vitro susceptibility tests become available. Acute, nonrecurrent vaginitis may require no additional therapy; however, women suffering from RVVC will of necessity require a maintenance antifungal regimen. Possible alternatives to weekly fluconazole are daily ketoconazole or itraconazole 100–200 mg, provided that susceptibility is confirmed in vitro. As per standard protocols, the maintenance daily regimens are continued for at least 6 months. In the event of frequently reported azole cross-resistance, no oral azoles are likely reasonable safe alternative agents. In this scenario, long-term maintenance therapy can be achieved with topical boric acid or nystatin for the same long-term duration, but little published data are available. Similarly, daily combination therapy with boric acid and nystatin is effective for symptomatic recurrent VVC due to C. glabrata although such cases are rare.
7.4 Refractory Candidemia and Disseminated Candidiasis
The incidence of bloodstream infections (BSIs) due to Candida spp. has increased worldwide, with accompanying significant mortality. Fortunately, in parallel with this increase has been an increase in the therapeutic armamentarium for candidemia (Box 66.4). The purpose of this chapter is not to review management of candidemia (see reviews [96, 114, 214]). Drug resistance is monitored by a variety of study organizations in multiple countries. Perhaps the most comprehensive antifungal susceptibility monitoring organization is the SENTRY system receiving in excess of 2000 bloodstream Candida isolates annually from all over the world [63]. Compiled data are shown in Table 66.3. Nevertheless, given the proportional and occasionally found absolute increase in cases of invasive candidiasis and candidemia due to non-albicans Candida species especially C. glabrata, together with the availability of safe and in the past predictable effective echinocandins, guidelines from national and international infectious disease societies have recently been issued which acknowledge the reduced azole susceptibility of non-albicans Candida species. Hence, until information of the identity of the Candida species responsible for the bloodstream infection is available, echinocandins are considered drugs of first choice to be prescribed [96].
Box 66.4. First-Line Antifungal Drug Therapy of Candidemia (Parenteral)
-
1.
Amphotericin B (conventional deoxycholate)
-
2.
Lipid formulation AmB
-
3.
Fluconazole (400 mg/day)
-
4.
Fluconazole (800 mg/day)
-
5.
Itraconazole
-
6.
Voriconazole
-
7.
Caspofungin
-
8.
Amphotericin B + flucytosine
-
9.
Amphotericin B + fluconazole
7.4.1 C. albicans
Despite the widespread use of fluconazole over the last 15 years, fluconazole resistance in C. albicans blood isolates remains below 5 %, with no evidence of a progressive increased resistance with time or associated with a specific geographic area [60]. It is not fear of an azole-resistant strain of C. albicans that drives principles of antifungal drug selection. Candidemia due to drug-resistant C. albicans is rare, but has been rarely reported in patients with hematologic malignancy [100]. However, C. albicans is no longer the most prevalent Candida species responsible for BSI, and rarely is drug resistance a management issue. Should an azole-resistant C. albicans isolate be responsible for the candidemia, the clinical manifestations include persistent candidemia on fluconazole therapy, relapsing candidemia or possibly increased mortality, and finally breakthrough candidemia. In the last decade, results of at least five randomized prospective controlled studies have been published involving fluconazole and other antifungal drugs [215–220]. Attempts have been made to correlate clinical outcome with in vitro MICs. In none of these studies has C. albicans antifungal resistance, specifically fluconazole resistance emerged as a cause of drug failure [216, 217]. The lack of fluconazole resistance in C. albicans BSI isolates after all these years remains reassuring, but the altered epidemiology is less so. In contrast to other studies, correlation between in vitro susceptibility and response to fluconazole therapy has been demonstrated, but rarely is persistent fungemia due to azole-resistant C. albicans but rather non-albicans Candida species [221].
7.4.2 C. glabrata
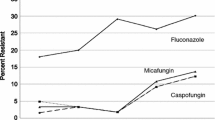
As evident in Table 66.2, candidemia due to C. glabrata has increased especially in North America and Europe. Fluconazole resistance in bloodstream C. glabrata isolates is evident in 7–10 % of strains, with an addition of 27–30 % of isolates considered S-DD indicating reduced fluconazole susceptibility of C. glabrata isolates. Accordingly, only 50–70 % of C. glabrata bloodstream isolates are highly susceptible to fluconazole. Several studies involving C. glabrata have shown a similar susceptibility pattern [63, 64]. Documented failure or suboptimal response to fluconazole and other antifungals has been forthcoming in some studies and is impressively present in others [221]. When failure was always apparent, this may simply reflect small numbers of patients with C. glabrata fungemia, i.e., some published studies have lacked the power to show any differences in outcome by Candida species.
Supporting the in vitro data are numerous case reports of fluconazole failure to eradicate C. glabrata fungemia subsequently responsive to parenteral polyene or echinocandin therapy as well as retrospective analysis of patients with persistent candidemia [151, 221]. Accordingly, most experts would recommend avoiding any azoles, including voriconazole, initially in patients with candidemia caused by C. glabrata and initiate therapy with an echinocandin. Until the Candida isolate (species) is identified and species identity is becoming more and more rapidly established, then given the increased likelihood of C. glabrata and other reduced fluconazole susceptibilities, selection should include the possibility and commence with an echinocandin. In candidemia patients doing well on azoles, continued therapy with the azole would be perfectly reasonable.
8 Adjuvant Therapy for Resistant Candidiasis
The use of immune and nonimmune adjuvants to treat refractory candidiasis is almost exclusively seen in patients with AIDS or chronic mucocutaneous candidiasis (CMC). Even with the latest generation of azoles (voriconazole, posaconazole) and polyene and echinocandin use, refractory mucosal disease is still reported due to resistant C. albicans, C. glabrata, and rarely other Candida species. There have been anecdotal successes reported with immunostimulators mainly recombinant human granulocyte-macrophage colony-stimulating factor (rhu GM-CSF) [222, 223]. Also, interferon gamma has occasionally been given [196]. Unfortunately, investigators tend to publish only successful therapeutic endeavors and failures are more frequent [224, 225]. Even so, long-term use and success of these growth factors have not been forthcoming especially associated with CMC. The use of these agents, given these expenses, requires the performance of randomized controlled studies which are unlikely given the current infrequency of these refractory cases. The value of GM-CSF in invasive candidiasis has not been demonstrated but may have a role in persistently neutropenic patients. Monoclonal antibodies were shown to prevent disseminated candidiasis in a mouse model and have been the bases for vaccine development. Likewise, the administration of anti-Candida heat shock antibodies may have an adjuvant role together with antifungals for resistant or refractory candidemia.
9 Prevention of Antifungal Resistance in Candida Species
In general, standard principles of infection control that apply to all microorganisms and particularly nosocomial infections should be applied to prevent antifungal resistance.
Avoidance of prophylactic or suppressive therapy and a preference for repeated short course of azoles for OPC in the late stages of AIDS are an attractive but unproved measure for delaying the appearance of azole resistance. In a study conducted in patients with recurrent OPC and AIDS, episodic fluconazole therapy was compared to continuous fluconazole therapy aimed at evaluating likelihood of inducing fluconazole resistance and refractory oropharyngeal candidiasis [98]. The study failed to show a difference in the two arms with regard to selection or induction of azole resistance. This somewhat disappointing result may reflect the fact that the study was conducted during the HAART era with relatively few individuals presenting with refractory mucosal disease, with advanced immunodeficiency and unavailability of HAART therapy. The study outcome is in sharp contrast to clinical experience obtained in the pre-HAART era.
It goes without saying that all unnecessary use of azoles should be avoided, whether as prophylaxis or therapy. Many clinicians prescribe a lower than recommended prophylactic dose of oral fluconazole in neutropenic patients, i.e., 100 mg vs. 400 mg daily. To date, no evidence has emerged of increased fluconazole resistance as a specific consequence of this reduced daily dose. Nevertheless, many experts advise against the use of azole prophylaxis in neutropenia of short duration. Paterson suggested that combining oral amphotericin B with azoles may prevent the emergence of resistant Candida species in neutropenic patients; however, oral amphotericin B is poorly tolerated and noncompliance is common [226].
Studies have indicated that most Candida species are carried and readily transferred manually by nursing physicians and other medical personnel [227]. Accordingly, adherence to strict handwashing principles applies equally to Candida and specifically the transfer of resistant strains of C. albicans and other Candida species [228, 229]. In particular, C. parapsilosis is frequently isolated from the hands in contrast to C. glabrata which appears to be endogenously acquired from GIT carriage only. Isolation of patients with resistant strains of Candida is not indicated in this era of universal precautions. Perhaps, the most controversial is the use of antifungal prophylaxis in selected high-risk patients in intensive care units. Several studies suggest limited benefit and only in selected high-risk ICU patients [230, 231, 232].
10 Conclusion and Perspective
During the last two decades, enormous strides have been made in understanding the subcellular, molecular, and genetic basis of antifungal resistance. All in all, clinically refractory candidiasis is uncommon. The explosion in clinically resistant cases of OPC and EC early in the AIDS epidemic has not stood the test of time with the arrival of antiretroviral therapy. Of course, clinically resistant cases still occur and remain a therapeutic challenge, but the majority of cases of mucosal disease are caused by azole-sensitive Candida albicans. There has been an increase in non-albicans Candida species causing invasive candidiasis. Much, but not all, evidence points to widespread prophylactic, empirical, and therapeutic use of fluconazole. Nevertheless, blood isolates of C. albicans remain remarkably and predictably susceptible to fluconazole and other azoles, and this is a worldwide experience. There is no doubt that certain Candida species are less susceptible and/or resistant to fluconazole and show cross-resistance to all azoles. This species-specific (C. krusei, C. glabrata) azole resistance has a major influence in antifungal drug selection. These two species not only expose vulnerability of the azole class but require higher doses of polyenes. As such, fungal susceptibility tests in the past were rarely available and infrequently and selectively used. This however has changed with increased availability. The newer generations of azoles are often active against non-albicans Candida species (C. glabrata and C. krusei) and as such offer early confident broad-spectrum therapy. Moreover, they are frequently active against fluconazole-resistant C. albicans. The echinocandins have further eased the concern of azole resistance in candidiasis, but time has yet to determine the potential for echinocandin-acquired resistance in candidiasis.
References
Edmond MB, Wallace SE, McClish DK, Pfaller MA, Jones RN, Wenzel RP. Nosocomial bloodstream infections in United States hospitals: a three-year analysis. Clin Infect Dis. 1999;29(2):239–44.
Trick WE, Fridkin SK, Edwards JR, Hajjeh RA, Gaynes RP. Secular trend of hospital-acquired candidemia among intensive care unit patients in the United States during 1989–1999. Clin Infect Dis. 2002;35(5):627–30.
Jarvis WR. Epidemiology of nosocomial fungal infections, with emphasis on Candida species. Clin Infect Dis. 1995;20(6):1526–30.
Kao AS, Brandt ME, Pruitt WR, Conn LA, Perkins BA, Stephens DS, et al. The epidemiology of candidemia in two United States cities: results of a population-based active surveillance. Clin Infect Dis. 1999;29(5):1164–70.
Baily GG, Perry FM, Denning DW, Mandal BK. Fluconazole-resistant candidosis in an HIV cohort. AIDS. 1994;8(6):787–92.
Kontoyiannis DP, Lewis RE. Antifungal drug resistance of pathogenic fungi. Lancet. 2002;359(9312):1135–44.
Perea S, Patterson TF. Antifungal resistance in pathogenic fungi. Clin Infect Dis. 2002;35(9):1073–80.
Yeo E, Alvarado T, Fainstein V, Bodey GP. Prophylaxis of oropharyngeal candidiasis with clotrimazole. J Clin Oncol. 1985;3(12):1668–71.
Klein RS, Harris CA, Small CB, Moll B, Lesser M, Friedland GH. Oral candidiasis in high-risk patients as the initial manifestation of the acquired immunodeficiency syndrome. N Engl J Med. 1984;311(6):354–8.
Coleman DC, Bennett DE, Sullivan DJ, Gallagher PJ, Henman MC, Shanley DB, et al. Oral Candida in HIV infection and AIDS: new perspectives/new approaches. Crit Rev Microbiol. 1993;19(2):61–82.
Hay RJ, Clayton YM. Fluconazole in the management of patients with chronic mucocutaneous candidosis. Br J Dermatol. 1988;119(5):683–4.
Horsburgh Jr CR, Kirkpatrick CH. Long-term therapy of chronic mucocutaneous candidiasis with ketoconazole: experience with twenty-one patients. Am J Med. 1983;74(1B):23–9.
Sobel JD, Faro S, Force RW, Foxman B, Ledger WJ, Nyirjesy PR, et al. Vulvovaginal candidiasis: epidemiologic, diagnostic, and therapeutic considerations. Am J Obstet Gynecol. 1998;178(2):203–11.
Fridkin SK, Jarvis WR. Epidemiology of nosocomial fungal infections. Clin Microbiol Rev. 1996;9(4):499–511.
Hachem R, Hanna H, Kontoyiannis D, Jiang Y, Raad I. The changing epidemiology of invasive candidiasis: Candida glabrata and Candida krusei as the leading causes of candidemia in hematologic malignancy. Cancer. 2008;112:2493–9.
Diekema D, Arbefeville S, Boyken L, Kroeger J, Pfaller M. The changing epidemiology of healthcare—associated candidemia over three decades. Diagn Microbiol Infect Dis. 2012;73:45–8.
Pfaller M, Neofytos D, Diekema D, Azie N, et al. Epidemiology and outcomes of candidemia in 3648 patients: data from the Prospective Antifungal Therapy (Path Alliance) registry, 2004–2008. Diagn Microbiol Infect Dis. 2012;74:323–31.
Fisher-Hoch SP, Hutwagner L. Opportunistic candidiasis: an epidemic of the 1980s. Clin Infect Dis. 1995;21(4):897–904.
Rangel-Frausto MS, Wiblin T, Blumberg HM, Saiman L, Patterson J, Rinaldi M, et al. National epidemiology of mycoses survey (NEMIS): variations in rates of bloodstream infections due to Candida species in seven surgical intensive care units and six neonatal intensive care units. Clin Infect Dis. 1999;29(2):253–8.
Wenzel RP, Edmond MB. Severe sepsis—national estimates. Crit Care Med. 2001;29(7):1472–4.
Blumberg HM, Jarvis WR, Soucie JM, Edwards JE, Patterson JE, Pfaller MA, et al. Risk factors for candidal bloodstream infections in surgical intensive care unit patients: the NEMIS prospective multicenter study. The National Epidemiology of Mycosis Survey. Clin Infect Dis. 2001;33(2):177–86.
Bross J, Talbot GH, Maislin G, Hurwitz S, Strom BL. Risk factors for nosocomial candidemia: a case-control study in adults without leukemia. Am J Med. 1989;87(6):614–20.
Pappas PG, Rex JH, Lee J, Hamill RJ, Larsen RA, Powderly W, et al. A prospective observational study of candidemia: epidemiology, therapy, and influences on mortality in hospitalized adult and pediatric patients. Clin Infect Dis. 2003;37(5):634–43.
Gudlaugsson O, Gillespie S, Lee K, Vande Berg J, Hu J, Messer S, et al. Attributable mortality of nosocomial candidemia, revisited. Clin Infect Dis. 2003;37(9):1172–7.
Wey SB, Mori M, Pfaller MA, Woolson RF, Wenzel RP. Hospital-acquired candidemia. The attributable mortality and excess length of stay. Arch Intern Med. 1988;148(12):2642–5.
Girmenia C, Martino P, De Bernardis F, Gentile G, Boccanera M, Monaco M, et al. Rising incidence of Candida parapsilosis fungemia in patients with hematologic malignancies: clinical aspects, predisposing factors, and differential pathogenicity of the causative strains. Clin Infect Dis. 1996;23(3):506–14.
Gumbo T, Isada CM, Hall G, Karafa MT, Gordon SM. Candida glabrata Fungemia. Clinical features of 139 patients. Medicine (Baltimore). 1999;78(4):220–7.
Merz WG, Karp JE, Schron D, Saral R. Increased incidence of fungemia caused by Candida krusei. J Clin Microbiol. 1986;24(4):581–4.
Nucci M, Queiroz-Telles F, Tobon AM, Restepo A, Colombo AL. Epidemiology of opportunistic fungal infections in Latin America. Clin Infect Dis. 2010;51:561–70.
Shah DN, Yau R, Lasco TM, Weston J, et al. Impact of prior inappropriate fluconazole dosing on isolation of fluconazole-nonsusceptible Candida species in hospitalized patients with candidemia. Antimicrob Agents Chemother. 2012;56:3239–43.
Gamacho-Montero J, Diaz-Martin A, Garcia-Cabrera E, et al. Risk factors for fluconazole-resistant candidemia. Antimicob Agents Chemother. 2010;54:3149–54.
Pfaller MA. Antifungal drug resistance: mechanisms, epidemiology and consequences for treatment. Am J Med. 2012;125:S3–13.
Ghannoum MA, Rice LB. Antifungal agents: mode of action, mechanisms of resistance, and correlation of these mechanisms with bacterial resistance. Clin Microbiol Rev. 1999;12(4):501–17.
Masia Canuto M, Gutierrez Rodero F. Antifungal drug resistance to azoles and polyenes. Lancet Infect Dis. 2002;2(9):550–63.
Bartizal K, Gill CJ, Abruzzo GK, Flattery AM, Kong L, Scott PM, et al. In vitro preclinical evaluation studies with the echinocandin antifungal MK-0991 (L-743,872). Antimicrob Agents Chemother. 1997;41(11):2326–32.
Rex JH, Pfaller MA, Galgiani JN, Bartlett MS, Espinel-Ingroff A, Ghannoum MA, et al. Development of interpretive breakpoints for antifungal susceptibility testing: conceptual framework and analysis of in vitro-in vivo correlation data for fluconazole, itraconazole, and candida infections. Subcommittee on Antifungal Susceptibility Testing of the National Committee for Clinical Laboratory Standards. Clin Infect Dis. 1997;24(2):235–47.
Rex JH, Pfaller MA, Walsh TJ, Chaturvedi V, Espinel-Ingroff A, Ghannoum MA, et al. Antifungal susceptibility testing: practical aspects and current challenges. Clin Microbiol Rev. 2001;14(4):643–58.
Rex JH, Pfaller MA, Rinaldi MG, Polak A, Galgiani JN. Antifungal susceptibility testing. Clin Microbiol Rev. 1993;6(4):367–81.
Rex JH, Nelson PW, Paetznick VL, Lozano-Chiu M, Espinel-Ingroff A, Anaissie EJ. Optimizing the correlation between results of testing in vitro and therapeutic outcome in vivo for fluconazole by testing critical isolates in a murine model of invasive candidiasis. Antimicrob Agents Chemother. 1998;42(1):129–34.
Pfaller MA, Andes D, Diekema DJ, Espinol Ingroff A, Sheehan D. Wild-type MIC distributions, epidemiological epidemiological cutoff values and species-specific clinical breakpoints for fluconazole and Candida: time for harmonization of CLSI and EUCAST broth microdilution methods. Drug Resist Updat. 2010;13:180–95.
Espinel-Ingroff A, Warnock DW, Vazquez JA, Arthington-Skaggs BA. In vitro antifungal susceptibility methods and clinical implications of antifungal resistance. Med Mycol. 2000;38 Suppl 1:293–304.
Clancy CJ, Kauffman CA, Morris A, et al. Correlation of fluconazole MIC and response to therapy for patients with candidemia due to C. albicans and non-C. albicans spp: results of a multicenter prospective study of candidemia. In: Proceedings of the 36th annual meeting of the Infectious Diseases Society of America; 1998.
National Committee for Clinical Laboratory Standards. Reference method for broth dilution antifungal susceptibility testing of yeasts: approved standards. Wayne, PA: National Committee for Clinical Laboratory Standards; 1997.
Odds FC, Motyl M, Andrade R, Bille J, Canton E, Cuenca-Estrella M, et al. Interlaboratory comparison of results of susceptibility testing with caspofungin against Candida and Aspergillus species. J Clin Microbiol. 2004;42(8):3475–82.
Pfaller MA, Boyken L, Hollis RJ, Kroeger J, Messer SA, et al. Wild-type MIC distributions and epidemiological cutoff values for the echinocandins and Candida spp. J Clin Microbiol. 2010;48:52–6.
Moosa MY, Sobel JD, Elhalis H, Du W, Akins RA. Fungicidal activity of fluconazole against Candida albicans in a synthetic vagina-simulative medium. Antimicrob Agents Chemother. 2004;48(1):161–7.
Pfaller MA, Diekema DJ, Andes D, Arendrup MC, Brown SD, et al. Clinical breakpoints for the echinocandins and Candida revisited: integration of molecular, clinical, and microbiological data to arrive at species-specific interpretive criteria. Drug Resist Updat. 2011;14:164–76.
Espinel-Ingroff A, Pfaller M, Erwin ME, Jones RN. Interlaboratory evaluation of Etest method for testing antifungal susceptibilities of pathogenic yeasts to five antifungal agents by using Casitone agar and solidified RPMI 1640 medium with 2 % glucose. J Clin Microbiol. 1996;34(4):848–52.
Peyron F, Favel A, Michel-Nguyen A, Gilly M, Regli P, Bolmstrom A. Improved detection of amphotericin B-resistant isolates of Candida lusitaniae by Etest. J Clin Microbiol. 2001;39(1):339–42.
Rex JH, Cooper Jr CR, Merz WG, Galgiani JN, Anaissie EJ. Detection of amphotericin B-resistant Candida isolates in a broth-based system. Antimicrob Agents Chemother. 1995;39(4):906–9.
Warnock DW, Johnson EM, Rogers TR. Multi-centre evaluation of the Etest method for antifungal drug susceptibility testing of Candida spp. and Cryptococcus neoformans. BSAC Working Party on Antifungal Chemotherapy. J Antimicrob Chemother. 1998;42(3):321–31.
Pfaller MA, Messer SA, Bolmstrom A. Evaluation of Etest for determining in vitro susceptibility of yeast isolates to amphotericin B. Diagn Microbiol Infect Dis. 1998;32(3):223–7.
Arendrup M, Lundgren B, Jensen IM, Hansen BS, Frimodt-Moller N. Comparison of Etest and a tablet diffusion test with the NCCLS broth microdilution method for fluconazole and amphotericin B susceptibility testing of Candida isolates. J Antimicrob Chemother. 2001;47(5):521–6.
Pfaller MA, Diekema DJ, Ostrosky-Zeichner L, Rex JH, Alexander BD, et al. Correlation of MIC with outcome for Candida species tested against caspofungin, anidulafungin, and micafungin: analysis and proposal for interpretive MIC breakpoints. J Clin Microbiol. 2008;46:2620–9.
Garcia-Effron G, Lee S, Park S, Cleary JD, Perlin DS. Effect of Candida glabrata FKS1 and FKS2 mutations on echinocandin sensitivity and kinetics of 1,3-beta-D-glucan synthase: implication for the existing susceptibility breakpoint. Antimicrob Agents Chemother. 2009;53:3690–9.
Andes D, Diekema DJ, Pfaller MA, Bohrmuller J, Marchillo K, et al. In vivo comparison of the pharmacodynamic targets for echinocandin drugs against Candida species. Antimicrob Agents Chemother. 2010;54:2497–506.
Clancy CJ, Nguyen MH. Correlation between in vitro susceptibility determined by E test and response to therapy with amphotericin B: results from a multicenter prospective study of candidemia. Antimicrob Agents Chemother. 1999;43(5):1289–90.
Nguyen MH, Clancy CJ, Yu VL, Yu YC, Morris AJ, Snydman DR, et al. Do in vitro susceptibility data predict the microbiologic response to amphotericin B? Results of a prospective study of patients with Candida fungemia. J Infect Dis. 1998;177(2):425–30.
Ostrosky-Zeichner L, Rex JH, Pappas PG, Hamill RJ, Larsen RA, Horowitz HW, et al. Antifungal susceptibility survey of 2,000 bloodstream Candida isolates in the United States. Antimicrob Agents Chemother. 2003;47(10):3149–54.
Pfaller MA, Diekema DJ. Twelve years of fluconazole in clinical practice: global trends in species distribution and fluconazole susceptibility of bloodstream isolates of Candida. Clin Microbiol Infect. 2004;10 Suppl 1:11–23.
Pfaller MA, Diekema DJ, Messer SA, Hollis RJ, Jones RN. In vitro activities of caspofungin compared with those of fluconazole and itraconazole against 3,959 clinical isolates of Candida spp., including 157 fluconazole-resistant isolates. Antimicrob Agents Chemother. 2003;47(3):1068–71.
Pfaller MA, Jones RN, Castanheira M. Regional data analysis of Candida non-albicans strains collected in United States medical sites over a 6-year period, 2006–2011. Mycoses. 2014;57:602–11.
Pfaller MA, Diekema DJ. Epidemiology of invasive candidiasis: a persistent public health problem. Clin Microbiol Rev. 2007;20(1):133–63.
Pfaller MA, Diekema DJ, Jones RN, Messer SA, Hollis RJ. Trends in antifungal susceptibility of Candida spp. isolated from pediatric and adult patients with bloodstream infections: SENTRY Antimicrobial Surveillance Program, 1997 to 2000. J Clin Microbiol. 2002;40(3):852–6.
Antoniadou A, Torres HA, Lewis RE, Thornby J, Bodey GP, Tarrand JP, et al. Candidemia in a tertiary care cancer center: in vitro susceptibility and its association with outcome of initial antifungal therapy. Medicine (Baltimore). 2003;82(5):309–21.
Xu J, Ramos AR, Vilgalys R, Mitchell TG. Clonal and spontaneous origins of fluconazole resistance in Candida albicans. J Clin Microbiol. 2000;38(3):1214–20.
Xiao L, Madison V, Chau AS, Loebenberg D, Palermo RE, McNicholas PM. Three-dimensional models of wild-type and mutated forms of cytochrome P450 14alpha-sterol demethylases from Aspergillus fumigatus and Candida albicans provide insights into posaconazole binding. Antimicrob Agents Chemother. 2004;48(2):568–74.
Hoban DJ, Zhanel GG, Karlowsky JA. In vitro susceptibilities of Candida and Cryptococcus neoformans isolates from blood cultures of neutropenic patients. Antimicrob Agents Chemother. 1999;43(6):1463–4.
Hazen KC, Baron EJ, Colombo AL, Girmenia C, Sanchez-Sousa A, del Palacio A, et al. Comparison of the susceptibilities of Candida spp. to fluconazole and voriconazole in a 4-year global evaluation using disk diffusion. J Clin Microbiol. 2003;41(12):5623–32.
Bodey GP, Mardani M, Hanna HA, Boktour M, Abbas J, Girgawy E, et al. The epidemiology of Candida glabrata and Candida albicans fungemia in immunocompromised patients with cancer. Am J Med. 2002;112(5):380–5.
Fidel Jr PL, Vazquez JA, Sobel JD. Candida glabrata: review of epidemiology, pathogenesis, and clinical disease with comparison to C. albicans. Clin Microbiol Rev. 1999;12(1):80–96.
Safdar A, Chaturvedi V, Koll BS, Larone DH, Perlin DS, Armstrong D. Prospective, multicenter surveillance study of Candida glabrata: fluconazole and itraconazole susceptibility profiles in bloodstream, invasive, and colonizing strains and differences between isolates from three urban teaching hospitals in New York City (Candida Susceptibility Trends Study, 1998 to 1999). Antimicrob Agents Chemother. 2002;46(10):3268–72.
Ghannoum MA, Okogbule-Wonodi I, Bhat N, Sanati H. Antifungal activity of voriconazole (UK-109,496), fluconazole and amphotericin B against hematogenous Candida krusei infection in neutropenic guinea pig model. J Chemother. 1999;11(1):34–9.
Imhof A, Balajee SA, Fredricks DN, Englund JA, Marr KA. Breakthrough fungal infections in stem cell transplant recipients receiving voriconazole. Clin Infect Dis. 2004;39(5):743–6.
Stiller RL, Bennett JE, Scholer HJ, Wall M, Polak A, Stevens DA. Susceptibility to 5-fluorocytosine and prevalence of serotype in 402 Candida albicans isolates from the United States. Antimicrob Agents Chemother. 1982;22(3):482–7.
Ellis D. Amphotericin B: spectrum and resistance. J Antimicrob Chemother. 2002;49 Suppl 1:7–10.
Powderly WG, Kobayashi GS, Herzig GP, Medoff G. Amphotericin B-resistant yeast infection in severely immunocompromised patients. Am J Med. 1988;84(5):826–32.
Nolte FS, Parkinson T, Falconer DJ, Dix S, Williams J, Gilmore C, et al. Isolation and characterization of fluconazole- and amphotericin B-resistant Candida albicans from blood of two patients with leukemia. Antimicrob Agents Chemother. 1997;41(1):196–9.
Merz WG, Sandford GR. Isolation and characterization of a polyene-resistant variant of Candida tropicalis. J Clin Microbiol. 1979;9(6):677–80.
Sterling TR, Gasser Jr RA, Ziegler A. Emergence of resistance to amphotericin B during therapy for Candida glabrata infection in an immunocompetent host. Clin Infect Dis. 1996;23(1):187–8.
Hawkins JL, Baddour LM. Candida lusitaniae infections in the era of fluconazole availability. Clin Infect Dis. 2003;36(2):e14–8.
Krcmery Jr V, Oravcova E, Spanik S, Mrazova-Studena M, Trupl J, Kunova A, et al. Nosocomial breakthrough fungaemia during antifungal prophylaxis or empirical antifungal therapy in 41 cancer patients receiving antineoplastic chemotherapy: analysis of aetiology risk factors and outcome. J Antimicrob Chemother. 1998;41(3):373–80.
Conly J, Rennie R, Johnson J, Farah S, Hellman L. Disseminated candidiasis due to amphotericin B-resistant Candida albicans. J Infect Dis. 1992;165(4):761–4.
Pappagianis D, Collins MS, Hector R, Remington J. Development of resistance to amphotericin B in Candida lusitaniae infecting a human. Antimicrob Agents Chemother. 1979;16(2):123–6.
Guinet R, Chanas J, Goullier A, Bonnefoy G, Ambroise-Thomas P. Fatal septicemia due to amphotericin B-resistant Candida lusitaniae. J Clin Microbiol. 1983;18(2):443–4.
Barchiesi F, Spreghini E, Tomassetti S, Della Vittoria A, Arzeni D, Manso E, et al. Effects of caspofungin against Candida guilliermondii and Candida parapsilosis. Antimicrob Agents Chemother. 2006;50(8):2719–27.
Kartsonis N, Killar J, Mixson L, Hoe CM, Sable C, Bartizal K, et al. Caspofungin susceptibility testing of isolates from patients with esophageal candidiasis or invasive candidiasis: relationship of MIC to treatment outcome. Antimicrob Agents Chemother. 2005;49(9):3616–23.
Park S, Kelly R, Kahn JN, Robles J, Hsu MJ, Register E, et al. Specific substitutions in the echinocandin target Fks1p account for reduced susceptibility of rare laboratory and clinical Candida sp. isolates. Antimicrob Agents Chemother. 2005;49(8):3264–73.
Hakki M, Staab JF, Marr KA. Emergence of a Candida krusei isolate with reduced susceptibility to caspofungin during therapy. Antimicrob Agents Chemother. 2006;50(7):2522–4.
Cheung C, Guo Y, Gialanella P, Feldmesser M. Development of candidemia on caspofungin therapy: a case report. Infection. 2006;34(6):345–8.
Hernandez S, Lopez-Ribot JL, Najvar LK, McCarthy DI, Bocanegra R, Graybill JR. Caspofungin resistance in Candida albicans: correlating clinical outcome with laboratory susceptibility testing of three isogenic isolates serially obtained from a patient with progressive Candida esophagitis. Antimicrob Agents Chemother. 2004;48(4):1382–3.
Laverdiere M, Lalonde RG, Baril JG, Sheppard DC, Park S, Perlin DS. Progressive loss of echinocandin activity following prolonged use for treatment of Candida albicans oesophagitis. J Antimicrob Chemother. 2006;57(4):705–8.
Moudgal V, Little T, Boikov D, Vazquez JA. Multiechinocandin- and multiazole-resistant Candida parapsilosis isolates serially obtained during therapy for prosthetic valve endocarditis. Antimicrob Agents Chemother. 2005;49(2):767–9.
Wingard JR, Merz WG, Rinaldi MG, Johnson TR, Karp JE, Saral R. Increase in Candida krusei infection among patients with bone marrow transplantation and neutropenia treated prophylactically with fluconazole. N Engl J Med. 1991;325(18):1274–7.
Sobel JD, Zervos M, Reed BD, Hooton T, Soper D, Nyirjesy P, et al. Fluconazole susceptibility of vaginal isolates obtained from women with complicated Candida vaginitis: clinical implications. Antimicrob Agents Chemother. 2003;47(1):34–8.
Kauffman C, et al. Clinical practice guideline for the management of candida: 2014 update by the Infectious Diseases Society of America. Clin Infect Dis. 2009;48(5):503–35.
Ben Ami R, Olshtain-Pops K, Krieger M, Oren I, et al. Antibiotic exposure as a risk factor for fluconazole-resistant Candida bloodstream infection. Antimicrob Agents Chemother. 2012;56:2518–23.
Andes DR, Safdar N, Baddley JW, Playforde G, Reboli AC. Impact of treatment strategy on outcomes in patients with candidemia and other forms of invasive candidiasis. Clin Infect Dis. 2012;54:1110–22.
Clevelar AP, Farley MM, Harrison LF, et al. Changes in incidence and antifungal drug resistance in candidemia; results from population based laboratory surveillance in Atlanta and Baltimore, 2008–2012. Clin Infect Dis. 2012;55:1352–61.
Marr KA, White TC, van Burik JA, Bowden RA. Development of fluconazole resistance in Candida albicans causing disseminated infection in a patient undergoing marrow transplantation. Clin Infect Dis. 1997;25(4):908–10.
Gleason TG, May AK, Caparelli D, Farr BM, Sawyer RG. Emerging evidence of selection of fluconazole-tolerant fungi in surgical intensive care units. Arch Surg. 1997;132(11):1197–201; discussion 202.
Law D, Moore CB, Denning DW. Amphotericin B resistance testing of Candida spp.: a comparison of methods. J Antimicrob Chemother. 1997;40(1):109–12.
Maenza JR, Keruly JC, Moore RD, Chaisson RE, Merz WG, Gallant JE. Risk factors for fluconazole-resistant candidiasis in human immunodeficiency virus-infected patients. J Infect Dis. 1996;173(1):219–25.
Fichtenbaum CJ, Powderly WG. Refractory mucosal candidiasis in patients with human immunodeficiency virus infection. Clin Infect Dis. 1998;26(3):556–65.
Fichtenbaum CJ, Koletar S, Yiannoutsos C, Holland F, Pottage J, Cohn SE, et al. Refractory mucosal candidiasis in advanced human immunodeficiency virus infection. Clin Infect Dis. 2000;30(5):749–56.
Fichtenbaum CJ, Zackin R, Rajicic N, Powderly WG, Wheat LJ, Zingman BS. Amphotericin B oral suspension for fluconazole-refractory oral candidiasis in persons with HIV infection. AIDS. 2000;14(7):845–52.
Cartledge JD, Midgley J, Gazzard BG. Non-albicans oral candidosis in HIV-positive patients. J Antimicrob Chemother. 1999;43(3):419–22.
Goldman M. Randomized study of long-term chronic suppressive fluconazole vs. episodic fluconazole for patients with advanced HIV infection and history of oropharyngeal candidiasis. In: 42nd interscience conference on antimicrobial agents and chemotherapy. San Diego, CA: American Society for Microbiology; 2002.
Sobel JD, Ohmit SE, Schuman P, Klein RS, Mayer K, Duerr A, et al. The evolution of Candida species and fluconazole susceptibility among oral and vaginal isolates recovered from human immunodeficiency virus (HIV)-seropositive and at-risk HIV-seronegative women. J Infect Dis. 2001;183(2):286–93.
Revankar SG, Dib OP, Kirkpatrick WR, McAtee RK, Fothergill AW, Rinaldi MG, et al. Clinical evaluation and microbiology of oropharyngeal infection due to fluconazole-resistant Candida in human immunodeficiency virus-infected patients. Clin Infect Dis. 1998;26(4):960–3.
Hope W, Morton A, Eisen DP. Increase in prevalence of nosocomial non-Candida albicans candidaemia and the association of Candida krusei with fluconazole use. J Hosp Infect. 2002;50(1):56–65.
Goff DA, Koletar SL, Buesching WJ, Barnishan J, Fass RJ. Isolation of fluconazole-resistant Candida albicans from human immunodeficiency virus-negative patients never treated with azoles. Clin Infect Dis. 1995;20(1):77–83.
Iwen PC, Kelly DM, Reed EC, Hinrichs SH. Invasive infection due to Candida krusei in immunocompromised patients not treated with fluconazole. Clin Infect Dis. 1995;20(2):342–7.
Nguyen MH, Peacock Jr JE, Morris AJ, Tanner DC, Nguyen ML, Snydman DR, et al. The changing face of candidemia: emergence of non-Candida albicans species and antifungal resistance. Am J Med. 1996;100(6):617–23.
Abi-Said D, Anaissie E, Uzun O, Raad I, Pinzcowski H, Vartivarian S. The epidemiology of hematogenous candidiasis caused by different Candida species. Clin Infect Dis. 1997;24(6):1122–8.
Chandra J, Kuhn DM, Mukherjee PK, Hoyer LL, McCormick T, Ghannoum MA. Biofilm formation by the fungal pathogen Candida albicans: development, architecture, and drug resistance. J Bacteriol. 2001;183(18):5385–94.
Perumal P, Mekala S, Chaffin WL. Role for cell density in antifungal drug resistance in Candida albicans biofilms. Antimicrob Agents Chemother. 2007;51(7):2454–63.
Nett J, Lincoln L, Marchillo K, Massey R, Holoyda K, Hoff B, et al. Putative role of beta-1,3 glucans in Candida albicans biofilm resistance. Antimicrob Agents Chemother. 2007;51(2):510–20.
Shuford JA, Rouse MS, Piper KE, Steckelberg JM, Patel R. Evaluation of caspofungin and amphotericin B deoxycholate against Candida albicans biofilms in an experimental intravascular catheter infection model. J Infect Dis. 2006;194(5):710–3.
Dick JD, Merz WG, Saral R. Incidence of polyene-resistant yeasts recovered from clinical specimens. Antimicrob Agents Chemother. 1980;18(1):158–63.
White TC, Marr KA, Bowden RA. Clinical, cellular, and molecular factors that contribute to antifungal drug resistance. Clin Microbiol Rev. 1998;11(2):382–402.
Yoon SA, Vazquez JA, Steffan PE, Sobel JD, Akins RA. High-frequency, in vitro reversible switching of Candida lusitaniae clinical isolates from amphotericin B susceptibility to resistance. Antimicrob Agents Chemother. 1999;43(4):836–45.
Dube MP, Heseltine PN, Rinaldi MG, Evans S, Zawacki B. Fungemia and colonization with nystatin-resistant Candida rugosa in a burn unit. Clin Infect Dis. 1994;18(1):77–82.
Blot S, Janssens R, Claeys G, Hoste E, Buyle F, De Waele JJ, et al. Effect of fluconazole consumption on long-term trends in candidal ecology. J Antimicrob Chemother. 2006;58(2):474–7.
Marchetti O, Bille J, Fluckiger U, Eggimann P, Ruef C, Garbino J, et al. Epidemiology of candidemia in Swiss tertiary care hospitals: secular trends, 1991–2000. Clin Infect Dis. 2004;38(3):311–20.
Mathema B, Cross E, Dun E, Park S, Bedell J, Slade B, et al. Prevalence of vaginal colonization by drug-resistant Candida species in college-age women with previous exposure to over-the-counter azole antifungals. Clin Infect Dis. 2001;33(5):E23–7.
Dorrell L, Edwards A. Vulvovaginitis due to fluconazole resistant Candida albicans following self treatment with non-prescribed triazoles. Sex Transm Infect. 2002;78(4):308–9.
Sobel JD, Wiesenfeld HC, Martens M, Danna P, Hooton TM, Rompalo A, et al. Maintenance fluconazole therapy for recurrent vulvovaginal candidiasis. N Engl J Med. 2004;351(9):876–83.
Donders GGG, Bellen G, Byttebier G, et al. Individualized decreasing-dose maintenance fluconazole regimen for recurrent vulvovaginal candidiasis (ReCiDiFtrial). Am J Obstet Gynecol. 2008;199:613–9.
Schuman P, Sobel JD, Ohmit SE, Mayer KH, Carpenter CC, Rompalo A, et al. Mucosal candidal colonization and candidiasis in women with or at risk for human immunodeficiency virus infection. HIV Epidemiology Research Study (HERS) Group. Clin Infect Dis. 1998;27(5):1161–7.
Vazquez JA, Sobel JD, Peng G, Steele-Moore L, Schuman P, Holloway W, et al. Evolution of vaginal Candida species recovered from human immunodeficiency virus-infected women receiving fluconazole prophylaxis: the emergence of Candida glabrata? Terry Beirn Community Programs for Clinical Research in AIDS (CPCRA). Clin Infect Dis. 1999;28(5):1025–31.
Myoken Y, Kyo T, Fujihara M, Sugata T, Mikami Y. Clinical significance of breakthrough fungemia caused by azole-resistant Candida tropicalis in patients with hematologic malignancies. Haematologica. 2004;89(3):378–80.
Goldman M, Cloud GA, Smedema M, LeMonte A, Connolly P, McKinsey DS, et al. Does long-term itraconazole prophylaxis result in in vitro azole resistance in mucosal Candida albicans isolates from persons with advanced human immunodeficiency virus infection? The National Institute of Allergy and Infectious Diseases Mycoses study group. Antimicrob Agents Chemother. 2000;44(6):1585–7.
Muller FM, Weig M, Peter J, Walsh TJ. Azole cross-resistance to ketoconazole, fluconazole, itraconazole and voriconazole in clinical Candida albicans isolates from HIV-infected children with oropharyngeal candidosis. J Antimicrob Chemother. 2000;46(2):338–40.
Cartledge JD, Midgley J, Gazzard BG. Clinically significant azole cross-resistance in Candida isolates from HIV-positive patients with oral candidosis. AIDS. 1997;11(15):1839–44.
Cuenca-Estrella M, Lee-Yang W, Ciblak MA, Arthington-Skaggs BA, Mellado E, Warnock DW, et al. Comparative evaluation of NCCLS M27-A and EUCAST broth microdilution procedures for antifungal susceptibility testing of candida species. Antimicrob Agents Chemother. 2002;46(11):3644–7.
Vazquez JA, Lundstrom T, Dembry L, Chandrasekar P, Boikov D, Parri MB, et al. Invasive Candida guilliermondii infection: in vitro susceptibility studies and molecular analysis. Bone Marrow Transplant. 1995;16(6):849–53.
Makarova NU, Pokrowsky VV, Kravchenko AV, Serebrovskaya LV, James MJ, McNeil MM, et al. Persistence of oropharyngeal Candida albicans strains with reduced susceptibilities to fluconazole among human immunodeficiency virus-seropositive children and adults in a long-term care facility. J Clin Microbiol. 2003;41(5):1833–7.
Davies A, Brailsford S, Broadley K, Beighton D. Resistance amongst yeasts isolated from the oral cavities of patients with advanced cancer. Palliat Med. 2002;16(6):527–31.
Stevens DA, Stevens JA. Cross-resistance phenotypes of fluconazole-resistant Candida species: results with 655 clinical isolates with different methods. Diagn Microbiol Infect Dis. 1996;26(3–4):145–8.
Cuenca-Estrella M, Diaz-Guerra TM, Mellado E, Monzon A, Rodriguez-Tudela JL. Comparative in vitro activity of voriconazole and itraconazole against fluconazole-susceptible and fluconazole-resistant clinical isolates of Candida species from Spain. Eur J Clin Microbiol Infect Dis. 1999;18(6):432–5.
Cuenca-Estrella M, Mellado E, Diaz-Guerra TM, Monzon A, Rodriguez-Tudela JL. Susceptibility of fluconazole-resistant clinical isolates of Candida spp. to echinocandin LY303366, itraconazole and amphotericin B. J Antimicrob Chemother. 2000;46(3):475–7.
Bachmann SP, Patterson TF, Lopez-Ribot JL. In vitro activity of caspofungin (MK-0991) against Candida albicans clinical isolates displaying different mechanisms of azole resistance. J Clin Microbiol. 2002;40(6):2228–30.
Pelletier R, Loranger L, Marcotte H, De Carolis E. Voriconazole and fluconazole susceptibility of Candida isolates. J Med Microbiol. 2002;51(6):479–83.
Tsai HF, Sammons LR, Zhang X, Suffis SD, et al. Microarray and molecular analyses of the azole resistance mechanism in Candida glabrata oropharyngeal isolates. Antimicrob Agents Chemother. 2010;54:3308–17.
Sanguinetti M, Posteraro B, Fiori B, Ranno S, Torelli R, Fadda G. Mechanisms of azole resistance in clinical isolates of Candida glabrata collected during a hospital survey of antifungal resistance. Antimicrob Agents Chemother. 2005;49(2):668–79.
Pfaller MA, Diekema DJ. Azole antifungal drug cross-resistance: mechanisms, epidemiology, and clinical significance. J Invasive Fungal Infect. 2007;1(3):74–92.
McKinsey DS, Wheat LJ, Cloud GA, Pierce M, Black JR, Bamberger DM, et al. Itraconazole prophylaxis for fungal infections in patients with advanced human immunodeficiency virus infection: randomized, placebo-controlled, double-blind study. National Institute of Allergy and Infectious Diseases Mycoses Study Group. Clin Infect Dis. 1999;28(5):1049–56.
Andes D, Lepak A, Nett J, Lincoln L, Marchillo K. In vivo fluconazole pharmacodynamics and resistance development in a previously susceptible Candida albicans population examined by microbiologic and transcriptional profiling. Antimicrob Agents Chemother. 2006;50(7):2384–94.
Andes D, Forrest A, Lepak A, Nett J, Marchillo K, Lincoln L. Impact of antimicrobial dosing regimen on evolution of drug resistance in vivo: fluconazole and Candida albicans. Antimicrob Agents Chemother. 2006;50(7):2374–83.
Kovacicova G, Krupova Y, Lovaszova M, Roidova A, Trupl J, Liskova A, et al. Antifungal susceptibility of 262 bloodstream yeast isolates from a mixed cancer and non-cancer patient population: is there a correlation between in-vitro resistance to fluconazole and the outcome of fungemia? J Infect Chemother. 2000;6(4):216–21.
Lee SC, Fung CP, Huang JS, Tsai CJ, Chen KS, Chen HY, et al. Clinical correlates of antifungal macrodilution susceptibility test results for non-AIDS patients with severe Candida infections treated with fluconazole. Antimicrob Agents Chemother. 2000;44(10):2715–8.
Clancy CJ, Yu VL, Morris AJ, Snydman DR, Nguyen MH. Fluconazole MIC and the fluconazole dose/MIC ratio correlate with therapeutic response among patients with candidemia. Antimicrob Agents Chemother. 2005;49(8):3171–7.
Pfaller MA, Castanheira M, Lockhart SR, Ahlquist AM, Messer SA, et al. Frequency of decreased susceptibility and resistance to echinocandins among fluconazole-resistant bloodstream isolates of Candida glabrata. J Clin Microbiol. 2012;50:1199–203.
Castanheira M, Woosley LN, Messer SA, Diekema DJ, Jones RN, et al. Frequency of fks mutations among Candida glabrata isolates from a 10-year global collection of bloodstream infection isolates. Antimicrob Agents Chemother. 2014;58:577–80.
Garcia-Effron G, Kontoyiannis DP, Lewis RE, Perlin DS. Caspofungin-resistant Candida tropicalis strains causing breakthrough fungemia in patients at high risk for hematologic malignancies. Antimicrob Agents Chemother. 2008;52:4181–3.
Garcia-Effron G, Park S, Perlin DS. Correlating echinocandin MIC and kinetic inhibition of fks1 mutant glucan synthases for Candida albicans: implications for interpretive breakpoints. Antimicrob Agents Chemother. 2009;53L:112–22.
Cleary JD, Garcia-Effron G, Chapman SW, Perlin DS. Reduced Candida glabrata susceptibility secondary to an FKS1 mutation developed during candidemia treatment. Antimicrob Agents Chemother. 2008;52:2263–5.
Garcia-Effron G, Chua DJ, Tomada JR, DiPersio J, Perlin DS, et al. Novel FKS mutations associated with echinocandin resistance in Candida species. Antimicrob Agents Chemother. 2010;54:2225–7.
Kahn JN, Garcia-Effron G, Hsu MJ, Park S, Marr KA, et al. Acquired echinocandin resistance in a Candida krusei isolate due to modification of glucan synthase. Antimicrob Agents Chemother. 2007;51:1876–8.
Miller CD, Lomaestro BW, Park S, Perlin DS. Progressive esophagitis caused by Candida albicans with reduced susceptibility to caspofungin. Pharmacotherapy. 2006;26:877–80.
Pfeiffer CD, Garcia-Effron G, Zaas AK, Perfect JR, Perlin DS, et al. Breakthrough invasive candidiasis in patients on micafungin. J Clin Microbiol. 2010;48:2373–80.
Thompson 3rd GR, Wiederhold NP, Vallor AC, Villareal NC, Lewis 2nd JS, et al. Development of caspofungin resistance following prolonged therapy for invasive candidiasis secondary to Candida glabrata infection. Antimicrob Agents Chemother. 2008;52:3783–5.
Lewis 2nd JS, Wiederhold NP, Wickes BL, Patterson TF, Jorgensen JH. Rapid emergence of echinocandin resistance in Candida glabrata resulting in clinical and microbiologic failure. Antimicrob Agents Chemother. 2013;57(9):4559–61.
Dannaoui E, Desnos-Ollivier M, Garcia-Hermoso D, Grenouillet F, Cassaing S, et al. Candida spp. with acquired echinocandin resistance, France, 2004–2010. Emerg Infect Dis. 2012;18:86–90.
Alexander BD, Johnson MD, Pfeiffer CD, et al. Increasing echinocandin resistance in Candida glabrata: clinical failures correlates with presence of FKS mutations and elevated minimum inhibitory concentrations. Clin Infect Dis. 2013;56:1724–32.
Perlin DS. Current perspectives on echinocandin class drugs. Future Microbiol. 2011;6:441–57.
Pham CD, Iqbal N, Bolden CB, Kuykendall RJ, Harrison LH, et al. Role of FKS Mutations in Candida glabrata: MIC values, echinocandin resistance, and multidrug resistance. Antimicrob Agents Chemother. 2014;58:4690–6.
Niimi K, Maki K, Ikeda F, Holmes AR, Lamping E, et al. Overexpression of Candida albicans CDR1, CDR2, or MDR1 does not produce significant changes in echinocandin susceptibility. Antimicrob Agents Chemother. 2006;50:1148–55.
Katiyar S, Phaller M, Edlind T. Candida albicans and Candida glabrata clinical isolates exhibiting reduced echinocandin susceptibility. Antimicrob Agents Chemother. 2006;50:2892–4.
Arendrup MC, Perlin DS, Jensen RH, Howard SJ, Goodwin J, et al. Differential In vivo activity of Anidulafungin, Caspofungin and Micafungin against C. glabrata with and without FKS resistance mutations. Antimicrob Agents Chemother. 2012;56(5):2435–42.
Wiederhold NP, Najvar LK, Bocanegra RA, Kirkpatrick WR, Patterson TF. Caspofungin dose escalation for invasive candidiasis due to resistant Candida albicans. Antimicrob Agents Chemother. 2011;55:3254–60.
Shields RK, Nguyen MH, Press EG, Kwa AL, Cheng S, et al. The presence of an FKS mutation rather than MIC is an independent risk factor for failure of echinocandin therapy among patients with invasive candidiasis due to Candida glabrata. Antimicrob Agents Chemother. 2012;56:4862–9.
Beyda ND, John J, Kilic A, Alam MJ, Lasco TM, et al. FKS mutant Candida glabrata: risk factors and outcomes in patients with candidemia. Clin Infect Dis. 2014;59:819–25.
Foldi R, Szilagyi J, Kardos G, Berenyi R, Kovacs R, et al. Effect of 50 % human serum on the killing activity of micafungin against eight Candida species using time-kill methodology. Diagn Microbiol Infect Dis. 2012;73:338–42.
d’Enfert C. Biofilms and their role in the resistance of pathogenic Candida to antifungal agents. Curr Drug Targets. 2006;7:465–70.
Desai JV, Bruno VM, Ganguly S, Stamper RJ, Mitchell KF, et al. Regulatory role of glycerol in Candida albicans biofilm formation. MBio. 2013;4: e00637-12.
Maenza JR, Merz WG, Romagnoli MJ, Keruly JC, Moore RD, Gallant JE. Infection due to fluconazole-resistant Candida in patients with AIDS: prevalence and microbiology. Clin Infect Dis. 1997;24(1):28–34.
Laguna F, Rodriguez-Tudela JL, Martinez-Suarez JV, Polo R, Valencia E, Diaz-Guerra TM, et al. Patterns of fluconazole susceptibility in isolates from human immunodeficiency virus-infected patients with oropharyngeal candidiasis due to Candida albicans. Clin Infect Dis. 1997;24(2):124–30.
Quereda C, Polanco AM, Giner C, Sanchez-Sousa A, Pereira E, Navas E, et al. Correlation between in vitro resistance to fluconazole and clinical outcome of oropharyngeal candidiasis in HIV-infected patients. Eur J Clin Microbiol Infect Dis. 1996;15(1):30–7.
Vazquez JA. Therapeutic options for the management of oropharyngeal and esophageal candidiasis in HIV/AIDS patients. HIV Clin Trials. 2000;1(1):47–59.
Sangeorzan JA, Bradley SF, He X, Zarins LT, Ridenour GL, Tiballi RN, et al. Epidemiology of oral candidiasis in HIV-infected patients: colonization, infection, treatment, and emergence of fluconazole resistance. Am J Med. 1994;97(4):339–46.
Martins MD, Lozano-Chiu M, Rex JH. Declining rates of oropharyngeal candidiasis and carriage of Candida albicans associated with trends toward reduced rates of carriage of fluconazole-resistant C. albicans in human immunodeficiency virus-infected patients. Clin Infect Dis. 1998;27(5):1291–4.
Zingman BS. Resolution of refractory AIDS-related mucosal candidiasis after initiation of didanosine plus saquinavir. N Engl J Med. 1996;334(25):1674–5.
Gruber A, Lukasser-Vogl E, Borg-von Zepelin M, Dierich MP, Wurzner R. Human immunodeficiency virus type 1 gp160 and gp41 binding to Candida albicans selectively enhances candidal virulence in vitro. J Infect Dis. 1998;177(4):1057–63.
Cassone A, De Bernardis F, Torosantucci A, Tacconelli E, Tumbarello M, Cauda R. In vitro and in vivo anticandidal activity of human immunodeficiency virus protease inhibitors. J Infect Dis. 1999;180(2):448–53.
Tacconelli E, Bertagnolio S, Posteraro B, Tumbarello M, Boccia S, Fadda G, et al. Azole susceptibility patterns and genetic relationship among oral Candida strains isolated in the era of highly active antiretroviral therapy. J Acquir Immune Defic Syndr. 2002;31(1):38–44.
Barchiesi F, Maracci M, Radi B, Arzeni D, Baldassarri I, Giacometti A, et al. Point prevalence, microbiology and fluconazole susceptibility patterns of yeast isolates colonizing the oral cavities of HIV-infected patients in the era of highly active antiretroviral therapy. J Antimicrob Chemother. 2002;50(6):999–1002.
Bagg J, Sweeney MP, Lewis MA, Jackson MS, Coleman D, Al MA, et al. High prevalence of non-albicans yeasts and detection of anti-fungal resistance in the oral flora of patients with advanced cancer. Palliat Med. 2003;17(6):477–81.
Silverman Jr S, Luangjarmekorn L, Greenspan D. Occurrence of oral Candida in irradiated head and neck cancer patients. J Oral Med. 1984;39(4):194–6.
Rautemaa R, Richardson M, Pfaller M, Koukila-Kahkola P, Perheentupa J, Saxen H. Decreased susceptibility of Candida albicans to azole antifungals: a complication of long-term treatment in autoimmune polyendocrinopathy-candidiasis-ectodermal dystrophy (APECED) patients. J Antimicrob Chemother. 2007;60(4):889–92.
Darouiche RO. Oropharyngeal and esophageal candidiasis in immunocompromised patients: treatment issues. Clin Infect Dis. 1998;26(2):259–72; quiz 73–4.
Martins MD, Rex JH. Fluconazole suspension for oropharyngeal candidiasis unresponsive to tablets. Ann Intern Med. 1997;126(4):332–3.
Grim SA, Smith KM, Romanelli F, Ofotokun I. Treatment of azole-resistant oropharyngeal candidiasis with topical amphotericin B. Ann Pharmacother. 2002;36(9):1383–6.
Eichel M, Just-Nubling G, Helm EB, Stille W. [Itraconazole suspension in the treatment of HIV-infected patients with fluconazole-resistant oropharyngeal candidiasis and esophagitis]. Mycoses. 1996;39 Suppl 1:102–6.
Ruhnke M, Schmidt-Westhausen A, Trautmann M. In vitro activities of voriconazole (UK-109,496) against fluconazole-susceptible and -resistant Candida albicans isolates from oral cavities of patients with human immunodeficiency virus infection. Antimicrob Agents Chemother. 1997;41(3):575–7.
Skiest DJ, Vazquez JA, Anstead GM, et al. Posaconazole for the treatment of azole-refractory oropharyngeal and esophageal candidiasis in subjects with HIV infection. Clin Infect Dis. 2007;44:607–14.
Arathoon EG, Gotuzzo E, Noriega LM, Berman RS, DiNubile MJ, Sable CA. Randomized, double-blind, multicenter study of caspofungin versus amphotericin B for treatment of oropharyngeal and esophageal candidiases. Antimicrob Agents Chemother. 2002;46(2):451–7.
Barbaro G, Barbarini G, Calderon W, Grisorio B, Alcini P, Di Lorenzo G. Fluconazole versus itraconazole for candida esophagitis in acquired immunodeficiency syndrome. Candida Esophagitis. Gastroenterology. 1996;111(5):1169–77.
Hegener P, Troke PF, Fatkenheuer G, Diehl V, Ruhnke M. Treatment of fluconazole-resistant candidiasis with voriconazole in patients with AIDS. AIDS. 1998;12(16):2227–8.
Villanueva A, Arathoon EG, Gotuzzo E, Berman RS, DiNubile MJ, Sable CA. A randomized double-blind study of caspofungin versus amphotericin for the treatment of candidal esophagitis. Clin Infect Dis. 2001;33(9):1529–35.
Villanueva A, Gotuzzo E, Arathoon EG, Noriega LM, Kartsonis NA, Lupinacci RJ, et al. A randomized double-blind study of caspofungin versus fluconazole for the treatment of esophageal candidiasis. Am J Med. 2002;113(4):294–9.
Ally R, Schurmann D, Kreisel W, Carosi G, Aguirrebengoa K, Dupont B, et al. A randomized, double-blind, double-dummy, multicenter trial of voriconazole and fluconazole in the treatment of esophageal candidiasis in immunocompromised patients. Clin Infect Dis. 2001;33(9):1447–54.
Krause DS, Simjee AE, van Rensburg C, Viljoen J, Walsh TJ, Goldstein BP, et al. A randomized, double-blind trial of anidulafungin versus fluconazole for the treatment of esophageal candidiasis. Clin Infect Dis. 2004;39(6):770–5.
Sobel JD, Vazquez JA. Symptomatic vulvovaginitis due to fluconazole-resistant Candida albicans in a female who was not infected with human immunodeficiency virus. Clin Infect Dis. 1996;22(4):726–7.
Sobel JD, Chaim W, Nagappan V, Leaman D. Treatment of vaginitis caused by Candida glabrata: use of topical boric acid and flucytosine. Am J Obstet Gynecol. 2003;189(5):1297–300.
Horowitz BJ. Topical flucytosine therapy for chronic recurrent Candida tropicalis infections. J Reprod Med. 1986;31(9):821–4.
White DJ, Habib AR, Vanthuyne A, Langford S, Symonds M. Combined topical flucytosine and amphotericin B for refractory vaginal Candida glabrata infections. Sex Transm Infect. 2001;77(3):212–3.
Singh S, Sobel JD, Bhargava P, Boikov D, Vazquez JA. Vaginitis due to Candida krusei: epidemiology, clinical aspects, and therapy. Clin Infect Dis. 2002;35(9):1066–70.
Sobel JD. Pathogenesis and treatment of recurrent vulvovaginal candidiasis. Clin Infect Dis. 1992;14 Suppl 1:S148–53.
Spinillo A, Colonna L, Piazzi G, Baltaro F, Monaco A, Ferrari A. Managing recurrent vulvovaginal candidiasis. Intermittent prevention with itraconazole. J Reprod Med. 1997;42(2):83–7.
Fong IW. The value of chronic suppressive therapy with itraconazole versus clotrimazole in women with recurrent vaginal candidiasis. Genitourin Med. 1992;68(6):374–7.
Guaschino S, De Seta F, Sartore A, Ricci G, De Santo D, Piccoli M, et al. Efficacy of maintenance therapy with topical boric acid in comparison with oral itraconazole in the treatment of recurrent vulvovaginal candidiasis. Am J Obstet Gynecol. 2001;184(4):598–602.
Eggimann P, Garbino J, Pittet D. Management of Candida species infections in critically ill patients. Lancet Infect Dis. 2003;3(12):772–85.
Mora-Duarte J, Betts R, Rotstein C, Colombo AL, Thompson-Moya L, Smietana J, et al. Comparison of caspofungin and amphotericin B for invasive candidiasis. N Engl J Med. 2002;347(25):2020–9.
Rex JH, Pappas PG, Karchmer AW, Sobel J, Edwards JE, Hadley S, et al. A randomized and blinded multicenter trial of high-dose fluconazole plus placebo versus fluconazole plus amphotericin B as therapy for candidemia and its consequences in nonneutropenic subjects. Clin Infect Dis. 2003;36(10):1221–8.
Rex JH, Bennett JE, Sugar AM, Pappas PG, van der Horst CM, Edwards JE, et al. A randomized trial comparing fluconazole with amphotericin B for the treatment of candidemia in patients without neutropenia. Candidemia Study Group and the National Institute. N Engl J Med. 1994;331(20):1325–30.
Phillips P, Shafran S, Garber G, Rotstein C, Smaill F, Fong I, et al. Multicenter randomized trial of fluconazole versus amphotericin B for treatment of candidemia in non-neutropenic patients. Canadian Candidemia Study Group. Eur J Clin Microbiol Infect Dis. 1997;16(5):337–45.
Kulberg BJ, Sobel JD, Ruhnke N, Pappas PG et al. A randomized, prospective, multicenter study of voriconazole versus a regimen of amphotericin B followed by fluconazole in the treatment of candidemia in non-neutropenic patients. Lancet 2005;366:1435–42.
Anaissie EJ, Vartivarian SE, Abi-Said D, Uzun O, Pinczowski H, Kontoyiannis DP, et al. Fluconazole versus amphotericin B in the treatment of hematogenous candidiasis: a matched cohort study. Am J Med. 1996;101(2):170–6.
Clancy CJ, Staley B, Nguyen MH. In vitro susceptibility of breakthrough Candida bloodstream isolates correlates with daily and cumulative doses of fluconazole. Antimicrob Agents Chemother. 2006;50(10):3496–8.
Vazquez JA, Gupta S, Villanueva A. Potential utility of recombinant human GM-CSF as adjunctive treatment of refractory oropharyngeal candidiasis in AIDS patients. Eur J Clin Microbiol Infect Dis. 1998;17(11):781–3.
Swindells S. Pilot study of adjunctive GM-CSF (yeast derived) for fluconazole-resistant oral candidiasis in HIV-infection. Infect Dis Clin Pract. 1997;6:278–9.
Poynton CH, Barnes RA, Rees J. Interferon gamma and granulocyte-macrophage colony-stimulating factor for the treatment of hepatosplenic candidosis in patients with acute leukemia. Clin Infect Dis. 1998;26(1):239–40.
Rokusz L, Liptay L, Kadar K. Successful treatment of chronic disseminated candidiasis with fluconazole and a granulocyte-macrophage colony-stimulating factor combination. Scand J Infect Dis. 2001;33(10):784–6.
Paterson PJ, McWhinney PH, Potter M, Kibbler CC, Prentice HG. The combination of oral amphotericin B with azoles prevents the emergence of resistant Candida species in neutropenic patients. Br J Haematol. 2001;112(1):175–80.
Fowler SL, Rhoton B, Springer SC, Messer SA, Hollis RJ, Pfaller MA. Evidence for person-to-person transmission of Candida lusitaniae in a neonatal intensive-care unit. Infect Control Hosp Epidemiol. 1998;19(5):343–5.
Burnie JP, Lee W, Williams JD, Matthews RC, Odds FC. Control of an outbreak of systemic Candida albicans. Br Med J (Clin Res Ed). 1985;291(6502):1092–3.
Lupetti A, Tavanti A, Davini P, Ghelardi E, Corsini V, Merusi I, et al. Horizontal transmission of Candida parapsilosis candidemia in a neonatal intensive care unit. J Clin Microbiol. 2002;40(7):2363–9.
Ostrosky-Zeichner L, Shoham S, Vazquez J, Reboli A, et al. A randomized double-blind, placebo-controlled trial of caspofungin prophylaxis followed by preemptive therapy for invasive candidiasis in high-risk adults in the critical care setting. Clin Infect Dis. 2014;55:1219–26.
Ables A, Blumer NA, Valainis GT. Fluconazole prophylaxis of severe Candida infection in trauma and postsurgical patients: a prospective, double blind, randomized, placebo-controlled trial. Infect Dis Clin Pract. 2000;9:169–73.
Garbino T. Fluconazole prevents severe Candida spp. infections in high risk critically ill patients. Washington, DC: American Society of Microbiology; 1997.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2017 Springer International Publishing AG
About this chapter
Cite this chapter
Sobel, J.D., Akins, R.A. (2017). The Role of Resistance in Candida Infections: Epidemiology and Treatment. In: Mayers, D., Sobel, J., Ouellette, M., Kaye, K., Marchaim, D. (eds) Antimicrobial Drug Resistance. Springer, Cham. https://doi.org/10.1007/978-3-319-47266-9_18
Download citation
DOI: https://doi.org/10.1007/978-3-319-47266-9_18
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-47264-5
Online ISBN: 978-3-319-47266-9
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)