Abstract
This abstract proposes a modeling methodology that enables reconstructing ankle joint mechanical function from muscle motor unit spike trains decomposed from high-density electromyography signals. The abstract outlines methods and results and discusses the implication that this approach can have for enhancing our understanding of the neuro-mechanical processes underlying human movement.
Access provided by CONRICYT-eBooks. Download conference paper PDF
Similar content being viewed by others
Keywords
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
1 Introduction
Human movement emerges from the coordinated interaction between the neuromuscular and the musculoskeletal systems [1]. Despite the knowledge of the mechanisms underlying movement neuromuscular and musculoskeletal functions, there currently is no relevant understanding of their interplay. Observations of movement neural function alone cannot explain how neural structures (i.e. motor neurons) elicit mechanical forces [1, 2]. Similarly, observations of movement mechanical function alone cannot explain mechanical variables tightly connected to muscle co-excitation such as joint stiffness [3], damping or compressive forces [4, 5], which are central in the execution of movements [1, 2]. A possible way to address this problem is given by neural data-driven musculoskeletal modeling [2, 6]. This enables simulating the dynamics of the musculoskeletal system as controlled by output of the nervous system [7].
In this abstract we show how this methodology can be used to record muscle electrophysiological signals non-invasively (i.e. high-density electromyograms), extract motor unit dynamics from noisy and high-interference recordings (i.e. using blind-source separation), and reconstruct the non-linear transformations that lead to production of isometric ankle joint moments. This opens up new avenues for linking movement neurocellular function (i.e. motor neuron) with the emerging mechanical function at the limb level and therefore study movement at the interacting neuro-mechanical levels.
2 Methods
The University Medical Center Göttingen Ethical Committee approved all experimental procedures. Four healthy male participants volunteered after signing informed content forms. First, static poses were recorded from all participants using a seven-camera motion capture system (Qualisys, Göteborg, Sweden) and 27 retroreflective markers [8–10]. Then, each participant performed a series of isometric ankle plantar-dorsi flexion tasks. In this, participants were required to track monitor-displayed ankle joint moments spanning a range of plantar-flexion and dorsi-flexion maximal voluntary contraction percentages (%MVC), i.e. from 20 %MVC to 90 %MVC. Tasks were repeated at ankle anatomical position as well as at 10° dorsi-flexion and 10° plantar-flexion. High-density electromyograms (EMG) were recorded using a multi-channel amplifier (OTBioelettronica, Torino, Italy). 64-channel grid electrodes were individually placed on the gastrocnemius lateralis/medialis, peroneus group, soleus, and tibialis anterior muscles. Experimental ankle joint angular moments and positions were measured using a dynamometer (Biodex Medical Systems, Inc., NY, USA).
High-density EMG signals were decomposed into the constituent motor unit discharges using the Blind source separation algorithm based on Convolution Kernel Compensation method [11]. Motion data recorded during the static poses were used to build a personalized model of the musculoskeletal geometry that matched each individual’s anthropometry. This was done via linear scaling in OpenSim. The subject-specific geometry model was then used as part of an open-loop neural data-driven musculoskeletal modeling formulation [8, 12, 13]. The model received input motor unit spike trains and isometric ankle positions. Discrete spike trains were processed by a twitch model based on a time-history dependent recursive filter and a non-linear transfer function [14, 15]. This generated continuous muscle fiber contractile responses, or fiber activation, to the input spike trains. These were used in conjunction with isometric join angle information to solve for the dynamic equilibrium between muscle fibers and series elastic tendons in the production of muscle-tendon force and joint moments. The model was initially calibrated on a set of 20 %MVC trials. Calibration matched the individual’s force using an optimization procedure that minimally adjusted internal parameters in order to minimize the difference between predicted and reference joint moments about the ankle plantar-dorsi flexion. The model was then blindly validated on novel trials and %MVC conditions not used to for calibration. During validation the model was operated in open-loop and driven by muscle spike trains, i.e. with no information on the experimental joint moments.
3 Results
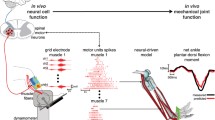
Figure 1 shows the process of neuro-mechanical transformation during which discrete spike trains are converted into continuous fiber activations and subsequently translated into mechanical tendon force. Figure 1 also shows how neurally controlled muscle-tendon forces, computed across all muscle-tendon units in the model, enable the blinded reconstruction of the net ankle plantar-dorsi flexion moments with substantial accuracy during validation trials.
(left-up) Subject-specific model of the musculoskeletal geometry. The model displays the three ankle joint positions investigated in the study as well as cumulative spike trains (CSTs) used for muscle neural control. (left-down) CST decomposed from the soleus high-density electromyograms during 60 %MVC contraction, reconstructed continuous whole-muscle activation and non-linear force transfer on the tendon. (right) Measured joint moment (black) and those blindly predicted via input CSTs (red)
4 Conclusion
The reliable simulation and determination of the processes underlying the neural control of musculoskeletal forces is central to understand human movement. In this abstract we provided preliminary results that support the possibility of reconstructing individuals’ ankle joint mechanical function from motor unit spike trains decoded in vivo in the intact human. Motor units represent the interface between movement neural and muscular levels because they contribute to transduce neural inputs into mechanical outputs, i.e. motor neuron action potentials directly result in innervated muscle fiber action potentials and therefore in mechanical force. Therefore, our proposed approach provides a window to movement neuro-mechanics.
The availability of neural data-driven modelling methods will enable understanding the interplay underlying in vivo movement function, pathology and recovery with implications to the design of personalized rehabilitation interventions and clinically viable neural interfaces [2].
References
R.M. Enoka, Neuromechanics of Human Movement, 4th edn. Human Kinetics Publishers, Inc. (2008)
M. Sartori, D.G. Llyod, D. Farina, Neural data-driven musculoskeletal modeling for personalized neurorehabilitation technologies. IEEE Trans. Biomed. Eng. 63(5), 879–893 (2016)
M. Sartori, M. Maculan, C. Pizzolato, M. Reggiani, D. Farina, Modeling and simulating the neuromuscular mechanisms regulating ankle and knee joint stiffness during human locomotion. J. Neurophysiol. 114, 2509–2527 (2015)
P. Gerus, M. Sartori, T.F. Besier, B.J. Fregly, S.L. Delp, S.A. Banks, M.G. Pandy, D.D. D’Lima, D.G. Lloyd, Subject-specific knee joint geometry improves predictions of medial tibiofemoral contact forces. J. Biomech. 46(16), 2778–2786 (2013)
J. Fernandez, M. Sartori, D.G. Lloyd, J. Munro, V. Shim, Bone remodelling in the natural acetabulum is influenced by muscle force-induced bone stress. Int. J. Numer. Method. Biomed. Eng. 30(1), 28–41 (2014)
M. Sartori, D. Farina, D.G. Lloyd, Hybrid neuromusculoskeletal modeling to best track joint moments using a balance between muscle excitations derived from electromyograms and optimization. J. Biomech. 47(15), 3613–3621 (2014)
J. Fernandez, J. Zhang, T. Heidlauf, M. Sartori, T. Besier, O. Röhrle, D. Lloyd, Multiscale musculoskeletal modelling, data–model fusion and electromyography-informed modelling. Interface Focus 6(2), 20150084 (2016)
M. Sartori, M. Reggiani, D. Farina, D.G. Lloyd, EMG-driven forward-dynamic estimation of muscle force and joint moment about multiple degrees of freedom in the human lower extremity. PLoS ONE 7(12), 1–11 (2012)
M. Sartori, L. Gizzi, D.G. Lloyd, D. Farina, A musculoskeletal model of human locomotion driven by a low dimensional set of impulsive excitation primitives. Front. Comput. Neurosci 7, 79 (2013)
A. Mantoan, C. Pizzolato, M. Sartori, Z. Sawacha, C. Cobelli, M. Reggiani, MOtoNMS: A MATLAB toolbox to process motion data for neuromusculoskeletal modeling and simulation. Source Code Biol. Med. 10(1), 1–12 (2015)
A. Holobar, D. Zazula, Multichannel blind source separation using convolution kernel compensation. IEEE Trans. Signal Process. 55(9), 4487–4496 (2007)
M. Sartori, M. Reggiani, E. Pagello, D.G. Lloyd, Modeling the human knee for assistive technologies. IEEE Trans. Biomed. Eng. 59(9), 2642–2649 (2012)
C. Pizzolato, D.G. Lloyd, M. Sartori, E. Ceseracciu, T.F. Besier, B.J. Fregly, M. Reggiani, CEINMS: a toolbox to investigate the influence of different neural control solutions on the prediction of muscle excitation and joint moments during dynamic motor tasks. J. Biomech. (2015)
H.S. Milner-Brown, R.B. Stein, R. Yemm, The contractile properties of human motor units during voluntary isometric contractions. J. Physiol. 228(2), 285–306 (1973)
K. Manal, T.S. Buchanan, A one-parameter neural activation to muscle activation model: estimating isometric joint moments from electromyograms. J. Biomech. 36(8), 1197–1202 (2003)
Acknowledgments
This work was supported by the ERC Advanced Grant DEMOVE [267888].
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2017 Springer International Publishing AG
About this paper
Cite this paper
Sartori, M., Yavuz, U.S., Frömmel, C., Farina, D. (2017). From Spiking Motor Units to Joint Function. In: Ibáñez, J., González-Vargas, J., Azorín, J., Akay, M., Pons, J. (eds) Converging Clinical and Engineering Research on Neurorehabilitation II. Biosystems & Biorobotics, vol 15. Springer, Cham. https://doi.org/10.1007/978-3-319-46669-9_208
Download citation
DOI: https://doi.org/10.1007/978-3-319-46669-9_208
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-46668-2
Online ISBN: 978-3-319-46669-9
eBook Packages: EngineeringEngineering (R0)