Abstract
Hair follicles contain nestin-expressing pluripotent stem cells which originate above the bulge area of the follicle, below the sebaceous gland. We have termed these cells hair follicle-associated pluripotent (HAP) stem cells. We have established efficient cryopreservation methods of the hair follicle that maintain the pluripotency of HAP stem cells as well as hair growth. We cryopreserved the whole hair follicle by slow-rate cooling in TC-Protector medium or in DMSO-containing medium and storage in liquid nitrogen or at −80 °C. After thawing and culture of the cryopreserved whisker follicles, growing HAP stem cells formed hair spheres. The hair spheres contained cells that differentiated to neurons, glial cells, and other cell types. The hair spheres derived from slow-cooling cryopreserved hair follicles were as pluripotent as hair spheres from fresh hair follicles. We have also previously demonstrated that cryopreserved mouse whisker hair follicles maintain their hair-growth potential. DMSO better cryopreserved mouse whisker follicles compared to glycerol. DMSO-cryopreserved hair follicles also maintained the HAP stem cells, evidenced by P75ntr expression. Subcutaneous transplantation of DMSO-cryopreserved hair follicles in nude mice resulted in extensive hair fiber growth over 8 weeks, indicating the functional recovery of hair-shaft growth of cryopreserved hair follicles. HAP stem cells can be used for nerve and spinal-cord repair. This biobanking of hair follicles can allow each patient the potential for their own stem cell use for regenerative medicine or hair transplantation.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
16.1 Introduction
Nestin-expressing stem cells of the hair follicle , discovered by our laboratory [1], have been shown to be able to form outer root sheaths of the follicle as well as neurons and many other non-follicle cell types [2]. We have termed the nestin-expressing stem cells of the hair follicle hair follicle-associated pluripotent (HAP) stem cells. We have shown that the HAP stem cells from the hair follicle can effect the repair of peripheral-nerve and spinal-cord injury [2–5]. The hair-follicle stem cells differentiate into neuronal and glial cells after transplantation to the injured peripheral nerve and spinal cord, and enhance injury repair and locomotor recovery [2–5].
When the excised hair follicles with their nerve stump were placed in Gelfoam ® 3D histoculture, HAP stem cells grew and extended the hair follicle nerve which consisted of βIII-tubulin-positive fibers with F-actin expression at the tip. These findings indicate that βIII-tubulin-positive fibers elongating from the whisker follicle sensory-nerve stump were growing axons. The growing whisker sensory nerve was highly enriched in HAP stem cells, which appeared to play a major role in its elongation and interaction with other nerves in 3D Gelfoam® histoculture, including the sciatic nerve, the trigeminal nerve, and the trigeminal nerve ganglion. These results suggest that a major function of the HAP stem cells in the hair follicle is for growth of the follicle sensory nerve [6].
Recently, we have shown that HAP stem cells can differentiate into beating cardiac muscle cells [7, 8]. HAP stem cells have critical advantages for regenerative medicine over embryonic stem (ES) cells and induced pluripotent stem (iPS) cells in that they are highly accessible from each patient, thereby eliminating immunological issues since they are autologous, require no genetic manipulation, are non-tumorigenic, and do not present ethical issues for regenerative medicine [9].
Efficient cryopreservation methods of the hair follicle which maintained the pluripotency of HAP stem cells and hair shaft growth are presented here [8].
16.2 Materials and Methods
16.2.1 Mice
Transgenic C57/B6-green fluorescent protein (GFP) mice [10] and transgenic mice with nestin-driven (ND)-GFP and non-transgenic (nu/nu) nude mice (AntiCancer, Inc., San Diego, CA) were used in these studies [11, 12]. The C57/B6-GFP mice expressed the Aequorea victoria GFP under the control of the chicken β-actin promoter and cytomegalovirus enhancer. ND-GFP mice express GFP under control of the nestin regulatory element. All of the tissues from this transgenic line, with the exception of erythrocytes and hair, were fluorescent green under excitation light. All animal experiments were conducted according to the Guidelines for Animal Experimentation at the Kitasato University [13].
16.2.2 Isolation of Vibrissa Hair Follicles
To isolate the vibrissa follicles from mice, the upper lip containing the vibrissa pad was cut under anesthesia and the inner surface was exposed. Entire vibrissa hair follicles were dissected under a binocular microscope and plucked from the pad by pulling them gently by the neck with fine forceps. The isolated vibrissae were washed in Dulbecco’s modified Eagle’s medium (DMEM)/F12 (GIBCO-BRL) with 2 % B-27 (GIBCO-BRL) and 50 μg/mL gentamicin (Gibco-BRL). The follicles were divided into three parts using a surgical knife and fine forceps under a binocular microscope as previously described [14]. All surgical procedures were done under a sterile environment.
16.2.3 Hair-Follicle and Hair-Sphere Culture
The upper part of the vibrissa hair follicle was isolated and cultured in DMEM with 10 % fetal bovine serum (FBS). After 4 weeks of culture, cells growing out from the upper follicle were treated enzymatically with Accumax (Innovative Cell Technologies, Inc.) to detach them. The detached cells were then transferred to non-adhesive culture dishes with DMEM/F12 containing 2 % B-27. After 1 week of culture, the growing cells formed hair spheres containing nestin-expressing HAP stem cells. After the change of medium to DMEM containing 10 % FBS and 2 days of additional culture, the HAP stem cells differentiated to β-III tubulin-positive neurons, glial fibrillary acidic protein (GFAP)-positive glial cells, K15-positive keratinocytes, and smooth muscle actin (SMA)-positive smooth muscle cells [13, 14].
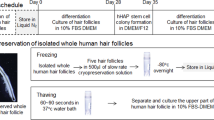
16.2.4 Cryopreservation of the Whole Hair Follicle
Five whole vibrissa follicles were transferred to cryovials, and TC-Protector medium (DS Pharma Biomedical Co.) was added (500 μL). Eighteen cryovials containing the vibrissa follicles were stored in a −80 °C freezer overnight and then transferred to a liquid nitrogen tank. Three mice were used for this method for three independent experiments involving one mouse each.
The cryopreserved vibrissa follicles were thawed at 37 °C in a water bath for 60–90 s (slow recovery) with gentle shaking and separated in three parts (upper, middle, and lower). The upper part of hair follicle was isolated and cultured in DMEM with 10 % FBS [13].
Whisker hair follicles were also cryopreserved in either 10 % glycerol or DMSO with 90 % FBS added (1 ml) as the freezing medium. For short time periods, cryovials containing hair follicles were stored in an −80 °C freezer [12].
16.2.5 Cryopreservation of Hair Spheres
The upper part of hair follicle was isolated and cultured in DMEM with 10 % FBS. After 4 weeks of culture, cells that grew out from the follicles were transferred to DMEM/F12 with 2 % B-27 as described above. After 1 week of culture, hair spheres formed. Fifty spheres were transferred to cryovials and TC-Protector medium was added (500 μL). Nine cryovials with spheres were stored in a −80 °C freezer overnight and then transferred to a liquid-nitrogen tank. The stored spheres were thawed at 37 °C in a water bath with gentle shaking. The spheres were cultured in DMEM with 10 % FBS [13].
16.2.6 Immunohistochemistry of Hair Follicles
Hair follicles were fixed in 4 % paraformaldehyde overnight at 4 °C, after dehydration in 15 % and then 30 % sucrose overnight at 4 °C. Tissues were then embedded in the OTC freezing compound (Thermo Scientific, Kalamazoo, MI) and frozen sections of 10 μm were prepared with a CM1850 cryostat (Leica, Buffalo Grove, IL). The frozen sections were washed with PBS three times. After incubation with 0.4 % Triton X-100 for 10 min, the sections were incubated with 20 % BSA in PBS containing 0.4 % Triton X-100 for 1 h at room temperature (RT). Primary antibody, anti- p75NTR (rabbit, 1:400, Cell Signaling), was applied at 4 °C overnight. After three washes in PBS, secondary antibody, Alexa Fluor® 555-conjugated anti-rabbit (1:100, Jackson Laboratories), was applied in PBS at RT for 1 h. After three washes in PBS, the nuclei were stained with DAPI (1:2000, Invitrogen) for 5 min. Images were obtained with a confocal FV1000 laser-scanning microscope [12].
16.2.7 Immunohistochemistry of Hair Spheres
After a 1-week culture of hair spheres in DMEM with 10 % FBS, the hair spheres were stained in individual wells with the following antibodies: anti-III-β-tubulin mAb (1:500, Tuj1 clone, Covance Research Products); anti-GFAP chicken polyclonal Ab (1:200, Abcam); anti-SMA mAb (1:400, Lab Vision); and anti-K15 mAb (1:100, Lab Vision). Secondary antibodies used were as follows: Alexa Fluor 568-labeled goat anti-mouse IgG (1:400, Molecular Probes) was used for anti-III-β-tubulin, anti-SMA, and anti-K15 and Alexa Fluor 568-labeled goat anti-chicken IgG (1:1000, Molecular Probes) was used for anti-GFAP. For quantification of the percentage of cells producing a given marker protein, at least four fields were photographed in any given experiment. The number of positive cells was determined relative to the total number of cells with 4′,6-diamidino-2-phenylindole dihydrochloride (Molecular Probes) nuclear staining [12].
16.2.8 Expression of Stem-Cell Marker Genes
The mRNA levels of stem-cell marker genes (nestin, Sox2, SSEA-1) were examined using real-time polymerase chain reaction (RT-PCR). After 1 week of culture in DMEM/F12 containing 2 % B-27, the growing cells formed hair spheres. Total RNA from 100 hair spheres was extracted using the RNeasy Plus Mini kit (Qiagen). c-DNA was synthesized by high-capacity RNA with a cDNA kit (ABI). Real-time PCR on CFX96 (Bio-Rad) with TaqMan Gene Expression Assays (ABI) and TaqMan Probes were used as follows: r18s:Hs99999901_s1; Nestin: Mm00450205_m1; Sox2: Mm03053810_s1; and SSEA-1: Mm00487448_s1. The mRNA levels were normalized by comparison with r18s.
Western blot analysis was also used to detect the expression of SSEA-1. After 1 week of culture in DMEM/F12 containing 2 % B-27, the growing cells formed hair spheres. Total proteins (30 μg/well) from hair spheres were subjected to 4–20 % SDS-PAGE (sodium dodecyl sulfate-polyacrylamide gel electrophoresis) and then transferred to Immobilon-P membranes (Millipore Corporation). SSEA-1 was detected by an anti-SSEA-1 primary antibody (1:250, BioLegend), followed by a mixture of peroxidase-conjugated anti-mouse IgA, IgG and IgM (Chemicon) with enhanced chemiluminescence plus a Western blotting detection system (Amersham Biosciences). Results are expressed as mean±standard deviation (SD) for three samples each.
16.2.9 Gelfoam® Histoculture of Whisker Hair Follicles
After 1 week, the cryopreserved hair follicles were thawed at 37 °C in a water bath with gentle shaking. The hair follicles were washed at DMEM medium for in vitro culture and in vivo transplantation. The cryopreserved hair follicles or fresh-isolated hair follicles were carefully placed on sterile Gelfoam® (Pharmacia and Upjohn Co., Kalamazoo, MI), hydrated with DMEM-F12 (GIBCO, Life Technologies, Grand Island, NY) containing B-27 (2.5 %) (GIBCO), N2 (1 %) (GIBCO) and 1 % penicillin and streptomycin (GIBCO). The Gelfoam® follicles cultures [6, 11, 12, 15] were incubated at 37 °C, 5 % CO2. The medium was changed every other day. High-resolution images of hair follicle growth on Gelfoam® were obtained with a confocal laser-scanning microscope (Fluoview FV1000, Olympus, Tokyo, Japan) [6, 11, 12, 15].
16.2.10 16.2.10 Subcutaneous Transplantation of Hair Follicles
Non-transgenic nude mice were anesthetized with a ketamine mixture (intramuscular injection of a 0.02 ml solution of 20 mg/kg ketamine, 15.2 mg/kg xylazine, and 0.48 mg/kg acepromazine maleate). Thawed hair follicles were transplanted into the subcutis on the right flank of nude mice and fresh isolated hair follicles were transplanted to the left flank as controls. A skin flap was made to expose the growing hair follicle every 2 weeks (2, 4, 6, and 8 weeks) in order to observe hair growth . Images were obtained with a Dino-Lite microscope portable imager (AM4113TGFBW Dino-Lite Premier; AnMo Electronics Corporation, Taiwan) [12, 16].
16.2.11 16.2.10 Measurement of Hair Shaft Length
The in vivo hair follicle images obtained with the Dino-Lite were used to determine the length of each hair shaft using Image Pro Plus 6.0 software. The average length of the ten longest hair follicles in each group are presented as mean±SEM [12].
16.2.12 16.2.10 Statistical Analysis
All experiments were conducted in three independent cultures. Results are expressed as mean±SD. P-values were calculated with the paired Student’s t-test. Group differences were obtained using the ANOVA test. The significant level for all tests was P < 0.05 [12].
16.3 Results and Discussion
16.3.1 Effects of Cryopreservation of the Whisker Hair Follicle and HAP Stem Cells
After thawing and 4 weeks culture in DMEM with 10 % FBS, the upper parts of hair follicles were placed in dishes. The attachment rate of the hair follicles and number of cells growing out from the follicle were quantified as follows: 95.2 % ± 7.7 % of fresh hair follicles (nonfrozen control) attached and 5.9 ± 0.9 × 104 cells per hair follicle grew out. 90.8 % ± 13.3 % of hair follicles cryopreserved by the slow-rate cooling method attached and 3.7 ± 1.3 × 104cells per hair follicle grew out [13].
The upper part of the fresh vibrissa had 38.0 ± 9.1 hair spheres growing, with each hair sphere containing ∼1 × 102 nestin-expressing HAP stem cells. Slow-rate cooling-cryopreserved hair follicles formed 41.6 ± 5.9 hair spheres per upper part of hair follicle, with each hair sphere containing ∼1 × 102 nestin-expressing HAP stem cells [13].
With hair spheres derived from fresh hair follicles, ∼4.3 % ± 3.6 % cells differentiated to anti-III-β-tubulin-positive neurons; 30.2 % ± 8.1 % of the cells differentiated to GFAP-positive glial cells; 40.2 % ± 8.0 % of the cells differentiated to SMA-positive smooth muscle cells; and 7.2 % ± 5.7 % of the cells differentiated to K15-positive keratinocytes. With hair spheres derived from slow-rate cooling-cryopreserved hair follicles, ∼12.6 % ± 3.2 % of the cells differentiated to anti-III-β-tubulin-positive neurons; 34.0 % ± 6.6 % of the cells differentiated to GFAP-positive glial cells; 63.7 % ± 10.0 % of the cells differentiated to SMA-positive smooth muscle cells; and 11.0 % ± 2.1 % of the cells differentiated to K15-positive keratinocytes [13].
Nestin RNA levels, relative to r18s, were 1.00 ± 0.11 in fresh hair follicles and 1.01 ± 0.02 in slow-rate cooled hair follicles. Sox2 mRNA levels, relative to r18s, were 1.00 ± 0.02 in fresh hair follicles and 0.81 ± 0.07 in slow-rate cooled hair follicles. SSEA-1 mRNA levels, relative to r18s, were 1.00 ± 0.02 in fresh hair follicles and 0.90 ± 0.03 in slow-rate cooled hair follicles. Thus, the mRNA levels of stem-cell marker genes were maintained in the slow-rate cooling method [13].
Western blot analysis was performed to detect the expression of SSEA-1. The protein levels of SSEA-1 were maintained in the slow-rate cooling method [13].
16.3.2 Cryopreservation of Hair Spheres
Fresh hair spheres completely attached when placed in plastic dishes. In contrast, after slow-rate cooling cryopreservation, 5.4 % ± 3.2 % of the hair spheres survived. With vitrification rapid-cooled cryopreservation, ~12.5 % + 10.8 % of the hair spheres survived. However, after thawing, slow rate cooled and vitrification rapid-cooled hair spheres did not differentiate. Our results demonstrate that slow-rate cooling cryopreservation of hair follicles preserves the same attachment rate and production of pluripotent hair spheres as fresh hair follicles [13].
16.3.3 Whisker Hair Follicle and HAP Stem Cell Behavior in Gelfoam® Histoculture After Cryopreservation
After thawing and culture on Gelfoam® for 4 weeks, the ND-GFP fluorescence of the HAP stem cells was measured. Confocal real-time 3D images of ND-GFP HAP hair follicle on Gelfoam® showed that ND-GFP HAP stem-cell-fluorescence in fresh hair follicle had dramatic increases at 10, 16 and 26 days. The increases in HAP stem cell ND-GFP fluorescence after DMSO cryopreservation of hair follicles was slower than in fresh follicles. Glycerol-cryopreserved follicles had less increases in ND-GFP fluorescence than the DMSO-preserved follicles (Fig. 16.1). At day 26, 2/6 hair follicles cryopreserved in glycerol recovered to grow ND-GFP HAP stem cells, 2/6 had poor growth, and 2/6 were consider dead. In contrast, 6/6 hair follicles frozen in DMSO recovered [12].
After 26 days in Gelfoam® histoculture, 3D confocal images showed that ND-GFP HAP stem cell outgrowth occurred both at the upper and lower part of fresh hair follicles. DAPI nuclear staining showed that the fresh hair follicles maintained normal structures in the hair shaft, inner root sheath, and outer root sheath, and a large amount of ND-GFP HAP stem cells grew around the bulb area. In DMSO-cryopreserved follicles, ND-GFP 3D images indicated similar HAP-stem cell outgrowth both in the upper and lower part of hair follicle, but with less cell outgrowth than in fresh hair follicles. With glycerol-cryopreservation, the recovered hair follicles had less ND-GFP HAP stem cells. The cultured hair follicles had ND-GFP HAP stem cells mostly in the outer root sheath and the bulb area after both DMSO- and glycerol-cryopreservation. In contrast, the inner structure of cultured hair follicles had little DAPI nuclear staining remained after either method of cryopreservation. These results indicate cell death of the inner part and structural damage of the frozen hair follicle in both cryopreservation media. The bulb area of the hair follicle had ND-GFP-nestin HAP stem cells growing out, and the dermal papilla area maintained DAPI nuclear staining after both cryopreservation methods. These results suggest the survival of bulb area and dermal papilla cells in both DMSO and glycerol-cryopreserved hair follicles. p75NTR immunostaining also indicated ND-GFP HAP stem cells were maintained in both conditions of cryopreservation [12].
16.3.4 Functional Recovery of Cryopreserved Hair Follicles After Subcutaneous Transplantation
After the hair follicles were cryopreserved in DMSO for 1 week at −80 °C, the hair follicles were thawed and transplanted subcutaneously in nude mice. Each flank was transplanted with five to six hair follicles and a total of three mice were used. Eight of 15 transplanted cryopreserved hair follicles grew out extensive long hair shafts (Fig. 16.2) [12].
In fresh hair follicles, the hair follicle established blood vessel connection with the host nude mice and grew rapidly by week 2 (1.26 mm ± 0.15 mm). At week 4, the average of the ten longest hair-shaft length was 3.82 mm ± 0.31 mm. At week 6, the average of the ten longest hair-shaft length was 5.73 mm ± 0.31 mm), and at week 8, the hair shafts length was 7.66 mm ± 0.63 mm. The DSMO-cryopreserved hair follicles also established blood vessel connections with the host nude mice as indicated by red blood in the lower part and upper part of the hair follicle. At week 4, there were more blood vessels surrounding the hair follicles. There were obvious hair roots at the bulb area as well as longer hair shafts shaft at week 4, with the longest length of 1.88 mm ± 0.55 mm, compared to week 2, when the longest hair shaft length was 0.85 mm ± 0.07 mm. These results indicate that the DSMO-cryopreserved hair follicle started to rapidly regrow hair. At week 6, the longest hair shafts grew up to 2.85 mm ± 0.66 mm and more hair roots in the bulb area were observed, as well as long hair shafts growing out. Eventually, at week 8, there were a large amount of long hair shafts with the longest with a length of 4.92 mm ± 0.97 mm. Our results showed that DSMO-cryopreserved hair follicles maintained the ability to produce hair shafts after subcutaneous transplantation [12].
16.4 Conclusions
Hair follicles have important potential including hair transplantation and use of HAP stem cells for regenerative medicine for nerves, spinal cord, heart and other applications .
The present chapter suggests that all these potential applications are maintained after hair follicle cryopreservation. Each potential patient can, therefore, have a bio-bank of their own hair follicles.
Abbreviations
- HAP:
-
Hair follicle-associated pluripotent
- ES:
-
Embryonic stem
- GFP:
-
Green fluorescent protein
- ND-GFP:
-
Nestin-driven-GFP
- SMA:
-
Smooth muscle actin
- GFAP:
-
Glial fibrillary acidic protein
- RT-PCR:
-
Real-time polymerase chain reaction
References
Li L, Mignone J, Yang M, Matic M, Penman S, Enikolopov G, Hoffman RM (2003) Nestin expression in hair follicle sheath progenitor cells. Proc Natl Acad Sci U S A 100:9958–9961
Amoh Y, Li L, Katsuoka K, Penman S, Hoffman RM (2005) Multipotent nestin-positive, keratin-negative hair-follicle-bulge stem cells can form neurons. Proc Natl Acad Sci U S A 102:5530–5534
Amoh Y, Li L, Katsuoka K, Hoffman RM (2008) Multipotent hair follicle stem cells promote repair of spinal cord injury and recovery of walking function. Cell Cycle 7:1865–1869
Liu F, Uchugonova A, Kimura H, Zhang C, Zhao M, Zhang L, Koenig K, Duong J, Aki R, Saito N, Mii S, Amoh Y, Katsuoka K, Hoffman RM (2011) The bulge area is the major hair follicle source of nestin-expressing pluripotent stem cells which can repair the spinal cord compared to the dermal papilla. Cell Cycle 10:830–839
Amoh Y, Kanoh M, Niiyama S, Hamada Y, Kawahara K, Sato Y, Hoffman RM, Katsuoka K (2009) Human hair follicle pluripotent stem (hfPS) cells promote regeneration of peripheral-nerve injury: an advantageous alternative to ES and iPS cells. J Cell Biochem 107:1016–1020
Mii S, Duong J, Tome Y, Uchugonova A, Liu F, Amoh Y, Saito N, Katsuoka K, Hoffman RM (2013) The role of hair follicle nestin-expressing stem cells during whisker sensory-nerve growth in long-term 3D culture. J Cell Biochem 114:1674–1684
Yashiro M, Mii S, Aki R, Hamada Y, Arakawa N, Kawahara K, Hoffman RM, Amoh Y (2015) From hair to heart: nestin-expressing hair-follicle-associated pluripotent (HAP) stem cells differentiate to beating cardiac muscle cells. Cell Cycle 14:2362–2366
Yamazaki A, Yashiro M, Mii S, Aki R, Hamada Y, Arakawa N, Kawahara K, Hoffman RM, Amoh Y (2016) Isoproterenol directs hair follicle-associated pluripotent (HAP) stem cells to differentiate in vitro to cardiac muscle cells which can be induced to form beating heart muscle tissue sheets. Cell Cycle 15:760–765
Hoffman RM (2016) Chap. 1: Introduction to hair-follicle-associated pluripotent (HAP) stem cells. In: Hoffman RM (ed) Multipotent stem cells of the hair follicle: methods and protocols, (Walker JM, series ed.). Methods Mol Biol 1453:0–5. Humana Press (Springer Science+Business Media), New York. doi:10.1007/978-1-4939-3786-8_1
Okabe M, Ikawa M, Kominami K, Nakanishi T, Nishimune Y (1997) ‘Green mice’ as a source of ubiquitous green cells. FEBS Lett 407:313–319
Duong J, Mii S, Uchugonova A, Liu F, Moossa AR, Hoffman RM (2012) Real-time confocal imaging of trafficking of nestin-expressing multipotent stem cells in mouse whiskers in long-term 3-D histoculture. In Vitro Cell Dev Biol Anim 48:301–305
Cao W, Li L, Tran B, Kajiura S, Amoh Y, Liu F, Hoffman RM (2015) Extensive hair shaft growth after mouse whisker follicle isolation, cryopreservation and transplantation in nude mice. PLoS ONE 10:e0145997
Kajiura S, Mii S, Aki R, Hamada Y, Arakawa N, Kawahara K, Li L, Katsuoka K, Hoffman RM, Amoh Y (2015) Cryopreservation of the hair follicle maintains pluripotency of nestin-expressing hair follicle-associated pluripotent stem cells. Tissue Eng Part C 21:825–831
Amoh Y, Mii S, Aki R, Hamada Y, Kawahara K, Hoffman RM, Katsuoka K (2012) Multipotent nestin-expressing stem cells capable of forming neurons are located in the upper, middle and lower part of the vibrissa hair follicle. Cell Cycle 11:3513–3517
Mii S, Uehara F, Yano S, Tran B, Miwa S, Hiroshima Y, Amoh Y, Katsuoka K, Hoffman RM (2013) Nestin-expressing stem cells promote nerve growth in long-term 3-dimensional Gelfoam®-supported histoculture. PLoS ONE 8:e67153
Hiroshima Y, Maawy A, Sato S, Murakami T, Uehara F, Miwa S, Yano S, Momiyama M, Chishima T, Tanaka K, Bouvet M, Endo I, Hoffman RM (2014) Hand-held high-resolution fluorescence imaging system for fluorescence-guided surgery of patient and cell-line pancreatic tumors growing orthotopically in nude mice. J Surg Res 187:510–517
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2016 Springer International Publishing Switzerland
About this chapter
Cite this chapter
Hoffman, R.M., Kajiura, S., Cao, W., Liu, F., Amoh, Y. (2016). Cryopreservation of Hair-Follicle Associated Pluripotent (HAP) Stem Cells Maintains Differentiation and Hair-Growth Potential. In: Karimi-Busheri, F., Weinfeld, M. (eds) Biobanking and Cryopreservation of Stem Cells. Advances in Experimental Medicine and Biology, vol 951. Springer, Cham. https://doi.org/10.1007/978-3-319-45457-3_16
Download citation
DOI: https://doi.org/10.1007/978-3-319-45457-3_16
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-45455-9
Online ISBN: 978-3-319-45457-3
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)