Abstract
The incidence of thyroid cancer is rising, and this rise in incidence is seen within all genders, races, and socioeconomic (SES) classes. The etiology of this rise in incidence remains unclear. It is known that the diagnostic cascade for thyroid cancer starts with identifying a thyroid nodule and subsequently undergoing ultrasound-guided fine needle aspiration (FNA). In a small subset of thyroid cancer cases, there is an identifiable cancer risk factor: exposure to ionizing radiation or family history. However, for the overwhelming majority of thyroid cancer patients, there is no clear risk factor for their cancer. Although some speculate that a new or previously unidentified risk factor may explain the rising incidence of thyroid cancer, the majority of the existing data supports the theory of overdiagnosis of indolent disease. Regardless of the etiology of the rise in thyroid cancer incidence, there are implications for management. Differentiating the indolent disease from the potentially life-threatening disease is complicated and requires both a greater understanding of the pathogenesis and of the implications of the thyroid cancer epidemic.
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
Keywords
- Thyroid cancer
- Incidence
- Risk factor
- Radiation
- Family history
- Obesity
- Diabetes
- Iodine
- Autoimmune thyroid
- Overdiagnosis
Rise in Thyroid Cancer Incidence
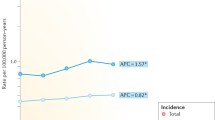
The incidence of thyroid cancer has tripled over the past 30 years (Fig. 1.1), with thyroid cancer now the eighth most common cancer in the United States and the fifth most common cancer in women [1–3]. Although thyroid cancers of all sizes have increased in incidence, 87 % of the rise in thyroid cancer is attributed to small papillary thyroid cancers (2 cm or smaller), which have an excellent prognosis [1]. It is estimated that in 2015, there will be 62,450 new cases of thyroid cancer but only 1,950 deaths [4]. Because of this rising incidence, thyroid cancer is projected to be the fourth most common cancer by 2030 [2, 5–9]. Not only has the incidence risen in the United States, the rise in thyroid cancer incidence has been seen across the world [10]. This rise in thyroid cancer incidence is most marked in Korea, where thyroid cancer is now the most common cancer and the incidence is close to 70/100,000 [11]. This worldwide unexplained rise in thyroid cancer incidence remains a major concern for physicians treating thyroid cancer.
Based on SEER data, the number of new cases of thyroid cancer was 13.5 per 100,000 men and women per year. The number of deaths was 0.5 per 100,000 men and women per year. These rates are age adjusted and based on 2008–2012 cases and deaths [5]
The greatest rise in thyroid cancer incidence has been seen in women [12]. Women represent close to 75 % of all thyroid cancer cases and the incidence has risen in both men and women but at a greater rate in women. From 1980 to 1983 versus 2003 to 2005, papillary thyroid cancer rates tripled among white and black females and doubled among white and black males [12]. Although two-thirds of thyroid cancers occur in patients < age 55, the fastest rise in incidence has been seen in adults over age 65 [13, 14]. Older adults have the highest incidence of thyroid cancer per 100,000, with 25.84 new cases diagnosed in patients ages 65–74 years versus 15.16 diagnosed in patients ages 20–49 [5]. Adults aged ≥ 65 also have the greatest growth in incidence with an annual percentage change of 8.8 % versus 6.4 % for those aged < 65 years [5, 15]. The rise in incidence has been seen across race groups, but incidence rates tend to be higher among whites than blacks and among white non-Hispanics than white Hispanics and Asian Pacific Islanders [12]. Historically, thyroid cancer diagnosis has been more common in cohorts with higher socioeconomic status (SES). Based on Surveillance, Epidemiology and End Results (SEER) data from 497 counties in the United States, county papillary thyroid cancer incidence positively correlates with rates of college education, white-collar employment, and family income [15].
The Origin of Thyroid Cancer
Diagnosing thyroid cancer usually starts with identifying a thyroid nodule and/or occasionally lateral neck mass. Between 20 and 70 % of adults have thyroid nodules with older adults having a higher prevalence than younger [16, 17]. In patients with thyroid nodules, male gender, younger age, and high-risk ultrasound characteristics, such as irregular borders, solid, hypoechoic, larger size, and microcalcifications, are associated with greater likelihood of thyroid cancer [18–21].
The majority of thyroid cancers are identified with fine-needle aspiration of a thyroid nodule. Of the nodules that undergo fine needle aspiration (FNA), only 5–8 % are thyroid cancer [22–24]. Although most cancers are diagnosed by FNA, 6–21% of the thyroid operations planned for treatment of benign disease have incidental discovery of thyroid cancer postoperatively [25–27].
The most common thyroid cancer diagnosed is papillary thyroid cancer, which represents 85 % of all thyroid cancers [28]. Additional thyroid cancers include other well-differentiated cancers, such as follicular and Hürthle cell which represent approximately 10 and 3 % of thyroid cancers, respectively [28]. Medullary thyroid cancer arises from c-cells and accounts for less than 5 % of all thyroid cancers [28–30]. Anaplastic is rare and deadly and represents only 1 % of all thyroid cancers [31, 32].
Risk Factors for Thyroid Cancer
As shown in Table 1.1, there are two accepted risk factors for well-differentiated thyroid cancer: ionizing radiation and family history. Ionizing radiation is thought to cause cancer through somatic mutations and DNA strand breaks [14, 33]. When catastrophic events such as Chernobyl happen, the risk of thyroid cancer is dose and age related [34]. Children and young adults under age 20 years are most susceptible to radiation-induced thyroid cancers [14, 35, 36]. Similarly, children who underwent radiation therapy for childhood cancers, acne, treatment of enlarged thymus, etc. also have an increased risk for thyroid cancer [37]. In addition, to radiation exposure, familial nonmedullary thyroid cancer does exist. If two or more first-degree relatives have well-differentiated thyroid cancer, then it is presumed to be hereditary. However, this hereditary form of well-differentiated thyroid cancer cannot be tracked with genetic testing and is thought to represent just over 5 % of all well-differentiated thyroid cancers [38–41]. Recently, a germline variant in HABP2 was identified in familial nonmedullary thyroid cancer [41]. Therefore, for the majority of patients with well-differentiated thyroid cancer, there is no clear etiology of their thyroid cancer and the cancer thought to be sporadic.
In comparison, for medullary thyroid cancer, up to 1–7% of patients with apparently sporadic medullary thyroid cancer end up having germline mutations and associated syndromes MEN2A and MEN2B [42, 43]. Genetic testing can identify the RET mutation involved in development of the medullary thyroid cancer, and then subsequent testing can identify family members at risk. In addition to germline mutations, up to half of patients with sporadic medullary thyroid cancer patients have an unidentified somatic RET mutation [44].
There are no clear risk factors for anaplastic thyroid cancer. However, anaplastic thyroid cancer is thought to arise from a well-differentiated thyroid cancer, and it is more common in older adults [32]. There is an accepted “second hit” hypothesis that well-differentiated thyroid cancers typically need a second mutation, often p53, to develop anaplastic thyroid cancer [31].
Proposed Explanations for the Rise in Thyroid Cancer Incidence
Table 1.2 illustrates the two broad theories to explain the rise in thyroid cancer incidence [14]. One theory is that new or previously unidentified risk factors for thyroid cancer explain the rise in incidence. These proposed risk factors would include radiation exposure outside of known catastrophic events or treatment of childhood cancers, obesity/diabetes, autoimmune thyroid disease, and iodine deficiency or excess. Another conflicting theory is that we have detection bias or in essence overdiagnosis leading to the rise in thyroid cancer incidence. In principle, there is a large reservoir of indolent thyroid cancer, and the more we “look,” the more we find. Based on this overdiagnosis theory, increased use of imaging, FNA, surgery, and pathologic inspection leads to detection of cancers that otherwise may have been unidentified. In the following section, we will explain both conflicting theories.
Explanation 1: New Risk Factor
Exposure to ionizing radiation is an accepted risk factor for well-differentiated thyroid cancer. However, typically patients aged 20 years and younger are most at risk, and in the absence of a recent catastrophic world event, such as Chernobyl or the 2011 reactor meltdown in Fukushima, Japan, this seems like an unlikely explanation for the worldwide rise in thyroid cancer incidence [14, 36]. Although some do worry that the background level of radiation exposure, especially due to increased use of CT scans, has increased and may contribute to the rise in thyroid cancer incidence, it has been estimated that pediatric CT scans account for <1% of the increased incidence of thyroid cancer [14]. Obesity and diabetes mellitus are increasingly common problems in well-developed countries. In parallel to the thyroid cancer epidemic is the obesity and diabetes epidemic [12, 45, 46]. Thus, it has been hypothesized that the thyroid cancer epidemic may be related to the rise in obesity and diabetes mellitus. A pooled analysis of five prospective studies found that obesity is an independent risk factor for thyroid cancer [47]. However, there is more conflicting data when evaluating diabetes and its relationship to thyroid cancer [48, 49]. To-date although correlations between both obesity and diabetes mellitus with thyroid cancer have been noted, causality has not been shown [47, 48].
Autoimmune thyroid disease, specifically Hashimoto’s thyroiditis and Graves’ disease, are common benign thyroid conditions that are similar to the thyroid cancer in the fact that they are far more common in women. There has been longstanding controversy about whether or not autoimmune thyroid disease is associated with thyroid cancer. Surgical studies have suggested a clear association, whereas studies based on FNA samples or antibodies have been less supportive [50–53]. Although thyroid cancer incidence has been rising, there is no clear data that autoimmune thyroid disease is more prevalent now than 30 years ago. Thus, this is an unlikely explanation for the recent rise in thyroid cancer incidence.
Iodine deficiency or excess is known to affect the proportions of thyroid cancer types in the world, as iodine deficiency is associated with a higher proportion of follicular thyroid cancer [54, 55]. However, the relationship between iodine levels and the recent pattern of diagnosing smaller papillary thyroid cancers is unclear [55].
Theory 2: Overdiagnosis
Suggesting that we may be tapping into a reservoir of indolent disease, the greatest rise in incidence has been in low-risk disease. Eighty-seven percent of the increase in thyroid cancer incidence is attributed to papillary thyroid cancers measuring 2 cm or smaller [1]. Since most disease detected is small, the prognosis for most patients is excellent [56]. Mortality for thyroid cancer has consistently been around 0.5 per 100,000 population [5]. Five-year disease-specific survival for localized disease is 99.8%, and for regional disease, it is 97% [2, 9]. Finally, thorough autopsy studies have revealed incidental small thyroid cancers in up to 36% of adults who die from another cause [57]. If pathologic slicing of thyroid specimens were finer, some have suggested that the actual prevalence may be as high as 100% [57]. This implies that there may be a bottomless reservoir of indolent disease. The fact that the rise in thyroid cancer incidence is primarily due to increased detection of low-risk disease and the fact that thyroid cancer is a common incidental finding on autopsy studies support the hypothesis that overdiagnosis may play an important role in the rise of thyroid cancer incidence in developed countries.
Excess thyroid imaging may be one of the reasons for the overdiagnosis of small thyroid cancers. Since at least half of all adults have thyroid nodules, increased imaging can lead to incidental nodule discovery [16, 17, 58, 59]. The rates of detection of thyroid nodules are 67% with thyroid ultrasound (US), 16% with CT and MRI, 9% with carotid duplex, and 3% with positron emission tomography (PET) or PET/CT [59–63]. Over the past 15 years, there has been a rise in the use of imaging studies that are associated with incidental thyroid nodule discovery. Based on data from a large health plan, between 1997 and 2006 use of US increased 40%, use of computed tomography (CT) doubled, and use of magnetic resonance imaging (MRI) tripled [64]. In addition to the rise in use of imaging associated with incidental cancer discovery, thyroid US is increasingly becoming an extension of the physical exam. When there is a palpable nodule, thyroid US is the preferred method of imaging [19]. Neck imaging to evaluate thyroid nodules and symptoms and neck imaging performed for other reasons have contributed to the rise in thyroid cancer incidence [65, 66].
Related to more imaging, greater use of thyroid FNA also leads to more cancer diagnosis. Use of thyroid FNAs more than doubled between 2006 and 2011 [67]. Since approximately 5–8% of thyroid nodules undergoing FNA are cancer and 20% are indeterminate, more FNAs will result in more cancer diagnoses [22–24].
More surgery may also play a role in the thyroid cancer epidemic. The number of thyroid surgeries being performed in the United States increased by 39% between 1996 and 2006 with one-third of the surgeries performed for thyroid cancer [68, 69]. Between 6 and 21% of thyroid surgeries that are planned for treatment of benign disease have an incidental discovery of thyroid cancer postoperatively [25–27]. Therefore, more thyroid surgery will result in more detection of more low-risk thyroid cancer, thus contributing to the rise in thyroid cancer incidence.
In addition to surgery, in recent years pathologic evaluation has become more detailed. Currently pathologists examine the entire thyroid and 14 versus five descriptive areas are reported [14, 70, 71]. This too could potentially contribute to the rise in thyroid cancer incidence as more small incidental cancers are detected.
Conclusion
There has been a rise in thyroid cancer incidence. Although there is an ongoing debate as to whether a new risk factor versus overdiagnosis explains the rise in incidence, most data support overdiagnosis playing a significant role in the developed countries for this finding. Regardless of etiology, this rise in thyroid cancer incidence has implications for patients, physicians, and society. As we diagnose more thyroid cancer, it becomes increasingly important to understand which patients need intensive treatment to prevent poor outcome, including death and recurrence, and which patients have low-risk disease and need minimal intervention or surveillance only.
References
Davies L, Welch HG. Increasing incidence of thyroid cancer in the United States, 1973–2002. JAMA. 2006;295(18):2164–7.
Simard EP, Ward EM, Siegel R, Jemal A. Cancers with increasing incidence trends in the United States: 1999 through 2008. CA Cancer J Clin. 2012;62(2):118–28.
https://www.cancer.gov/types/common-cancers National Cancer Institute. Common Cancer Types. Feb. 1, 2006.
American Cancer Society Cancer Facts and Figures 2015 Atlanta, Ga: American Cancer Society. Available from: http://www.cancer.org/acs/groups/content/@editorial/documents/document/acspc-044552.pdf. Cited 2015.
Surveillance, Epidemiology, and End Results Program (SEER). Available from: www.seer.cancer.gov. Cited 2015.
National Cancer Institute at the National Institutes of Health; Common Cancer Types. Available from: http://www.cancer.gov/cancertopics/types/commoncancers. Cited 2015.
Rahib L, Smith BD, Aizenberg R, Rosenzweig AB, Fleshman JM, Matrisian LM. Projecting cancer incidence and deaths to 2030: the unexpected burden of thyroid, liver, and pancreas cancers in the United States. Cancer Res. 2014;74(11):2913–21.
Esserman LJ, Thompson Jr IM, Reid B. Overdiagnosis and overtreatment in cancer: an opportunity for improvement. JAMA. 2013;310(8):797–8.
Siegel R, Naishadham D, Jemal A. Cancer statistics, 2012. CA Cancer J Clin. 2012;62(1):10–29.
Brito JP, Morris JC, Montori VM. Thyroid cancer: zealous imaging has increased detection and treatment of low risk tumours. BMJ. 2013;347:f4706.
Ahn HS, Kim HJ, Welch HG. Korea’s thyroid-cancer “epidemic”--screening and overdiagnosis. N Engl J Med. 2014;371(19):1765–7.
Enewold L, Zhu K, Ron E, Marrogi AJ, Stojadinovic A, Peoples GE, et al. Rising thyroid cancer incidence in the United States by demographic and tumor characteristics, 1980–2005. Cancer Epidemiol Biomarkers Prev. 2009;18(3):784–91.
The American Cancer Society. Available from: www.cancer.org. Cited 2015.
Davies L, Morris L, Haymart M, Chen A, Goldenberg D, Morris J, et al. AACE Endocrine Surgery Scientific Committee Disease Review Statement: on the Increasing Incidence of Thyroid Cancer. Endocr Pract. 2015;21(6):686–96.
Morris LG, Sikora AG, Tosteson TD, Davies L. The increasing incidence of thyroid cancer: the influence of access to care. Thyroid. 2013;23(7):885–91.
Tan GH, Gharib H. Thyroid incidentalomas: management approaches to nonpalpable nodules discovered incidentally on thyroid imaging. Ann Intern Med. 1997;126(3):226–31.
Rojeski MT, Gharib H. Nodular thyroid disease. Evaluation and management. N Engl J Med. 1985;313(7):428–36.
Smith-Bindman R, Lebda P, Feldstein VA, Sellami D, Goldstein RB, Brasic N, et al. Risk of thyroid cancer based on thyroid ultrasound imaging characteristics: results of a population-based study. JAMA Intern Med. 2013;173(19):1788–96.
Cooper DS, Doherty GM, Haugen BR, Kloos RT, Lee SL, Mandel SJ, et al. Revised American Thyroid Association management guidelines for patients with thyroid nodules and differentiated thyroid cancer. Thyroid. 2009;19(11):1167–214.
Fish SA, Langer JE, Mandel SJ. Sonographic imaging of thyroid nodules and cervical lymph nodes. Endocrinol Metab Clin North Am. 2008;37(2):401–17, ix.
Haugen BR, Alexander EK, Bible KC, Doherty GM, Mandel SJ, Nikiforov YE, et al. 2015 American Thyroid Association Management Guidelines for Adult Patients with Thyroid Nodules and Differentiated Thyroid Cancer: The American Thyroid Association Guidelines Task Force on Thyroid Nodules and Differentiated Thyroid Cancer. Thyroid. 2016;26(1):1–133.
Alexander EK, Kennedy GC, Baloch ZW, Cibas ES, Chudova D, Diggans J, et al. Preoperative diagnosis of benign thyroid nodules with indeterminate cytology. N Engl J Med. 2012;367(8):705–15.
Yassa L, Cibas ES, Benson CB, Frates MC, Doubilet PM, Gawande AA, et al. Long-term assessment of a multidisciplinary approach to thyroid nodule diagnostic evaluation. Cancer. 2007;111(6):508–16.
Gharib H, Papini E, Paschke R, Duick DS, Valcavi R, Hegedus L, et al. American Association of Clinical Endocrinologists, Associazione Medici Endocrinologi, and European Thyroid Association medical guidelines for clinical practice for the diagnosis and management of thyroid nodules: Executive Summary of recommendations. J Endocrinol Invest. 2010;33(5):287–91.
Deveci MS, Deveci G, LiVolsi VA, Gupta PK, Baloch ZW. Concordance between thyroid nodule sizes measured by ultrasound and gross pathology examination: effect on patient management. Diagn Cytopathol. 2007;35(9):579–83.
Haymart MR, Cayo M, Chen H. Papillary thyroid microcarcinomas: big decisions for a small tumor. Ann Surg Oncol. 2009;16(11):3132–9.
Ito Y, Higashiyama T, Takamura Y, Miya A, Kobayashi K, Matsuzuka F, et al. Prognosis of patients with benign thyroid diseases accompanied by incidental papillary carcinoma undetectable on preoperative imaging tests. World J Surg. 2007;31(8):1672–6.
Hundahl SA, Fleming ID, Fremgen AM, Menck HR. A National Cancer Data Base report on 53,856 cases of thyroid carcinoma treated in the U.S., 1985–1995. Cancer. 1998;83(12):2638–48.
Saad MF, Ordonez NG, Rashid RK, Guido JJ, Hill Jr CS, Hickey RC, et al. Medullary carcinoma of the thyroid. A study of the clinical features and prognostic factors in 161 patients. Medicine. 1984;63(6):319–42.
Giuffrida D, Gharib H. Current diagnosis and management of medullary thyroid carcinoma. Ann Oncol Off J Eur Soc Med Oncol/ESMO. 1998;9(7):695–701.
Smallridge RC, Marlow LA, Copland JA. Anaplastic thyroid cancer: molecular pathogenesis and emerging therapies. Endocr Relat Cancer. 2009;16(1):17–44.
Nagaiah G, Hossain A, Mooney CJ, Parmentier J, Remick SC. Anaplastic thyroid cancer: a review of epidemiology, pathogenesis, and treatment. J oncol. 2011;2011:542358.
Nikiforova MN, Stringer JR, Blough R, Medvedovic M, Fagin JA, Nikiforov YE. Proximity of chromosomal loci that participate in radiation-induced rearrangements in human cells. Science. 2000;290(5489):138–41.
Zablotska LB, Nadyrov EA, Rozhko AV, Gong Z, Polyanskaya ON, McConnell RJ, et al. Analysis of thyroid malignant pathologic findings identified during 3 rounds of screening (1997–2008) of a cohort of children and adolescents from belarus exposed to radioiodines after the Chernobyl accident. Cancer. 2015;121(3):457–66.
Schonfeld SJ, Lee C, Berrington de Gonzalez A. Medical exposure to radiation and thyroid cancer. Clin Oncol. 2011;23(4):244–50.
Furukawa K, Preston D, Funamoto S, Yonehara S, Ito M, Tokuoka S, et al. Long-term trend of thyroid cancer risk among Japanese atomic-bomb survivors: 60 years after exposure. Int J Cancer. 2013;132(5):1222–6.
de Vathaire F, Haddy N, Allodji R, Hawkins M, Guibout C, El-Fayech C, et al. Thyroid radiation dose and other risk factors of thyroid carcinoma following childhood cancer. J Clin Endocrinol Metab. 2015;100:4282–90. jc20151690.
Fallah M, Pukkala E, Tryggvadottir L, Olsen JH, Tretli S, Sundquist K, et al. Risk of thyroid cancer in first-degree relatives of patients with non-medullary thyroid cancer by histology type and age at diagnosis: a joint study from five Nordic countries. J Med Genet. 2013;50(6):373–82.
Sippel RS, Caron NR, Clark OH. An evidence-based approach to familial nonmedullary thyroid cancer: screening, clinical management, and follow-up. World J Surg. 2007;31(5):924–33.
Nose V. Familial non-medullary thyroid carcinoma: an update. Endocr Pathol. 2008;19(4):226–40.
Gara SK, Jia L, Merino MJ, Agarwal SK, Zhang L, Cam M, et al. Germline HABP2 mutation causing familial nonmedullary thyroid cancer. N Engl J Med. 2015;373(5):448–55.
Eng C, Mulligan LM, Smith DP, Healey CS, Frilling A, Raue F, et al. Low frequency of germline mutations in the RET proto-oncogene in patients with apparently sporadic medullary thyroid carcinoma. Clin Endocrinol (Oxf). 1995;43(1):123–7.
Elisei R, Romei C, Cosci B, Agate L, Bottici V, Molinaro E, et al. RET genetic screening in patients with medullary thyroid cancer and their relatives: experience with 807 individuals at one center. J Clin Endocrinol Metab. 2007;92(12):4725–9.
Elisei R, Cosci B, Romei C, Bottici V, Renzini G, Molinaro E, et al. Prognostic significance of somatic RET oncogene mutations in sporadic medullary thyroid cancer: a 10-year follow-up study. J Clin Endocrinol Metab. 2008;93(3):682–7.
Flegal KM, Carroll MD, Kuczmarski RJ, Johnson CL. Overweight and obesity in the United States: prevalence and trends, 1960–1994. Int J obes Relat Metab Disord J Int Assoc Study Obes. 1998;22(1):39–47.
Menke A, Casagrande S, Geiss L, Cowie CC. Prevalence of and trends in diabetes among adults in the United States, 1988–2012. JAMA. 2015;314(10):1021–9.
Kitahara CM, Platz EA, Freeman LE, Hsing AW, Linet MS, Park Y, et al. Obesity and thyroid cancer risk among U.S. men and women: a pooled analysis of five prospective studies. Cancer Epidemiol Biomarkers Prev. 2011;20(3):464–72.
Aschebrook-Kilfoy B, Sabra MM, Brenner A, Moore SC, Ron E, Schatzkin A, et al. Diabetes and thyroid cancer risk in the National Institutes of Health-AARP Diet and Health Study. Thyroid. 2011;21(9):957–63.
Kitahara CM, Platz EA, Beane Freeman LE, Black A, Hsing AW, Linet MS, et al. Physical activity, diabetes, and thyroid cancer risk: a pooled analysis of five prospective studies. Cancer Causes Control. 2012;23(3):463–71.
Ye ZQ, Gu DN, Hu HY, Zhou YL, Hu XQ, Zhang XH. Hashimoto’s thyroiditis, microcalcification and raised thyrotropin levels within normal range are associated with thyroid cancer. World J Surg Oncol. 2013;11:56.
Repplinger D, Bargren A, Zhang YW, Adler JT, Haymart M, Chen H. Is Hashimoto’s thyroiditis a risk factor for papillary thyroid cancer? J Surg Res. 2008;150(1):49–52.
Fiore E, Rago T, Scutari M, Ugolini C, Proietti A, Di Coscio G, et al. Papillary thyroid cancer, although strongly associated with lymphocytic infiltration on histology, is only weakly predicted by serum thyroid auto-antibodies in patients with nodular thyroid diseases. J Endocrinol Invest. 2009;32(4):344–51.
Anil C, Goksel S, Gursoy A. Hashimoto’s thyroiditis is not associated with increased risk of thyroid cancer in patients with thyroid nodules: a single-center prospective study. Thyroid. 2010;20(6):601–6.
Lawal O, Agbakwuru A, Olayinka OS, Adelusola K. Thyroid malignancy in endemic nodular goitres: prevalence, pattern and treatment. Eur J Surg Oncol Journal Eur Soc Surg Oncol Br Assoc Surg Oncol. 2001;27(2):157–61.
Feldt-Rasmussen U. Iodine and cancer. Thyroid. 2001;11(5):483–6.
Ho AS, Davies L, Nixon IJ, Palmer FL, Wang LY, Patel SG, et al. Increasing diagnosis of subclinical thyroid cancers leads to spurious improvements in survival rates. Cancer. 2015;121(11):1793–9.
Harach HR, Franssila KO, Wasenius VM. Occult papillary carcinoma of the thyroid. A “normal” finding in Finland. A systematic autopsy study. Cancer. 1985;56(3):531–8.
Davies L, Ouellette M, Hunter M, Welch HG. The increasing incidence of small thyroid cancers: where are the cases coming from? Laryngoscope. 2010;120(12):2446–51.
Ezzat S, Sarti DA, Cain DR, Braunstein GD. Thyroid incidentalomas. Prevalence by palpation and ultrasonography. Arch Intern Med. 1994;154(16):1838–40.
Jin J, McHenry CR. Thyroid incidentaloma. Best Pract Res Clin Endocrinol Metab. 2012;26(1):83–96.
Steele SR, Martin MJ, Mullenix PS, Azarow KS, Andersen CA. The significance of incidental thyroid abnormalities identified during carotid duplex ultrasonography. Arch Surg. 2005;140(10):981–5.
Cohen MS, Arslan N, Dehdashti F, Doherty GM, Lairmore TC, Brunt LM, et al. Risk of malignancy in thyroid incidentalomas identified by fluorodeoxyglucose-positron emission tomography. Surgery. 2001;130(6):941–6.
Youserm DM, Huang T, Loevner LA, Langlotz CP. Clinical and economic impact of incidental thyroid lesions found with CT and MR. AJNR Am J Neuroradiol. 1997;18(8):1423–8.
Smith-Bindman R, Miglioretti DL, Larson EB. Rising use of diagnostic medical imaging in a large integrated health system. Health Aff (Millwood). 2008;27(6):1491–502.
Brito JP, Al Nofal A, Montori VM, Hay ID, Morris JC. The impact of subclinical disease and mechanism of detection on the rise in thyroid cancer incidence: a population-based study in Olmsted County, Minnesota During 1935 Through 2012. Thyroid. 2015;25(9):999–1007.
Van den Bruel A, Francart J, Dubois C, Adam M, Vlayen J, De Schutter H, et al. Regional variation in thyroid cancer incidence in belgium is associated with variation in thyroid imaging and thyroid disease management. J Clin Endocrinol Metab. 2013;98(10):4063–71.
Sosa JA, Hanna JW, Robinson KA, Lanman RB. Increases in thyroid nodule fine-needle aspirations, operations, and diagnoses of thyroid cancer in the United States. Surgery. 2013;154(6):1420–6.
Sun GH, DeMonner S, Davis MM. Epidemiological and economic trends in inpatient and outpatient thyroidectomy in the United States, 1996–2006. Thyroid. 2013;23(6):727–33.
Loyo M, Tufano RP, Gourin CG. National trends in thyroid surgery and the effect of volume on short-term outcomes. Laryngoscope. 2013;123(8):2056–63.
Verkooijen HM, Fioretta G, Pache JC, Franceschi S, Raymond L, Schubert H, et al. Diagnostic changes as a reason for the increase in papillary thyroid cancer incidence in Geneva, Switzerland. Cancer Causes Control. 2003;14(1):13–7.
Ghossein R, Asa SL, Barnes L, Chan J, Harrison JB, Heffess CS, et al. College of American Pathologists, Northfield, IL. Protocol for the Examination of Specimens From Patients With Carcinomas of the Thyroid Gland. Based on AJCC/UICC TNM. 7th ed. 2011.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2017 Springer International Publishing Switzerland
About this chapter
Cite this chapter
Haymart, M.R., Esfandiari, N.H. (2017). Incidence and Epidemiology. In: Roman, S., Sosa, J., Solórzano, C. (eds) Management of Thyroid Nodules and Differentiated Thyroid Cancer. Springer, Cham. https://doi.org/10.1007/978-3-319-43618-0_1
Download citation
DOI: https://doi.org/10.1007/978-3-319-43618-0_1
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-43616-6
Online ISBN: 978-3-319-43618-0
eBook Packages: MedicineMedicine (R0)