Abstract
For suicide gene therapy, initially prodrug-converting enzymes (gene-directed enzyme-producing therapy, GDEPT) were employed to intracellularly metabolize non-toxic prodrugs into toxic compounds, leading to the effective suicidal killing of the transfected tumor cells. In this regard, the suicide gene therapy has demonstrated its potential for efficient tumor eradication. Numerous suicide genes of viral or bacterial origin were isolated, characterized, and extensively tested in vitro and in vivo, demonstrating their therapeutic potential even in clinical trials to treat cancers of different entities. Apart from this, growing efforts are made to generate more targeted and more effective suicide gene systems for cancer gene therapy. In this regard, bacterial toxins are an alternative to the classical GDEPT strategy, which add to the broad spectrum of different suicide approaches. In this context, lytic bacterial toxins, such as streptolysin O (SLO) or the claudin-targeted Clostridium perfringens enterotoxin (CPE) represent attractive new types of suicide oncoleaking genes. They permit as pore-forming proteins rapid and also selective toxicity toward a broad range of cancers. In this chapter, we describe the generation and use of SLO as well as of CPE-based gene therapies for the effective tumor cell eradication as promising, novel suicide gene approach particularly for treatment of therapy refractory tumors.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
Finding novel and efficient therapies that target malignancies is still important as the incidence of cancer diseases is constantly increasing. Conventional treatment modalities for cancer such as surgery, radiation therapy, and chemotherapy, which are usually combined for a better treatment effect, remain the therapeutic backbone of cancer therapy. However, these therapies do have their limitations, mainly caused by tumor heterogeneity and development of therapy refractory tumor cell populations. During the last decades, anticancer therapy has been continuously improved to overcome these drawbacks, but problems with adverse effects and drug resistance still constitute a main obstacle for successful cancer treatment. Therefore, alternative treatment options are urgently required to efficiently target and eradicate tumors.
Cancer gene therapy represents one such promising strategy, an approach where selective tumor cell killing and tumor growth inhibition can be achieved by introducing foreign nucleic acid (DNA or RNA) as therapeutic agent to tumor cells (Walther and Stein 1999). Genetic therapy can be approached from different directions, such as insertion of a normal gene into cancer cells to replace a mutated or altered gene (Lu et al. 2012; Senzer et al. 2013), selective eradication of tumor cells by suicide mechanisms, induced apoptosis using additive gene insertion (Di Stasi et al. 2011; Zarogoulidis et al. 2013), gene suppression by intervention of gene transcription and translation using, e.g., antisense-oligonucleotides (ASO) (Moulder et al. 2008; Fidias et al. 2009), micro-RNA (miRNA) (Croce 2009) or small interfering double-stranded RNA (siRNA) (Santel et al. 2010; Strumberg et al. 2012). Furthermore, many approaches involve inoculation of immune cells (namely engineered T cells) for immunotherapy. These cells are specifically modified to either replace the immune system to enhance the anti-tumoral response or to boost the patient’s own immune system to efficiently kill cancer cells (Kantoff et al. 2010; Sharpe and Mount 2015).
Gene transfer technology comprises a diverse set of therapeutic options and provides promising frontiers for treatment. During the last decades, a broad variety of viral and non-viral vectors have been developed (Gillet et al. 2009). In this regard, replicating and non-replicating viral vectors were improved using retroviral or DNA-virus technology platforms (e.g., lentivirus, adenovirus, AAV, Herpes simplex virus) to increase transfer efficiencies and to improve vector targeting and transgene expression complemented with transcriptional targeting or conditional gene (Walther and Stein 2000). Non-viral systems have entered a new level of quality represented by novel vector types (e.g., minicircle, miniplasmid, dumbbell-shaped minimalistic vectors, sleeping-beauty), transfer technologies including nanoparticles/lipofection and physical technologies (e.g., sonoporation, electroporation, particle bombardment/gene-gun, jet injection). One basic obstacle in cancer gene therapy is the specific targeting directly to a solid tumor. Since particularly in its advanced stages cancer is a metastasizing disease, systemic gene delivery is still one major challenge in cancer gene therapy. Insufficient selectivity and transfer efficiency especially for clinical applications are limiting factors for successful gene therapy and demand improvements in targeting of vector delivery, transgene transcription, and/or translation. In this context, local gene therapy of cancer for local control of the disease is still of some attractiveness and about 20 % of all clinical cancer gene therapy trials are performed as local viral or non-viral gene transfer (Walther et al. 2011b).
Apart from the development of improved transfer technologies, an appropriate therapeutic gene is decisive for a successful cancer treatment. The choice of the respective and most suited gene is often determined by the specific gene therapeutic strategy used for cancer treatment, such as immunogene therapy, suicide gene therapy, gene correction therapy, or gene suppression therapy.
Since long time of the evolution of cancer gene therapy bacterial toxins have complemented the list of therapeutic genes and are attractive candidates as they have demonstrated efficient cell killing capacity in several in vitro and in vivo studies and have shown their potential for effective cancer treatment (Richardson et al. 1999). In this chapter, we will focus on the bacterial toxin-based suicide gene therapy, which is currently gaining increasing attention as treatment option.
2 Suicide Gene Therapy for Cancer Treatment
The major application of suicide gene therapy is focused on treatment of cancer. For this, initially different so-called prodrug-converting enzymes (gene-directed enzyme-producing therapy, GDEPT) of bacterial or viral origin were used for expression in tumor cells, which convert non-toxic prodrugs into toxic metabolites to kill tumor cells and neighboring cells (bystander effect). Most prominent members of these suicide genes are still the cytosine deaminase (CD), Herpes simplex virus thymidine kinase (HSV-tk), cytochrome P450-2B1, and nitroreductase and variants thereof. The CD- and HSV-tk-expressing vectors have long entered clinical phases I and II (Zarogoulidis et al. 2013). Recent developments for these classical suicide genes are aiming at their optimization via mutated variants or fusion proteins for more efficient generation of the toxic metabolites. The term suicide gene therapy has meanwhile broadened toward the delivery of genes that are either directly toxic or pro-apoptotic (Fig. 1).
Targeted killing of cancer cells by using suicide gene therapy. a This approach involves the transfer of a therapeutic gene encoding a prodrug-activating enzyme into tumor cells followed by treatment with a specific prodrug. The expression of the therapeutic gene (prodrug-activating enzyme) enables the conversion of the inactive non-toxic prodrug into an active cytotoxic drug. Toxic metabolites can then pass to neighboring cancer cells causing cell killing via the bystander effect. b Direct cell killing is also possible if the inserted gene is expressed to produce a toxin-inducing cytotoxicity. As the transfected cells undergo cell death, the expressed toxin can affect neighboring non-transfected cells
Even though the ability to kill cancer cells is powerful, there are two major drawbacks of this enzyme-prodrug system: the mentioned bystander effect, which can cause unwanted side effects and a reduced effectiveness in slow-dividing cancer cells. As alternative, suicide gene therapy based on apoptotic genes, such as p53, Bax, or FasL, has been extensively studied but also revealed limitations as cancer cells develop resistance to apoptosis induction (Reed 2002; Igney and Krammer 2002).
Therefore, novel suicide gene therapeutics such as bacterial toxins came into focus, which can overcome the obstacles of resistance and proliferation dependence of the classical suicidal systems.
3 Bacterial Toxins in Cancer Therapy
The concept of using bacterial toxins as anticancer agents is actually not new as their therapeutic potential was recognized and explored almost 100 years ago (Richardson et al. 1999; Strebhardt and Ullrich 2008). Meanwhile, a continuously growing number of promising experimental in vitro and in vivo studies, using bacterial toxins for cancer treatment, has been published, which reveal their capability of effective cell killing (McCarthy 2006; Patyar et al. 2010; Felgner et al. 2016). In the last decades, the processing and manipulation of toxic bacterial proteins, such as diphtheria toxin, streptolysin O, or clostridium perfringens enterotoxin, and their encoding genes were facilitated, leading to the establishment of “toxin-based therapy” for cancer treatment introducing novel features to suicide gene therapy such as rapid and quite effective pore-forming cell lysis as novel oncoleaking strategy.
3.1 Diphtheria Toxin
One prominent bacterial toxin, which has been extensively used for therapeutic approaches including gene therapies, is the diphtheria toxin (DT). The DT, a 62-kDa exotoxin, secreted by pathogenic strains of Corynebacterium diphtheria, binds to the heparin-binding epidermal growth factor precursor (HB-EGF) on the cell surface (Louie et al. 1997). DT consists of 535 amino acids and belongs to the group of AB toxins as it can be cleaved into 2 major fragments (DTA and DTB). The fragment DTB mediates cell entry by binding to a surface receptors and subsequent translocation into cytoplasm by undergoing endocytosis. By contrast, DTA is responsible for the cytotoxic enzymatic activity and inactivates the ADP-ribosylation of elongation factor 2 (EF2), causing inhibition of protein syntheses and cell death (Thorburn et al. 2004; Deng and Barbieri 2008). It is known that the delivery of a single molecule of the catalytic DTA is sufficient to kill a cell, but it is not able to enter a neighboring cell in the absence of DTB (Yamaizumi et al. 1978) (Fig. 2).
Mechanism of action of diphtheria toxin. 1 The secreted toxin consists of three functional domains: the N-terminal catalytic domain (DTA), the translocation domain (T), which is bridged by a disulfide bond to the receptor-binding domain (R). 2 DT binds its receptor (heparin-binding epidermal growth factor precursor). 3 The cell surface furin protease cleaves the polypeptide chain between the C and T domains. 4 The toxin-receptor complex is internalized into clathrin-coated pit. 5 Inside the early endosome, furin protease cleaves toxin molecules and T domain undergoes conformational change, inserts into endosome membrane and forms a channel, which leads to translocation of catalytic domain into the cytoplasm, followed by reduction of the disulfide bond. 6 DTA inactivates eukariotic translation elongation factor 2 (eEF2) by ADP-ribosylation, causing inhibition of translation and consequently cell death
3.1.1 DT-Based Suicide Gene Therapy
As mentioned above, DTA is not able to enter a neighboring cell in the absence of DTB. Therefore, it only specifically kills the actual targeted cell, restricting its toxicity. These features of high therapeutic potency, the locally restricted toxic effect, the additional advantages of the evasion of anti-DT immunity, as it is endogenously expressed within the tumor, and the absence of cellular resistance to the toxin supports the great potential of DT-A as gene therapeutic agent.
Nevertheless, this potent bacterial toxin requires efficient and reliable selective targeting, mainly to avoid any unintended side effects on normal cells, which is an essential requirement for the use of the toxin in cancer gene therapy. Several attempts to limit the toxicity of DTA by using modified metallothionein promoter (Maxwell et al. 1986) or by replacement of the wild type DTA sequence with attenuated mutant variants of DTA (Maxwell et al. 1987) were still not able to generate targeted tumor cell killing. To minimize damage to healthy tissue, a specific targeting mechanism was an essential requirement to ensure further use of the toxin.
In the last decades, tissue- and tumor-specific promoter elements were identified, which are critically important for more effective and transcriptionally targeted application of gene therapy (Haviv and Blackwell 2001; Dorer and Nettelbeck 2009). With this knowledge, transcriptional targeting, a method based on positioning the therapeutic gene (e.g., suicide gene) under the transcriptional regulation of a promoter which is specifically or preferentially activated in targeted tumor tissue, was developed (Fukazawa et al. 2004; Saukkonen and Hemminki 2004; Danda et al. 2013). Until today, numerous tissue-specific promoters have been cloned, molecularly characterized, and applied for the controlled DTA expression in different cancer entities.
One example of such promoter is originated from the human H19 RNA gene that is highly expressed in a wide range of cancers and is important for cell proliferation, genetic instability, vascular angiogenesis, multiple drug resistance, metastasis as well as secondary tumor progression and dissemination (Matouk et al. 2013). Mizrahi et al. reported the use of then H19 gene promoter to drive the targeted expression of DTA in ovarian cancer and demonstrated significant tumor growth inhibition of ovarian cancer xenograft-bearing mice after intratumoral injection of DTA-H19 (Mizrahi et al. 2009). This great potential has been further confirmed in a variety of tumor entities, such as pancreatic cancer (Scaiewicz et al. 2010; Sorin et al. 2012), colon adenocarcinoma (Sorin et al. 2011), or primary lung cancer (Hasenpusch et al. 2011). Thus, DTA-H19 became a “multi-potent vector” (Smaldone and Davies 2010; Amit and Hochberg 2012; Amit et al. 2013) and has entered multiple clinical studies. A phase I and II clinical trial in patients with invasive bladder cancers, receiving intravesical DTA-H19 (namely BC-819) revealed partial and complete response rates as well as prevention of tumor recurrence in two-thirds of treated patients (Gofrit et al. 2014).
Another very recent example for transcriptionally targeted therapy of DT was shown by Tholey et al. They generated DNA-vector constructs with either the pancreatic cancer-specific mesothelin (MSLN) or Mucin 1 (MUC1) promoter linked to DTA coding sequence and combined it with a highly efficient and biodegradable polymer to deliver the vector DNA to pancreatic cancer cells (Tholey et al. 2015). MSLN and MUC1 gene promoters represent promising transcriptional control elements, as they are active at low level in normal cells and highly active a diversity of tumor types, particularly in pancreatic cancer cells, mainly in the most aggressive form that are typically resistant to conventional therapy (Singh and Bandyopadhyay 2007; Showalter et al. 2008; Winter et al. 2012). With this knowledge on promoter activities, MSLN and MUC1 promoter-driven DTA constructs were generated, demonstrating its specific and selective activity as it preferentially kills MSLN/MUC1-expressing pancreatic cancer cells in vitro. A further analysis of matched primary and metastatic tumors in patients showed the great potential of MUC1-targeted therapy as targeting strategy, since this expression is observed consistently in primary tumors and metastasis.
3.2 Streptolysin O
Apart from the strategy of, e.g., of intervention in protein translation by bacterial toxin like DTA, the approach of cell lysis by pore-forming toxins is of attractiveness to eradicate tumor cells. Particularly in light of additional immunostimulation, tumor cell lysis might add to tumor therapy as it deliberates tumor antigens, which could in turn contribute to the activation of the patient’s immune response against the tumor. One such pore-forming toxin is streptolysin O (SLO). SLO is a 62-kDa toxin secreted by many strains of Streptococcus bacteria and belongs to the family of pore-forming toxins called cholesterol-dependent cytolysins (CDCs) (Bhakdi et al. 1996). SLO consists of four domains D1–D4, which are rich in β-sheet proportions. The most important protein domains are D3 and D4, as domain D3 provides the transmembrane spanning regions for the toxin and domain D4 directly interacts with cholesterol of the cell membranes. After specifically binding to membrane cholesterols, SLO monomers oligomerize to form homotypic aggregates, which insert into membrane to form a large pore whose diameters can reach up to 35 nm (Shatursky et al. 1999; Sierig et al. 2003) (Fig. 3a).
a The mechanism of SLO action. SLO binds specifically to membrane cholesterol and oligomerizes to create a ring structure, which contains 45–50 units and inserts into the membrane to create a large pore, leading to loss of balance between in- and effluxes across the cell membrane. This pore formation further induces cytolysis. b The mechanism of CPE binding and mediated cytotoxicity. CPE binds directly to its receptor, preferably claudin-3 or claudin-4, at the plasma membrane of intestinal epithelium. The small CPE/claudin complexes may also include other claudins, e.g., claudin-1, via an indirect interaction. Six small complexes oligomerize to form a large hexameric complex (CH-1), which increases permeability of the cell membrane. CH-1 complexes eventually incorporate occluding, resulting in an even larger complex, namely CH-2, which disrupts epithelial tight junctions resulting in a breakdown of colloid-osmotic equilibrium of affected cells. In consequence, cells undergo cell death by lysis
3.2.1 SLO-Mediated Cytolytic Suicide Gene Therapy
While recombinant SLO protein has been used in several studies for its pore-forming properties, Yang et al. thought of exploiting this particular property of SLO to kill malignant tumor cells and to take the advantage to overcome the anti-apoptotic resistance of cancer cells as well proliferation rate dependence (Yang et al. 2006). Most bacterial toxins, such as diphtheria toxin or pseudomonas exotoxin, tested so far in suicide gene therapy acted “inside” the targeted tumor cell (Martín et al. 2000; Kawakami et al. 2001). Conversely, pore-forming toxins are known to act at the cell membrane and have formerly been used as immunotoxins or recombinant proteins for anticancer treatment. In the study of Yang et al., a conventional plasmid expression vector carrying the SLO gene was used in a liposome-mediated transfection system. Initially, HEK293T cells (human embryonic kidney fibroblasts) were transiently transfected with the SLO vector, leading to cell death caused by cell membrane permeabilization and disintegration. SLO secreted by bacteria usually creates large pores in target cell membrane, allowing large molecules to pass through. To determine whether the observed cytotoxicity in SLO transfected cells was caused by pore formation of expressed SLO lactate dehydrogenase (LDH) release, caspase activity was measured and cells were monitored under electron microscopy. High level of LDH but no caspase activation was observed, indicating that SLO protein expressed within HEK cells induces necrosis. In further studies, they extended their approach by developing an adenoviral expression vector as high-efficiency gene transfer system, which significantly reduced cell viability in several human cancer cell lines (cervical carcinoma cells, C33; lung carcinoma cells, A549; breast cancer cells, MCF-7; and prostate cancer cells, Hep3B). After SLO gene transfer, significant anti-tumoral activity by SLO-mediated cytotoxicity was observed in treated CA33 xenograft-bearing mice.
This study demonstrated the successful use of the SLO gene as anticancer agent in vitro and more importantly in vivo. However, these studies reveal limitations, as the use is limited to local treatment otherwise massive unwanted side effects could occur, since cholesterol is certainly also present in cell membranes of healthy normal cells. Therefore, further modifications like attachment of rather tumor-specific promoters upstream the SLO gene or changing adenoviral fiber proteins, which bind to specific tumor cell surface proteins, are required.
3.3 Clostridium Perfringens Enterotoxin
Another promising pore-forming bacterial toxin for the suicide gene therapy is the clostridium perfringens enterotoxin, which is produced by the anaerobic gram-positive bacterium Clostridium perfringens and mainly associated with food poisoning (Minton 2003; Johnson 1999). This 35-kDA single protein contains 319 amino acids with a unique primary sequence. CPE is a two-domain protein that consists of (1) C-terminal receptor-binding domain (amino acid residues 184-319), which recognizes and binds to certain members of the claudin family and an N-terminal domain that is involved in oligomerization and pore formation (Kitadokoro et al. 2011; Briggs et al. 2011).
The C-terminal fragment of CPE (c-CPE) reveals a high-affinity binding to its receptors, for example, claudin-3 or claudin-4; however, it is not able to initiate or form pores (Hanna et al. 1991). The N-terminal residues 80–160 also referred to as TM1 region consist of hydrophilic and hydrophobic amino acids, which resemble the β-loops, which then mediate membrane insertion and pore formation. The mechanism of action of CPE is initiated by the binding to its natural receptors, the transmembrane proteins claudin. In particular, claudin-3, claudin-4, claudin-6, claudin-6, and claudin-14 are proven CPE receptors (Katahira et al. 1997; Fujita et al. 2000; Lal-Nag et al. 2012; Shrestha and McClane 2013; Shrestha et al. 2016).
The claudin family consists of at least 27 proteins that are essential for tight junction formation in epithelial and endothelial cells. They also play an important role in controlling paracellular transport and maintenance of cell polarity (Gumbiner 1987). Claudins are comprised of four transmembrane domains; a C-terminal cytoplasmic tail; and two extracellular loops, ECL1 and ECL2 (Günzel and Fromm 2012). The binding of CPE to its claudin receptor triggers the formation of a “small complex” (90 kDa), containing CPE and the receptor (Tsukita and Furuse 2000; Smedley et al. 2007). This small complex by itself is not able to mediate cytoxicity, but several small complexes interact and oligomerize to a prepore on the membrane surface, which results in a 450-kDa “large complex”—named CH-1 complex (Robertson et al. 2007). The CH-1 complex that comprises a CPE hexamer and claudins subsequently forms a pore into the membrane, causing membrane permeability alterations, and permits a calcium influx, inducing cell death (Matsuda and Sugimoto 1979; Freedman et al. 2016). The morphological damage leads to exposure of the basolateral cell surface, allowing additional binding of the toxin to form an even larger ~600-kDa complex, known as CH-2, which consists of claudins as well as occludin (Singh et al. 2000) (Fig. 3b).
So far it is known that high CPE concentration causes formation of many pores, leading to a massive calcium influx and consequently to necrotic cell death, whereas low CPE concentration results in formation of low number of pores, rather causing apoptosis (Chakrabarti et al. 2003).
3.3.1 CPE-Based Oncoleaking Suicide Gene Therapy
Numerous studies have shown that certain cancer entities, particularly epithelial cancers, such as colon, breast, prostate, ovarian, and pancreatic cancer, possess a high expression of claudin-3 and/or claudin-4 (Rangel et al. 2003; Hewitt et al. 2006; Santin et al. 2007; Takala et al. 2007; Kominsky et al. 2007; Saeki et al. 2009; Neesse et al. 2012; Lu et al. 2013). Due to this fact, considerable effort has been made to develop a CPE-based approach for cancer therapy and its potential clinical benefit in targeting claudin-3- and claudin-4-expressing tumors has been evaluated. The application of recombinant CPE protein demonstrated a dose-dependent cell killing of claudin-3 and claudin-4-overexpressing pancreatic, breast or colon cancer cells in vitro and in vivo (Litkouhi et al. 2007; Saeki et al. 2009; Gao and McClane 2012; Kojima et al. 2012). In addition to that, the intratumoral application in tumor bearing mice did not induce unwanted toxin-associated side effects. However, the use of recombinant CPE protein requires repeated application of the toxin to achieve significant therapeutic effect (Michl et al. 2001; Kominsky et al. 2004). Alternatively, the gene transfer of a CPE-expressing vector could be sufficient to significantly prolong toxin availability and improve intratumoral dispersion and subsequently amplify the cytotoxic effect.
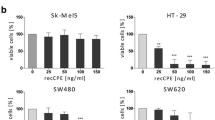
Based on this idea, we established an eukaryotic translation optimized CPE vector (optCPE), which combines both target specificity and efficient cytotoxicity (Walther et al. 2011a). The intracellular CPE expression and accumulation after gene transfer led to effective eradication of claudin-3 and claudin-4 high-expressing cells, such as the mammary carcinoma cell line MCF-7 or the human pancreatic cancer cells Panc1, whereas claudin-negative cells like the melanoma cell line Sk-Mel5 remained unaffected. This study further demonstrated that even though CPE is produced inside the transfected cell, its outside action of binding to the claudins and mediating pore formation and cell lysis is not hampered.
More importantly, it was shown that non-viral intratumoral gene transfer of CPE-expressing plasmid-vector does induce extensive tumor necrosis in HCT116 human colon carcinoma and in MCF-7 human mammary carcinoma xenotransplanted mice. This was associated with significant reduction in tumor growth and showed improved efficacy over treatments with the recombinant CPE, which was well tolerated by the animals.
In our very recent study, we employed the optCPE gene therapy to selectively eradicate claudin-3 and claudin-4-expressing pancreatic carcinomas and demonstrated again the successful use of this suicide gene therapy approach as CPE expression permitted rapid tumor destruction in vitro (Pahle et al. 2015).
In both studies, we observed the presence of released biological active CPE in the media of all transfected cells (claudin-positive and claudin-negative cells), suggesting a cytotoxicity-independent deliberation of CPE, which further supports the concept of bystander effect that strongly contributes to the efficiency of this gene therapy approach (Fig. 1b).
For the improved and more effective use of the toxin, the mode of cell death, induced by transfected CPE is of interest. The analysis on cell death mechanism revealed that delayed activation of the caspases 3/7 was induced, indicating rather CPE-mediated necrosis than apoptosis. This was further supported by the dramatic increase of LDH release after CPE transfection and appearance of necrotic cell morphology, such as cell membrane and nuclear rupture. As mentioned above and reported by others, cell death mechanism is dependent on CPE concentration, number of pores generated by CPE and claudin localization (tight junctions, cell membrane, cytoplasm) of targeted cell, which determines accessibility for CPE binding.
Taken together, CPE oncoleaking gene therapy is of great value for the targeted eradication of therapy refractory tumors, which is further improved by the bystander effect of this particular suicide approach.
4 Conclusions
As gene therapy comes of age, it has shown its efficacy for the treatment of cancer diseases reflected by the application of this strategy in clinical trials. In fact, more than 7 % of all gene therapy clinical trials worldwide are employing suicide approaches either as monotherapy or in combination with other, conventional therapies such as chemo- and radiotherapy. Numerous suicidal systems have been established and successfully employed and among them bacterial toxins might experience some thorough re-evaluation as potential tools for more effective and to some extend more targeted gene therapies. In this regard, pore-forming, oncoleaking bacterial toxins such as SLO or CPE hold promise for the efficient and rapid tumor cell killing. In particular CPE action is associated with selective tumor cell killing properties targeting the claudin-3 and claudin-4 tight junction proteins, which are often deregulated in epithelial cancers. As shown for meanwhile classical suicidal systems (e.g., CD, HSV-tk), bacterial toxins like CPE do also possess the feature of bystander effect, which is important if not essential for effective in vivo use of this suicide gene therapeutic. These initial studies for the use of bacterial toxins for oncoleaking suicide therapies might further promote the directed search for novel, similarly or even more effective and tumor-targeted toxins that can potentially be used for cancer gene therapy.
References
Amit D, Hochberg A (2012) Development of targeted therapy for a broad spectrum of cancers (pancreatic cancer, ovarian cancer, glioblastoma and HCC) mediated by a double promoter plasmid expressing diphtheria toxin under the control of H19 and IGF2-P4 regulatory sequences. Int J Clin Exp Med 5:296–305
Amit D, Tamir S, Hochberg A (2013) Development of targeted therapy for a broad spectrum of solid tumors mediated by a double promoter plasmid expressing diphtheria toxin under the control of IGF2-P4 and IGF2-P3 regulatory sequences. Int J Clin Exp Med 6:110–118. doi:10.1186/1479-5876-8-134
Bhakdi S, Bayley H, Valeva A et al (1996) Staphylococcal alpha-toxin, streptolysin-O, and Escherichia coli hemolysin: prototypes of pore-forming bacterial cytolysins. Arch Microbiol 165:73–79. doi:10.1007/s002030050300
Briggs DC, Naylor CE, Smedley JG et al (2011) Structure of the food-poisoning Clostridium perfringens enterotoxin reveals similarity to the aerolysin-like pore-forming toxins. J Mol Biol 413:138–149. doi:10.1016/j.jmb.2011.07.066
Chakrabarti G, Zhou X, McClane BA (2003) Death pathways activated in CaCo-2 cells by Clostridium perfringens enterotoxin. Infect Immun 71:4260–4270
Croce CM (2009) Causes and consequences of microRNA dysregulation in cancer. Nat Rev Genet 10:704–714. doi:10.1038/nrg2634
Danda R, Krishnan G, Ganapathy K et al (2013) Targeted expression of suicide gene by tissue-specific promoter and microRNA regulation for cancer gene therapy. PLoS ONE 8:e83398. doi:10.1371/journal.pone.0083398
Deng Q, Barbieri JT (2008) Molecular mechanisms of the cytotoxicity of ADP-ribosylating toxins. Annu Rev Microbiol 62:271–288. doi:10.1146/annurev.micro.62.081307.162848
Di Stasi A, Tey S-K, Dotti G et al (2011) Inducible apoptosis as a safety switch for adoptive cell therapy. N Engl J Med 365:1673–1683. doi:10.1056/NEJMoa1106152
Dorer DE, Nettelbeck DM (2009) Targeting cancer by transcriptional control in cancer gene therapy and viral oncolysis. Adv Drug Deliv Rev 61:554–571. doi:10.1016/j.addr.2009.03.013
Felgner S, Kocijancic D, Frahm M, Weiss S (2016) Bacteria in cancer therapy: renaissance of an old concept. Int J Microbiol 2016:1–14. doi:10.1155/2016/8451728
Fidias P, Pennell NA, Boral AL et al (2009) Phase I study of the c-raf-1 antisense oligonucleotide ISIS 5132 in combination with carboplatin and paclitaxel in patients with previously untreated, advanced non-small cell lung cancer. J Thorac Oncol 4:1156–1162. doi:10.1097/JTO.0b013e3181b2793f
Freedman J, Shrestha A, McClane B (2016) Clostridium perfringens Enterotoxin: action, genetics, and translational applications. Toxins (Basel) 8:73. doi:10.3390/toxins8030073
Fujita K, Katahira J, Horiguchi Y et al (2000) Clostridium perfringens enterotoxin binds to the second extracellular loop of claudin-3, a tight junction integral membrane protein. FEBS Lett 476:258–261
Fukazawa T, Maeda Y, Sladek FM, Owen-Schaub LB (2004) Development of a cancer-targeted tissue-specific promoter system. Cancer Res 64:363–369. doi:10.1158/0008-5472.can-03-2507
Gao Z, McClane B a (2012) Use of Clostridium perfringens enterotoxin and the enterotoxin receptor-binding domain (C-CPE) for cancer treatment: opportunities and challenges. J Toxicol 2012:981626. doi:10.1155/2012/981626
Gillet J-P, Macadangdang B, Fathke RL et al (2009) The development of gene therapy: from monogenic recessive disorders to complex diseases such as cancer. Methods Mol Biol Clift NJ 542:5–54
Gofrit ON, Benjamin S, Halachmi S et al (2014) DNA based therapy with diphtheria toxin-A BC-819: a phase 2b marker lesion trial in patients with intermediate risk nonmuscle invasive bladder cancer. J Urol 191:1697–1702. doi:10.1016/j.juro.2013.12.011
Gumbiner B (1987) Structure, biochemistry, and assembly of epithelial tight junctions. Am J Physiol 253:C749–C758
Günzel D, Fromm M (2012) Claudins and other tight junction proteins. Compr Physiol 2:1819–1852. doi:10.1002/cphy.c110045
Hanna PC, Mietzner TA, Schoolnik GK, McClane BA (1991) Localization of the receptor-binding region of Clostridium perfringens enterotoxin utilizing cloned toxin fragments and synthetic peptides. The 30 C-terminal amino acids define a functional binding region. J Biol Chem 266:11037–11043
Hasenpusch G, Pfeifer C, Aneja MK et al (2011) Aerosolized BC-819 inhibits primary but not secondary lung cancer growth. PLoS ONE 6:e20760. doi:10.1371/journal.pone.0020760
Haviv YS, Blackwell J (2001) Transcriptional regulation in cancer gene therapy. Isr Med Assoc J 3:517–522
Hewitt KJ, Agarwal R, Morin PJ (2006) The claudin gene family: expression in normal and neoplastic tissues. BMC Cancer 6:186. doi:10.1186/1471-2407-6-186
Igney FH, Krammer PH (2002) Death and anti-death: tumour resistance to apoptosis. Nat Rev Cancer 2:277–288. doi:10.1038/nrc776
Johnson EA (1999) Clostridial toxins as therapeutic agents: benefits of natur's most toxic proteins. Annu Rev Microbiol 53:551–75. doi:10.1146/annurev.micro.53.1.551
Kantoff PW, Higano CS, Shore ND et al (2010) Sipuleucel-T immunotherapy for castration-resistant prostate cancer. N Engl J Med 363:411–422. doi:10.1056/NEJMoa1001294
Katahira J, Inoue N, Horiguchi Y et al (1997) Molecular cloning and functional characterization of the receptor for Clostridium perfringens enterotoxin. J Cell Biol 136:1239–1247
Kawakami K, Kawakami M, Joshi BH, Puri RK (2001) Interleukin-13 receptor-targeted cancer therapy in an immunodeficient animal model of human head and neck cancer. Cancer Res 61:6194–6200
Kitadokoro K, Nishimura K, Kamitani S et al (2011) Crystal structure of Clostridium perfringens enterotoxin displays features of beta-pore-forming toxins. J Biol Chem 286:19549–19555. doi:10.1074/jbc.M111.228478
Kojima T, Kyuno D, Sawada N (2012) Targeting claudin-4 in human pancreatic cancer. Expert Opin Ther Targets 16:881–887. doi:10.1517/14728222.2012.708340
Kominsky SL, Tyler B, Sosnowski J et al (2007) Clostridium perfringens enterotoxin as a novel-targeted therapeutic for brain metastasis. Cancer Res 67:7977–7982. doi:10.1158/0008-5472.CAN-07-1314
Kominsky SL, Vali M, Korz D et al (2004) Clostridium perfringens enterotoxin elicits rapid and specific cytolysis of breast carcinoma cells mediated through tight junction proteins claudin 3 and 4. Am J Pathol 164:1627–1633
Lal-Nag M, Battis M, Santin a D, Morin PJ (2012) Claudin-6: a novel receptor for CPE-mediated cytotoxicity in ovarian cancer. Oncogenesis 1:e33. doi:10.1038/oncsis.2012.32
Litkouhi B, Kwong J, Lo C-M et al (2007) Claudin-4 overexpression in epithelial ovarian cancer is associated with hypomethylation and is a potential target for modulation of tight junction barrier function using a C-terminal fragment of Clostridium perfringens enterotoxin. Neoplasia 9:304–314
Louie GV, Yang W, Bowman ME, Choe S (1997) Crystal structure of the complex of diphtheria toxin with an extracellular fragment of its receptor. Mol Cell 1:67–78. doi:10.1016/S1097-2765(00)80008-8
Lu C, Stewart DJ, Lee JJ et al (2012) Phase I clinical trial of systemically administered TUSC2(FUS1)-nanoparticles mediating functional gene transfer in humans. PLoS ONE 7:e34833. doi:10.1371/journal.pone.0034833
Lu Z, Ding L, Lu Q, Chen Y-H (2013) Claudins in intestines: distribution and functional significance in health and diseases. Tissue barriers 1:e24978
Martín V, Cortés ML, de Felipe P et al (2000) Cancer gene therapy by thyroid hormone-mediated expression of toxin genes. Cancer Res 60:3218–3224
Matouk I, Raveh E, Ohana P et al (2013) The increasing complexity of the oncofetal h19 gene locus: functional dissection and therapeutic intervention. Int J Mol Sci 14:4298–4316. doi:10.3390/ijms14024298
Matsuda M, Sugimoto N (1979) Calcium-independent and dependent steps in action of Clostridiumperfringens enterotoxin on HeLa and Vero cells. Biochem Biophys Res Commun 91:629–636. doi:10.1016/0006-291X(79)91568-7
Maxwell F, Maxwell IH, Glode LM (1987) Cloning, sequence determination, and expression in transfected cells of the coding sequence for the tox 176 attenuated diphtheria toxin A chain. Mol Cell Biol 7:1576–1579
Maxwell IH, Maxwell F, Glode LM (1986) Regulated expression of a diphtheria toxin A-chain gene transfected into human cells: possible strategy for inducing cancer cell suicide. Cancer Res 46:4660–4.
McCarthy EF (2006) The toxins of William B. Coley and the treatment of bone and soft-tissue sarcomas. Iowa Orthop J 26:154–158
Michl P, Buchholz M, Rolke M et al (2001) Claudin-4: a new target for pancreatic cancer treatment using Clostridium perfringens enterotoxin. Gastroenterology 121:678–684. doi:10.1053/gast.2001.27124
Minton NP (2003) Clostridia in cancer therapy. Nat Rev Microbiol 1:237–242
Mizrahi A, Czerniak A, Levy T et al (2009) Development of targeted therapy for ovarian cancer mediated by a plasmid expressing diphtheria toxin under the control of H19 regulatory sequences. J Transl Med 7:69. doi:10.1186/1479-5876-7-69
Moulder SL, Symmans WF, Booser DJ et al (2008) Phase I/II study of G3139 (Bcl-2 antisense oligonucleotide) in combination with doxorubicin and docetaxel in breast cancer. Clin Cancer Res 14:7909–7916. doi:10.1158/1078-0432.CCR-08-1104
Neesse a, Griesmann H, Gress TM, Michl P (2012) Claudin-4 as therapeutic target in cancer. Arch Biochem Biophys 524:64–70. doi:10.1016/j.abb.2012.01.009
Pahle J, Aumann J, Kobelt D, Walther W (2015) Oncoleaking: use of the pore-forming clostridium perfringens enterotoxin (CPE) for suicide gene therapy. Methods Mol Biol 1317:69–85. doi:10.1007/978-1-4939-2727-2_5
Patyar S, Joshi R, Byrav DP et al (2010) Bacteria in cancer therapy: a novel experimental strategy. J Biomed Sci 17:21
Rangel LBA, Agarwal R, D’Souza T et al (2003) Tight junction proteins claudin-3 and claudin-4 are frequently overexpressed in ovarian cancer but not in ovarian cystadenomas. Clin Cancer Res 9:2567–2575
Reed JC (2002) Apoptosis-based therapies. Nat Rev Drug Discov 1:111–121. doi:10.1038/nrd726
Richardson MA, Ramirez T, Russell NC, Moye LA (1999) Coley toxins immunotherapy: a retrospective review. Altern Ther Health Med 5:42–47
Robertson SL, Smedley JG, Singh U et al (2007) Compositional and stoichiometric analysis of Clostridium perfringens enterotoxin complexes in Caco-2 cells and claudin 4 fibroblast transfectants. Cell Microbiol 9:2734–2755
Saeki R, Kondoh M, Kakutani H et al (2009) A novel tumor-targeted therapy using a claudin-4-targeting molecule. Mol Pharmacol 76:918–926
Santel A, Aleku M, Röder N et al (2010) Atu027 prevents pulmonary metastasis in experimental and spontaneous mouse metastasis models. Clin Cancer Res 16:5469–5480. doi:10.1158/1078-0432.CCR-10-1994
Santin AD, Bellone S, Siegel ER et al (2007) Overexpression of Clostridium perfringens enterotoxin receptors claudin-3 and claudin-4 in uterine carcinosarcomas. Clin Cancer Res 13:3339–3346
Saukkonen K, Hemminki A (2004) Tissue-specific promoters for cancer gene therapy. Expert Opin Biol Ther 4:683–96
Scaiewicz V, Sorin V, Fellig Y et al (2010) Use of H19 gene regulatory sequences in DNA-based therapy for pancreatic cancer. J Oncol 2010:178174. doi:10.1155/2010/178174
Senzer N, Nemunaitis J, Nemunaitis D et al (2013) Phase I study of a systemically delivered p53 nanoparticle in advanced solid tumors. Mol Ther 21:1096–1103. doi:10.1038/mt.2013.32
Sharpe M, Mount N (2015) Genetically modified T cells in cancer therapy: opportunities and challenges. Dis Model Mech 8:337–350. doi:10.1242/dmm.018036
Shatursky O, Heuck AP, Shepard LA et al (1999) The mechanism of membrane insertion for a cholesterol-dependent cytolysin. Cell 99:293–299. doi:10.1016/S0092-8674(00)81660-8
Showalter S, Huang Y-H, Witkiewicz A et al (2008) Nanoparticulate delivery of diphtheria toxin DNA effectively kills mesothelin expressing pancreatic cancer cells. Cancer Bio Ther 7:1584–1590
Shrestha A, McClane BA (2013) Human claudin-8 and -14 are receptors capable of conveying the cytotoxic effects of Clostridium perfringens enterotoxin. MBio. doi:10.1128/mBio.00594-12
Shrestha A, Uzal FA, McClane BA (2016) The interaction of Clostridium perfringens enterotoxin with receptor claudins. Anaerobe 41:18–26. doi:10.1016/j.anaerobe.2016.04.011
Sierig G, Cywes C, Wessels MR, Ashbaugh CD (2003) Cytotoxic effects of streptolysin o and streptolysin s enhance the virulence of poorly encapsulated group a streptococci. Infect Immun 71:446–455
Singh R, Bandyopadhyay D (2007) MUC1: a target molecule for cancer therapy. Cancer Biol Ther 6:481–486
Singh U, Van Itallie CM, Mitic LL et al (2000) CaCo-2 cells treated with Clostridium perfringens enterotoxin form multiple large complex species, one of which contains the tight junction protein occludin. J Biol Chem 275:18407–18417
Smaldone MC, Davies BJ (2010) BC-819, a plasmid comprising the H19 gene regulatory sequences and diphtheria toxin A, for the potential targeted therapy of cancers. Curr Opin Mol Ther 12:607–616
Smedley JG, Uzal FA, McClane BA (2007) Identification of a prepore large-complex stage in the mechanism of action of Clostridium perfringens enterotoxin. Infect Immun 75:2381–2390
Sorin V, Ohana P, Gallula J et al (2012) H19-promoter-targeted therapy combined with gemcitabine in the treatment of pancreatic cancer. ISRN Oncol 2012:351750. doi:10.5402/2012/351750
Sorin V, Ohana P, Mizrahi A et al (2011) Regional therapy with DTA-H19 vector suppresses growth of colon adenocarcinoma metastases in the rat liver. Int J Oncol 39:1407–1412
Strebhardt K, Ullrich A (2008) Paul Ehrlich’s magic bullet concept: 100 years of progress. Nat Rev Cancer 8:473–480. doi:10.1038/nrc2394
Strumberg D, Schultheis B, Traugott U et al (2012) Phase I clinical development of Atu027, a siRNA formulation targeting PKN3 in patients with advanced solid tumors. Int J Clin Pharmacol Ther 50:76–78
Takala H, Saarnio J, Wiik H, Soini Y (2007) Claudins 1, 3, 4, 5 and 7 in esophageal cancer: loss of claudin 3 and 4 expression is associated with metastatic behavior. APMIS Acta Pathol Microbiol Immunol Scand 115:838–847
Tholey RM, Lal S, Jimbo M et al (2015) MUC1 promoter-driven DTA as a targeted therapeutic strategy against pancreatic cancer. Mol Cancer Res 13:439–448. doi:10.1158/1541-7786.MCR-14-0199
Thorburn A, Thorburn J, Frankel AE (2004) Induction of apoptosis by tumor cell-targeted toxins. Apoptosis 9:19–25. doi:10.1023/B:APPT.0000012118.95548.88
Tsukita S, Furuse M (2000) Pores in the wall: claudins constitute tight junction strands containing aqueous pores. J Cell Biol 149:13–16
Walther W, Petkov S, Kuvardina ON et al (2011a) Novel Clostridium perfringens enterotoxin suicide gene therapy for selective treatment of claudin-3- and -4-overexpressing tumors. Gene Ther 19:494–503. doi:10.1038/gt.2011.136
Walther W, Schlag PM, Stein U (2011b) Local gene delivery for therapy of solid tumors. Drug delivery in oncology. Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim, pp 1391–1413
Walther W, Stein U (1999) Therapeutic genes for cancer gene therapy. Mol Biotechnol 13:21–28. doi:10.1385/MB:13:1:21
Walther W, Stein U Walther W, Stein U (2000) Viral vectors for gene transfer: a review of their use in the treatment of human diseases. Drugs 60:249–271. jourlib.org. http://www.jourlib.org/references/5588026. Accessed 30 Sep 2015
Winter JM, Tang LH, Klimstra DS et al (2012) A novel survival-based tissue microarray of pancreatic cancer validates MUC1 and mesothelin as biomarkers. PLoS ONE 7:e40157. doi:10.1371/journal.pone.0040157
Yamaizumi M, Mekada E, Uchida T et al (1978) One molecule of diphtheria toxin fragment a introduced into a cell can kill the cell. Cell 15:245–250. doi:10.1016/0092-8674(78)90099-5
Yang WS, Park S-O, Yoon A-R et al (2006) Suicide cancer gene therapy using pore-forming toxin, streptolysin O. Mol Cancer Ther 5:1610–1619. doi:10.1158/1535-7163.MCT-05-0515
Zarogoulidis P, Darwiche K, Sakkas A et al (2013) Suicide gene therapy for cancer—current strategies. J Genet Syndr gene Ther 9(4pii):16849. doi:10.4172/2157-7412.1000139
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2016 Springer International Publishing Switzerland
About this chapter
Cite this chapter
Pahle, J., Walther, W. (2016). Bacterial Toxins for Oncoleaking Suicidal Cancer Gene Therapy. In: Walther, W. (eds) Current Strategies in Cancer Gene Therapy. Recent Results in Cancer Research, vol 209. Springer, Cham. https://doi.org/10.1007/978-3-319-42934-2_7
Download citation
DOI: https://doi.org/10.1007/978-3-319-42934-2_7
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-42932-8
Online ISBN: 978-3-319-42934-2
eBook Packages: MedicineMedicine (R0)