Abstract
The great advances in mPCa management recently obtained with the second-line treatments have highlighted the need to identify and exploit surrogate endpoints for survival of patients and effectiveness of treatment. These surrogate endpoints could be also used to reduce size and costs of pivotal trials of new molecules and the time from the benchtop to the patients’ bedside. A large consensus is emerging in the scientific community that circulating tumor cells (CTCs) meet the criteria required for a surrogate endpoint, particularly in mPCa.
The main open question about the clinical management of mPCa is discussed, with the intent to underscore how the extended use of CTC detection and characterization can offer further benefit to these patients.
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
Keywords
- Overall Survival
- Androgen Receptor
- Radical Prostatectomy
- Circulate Tumor Cell
- Androgen Receptor Expression
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
5.1 CTCs as Prognostic and Predictive Biomarker
The process by which we are finally able to license any clinical-pathological parameter as a biomarker passes through some mandatory steps, namely, analytical validity, clinical validity and, hopefully, clinical utility [1].
In particular, the clinical validity defines a test that is clinically usable [1], on the basis of reliability, accuracy, and needed sensitivity and on specific and predictive value for impacting patient care. On the other hand, with clinical utility, we refer to the ability of a test to be used into the medical practice, because of an improved benefit or reduction in cost beyond the best available test.
CTCs could affect the clinical utility in PCa in different manner:
-
1.
From changing treatment decision (stopping a therapy that does not work or on the contrary, continuing a therapy beneficial for patients)
-
2.
Improving tolerability of a systemic regimen
-
3.
Improving survival (improving treatment selection and reduction in toxicity)
-
4.
Improving cost-effectiveness (with reduction of ineffective drug explosion time) [2]
In European countries, during the last decade, the 5-year relative survival percentages for PCa steadily increased from 73.4 % in 1999–2001 to 83.4 % in 2005–2007 [3]. This encouraging result is undoubtable due to the extensive use of PSA screening and the radical prostatectomy, despite the other side of the coin being that the number needed to treat to prevent one death at 18 years of follow-up was eight men [4], a relevant rate of overdiagnosis and overtreatment.
However, due to the nonnegligible risk of the incidence of distant metastases over the next 18 years after the first diagnosis (a cumulative incidence of 26.1 % in the radical prostatectomy group and of 38.3 % in the watchful waiting group, respectively) [4], the need to improve our capacity to stratify PCa patients according their risk of disease recurrence remains high.
This is particularly relevant for a public, universalistic health system like the European one. Indeed, because of the expected increase of life expectancy and incidence of PCa, we can expect that the disease’s economic burden in Europe will also increase substantially. It is estimated that the total economic costs of PCa in Europe exceed € 8.43 billion [5], with a high proportion of the costs of PCa care occurring in the first year after diagnosis. In European countries with available data (UK, Germany, France, Italy, Spain, the Netherlands), this amounted to € 106.7–179.0 million for all PCa patients diagnosed in 2006.
The first analytically and clinically validated CTC detection platform was the CellSearch® system. In a first published clinical study, the platform was tested in patients (n = 964) from different cancers and in 324 healthy donors or benign disease samples. In 123 patients (188 samples) affected by metastatic prostate cancer, 77 samples showed more than 5 CTCs/7.5 ml of peripheral blood. Based on the absence of CTCs in healthy controls, a high specificity (>99 %) using a cutoff of a single CTC was observed [6]. In mPCa the number of CTCs detected per 7.5 mL of whole blood can range widely depending on the context.
Speaking about PCa, Moreno et al. firstly reported in 2001 that CTC levels can be quantified in the circulation of these patients and that the change of the numbers of CTCs correlates with disease progression with no diurnal variations [7]. In 2007, Danila and colleagues reported that the number of CTCs before therapy provides unique information relative to prognosis and that the shedding of cells into the circulation represents an intrinsic property of the tumor, based on the extent of the disease [8].
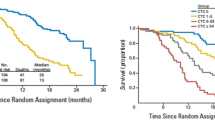
In 2008, the results of the first important trial that studied the association of CTC count with overall survival (OS) in castration-resistant prostate cancer (CRPC) (IMMC-38, NCT00133900) were published. In this trial, 276 patients affected by CRPC were prospectively evaluated; the CTC counting was performed at diagnosis and after initiation of treatment with cytotoxic chemotherapy. This study demonstrated that an unfavorable CTC level, defined as a value equal or higher than 5 cells/7.5 mL, was associated with a shorter median overall survival at all predefined time points (6.7–9.5 months vs. 19.6–20.7 months; HR, 3.6–6.5; P < 0.0001) [9].
At baseline, 57 % of patients had an unfavorable CTC count with a decreased median survival of 11.5 months; this finding was significantly lower when compared with 21.7 months for patients with a favorable CTC count (defined as CTC level lower than 5 cells/7.5 mL). Patients converting from unfavorable CTC level at baseline to favorable CTC count after treatment had a corresponding improvement in median OS (from 6.8 month to 21.3 month, respectively). The CTC count prior to and following initiation of treatment was the strongest prognostic factor, superior to prostate-specific antigen (PSA) and many established prognostic variables.
In the next paragraphs, we will address the main open question about the clinical management of mPCa, with the intent to underscore how the extended use of CTC detection and characterization can offer further benefit to these patients.
5.2 Can We Use the Enumeration of CTCs for Planning PCa Prevention Strategies?
Increasing age, ethnic origin, and heredity have been associated with higher risk of developing clinical PCa. However, if the frequency of incidentally- and autopsy-detected cancers is roughly the same in different parts of the world [10], the incidence of clinical PCa differs widely between different geographical areas. Notably, if Japanese men move from Japan to Hawaii, their risk of PCa increases; if they move to California, their risk increases even more, approaching that of American men [11].
These findings indicate that exogenous factors affect the risk of progression from latent to clinical PCa, including alimentary and sexual behavior, alcohol consumption, exposure to ultraviolet radiation, chronic inflammation [12, 13], and occupational exposure [13]. On this basis, PCa may be an ideal candidate for exogenous preventive measures that might include dietary and pharmacological prevention, particularly if we considered the high prevalence and long latency of this endocrine-dependent malignancy.
However, the availability of serum markers and the identification of prostatic intraepithelial lesions are mandatory to plan efficacious prevention. Indeed, if hereditary factors are important in determining the risk of developing clinical PCa, exogenous factors play a role in the risk of progression. Unfortunately, due to the lack of a reliable marker for identifying these patients, there is, as yet, insufficient evidence to recommend lifestyle changes (such as a reduced intake of animal fat and an increased intake of fruit, cereals and vegetables) in order to decrease this last risk [14].
Unlike early breast cancer, in which prognostic and predictive impact of tumor cells in peripheral blood or bone marrow was largely provided [15–17], the role of CTCs in localized PCa is far from clear. Whereas some authors were unable to find a significantly higher number of CTCs in the setting of localized PCa [18], more recently close to 50 % of CTC-positive patients have been reported in men candidates to undergo radical prostatectomy because of positive biopsy for cancer [19]. If we can use CTC count to plan any prevention strategy at least in these patients remains an open question that deserves ad hoc designed studies.
5.3 Can We Use the Enumeration of CTCs for Choosing the Treatment of mPCa?
Discriminating among widely advanced disease versus locally advanced disease (clinical stage T3) drives the treatment choice of PCa. Generally, after a radical prostatectomy, the PSA level should be less than 0.2 ng/mL, and after radiation therapy the level should be less than 0.5 ng/mL [20]. The most common presentation of advanced PCa is a patient with a rising PSA level in whom initial local therapy has failed; this condition is defined as “biochemical failure,” and it determines a change of treatment.
Historically, systemic therapy for metastatic and advanced prostate cancer has included androgen suppression. In metastatic disease, this palliative therapy has yielded a median progression-free survival (PFS) of 18–20 months and an overall survival (OS) of 24–36 months. However, virtually all patients develop hormone-refractory disease.
Despite the steady decline in the incidence of newly diagnosed mPCa and microscopic lymph node metastasis, from 20 % in the 1970s to 3.4 % in the 1990s, the risk of extra-prostatic disease in patients with clinically localized disease remains high, at 30–60 %, despite initial treatment with intent to cure. In some cases of hormone-refractory prostate cancer, the prostate cancer may continue to exhibit hormone dependence; however, so far we cannot predict whether these patients may benefit from androgen withdrawal versus continued hormone therapy.
Indeed, despite the great effort employed, often rewarded by a net improving of clinical results, we still live in an imperfect world, so the main clinical and research unanswered question in CRPC has been to define and standardize progression as an objective end point, in order to optimize duration of any systemic therapy [21]
The definition of a rising PSA level is not consistent in the literature, but many agree that the occurrence of two consecutive PSA level elevations can be considered biochemical failure. Other important prognostic indicators include the PSA velocity, time to PSA nadir, time to PSA recurrence, and pattern of PSA recurrence. Denham et al. reported that the PSA doubling time and the time to biochemical failure could provide useful surrogate endpoints for prostate cancer-specific mortality, potentially meaning that the follow-up period in clinical trials can be significantly reduced. However, further studies are still needed [22].
Speaking about other clinical-pathological criteria (and putative surrogate endpoints), pretreatment Gleason score, clinical stage, PSA level, and percentage of positive core biopsy results have been found to be reliable predictors of failure following local therapy. Unfortunately, no means of identifying recurrences limited to the pelvis is reliable. Although a Gleason grade of 7 or less is associated with a better prognosis than a grade of 8 or more, if the PSA level rise occurs after 2 years following local treatment, the associated survival likelihood is greater than if the rise occurs before 2 years.
In a study, based on an evaluation of data from the Radiation Therapy and Oncology Group 92–02 randomized trial, Ray et al. determined that distant metastasis and general failure of clinical treatment at 3 years might be candidates as surrogate endpoints for prostate cancer-specific survival at 10 years, potentially shortening the duration of clinical trials for prostate cancer. According to investigators’ conclusions, these endpoints still need to be validated in other datasets [23].
On this basis, we should not be surprised if the decision algorithm for initiation of treatment for biochemical failure is controversial. Certain factors to consider include the type of local therapy previously instituted (if any), the patient’s life expectancy, the intention and likelihood of cure, the risk for increased morbidity, and the patient’s quality of life. So far, no guidelines have been set for treating patients with advanced PCa in whom local therapy has failed.
The enumeration of CTCs in the peripheral blood of mPCa patients might contribute to address this issue, and several clinical studies reported results concerning the potential of this parameter as surrogate marker of overall survival (OS) in mPCa (reviewed by de Bono et al. [24]).
For example, Goldkorn and colleagues [25] published the result of SWOG S0421 that addressed the prognostic and predictive value of CTC enumeration prospectively, in a large phase III cohort treated homogenously with docetaxel—the standard first-line chemotherapy for mCRPC. The authors could validate baseline CTC counts as prognosticator and demonstrated that rising CTCs at 3 weeks heralded significantly worse OS, potentially serving as an early metric to help redirect and optimize therapy in this clinical setting. The prognostic value of CTCs was also reported in metastatic hormone-sensitive PCa by SWOG S0925 [26], despite that the little number of evaluable patients (n = 39) included in the study requires to be confirmed in larger studies.
A comparison of individual prognostic value of CTCs and objective response criteria has been also prospectively conducted in mCRPC treated by first-line docetaxel [27]. The authors included morphological RECIST and clinical criteria, as well as PSA decline, for evaluating patients’ survival. This small pilot study (n = 33) offers the rationale to larger validation studies, and the authors concluded that CTC counts appear to be an earlier and more sensitive predictor for survival and treatment response than current objective response approaches. In other words, CTCs might provide complementary information for individualized treatment strategies.
Notably, the use of CTCs as an earlier surrogate marker of OS might contribute to reduce the time and the cost of clinical studies focused to identify men likely to respond to new available therapies. Indeed, CTC enumeration was included as an outcome measure into the abiraterone acetate phase III registration trial (COU-AA-301) in patients with mCRPC previously treated with docetaxel [28].
Similarly, CTC count was embedded as a biomarker endpoint into the AFFIRM trial that conducted to the approval of enzalutamide for post-chemotherapy CRPC, based on the OS benefit. In this trial, the higher rate of conversion from unfavorable to favorable CTC count and the lower conversion from favorable to unfavorable CTC count for enzalutamide relative to placebo were consistent with the observed OS benefit [29].
5.4 CTC Detection Methods: Looking to Consensus Criteria
The main reason of the CTC success as potential surrogate endpoint comes from afar and depends on a strong biological evidence, sustained by the robustness of detection methods.
In 1869, for the first time, CTCs were observed in the blood of a man with metastatic cancer by Thomas Ashworth, who postulated that “cells identical with those of the cancer itself being seen in the blood may tend to throw some light upon the mode of origin of multiple tumors existing in the same person.” A thorough comparison of the morphology of the circulating cells to tumor cells from different lesions led Ashworth to conclude that “One thing is certain, that if they [CTC] came from an existing cancer structure, they must have passed through the greater part of the circulatory system to have arrived at the internal saphena vein of the sound leg” [30].
In 1874 De Morgan postulated that cells derived from a primary tumor could escape and travel through environ tissue and invade new areas, using lymphatic or blood vessels [31].
Twenty years after Ashworth, Stephan Paget, a surgeon in the UK, proposed the “seed and soil” theory, the theory that suggests that a tumor cell – the seed – either sleeps or thrives within the unique environment of each organ [32].
The first systematic study using smears blood from cancer patients, in 1934, demonstrated the presence of CTC in 43 % of cases [33].
Only in 2003 the soil theory was verified, an analysis of CTCs that is a “seed” in the blood has been considered to be a very important field in clinical prediction. In 2004, a clinical study was reported showing the importance of CTC as a prognostic factor. Strong evidence for CTCs as prognostic markers has been documented for breast cancer [34], but CTC detection is also connected to metastatic relapse and progression in other tumor entities, including prostate, lung, and colorectal cancer.
The process of metastatic spread from the primary tumor site into distal organs is still not well understood. Recent studies suggest an early spread of tumor cells to lymph nodes or bone marrow (BM) referred as “disseminated tumor cells” (DTCs) or as “circulating tumor cells” (CTCs) when present in the peripheral blood (PB) [17, 35].
The rate of tumor cells that are released by cancer is not known, but different studies estimate that millions of cells are dispersed into the body. The evidence demonstrated that only few tumor cells are able to overcome the lack of cell matrix interaction and escape the immunosurveillance, thus, to survive in the bloodstream and reach a distant organ and eventually grow into a metastasis.
Only in 2007, for the first time, the American Society of Clinical Oncology (ASCO) cited CTC and DTC in recommendations on tumor markers. Recently, the American Joint Committee on Cancer has proposed a new category, M0(i+), for TNM staging in breast cancer (BC). This category is defined as “no clinical or radiographic evidence of distant metastases, but deposits of molecularly or microscopically detected tumor cells (no larger than 0.2 mm) in blood, bone marrow, or other non-regional nodal tissue in a patient without symptoms or signs of metastases.”
More recently, Bidard and colleagues demonstrated the clinical validity of the CTC assay, as performed by the CellSearch platform, reaching the level I of evidence by the pooled analysis of individual data obtained from close to two thousand European metastatic BC [36].
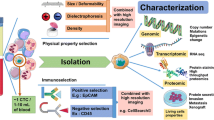
CTCs are very rare cells as only one CTC is contained in about 1 × 108 or 1 × 109 of blood cells in cancer patients’ blood, thus their detection and characterization requires highly sensitive and specific methods. To date, the only method FDA approved is the CellSearch system. This platform takes advantage of the fact that carcinomas derive from epithelial cells that are not normally found in the bloodstream. From 7.5 ml of blood, CTCs are immune-magnetically enriched with a specific antibody for epithelial cell adhesion molecule (EpCAM) coupled with ferrofluid. In a second step, the enriched cells were stained with a nucleic acid dye, DAPI, and a monoclonal antibody directed against cytokeratins (CK) 8, 18, and 19; in order to exclude contaminating leukocytes, an antibody that identifies CD45 is included. An automated microscope collects the images of any fluorescent event and proposes a photo gallery to a trained operator for the manual scoring of CTCs (see Fig. 5.1).
In this picture, it is possible to see CTCs with different morphology detected with CellSearch platform. CTCs are immune-magnetically enriched with a specific antibody for epithelial cell adhesion molecule (EpCAM) coupled with ferrofluid. In a second step, the enriched cells were stained with a nucleic acid dye, DAPI (in purple), and a monoclonal antibody directed against cytokeratins (CK) 8, 18, and 19 (in green)
Currently there are many methods in order to isolate and detect CTCs, follow you find an overview of strategies used to capture CTCs and specific examples from every kind (see Table 5.1).
Methods that use immunoaffinity purification strategy have proven to be an efficient way to capture CTCs and for this is the most widely used. They typically use anti-EpCAM antibodies but also other antibodies that recognized tumor-associated antigen, acting as capturing elements for CTCs from human whole blood. The main example is the CellSearch platform, but there are also CTC-chip, an array of 78,000 microspots coated with anti-EpCAM antibodies, Adna Test and Mag-Sweeper Isoflux that use a cocktail of antibodies specific to kind of cancer, and the GILUPI CellCollector® that is the first in vivo CTC isolation product worldwide which is CE approved. This device resembles a venous blood withdrawal. The GILUPI CellCollector® is placed directly into the bloodstream of a patient via an indwelling catheter (size 20 G, pink), remains in the arm vein for 30 min, and thus enables the capture of a large number of CTCs in vivo [37].
It is also known that tumor cells are a heterogeneous population, and EpCAM is not constantly expressed on them. Furthermore, it has been noted that circulating tumor microemboli (CTM) or CTCs with epithelial mesenchymal transition (EMT) which are attracting attention these years show no or weak expression of EpCAM, and therefore they are not detectable by the method above. For this reason, methods to isolate CTCs based on their physical properties, including density, size, deformability, and electrical properties have been developed.
Some groups use density gradient centrifugation methods for separating CTCs in mononuclear fraction based on cell density as centrifugation with Ficoll-Paque solution or OncoQuick (combine a porous filter for size-based separation in conjunction with gradient centrifugation). The isolation is in general followed by an RT-PCR specific for CK. The most promising method is leukapheresis in which white blood cells are separated from a sample of blood. In this way, a large volume of patient’s blood could be analyzed for CTCs; the result is an improvement in the number of CTCs isolated and in sensitivity for downstream analysis and characterization.
Microfiltration and microfluidics are also employed: with microfiltration CTCs are retained on the basis of size, assuming that CTCs are larger than leukocytes. The two main techniques are ISET [38] that uses a polycarbonate filter with 8 μm diameter circular pores for CTC enrichment and ScreenCell that uses circular track-etched filters; the pores’ range is 7.5–6.5 μm. This methods’ advantage is that CTCs can be isolated as living cells without fixation. Nowadays, inexpensive and convenient devices are available, but they are disadvantageous in that the blood samples have to be isolated in a short time after drawing. Recently De Wit and colleagues [39] were able to isolate CTCs onto a silicon membrane with 5 μm diameter circular pores. Using microfluidic tool to retain CTCs, the size and deformability of these cells can be explored.
The dielectrophoresis (DEP) exploits the electrical properties of CTCs, to discriminate them from leukocytes by applying a nonuniform electric field. Gupta and colleagues developed ApoStream instrument for file flow fractionation [40], and Manaresi and colleague [41] developed DEPArray, based on a microfluidic cartridge that contains an array of individually controllable electrodes, each with embedded sensors. This circuitry enables the creation of dielectrophoretic (DEP) cages around cells. After imaging, individual cells of interest are gently moved to specific locations on the cartridge, e.g. for cell-cell interaction studies or into the holding chamber for isolation and recovery.
Functional assay
CTCs could also be enriched by an approach that utilizes the functional aspect of CTCs as invasiveness and secretion of specifics protein. So far, only two technologies use this strategy, namely, EPISPOT and VitaAssayTM. By the first, membrane immune-captures specific proteins secreted near of the cells. The second method takes advantage of the propensity of cells to invade into collagenous matrices.
Notably, the numbers of CTCs reported vary widely between different platforms; for this reason, there is a need of a uniform, clear, and concordant definition of criteria for defining an event as a CTCs. About CellSearch platform, many studies have been performed, and all this show a high level of concordance also if the classification is operator dependent [42–44].
However, the same level of evidence has not been yet obtained for other different platforms; the studies are few, and the great majority of them are lacking of automation in the classification of CTCs.
Hopefully, this step will be overcome in a few years through the results of the CANCER-ID (IMI-JU-11-2013, EoL no. 115749-1, “Cancer treatment and monitoring through identification of circulating tumor cells and tumour related nucleic acids in blood”), an EU-founded project that, among other, is working to an open source computer program to identify CTCs from image obtained by different platforms. Indeed, the main purpose of the consortium, which so far collected 37 partners among academic and industry world, is to construct a consensus about the minimum criteria necessary and sufficient to define an event as a CTC (http://www.cancer-id.eu/).
5.5 Molecular Characterization of CTCs in mPCa
CTCs represent a source of tumor specimen useful for molecular studies without the invasiveness of a tumor biopsy; at the same time, by collecting sequential blood samples, CTC study allows longitudinal analyses in order to assess the effect or lack of effect of treatments.
Especially in prostate cancer (PCa), and into the age of target therapy, molecular characterization of CTCs should bring advances in the current lack of biomarkers specific for individualized treatment. Characterization of CRPC disease in clinical studies is challenging, for its heterogeneity and because often metastases are exclusive to the bone, a site which is difficult to reach.
A wide assortment of protein- and genome-based assay can be performed on CTCs. The most common ones are immunohistochemistry, immunofluorescence, gene-copy-number analysis using comparative genomic hybridization (aCGH), genomic sequencing analysis, epigenetic studies, and finally next-generation sequencing (NGS).
The common approach of immunophenotyping of CTCs is the complemented assay of enumeration; the only drawback is that the number of antibodies necessary to identify CTCs limits the number of characterizations. By using CellSearch system, it is possible to introduce an additional antibody conjugated to a fluorochrome in order to evaluate the CTC expression of specific antigens. Many studies in CRPC focus on the expression of androgen receptor (AR) by using an antibody directed against AR, and the presence of genomic AR amplification is then confirmed by FISH analysis [45].
This approach is aimed at monitoring the response to the AR targeting agents, like enzalutamide and abiraterone. In fact, prostate cancer could develop resistance to androgen receptor therapy by way of amplification, mutation, or spliced variant of AR or autocrine androgen synthesis [46–49].
M. Crespo [45] analyzed 94 samples from 48 patients affected by metastatic CRCP using CellSearch platform with an additional antibody specific for AR. In this study, the authors compared patients grouped by the absence of prior exposure vs. resistance to abiraterone or enzalutamide. A large intra- and inter-patient heterogeneity of AR expression in CTCs was observed. Crespo and colleagues did not observe a difference in nuclear AR expression in CTCs in CRCP, suggesting that there are no changes in nuclear AR expression following development of resistance to novel endocrine agents in CRCP. However, we observed that the antibody chosen by the authors did not distinguish AR full length from AR-V7 or other spliced isoforms of this protein.
Speaking about the expression of AR and AR-V7 variant, Miyamoto uses the CTC-iChip, a microfliudic device, in order to sequence RNA of 77 single CTCs from 13 PCa patients, of whom 11 were CRCP [50]. This study provided several important observations, firstly that about one-sixth of CTCs co-expressed more than one AR splice variant (AR-V7). This finding does not agree with the common opinion that several variants are co-expressed in tumor tissue and/or CTCs and that they may be competing with full-length AR (AR-FL) for dimerization, which is required for transcriptional activity. They also observed the presence of other AR variants, like AR-V1, AR-V3, and AR-V4 in 5 out of 11 patients and AR-V7 and AR-V12 in 8 out of 11 patients. These results revealed a more complex and heterogeneous pattern regarding AR spliced-variant expression in the CTC compartment that was not revealed in primary tumors. Interestingly, the researchers also observed an inverse relationship between glucocorticoid receptor (GR) and non-canonical Wnt signaling in enzalutamide-progressing patients; both these pathways can be activated in drug resistance in PCa, and the finding suggests the presence in a part of CTC population of an AR-independent drug resistance pathway. In the small group observed by Miyamoto, he did not find a substantial enrichment in AR-V7 expression in patients treated with enzalutamide compared with the cohort enzalutamide naïve.
This is in contrast with the Antonarakis and colleagues study that demonstrated that the resistance to treatment with enzalutamide and abiraterone was associated with expression of AR-V7 in CTCs. Notably, Antonorakis and colleagues studied the outcomes of 31 CRCP patients according to the presence of AR-V7 RNA, as detected by AlereTM CTC Adna Test. These authors concluded by proposing the presence of AR-V7 as a predictive biomarker for lack of clinical benefit of this target drug [51].
Genomic changes showed by CGH array and limited sequencing have been reported on CTCs isolated by using CellSearch platform. Analysis in pared tumors, metastasis, and CTCs suggests that most mutations detected in CTCs were present at a low level in the primary tumors [52].
By using different methods to count and isolate CTC (HD-CTC), Dago and colleagues characterized 41 CTCs collected at four clinical time points. They were able to demonstrate the emergence of distinct CTC subpopulations with specific molecular alterations that were associated to the clinical course of disease and the treatment with targeted ADT [53].
A study carried out with Epic CTC platform, a system without enrichment that spots nucleated cells onto glass microscope slides, revealed that CTCs and WBC are characterized by distinct PTEN and CEP10 genotypes, and CTCs showed an increased ploidy and a heterogeneous status of PTEN. By using FISH analysis, the authors [54] demonstrated a good correlation between PTEN in CTCs and in fresh tumor tissue. Notably, PTEN loss in CTCs (as well as in tumor biopsy) was associated with a worse prognosis.
Recently, a study also revealed that in metastatic neuroendocrine prostate cancer, the CTCs were heterogenic for CK and AR expressions; the expression of AR was much lower, and the presence of AR was localized into the cytoplasm, contrary to CRPC that show AR in the nucleus. This characteristic in addition to morphology has a diagnostic potential in distinguishing NEPC from CRPC [55].
Finally yet importantly, if we will be able to address the full molecular characterization of CTCs, we will probably realize the right concept of “liquid biopsy,” i.e., a minimally invasive procedure to investigate the malignancies throughout the disease course.
Conclusions
The great majority of the studies that we have briefly discussed here underscore the limits deriving from the need to enlarge the cohorts of patients studied and to receive an external validation as further independent confirmation. However, all of them indicate that CTC evaluation could provide information about disease heterogeneity, its clonal evolution, metastatic dissemination, and development of resistance to therapeutics in individual patient, throughout the continuum of the care. The study of this particular compartment of malignancy offers firstly the opportunity to design the patient treatment onto the biology of his/her disease.
References
Hayes DF (2015) Biomarker validation and testing. Mol Oncol 9(5):960–966
Zhang T, Armstrong AJ (2016) Clinical utility of circulating tumor cells in advanced prostate cancer. Curr Oncol Rep 18(1):3
De Angelis R, Sant M, Coleman MP, Francisci S, Baili P, Pierannunzio D, Trama A, Visser O, Brenner H, Ardanaz E et al (2014) Cancer survival in Europe 1999-2007 by country and age: results of EUROCARE – 5-a population-based study. Lancet Oncol 15(1):23–34
Bill-Axelson A, Holmberg L, Garmo H, Rider JR, Taari K, Busch C, Nordling S, Haggman M, Andersson SO, Spangberg A et al (2014) Radical prostatectomy or watchful waiting in early prostate cancer. N Engl J Med 370(10):932–942
Luengo-Fernandez R, Leal J, Gray A, Sullivan R (2013) Economic burden of cancer across the European Union: a population-based cost analysis. Lancet Oncol 14(12):1165–1174
Allard WJ, Matera J, Miller MC, Repollet M, Connelly MC, Rao C, Tibbe AG, Uhr JW, Terstappen LW (2004) Tumor cells circulate in the peripheral blood of all major carcinomas but not in healthy subjects or patients with nonmalignant diseases. Clin Cancer Res 10(20):6897–6904
Moreno JG, O’Hara SM, Gross S, Doyle G, Fritsche H, Gomella LG, Terstappen LW (2001) Changes in circulating carcinoma cells in patients with metastatic prostate cancer correlate with disease status. Urology 58(3):386–392
Danila DC, Heller G, Gignac GA, Gonzalez-Espinoza R, Anand A, Tanaka E, Lilja H, Schwartz L, Larson S, Fleisher M et al (2007) Circulating tumor cell number and prognosis in progressive castration-resistant prostate cancer. Clin Cancer Res 13(23):7053–7058
de Bono JS, Scher HI, Montgomery RB, Parker C, Miller MC, Tissing H, Doyle GV, Terstappen LW, Pienta KJ, Raghavan D (2008) Circulating tumor cells predict survival benefit from treatment in metastatic castration-resistant prostate cancer. Clin Cancer Res 14(19):6302–6309
Lawrentschuk N, Haider MA, Daljeet N, Evans A, Toi A, Finelli A, Trachtenberg J, Zlotta A, Fleshner N (2010) ‘Prostatic evasive anterior tumours’: the role of magnetic resonance imaging. BJU Int 105(9):1231–1236
Breslow N, Chan CW, Dhom G, Drury RA, Franks LM, Gellei B, Lee YS, Lundberg S, Sparke B, Sternby NH et al (1977) Latent carcinoma of prostate at autopsy in seven areas. The International Agency for Research on Cancer, Lyons, France. Int J Cancer 20(5):680–688
Nelson WG, De Marzo AM, Isaacs WB (2003) Prostate cancer. N Engl J Med 349(4):366–381
Leitzmann MF, Rohrmann S (2012) Risk factors for the onset of prostatic cancer: age, location, and behavioral correlates. Clin Epidemiol 4:1–11
Richman EL, Kenfield SA, Stampfer MJ, Giovannucci EL, Chan JM (2011) Egg, red meat, and poultry intake and risk of lethal prostate cancer in the prostate-specific antigen-era: incidence and survival. Cancer Prev Res (Phila) 4(12):2110–2121
Lucci A, Hall CS, Lodhi AK, Bhattacharyya A, Anderson AE, Xiao L, Bedrosian I, Kuerer HM, Krishnamurthy S (2012) Circulating tumour cells in non-metastatic breast cancer: a prospective study. Lancet Oncol 13(7):688–695
Rack B, Schindlbeck C, Juckstock J, Andergassen U, Hepp P, Zwingers T, Friedl TW, Lorenz R, Tesch H, Fasching PA et al (2014) Circulating tumor cells predict survival in early average-to-high risk breast cancer patients. J Natl Cancer Inst 106(5)
Braun S, Vogl FD, Naume B, Janni W, Osborne MP, Coombes RC, Schlimok G, Diel IJ, Gerber B, Gebauer G et al (2005) A pooled analysis of bone marrow micrometastasis in breast cancer. N Engl J Med 353(8):793–802
Davis JW, Nakanishi H, Kumar VS, Bhadkamkar VA, McCormack R, Fritsche HA, Handy B, Gornet T, Babaian RJ (2008) Circulating tumor cells in peripheral blood samples from patients with increased serum prostate specific antigen: initial results in early prostate cancer. J Urol 179(6):2187–2191; discussion 2191
Rossi E et al (2015) AACR Annu Meet poster #379
Amling CL, Bergstralh EJ, Blute ML, Slezak JM, Zincke H (2001) Defining prostate specific antigen progression after radical prostatectomy: what is the most appropriate cut point? J Urol 165(4):1146–1151
Armstrong AJ, Eisenberger MA, Halabi S, Oudard S, Nanus DM, Petrylak DP, Sartor AO, Scher HI (2012) Biomarkers in the management and treatment of men with metastatic castration-resistant prostate cancer. Eur Urol 61(3):549–559
Denham JW, Steigler A, Wilcox C, Lamb DS, Joseph D, Atkinson C, Matthews J, Tai KH, Spry NA, Christie D et al (2008) Time to biochemical failure and prostate-specific antigen doubling time as surrogates for prostate cancer-specific mortality: evidence from the TROG 96.01 randomised controlled trial. Lancet Oncol 9(11):1058–1068
Ray ME, Bae K, Hussain MH, Hanks GE, Shipley WU, Sandler HM (2009) Potential surrogate endpoints for prostate cancer survival: analysis of a phase III randomized trial. J Natl Cancer Inst 101(4):228–236
Mehra N, Zafeiriou Z, Lorente D, Terstappen LW, de Bono JS (2015) CCR 20th anniversary commentary: circulating tumor cells in prostate cancer. Clin Cancer Res 21(22):4992–4995
Goldkorn A, Ely B, Quinn DI, Tangen CM, Fink LM, Xu T, Twardowski P, Van Veldhuizen PJ, Agarwal N, Carducci MA et al (2014) Circulating tumor cell counts are prognostic of overall survival in SWOG S0421: a phase III trial of docetaxel with or without atrasentan for metastatic castration-resistant prostate cancer. J Clin Oncol 32(11):1136–1142
Yu EY, Li H, Higano CS, Agarwal N, Pal SK, Alva A, Heath EI, Lam ET, Gupta S, Lilly MB et al (2015) SWOG S0925: a randomized phase II study of androgen deprivation combined with cixutumumab versus androgen deprivation alone in patients with new metastatic hormone-sensitive prostate cancer. J Clin Oncol 33(14):1601–1608
Thalgott M, Heck MM, Eiber M, Souvatzoglou M, Hatzichristodoulou G, Kehl V, Krause BJ, Rack B, Retz M, Gschwend JE et al (2015) Circulating tumor cells versus objective response assessment predicting survival in metastatic castration-resistant prostate cancer patients treated with docetaxel chemotherapy. J Cancer Res Clin Oncol 141(8):1457–1464
Scher HI, Heller G, Molina A, Attard G, Danila DC, Jia X, Peng W, Sandhu SK, Olmos D, Riisnaes R et al (2015) Circulating tumor cell biomarker panel as an individual-level surrogate for survival in metastatic castration-resistant prostate cancer. J Clin Oncol 33(12):1348–1355
Fleisher M et al (2015) J Clin Oncol 33(suppl; abstr 5035)
Ashworth TR (1869) A case of cancer in which cells similar to those in the tumours were seen in the blood after death. Aust Med J 14:146
De Morgan C (1874) Observations on cancer: its pathology, and its relations to the organism and to other morbid growths. Lancet 103(2636):325–329
Paget S (1889) The distribution of secondary growths in cancer of the breast. 1989. Cancer Metastasis Rev 8(2):98–101
Pool EH, Dunlop GR (1934) Cancer cells in the blood stream. Am J Cancer 21:99–102
Cristofanilli M, Budd GT, Ellis MJ, Stopeck A, Matera J, Miller MC, Reuben JM, Doyle GV, Allard WJ, Terstappen LW et al (2004) Circulating tumor cells, disease progression, and survival in metastatic breast cancer. N Engl J Med 351(8):781–791
Braun S, Cevatli BS, Assemi C, Janni W, Kentenich CR, Schindlbeck C, Rjosk D, Hepp F (2001) Comparative analysis of micrometastasis to the bone marrow and lymph nodes of node-negative breast cancer patients receiving no adjuvant therapy. J Clin Oncol 19(5):1468–1475
Bidard FC, Peeters DJ, Fehm T, Nole F, Gisbert-Criado R, Mavroudis D, Grisanti S, Generali D, Garcia-Saenz JA, Stebbing J et al (2014) Clinical validity of circulating tumour cells in patients with metastatic breast cancer: a pooled analysis of individual patient data. Lancet Oncol 15(4):406–414
Saucedo-Zeni N, Mewes S, Niestroj R, Gasiorowski L, Murawa D, Nowaczyk P, Tomasi T, Weber E, Dworacki G, Morgenthaler NG et al (2012) A novel method for the in vivo isolation of circulating tumor cells from peripheral blood of cancer patients using a functionalized and structured medical wire. Int J Oncol 41(4):1241–1250
Vona G, Sabile A, Louha M, Sitruk V, Romana S, Schutze K, Capron F, Franco D, Pazzagli M, Vekemans M et al (2000) Isolation by size of epithelial tumor cells: a new method for the immunomorphological and molecular characterization of circulating tumor cells. Am J Pathol 156(1):57–63
de Wit S, van Dalum G, Lenferink AT, Tibbe AG, Hiltermann TJ, Groen HJ, van Rijn CJ, Terstappen LW (2015) The detection of EpCAM(+) and EpCAM(-) circulating tumor cells. Sci Rep 5:12270
Gupta V, Jafferji I, Garza M, Melnikova VO, Hasegawa DK, Pethig R, Davis DW (2012) ApoStream(™), a new dielectrophoretic device for antibody independent isolation and recovery of viable cancer cells from blood. Biomicrofluidics 6(2):24133
Bolognesi C, Forcato C, Buson G, Fontana F, Mangano C, Doffini A, Sero V, Lanzellotto R, Signorini G, Calanca A et al (2016) Digital sorting of pure cell populations enables unambiguous genetic analysis of heterogeneous formalin-fixed paraffin-embedded tumors by next generation sequencing. Sci Rep 6:20944
Riethdorf S, Fritsche H, Muller V, Rau T, Schindlbeck C, Rack B, Janni W, Coith C, Beck K, Janicke F et al (2007) Detection of circulating tumor cells in peripheral blood of patients with metastatic breast cancer: a validation study of the Cell Search system. Clin Cancer Res 13(3):920–928
Kraan J, Sleijfer S, Strijbos MH, Ignatiadis M, Peeters D, Pierga JY, Farace F, Riethdorf S, Fehm T, Zorzino L et al (2011) External quality assurance of circulating tumor cell enumeration using the Cell Search((R)) system: a feasibility study. Cytometry B Clin Cytom 80(2):112–118
Cummings J, Morris K, Zhou C, Sloane R, Lancashire M, Morris D, Bramley S, Krebs M, Khoja L, Dive C (2013) Method validation of circulating tumour cell enumeration at low cell counts. BMC Cancer 13:415
Crespo M, van Dalum G, Ferraldeschi R, Zafeiriou Z, Sideris S, Lorente D, Bianchini D, Rodrigues DN, Riisnaes R, Miranda S et al (2015) Androgen receptor expression in circulating tumour cells from castration-resistant prostate cancer patients treated with novel endocrine agents. Br J Cancer 112(7):1166–1174
Koivisto P, Kononen J, Palmberg C, Tammela T, Hyytinen E, Isola J, Trapman J, Cleutjens K, Noordzij A, Visakorpi T et al (1997) Androgen receptor gene amplification: a possible molecular mechanism for androgen deprivation therapy failure in prostate cancer. Cancer Res 57(2):314–319
Stanbrough M, Bubley GJ, Ross K, Golub TR, Rubin MA, Penning TM, Febbo PG, Balk SP (2006) Increased expression of genes converting adrenal androgens to testosterone in androgen-independent prostate cancer. Cancer Res 66(5):2815–2825
Dehm SM, Schmidt LJ, Heemers HV, Vessella RL, Tindall DJ (2008) Splicing of a novel androgen receptor exon generates a constitutively active androgen receptor that mediates prostate cancer therapy resistance. Cancer Res 68(13):5469–5477
Taylor BS, Schultz N, Hieronymus H, Gopalan A, Xiao Y, Carver BS, Arora VK, Kaushik P, Cerami E, Reva B et al (2010) Integrative genomic profiling of human prostate cancer. Cancer Cell 18(1):11–22
Miyamoto DT, Lee RJ, Stott SL, Ting DT, Wittner BS, Ulman M, Smas ME, Lord JB, Brannigan BW, Trautwein J et al (2012) Androgen receptor signaling in circulating tumor cells as a marker of hormonally responsive prostate cancer. Cancer Discov 2(11):995–1003
Antonarakis ES, Lu C, Wang H, Luber B, Nakazawa M, Roeser JC, Chen Y, Mohammad TA, Fedor HL, Lotan TL et al (2014) AR-V7 and resistance to enzalutamide and abiraterone in prostate cancer. N Engl J Med 371(11):1028–1038
Heitzer E, Auer M, Gasch C, Pichler M, Ulz P, Hoffmann EM, Lax S, Waldispuehl-Geigl J, Mauermann O, Lackner C et al (2013) Complex tumor genomes inferred from single circulating tumor cells by array-CGH and next-generation sequencing. Cancer Res 73(10):2965–2975
Dago AE, Stepansky A, Carlsson A, Luttgen M, Kendall J, Baslan T, Kolatkar A, Wigler M, Bethel K, Gross ME et al (2014) Rapid phenotypic and genomic change in response to therapeutic pressure in prostate cancer inferred by high content analysis of single circulating tumor cells. PLoS One 9(8):e101777
Punnoose EA, Ferraldeschi R, Szafer-Glusman E, Tucker EK, Mohan S, Flohr P, Riisnaes R, Miranda S, Figueiredo I, Rodrigues DN et al (2015) PTEN loss in circulating tumour cells correlates with PTEN loss in fresh tumour tissue from castration-resistant prostate cancer patients. Br J Cancer 113(8):1225–1233
Beltran H, Jendrisak A, Landers M, Mosquera JM, Kossai M, Louw J, Krupa R, Graf RP, Schreiber NA, Nanus DM et al (2016) The initial detection and partial characterization of circulating tumor cells in neuroendocrine prostate cancer. Clin Cancer Res 22(6):1510–1519
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2017 Springer International Publishing Switzerland
About this chapter
Cite this chapter
Rossi, E., Zamarchi, R. (2017). Circulating Tumor Cells (CTCs) and Metastatic Prostate Cancer (mPCa). In: Bertoldo, F., Boccardo, F., Bombardieri, E., Evangelista, L., Valdagni, R. (eds) Bone Metastases from Prostate Cancer . Springer, Cham. https://doi.org/10.1007/978-3-319-42327-2_5
Download citation
DOI: https://doi.org/10.1007/978-3-319-42327-2_5
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-42326-5
Online ISBN: 978-3-319-42327-2
eBook Packages: MedicineMedicine (R0)