Abstract
Why and how our immune system functions and sometimes dysfunctions? Immunologists are often surprised by the complexity of the human immune system’s performance. A brief exploration of the evolutionary history of the immune system might be able to provide insight for understanding this complexity of our important defense system and its role for human health.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Natural Killer Cell
- Adaptive Immunity
- Adaptive Immune System
- Immune System Function
- Natural Killer Cell Receptor
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
Why and how our immune system functions and sometimes dysfunctions? Immunologists are often surprised by the complexity of the human immune system’s performance. A brief exploration of the evolutionary history of the immune system might be able to provide insight for understanding this complexity of our important defense system and its role for human health.
Human immunity works through a complex, orchestrated, and many functional and organ-specific, though always interconnected, approaches. As from the evolution from simple organisms - as known especially from insects with a short life time (e.g. fruit fly) - to highly developed mammals, we know that two major immune system branches have evolved subsequently as a consequence of expanded life times and environmental challenges, the innate immunity and adaptive immunity. The coordinated efforts of the innate and adaptive immune branches normally guarantee an effective host defense against potentially harmful pathogens, to differentiate immune answers between self and nonself and hereby avoiding to harm the host. Innate immunity is the primary line of immune defense and yields an immediate nonspecific response, which is mediated mainly by neutrophils, monocytes, macrophages, dendritic cells (DCs), and natural killer (NK) cells, together with cytokines, defensins, and complement and acute phase reactants such as C-reactive protein (Akira et al. 2006; Medzhitov and Janeway 1997). Adaptive immunity, the so-called secondary line of defense, relies upon B and T lymphocytes which express antigen-specific surface receptors. There are two key components of the adaptive immune system: the humoral, antibody-mediated, and depending on B lymphocytes, and the cellular immunity as coordinated by T lymphocytes.
Innate immune mechanisms can be tracked back to almost the lowest level of the evolutionary tree of life, which indicates the importance of innate immunity in life surviving starting from the appearance of single-cell microorganisms on Earth more than 3.5 billion years ago (Kimbrell and Beutler 2001). The following evolution of diverse bacteria, archaea, and eukaryotes proceeded to the development of multicellular organisms (metazoans) that occurred around 600 million years ago. After the “cambrian explosion,” oxygen concentration and diversity of organisms had increased, and the diversity in metazoan species offered new host opportunities for microbial pathogens (Fig. 1.1).
The “cambrian explosion”: increase of the diversity and complexity of organisms as paralleled by the increase of oxygen in the atmosphere. Right graph green and red lines reflecting the anticipated lower and upper range of the oxygen concentration (cited figures as published by Falkowsky 2006 and Holland 2006)
On the same timescale, the diversity of microbial pathogens might explain the consecutive and remarkable varieties of innate defense mechanisms in plants and animals. Interestingly, a unifying element of innate immunity exists, which is the use of germline-encoded pattern recognition receptors for pathogens or damaged self-components, such as the Toll-like receptors, nucleotide-binding domain leucine-rich repeat (LRR)-containing receptors, and C-type lectin receptors (Buchmann 2014) [see also Chap. 3, part 3].
Adaptive immunity appeared in vertebrates around 500 million years ago with its unique feature of the somatic development of clonally diverse lymphocytes, each of which has a specific antigen recognition receptor that can trigger its activation. The existence of a highly diverse lymphocyte receptor repertoire allows vertebrates to recognize almost any potential pathogen or toxin and to mount antigen-specific responses to it (Cooper and Herrin 2010). Activated lymphocytes then engage in population expansion and differentiation into mature effector lymphocytes with cytotoxic and proinflammatory functions or into plasma cells that secrete antibodies. In addition, the population expansion and some long-existing antigen-primed cytotoxic lymphocytes and plasma cells provide protective memory to prevent from potentially detrimental consequences of the next invasion (Cooper and Herrin 2010).
T-cell-related cellular immune responses and B-cell-related humoral immune responses require the involvement of various phagocytic cells, dendritic cells (DCs), natural killer (NK) cells, and other types of innate immune cell and humoral components, but it is difficult to trace the evolutionary history of the extensive network of individual immune cell types like that in other systems such as myogenic cells (Yi et al. 2009, Cooper and Herrin 2010). Moreover, evolutionary processes are continually affecting the immune system. For example, we can see a rather recent evolution of very different types of NK cell receptors in mice and humans, which shared a common ancestor around 65 million years ago (Abi-Rached and Parham 2005). This kind of evolutionary changes increases the difficulty in deciphering some of the steps in the evolutionary history of immunity, for instance, the exact time when DC and NK cells entered the evolutionary scene remains a puzzle.
When reflecting the evolutionary history of immunity (see Fig. 1.2), the conclusion can be drawn that the high complexity of actions and interactions of the innate and adaptive immunity are the result of powerful and long-lasting selection and deselection processes, the increasing complexity, and life span of the organisms, which over the time has had to increase also the probability to efficiently distinguish between self and nonself and hereby combating pathogens (Flajnik and Kasahara 2010). However, the appearance of an adaptive immune system featuring a big randomly created receptor repertoire expressed by lymphocytes with proinflammatory potential would undoubtedly pose the danger of autoimmunity. Since we need to understand how the individual components of our complex immune system collaborate to activate protective immunity, a more general and “holistic” view is therefore important for the understanding of inflammatory and autoimmune diseases and for designing strategies to alleviate inappropriate or excessive immune responses. The importance of such understanding is of ultimate importance in our civilization in view of the fact that autoimmune and infectious causes of diseases are rising worldwide. The rise in the prevalence of allergic diseases has continued in the industrialized world for more than 50 years (from the American Academy of Allergy, Asthma & Immunology (AAAAI), Milwaukee/MI, USA); autoimmune disease prevalence is rising according to the National Institutes of Health (NIH, Bethesda/MD, USA), as well as the incidence of sepsis is increasing in all areas of the world where epidemiology studies have been conducted (Martin 2012).
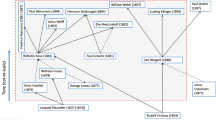
The evolution of the immune system (Compiled after Paul 2003)
It will be of key importance and of special interest how the further evolution and adaption processes of immune cells and immunity as a whole will occur in the coming hundreds and thousands of years. It should be considered also that since the gravitational environment on Earth might represent a key factor in the molecular homeostasis of the immune system and therefore optimal conditions for evolutionary development and adaptation, it has become even more interesting to investigate the “new immune system” when new living conditions occur and challenges are affecting our immune responses and evolution: life under conditions of reduced gravity in the hostile environment of space.
References
Abi-Rached L, Parham P (2005) Natural selection drives recurrent formation of activating killer cell immunoglobulin-like receptor and Ly49 from inhibitory homologues. J Exp Med 201:1319–1332
Akira S, Uematsu S, Takeuchi O (2006) Pathogen recognition and innate immunity. Cell 124:783–801
Buchmann K (2014) Evolution of innate immunity: clues from invertebrates via fish to mammals. Front Immunol 5:459
Cooper MD, Herrin BR (2010) How did our complex immune system evolve? Nat Rev Immunol 10(1):2–3. doi:10.1038/nri2686
Falkowski PG (2006) Evolution. Tracing oxygen’s imprint on earth’s metabolic evolution. Science 311(5768):1724–5
Flajnik MF, Kasahara M (2010) Origin and evolution of the adaptive immune system: genetic events and selective pressures. Nat Rev Genet 11:47–59
Holland HD (2006) The oxygenation of the atmosphere and oceans. Philos Trans R Soc Lond B Biol Sci 361(1470):903–15
Kimbrell DA, Beutler B (2001) The evolution and genetics of innate immunity. Nat Rev Genet 2:256–267
Martin GS (2012) Sepsis, severe sepsis and septic shock: changes in incidence, pathogens and outcomes. Expert Rev Anti Infect Ther 10:701–706
Medzhitov R, Janeway CA Jr (1997) Innate immunity: the virtues of a nonclonal system of recognition. Cell 91:295–298
Paul WE (2003) Fundamental immunology. Lippincott Williams & Wilkins, Philadelphia
Yi B, Bumbarger D, Sommer RJ (2009) Genetic evidence for pax-3 function in myogenesis in the nematode Pristionchus pacificus. Evol Dev 11:669–679
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Copyright information
© 2016 Springer International Publishing Switzerland
About this chapter
Cite this chapter
Yi, B., Thiel, M., Choukèr, A. (2016). The Immune System in Evolution. In: The Immune System in Space: Are we prepared?. SpringerBriefs in Space Life Sciences. Springer, Cham. https://doi.org/10.1007/978-3-319-41466-9_1
Download citation
DOI: https://doi.org/10.1007/978-3-319-41466-9_1
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-41464-5
Online ISBN: 978-3-319-41466-9
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)