Abstract
Purpose
Defining the site of recurrent disease early after definitive treatment for a localized prostate cancer is a critical issue as it may greatly influence the subsequent therapeutic strategy or patient management.
Methods
A systematic review of the literature was performed by searching Medline from January 1995 up to January 2011. Electronic searches were limited to the English language, and the keywords prostate cancer, radiotherapy [RT], high-intensity focused ultrasound [HIFU], cryotherapy [CRIO], transrectal ultrasound [TRUS], magnetic resonance imaging [MRI], PET/TC, and prostate biopsy were used.
Results
Despite the fact that diagnosis of a local recurrence is based on PSA values and kinetics, imaging by means of different techniques may be a prerequisite for effective disease management. Unfortunately, prostate cancer local recurrences are very difficult to detect by TRUS and conventional imaging that have shown limited accuracy at least at early stages. On the contrary, functional and molecular imaging, such as dynamic contrast-enhanced MRI (DCE-MRI) and diffusion-weighted imaging (DWI), offers the possibility of imaging molecular or cellular processes of individual tumors.
Recently, PET/CT, using 11C-choline, 18F-fluorocholine, or 11C-acetate, has been successfully proposed in detecting local recurrences as well as distant metastases. Nevertheless, in controversial cases, it is necessary to perform a biopsy of the prostatic fossa or a biopsy of the prostate to assess the presence of a local recurrence under guidance of MRI or TRUS findings.
Conclusion
It is likely that imaging will be extensively used in the future to detect and localize prostate cancer local recurrences before salvage treatment.
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
Keywords
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
1 Introduction
Recurrence after a definitive treatment for prostate cancer (PCa) (i.e., radical prostatectomy [RP], radiotherapy [RT], high-intensity focused ultrasound [HIFU], and cryotherapy [CT]) is defined by an increase in the serum value of PSA after reaching the nadir. Generally, the definition of recurrence after RP has relied on a single elevated PSA level, but the reported level of PSA which indicates failure after RP varies. Various PSA thresholds have been used, including >0.1, >0.2, >0.4, and >0.5 ng/ml [1–4]. After radiotherapy, the RTOG-ASTRO Phoenix consensus has assessed that a rise by 2 ng/ml or more above the nadir as standard definition of biochemical failure after RT (or 3 ng/ml after brachytherapy).
The PCa recurrences may be classified into four main categories: (1) PSA-only relapse, (2) local recurrence, (3) distant metastases (most commonly nodal or osseous), and (4) combination of local and distant recurrences. Detecting the site of recurrence is difficult, since an increasing PSA level is rarely associated with symptoms or findings at physical examination [5–10]. Thus, PSA doubling time (in months), PSA velocity (in ng/ml/year), and nomograms have been advocated in order to improve the discrimination between local or distant recurrence [11–13].
From a practical point of view, detecting the site of recurrence (local vs distant) is critical for defining the optimum treatment [14, 15]. For instance, patients submitted to RRP with a local recurrence with no detectable distant metastases may benefit from salvage radiotherapy with or without androgen deprivation (according to the Consensus Statement on Radiation Therapy of Prostate Cancer and to the Society of Therapeutic Radiation Oncologist guidelines), whereas those with distant disease may be treated with systemic treatments [16].
Currently, different imaging methods are suggested, together with the biopsy of prostatic fossa or prostate biopsy after treatment which may help the urologists and the oncologists to assess local recurrence in patient with PSA relapse after definitive treatments for PCa.
We have reviewed the available literature on this topic and analyzed all the advantages and disadvantages of all the available imaging techniques.
2 Material and Methods
A systematic review of the literature was performed by searching Medline from January 1995 up to January 2011. Electronic searches were limited to the English language, and the keywords prostate cancer, RT, HIFU, CT, transrectal ultrasound [TRUS], magnetic resonance imaging [MRI], PET/TC, and prostate biopsy were used.
2.1 Local Recurrence After Radical Prostatectomy
After surgery, it is possible to perform different imaging modalities to detect a local recurrence [17, 18]. Traditionally, transrectal ultrasound (TRUS) is easily performed, but it has shown to have many limitations when differentiating between recurrent or residual tumor and postsurgical scarring. Nevertheless, the biopsies of the prostatic fossa after RP are still executed under TRUS-guidance.
2.1.1 TRUS
Although several trials have shown that TRUS is better than digital rectal examination (DRE) for detecting local recurrence, it lacks specificity.
TRUS appearance of local recurrence of PCa in the prostatic fossa in patients with no clinical or biochemical evidence of recurrence after RP includes asymmetric thickening or fullness of the anastomosis, loss of the integrity of the retro-anastomotic fat plane, and/or the presence of hypoechoic lesion in the peri-anastomotic area, surrounded by a variable amount of tissue that is more prominent anteriorly and that is hypoechoic relative to the surrounding fat [19–21].
The most common TRUS-detectable lesion site is the vesico-urethral anastomosis (VUA) area. The other sites include the anterior and the posterior bladder neck and, less frequently, the retrovesical space (posterior to the bladder neck). Particularly, TRUS provides a substantial advantage compared with DRE in the PCa recurrence localized at the bladder neck, since these lesions may be more difficult to be palpable because of the anterior location or because of merging of the lesion with the bladder wall.
Thus, lesions that occupied more than one site within the prostatic fossa had a greater likelihood of having positive biopsy findings, and the lesions are more likely to be palpable compared with those that occupied one site, as reported by Leventis et al. [19].
In the last decade, several studies have addressed the clinical utility of TRUS in detecting local recurrence and described a statistically significant correlation between TRUS-suspected areas in the prostatic fossa and positive biopsies [20]. As indicated by Leventis et al. and Scattoni et al., local recurrences are more often hypoechoic (65 % of cases), whereas about 30 % of local recurrences are isoechoic with VUA appearance, with about 20 % of patients with a final positive biopsy [19, 22]. Unfortunately, the ability of TRUS to detect a local recurrence depends on the PSA levels. Scattoni et al. have demonstrated that TRUS was able to detect every biopsy-proven local recurrence lesion only with a PSA >2.0 ng/ml [22]; therefore, the use of TRUS is questionable.
2.1.2 TRUS-Guided Biopsies
Due to the low accuracy of TRUS in the detection of recurrent prostate cancer especially at low PSA levels, it may be useful to perform a prostatic fossa biopsy. The optimal biopsy strategy regarding location and number of cores has not been proved. Scattoni et al. [22] have suggested that a sampling with 6 cores in the VUA region is an efficient tool in the detection of local recurrence after RP, even with PSA <0.5 ng/ml. However, the likelihood of biopsy-proven local recurrence after RP has been reported to vary between 35 and 54 % with nearly a third of patients requiring two or more TRUS-guided biopsy sessions to obtain a final diagnosis [23, 24]. Different authors agree that increasing the core number do not markedly improve the detection rate of recurrence [19–21]. It has been suggested that a more easy sampling may be performed using an end-fire probe to guide the biopsy, with the final aim to direct the needle into the prostatic fossa at a more orthogonal angle. Conversely, side-fire or biplanar probes sample a longer segment of the retrotrigonal space and prostatic fossa, where local recurrence is less frequent but more often visible.
Several authors have tried to find further factors that may predict PCa recurrence detection in the prostatic fossa, but the results are still controversial. Shekarriz et al. [25] have demonstrated that the pathological stage or status of the margins following RP (including seminal vesicle involvement) or recurrence time may predict the results of VUA biopsy. Saleem et al. [26] have reported that the pathological stage, Gleason score, and PSA velocity are unhelpful in predicting biopsy results. Conversely, Scattoni et al. [22] have found that only an abnormal TRUS and DRE may be considered as significant predictors of PCa recurrence detection, while PSA, pathological stage, the Gleason score, margins’ status following RP, or the PSA elevation time have no correlation with a positive biopsy. Zietman et al. [27] reported that the likelihood of a positive rebiopsy is dependent on original tumor size and current PSA levels.
Unfortunately, the clinical utility of TRUS biopsy of the prostatic fossa is controversial and highly dependent to PSA levels. Shekarriz et al. [25] have reported that the higher the serum PSA level, the higher the positive biopsy rate, with a PSA cutoff of 1.0 ng/ml. TRUS biopsy of the VUA shows a low incidence of detection with a PSA level <0.5 ng/ml, as reported by both Saleem [26] and Connolly [28]. Similarly, Naya et al. [29] have, more recently, reported that none of the men with a serum PSA concentration of less than 0.5 ng/ml at biopsy who had normal results for both TRUS and DRE had a biopsy-proven local recurrence. On the contrary, Scattoni et al. [30] have documented that the sensitivity of a TRUS extends even to those patients with very low serum PSA levels since more than 70 % of the patients having a positive TRUS and PSA <0.5 ng/ml had a biopsy-proven local recurrence (Table 27.1).
While some studies have supported the need for histologic or radiographic confirmation of the recurrence before salvage radiotherapy, more recently, others demonstrated no differences in survival rates after RT between patients with PSA recurrence only and those with a documented local recurrence. A recent study has demonstrated that a biopsy of VUA before RT seems unnecessary for PSA ≤0.9 ng/ml. For higher values, a positive biopsy of VUA seems to always justify a salvage RT, which may not be recommendable, given the non-negligible risk of an already micrometastatic disease, if the biopsy results are negative [31].
In conclusion, TRUS biopsy of the prostatic fossa seems to be more accurate than TRUS in the detection of prostate cancer recurrence, even if its accuracy is highly correlated to PSA levels. Moreover, the clinical value of TRUS biopsy of the VAU remains in question.
2.1.3 MR Imaging
MR has a better diagnostic yield than TRUS and allows an evaluation of pelvic lymph node and bone status, with the detection of all sites of pelvic relapse in a single examination.
The administration of MR contrast medium, i.e., gadolinium, seems to improve further the overall accuracy. It theoretically allows detection of cancerous tissue in cases where morphological anomalies are not evidenced on unenhanced MR images and differentiation between tumor relapse and postoperative fibrosis or scar tissue.
MRI and dynamic contrast-enhanced MRI (DCE-MRI) can identify different site of local recurrence: VUA (52 %), retrovesical space (20 %), bladder neck (16 %), and circumferential areas (12 %).
Recurrences were, in most cases, slightly hyperintense to internal obturator muscle on T2-weighted sequences as found by Sella et al. [32] and in fewer cases markedly hyperintense on T2-weighted sequences.
Nodules that appear slightly hyperintense or markedly hyperintense on T2-weighted sequences may represent not only recurrences but also prostatic or seminal vesicle residues with different amounts of fibrosis.
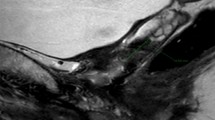
The peri-anastomotic fibrosis appears hypointense on T2w images, with absent enhancement on DCE-MRI images (Fig. 27.1). After DCE-MRI, all benign nodules showed signal enhancement of less than 50 % in the early phase, whereas all recurrences showed fast signal enhancement in the early phase followed by plateau or washout. Recurrences appear as lobulated masses with intermediate signal intensity on T2w images, enhancing after intravenous injection of contrast medium (Fig. 27.1).
A 56-year-old man with rising PSA after radical prostatectomy. (a, b) MR T2-weighted coronal and axial images with endorectal coil show a soft-tissue mass (arrowheads) anterior to the rectum. (c) Post-contrast dynamic image shows clear enhancement of the tissue. (d) PET/CT using 11C-choline image shows the uptake of the mass (arrowheads). The mass was proved to be a local recurrence by using transrectal US-guided biopsy
Silverman et al. [33] have achieved a high sensitivity and specificity (100 %) evaluating a group of patients with T1- and T2-weighted sequences and T1-weighted images with fat-suppression technique after gadolinium administration. All nodules showed signal enhancement after gadolinium administration, strengthening the suspicion that they were recurrences.
Also Sella et al. [32] have achieved a high sensitivity (95 %) and specificity (100 %) using T1- and T2-weighted sequences. All the local recurrences seen on MR images were isointense on T1-weighted sequences and slightly hyperintense to muscle on T2-weighted sequences. However, in the Sella study, the mean PSA level was 2.1 ng/ml, and in the Silverman study, 74 % had palpable recurrence and 88 % had a PSA >0.4 ng/ml; there would not have been any need of MRI to detect these recurrences. Therefore, the clinical benefit of current imaging is very low.
Casciani et al. [34] reported that MRI alone showed a poorer accuracy in detecting recurrences, probably due to the smaller size (between 0.4 and 3.0 cm) of the recurrences compared with those in the study of Silverman and the study of Sella et al. (0.7–3.8 cm and 0.8–4.5 cm, respectively). This comparison showed a statistically significant lower diagnostic accuracy of unenhanced eMR in comparison to CE-eMR (70 vs. 86 %), a statistically significant lower sensitivity (60 vs. 84 %) and no significant specificity differences (82 % vs. 89 %). Cosciani et al. [34] have supported the accuracy of eMR after RP providing high sensitivity of 84 % and specificity of 89.3 %, in patients with high PSA levels.
Recently, great interest has been shown to anatomic T2w imaging with functional MRI techniques such as DCE-MRI, DWI, and MR with spectroscopic imaging. In particular, DCE-MRI is useful for differentiating fibrosis in the prostatectomy fossa, remnants of normal prostatic tissue, and hyperplastic nodules from prostate cancer recurrence. DWI increases the accuracy of DCE-MRI well correlating with tissue cellularity of malignant tumors of the prostate.
In conclusion, MRI has proved to be useful at PSA values (generally higher than 1 ng/ml) for which the identified recurrence after RP cannot be treated with success.
Furthermore, MRI showed a limited clinical benefit in early diagnosis of recurrence after surgery since the lower detection limit is above 0.5 cm.
2.1.4 PET
Improvement about the detection of local recurrence may be reached by employing an imaging technique based on metabolism rather than an anatomic imaging technique. In this respect, PET may play a role, with the use of different tracers [35].
Few studies [36–51] have reported on the detection of local recurrence after RP with 18F-fluoro-2-deoxy-D-glucose (18FDG). Its use in PCa is limited by a low sensitivity. There is a modest glucose consumption by PCa cells, and the uptake of this medium in the recurrent tumor has been shown to be similar to the uptake in postoperative scar or benign prostate tissue. Moreover, 18FDG is highly excreted into urine. Thus, results have been particularly disappointing for the diagnosis of recurrences.
Promising results in the detection of recurrent PCa have been obtained with the newer PET tracers: 11C-acetate, 11C-choline (Ch-PET), and 18F-fluorocholine. Since Ch-PET is not rapidly excreted in urine, Ch-PET show clear images of the pelvic region and of the PCa and pelvic lymph node metastases in the absence of urinary radioactivity.
Generally, Ch-PET provides good sensitivity and specificity values in detecting distant and local recurrences after RP and RT, but only in patients with high PSA levels.
Only few studies have assessed the accuracy of PET in RP patients with low PSA values; most of them report a low sensitivity of PET in detecting local recurrence. In a recent study, also Vees et al. [52] did not recommended Ch-PET as a standard diagnostic tool if early relapse is suspected because the high levels of PSA (<1 ng/ml) needed to detect local residual or recurrent disease after RP in about half the patients.
Recently, Heinisch et al. [53] have recommended using a 18F-fluorocholine PET/CT at PSA levels of >5 ng/ml. By contrast, de Jong et al. have reported that Ch-PET cannot be used to visualize prostate cancer on restaging at a PSA level of <4.5 ng/ml. Rinnab et al. [54] recommend using PET, even at PSA levels of <2.5 ng/ml, because early detection of recurrence can be an advantage for patients with increasing PSA levels.
In integrated PET/CT (computed tomography), the focal uptake of choline can be more easily assigned to anatomical structures, with a better differentiation of physiological (rectum and bladder) uptake from residual/recurrent PCa, resulting in a higher accuracy.
Moreover, with respect to conventional imaging techniques, the most important advantage is the staging of the disease in one step. Rinnab et al. have reported an overall sensitivity and positive predictive value of 95 and 86 %. The overall specificity was 40 % with a negative predictive value of 67 %.
In conclusion, Ch-PET detection rate of recurrences increases together with the increase of PSA serum value, and, according to the current available data, the use of choline PET/CT cannot be recommended for PSA values lower than 1 ng/ml.
2.2 Local Recurrences After Radiotherapy
Diagnosing local recurrence after radiotherapy (RT) is challenging because of radiation-induced fibrosis and shrinkage of the prostate. The sensitivity and specificity of TRUS are reported to be 49 % and 57 %, respectively [55]. Prostate cancer visualization by MRI is also critical, because the tissue contrast between recurrent cancer and benign irradiated tissue is decreased as the recurrent cancer after radiation therapy demonstrates low signal intensity on T2w imaging [56].
Results for T2w imaging at 3 T using a phased-array coil showed a poor diagnostic performance in predicting recurrent cancer in patients with biochemical failure after radiation therapy [57].
DCE-MRI can predict locally recurrent cancer more accurately than T2w imaging showing a hypervascular area within the slow/low enhancement of postradiation fibrosis. DWI added to MRI examination protocol increases the accuracy of the technique showing focal low signal intensity relative to the surrounding prostate tissue on ADC maps [57].
After RT, MR spectroscopy imaging demonstrates intraprostatic voxels with no detectable peaks for choline, polyamines, creatine, and citrate (so-called metabolic atrophy). However, residual prostate cancer can still be identified by a relative increase in the (choline + creatine)/citrate ratio or by an increase in the choline peak with no detectable citrate.
Using these criteria, good correlations between spectroscopic data and biopsy findings have been reported [58]. However, for unclear reasons, some benign glands can exhibit high levels of choline after RT and cause false-positive findings [59].
The use of choline PET/CT can be recommended since local recurrence after RT is associated with PSA values greater than 2 ng/ml (Fig. 27.2).
A 78-year-old man with rising PSA after radiotherapy. (a) MR T2-weighted axial image shows low signal intensity of the irradiated tissue (arrowheads). (b) DWI image shows normal low signal intensity of prostate (arrowheads). (c, d) PET/CT using 11C-choline images confirm (c) low uptake of the prostate (asterisk) and (d) focal site of pathologic increase of 11C-choline uptake in the left internal iliac lymph node (arrows)
2.2.1 Biopsy After Radiotherapy
Biopsy is performed to identify a persistence or recurrence following RT, when PSA failure occurs according to Phoenix or ASTRO criteria. Biopsy is not a considered a gold standard of treatment efficacy, but is an independent predictor of outcome [60].
The role of biopsy after RT are (1) to provide pathological analysis and diagnosis of local recurrence, (2) to rule out local recurrence, and (3) to describe grade and tumor spread in the gland.
Biopsy mapping is indicated after not less than 24 months from the end of RT cycle. Crook et al. [60] recommended that biopsies should be performed at least 30–36 months following RT since false-negative results were observed in 19 % and false positive in 30 % when early biopsies at 12 months were performed.
The transrectal approach is usually performed; however, the transperineal route is preferred in patients with proctitis or previous events of post-RT rectorrhagia. Biopsy should be performed as random mapping (8–12 cores) to the whole prostatic gland and to the base of seminal vesicles. Target biopsy directed to visible nodules by TRUS or MRI should be performed due to the high probability of recurrence.
The information provided in the surgical pathology report of a prostate needle biopsy with carcinoma has become critical in the subsequent salvage therapy. Map distribution of cancer based on biopsy is important to assess tumor spread, and it is essential for planning the salvage therapy [61].
In conclusion, histologically proved local relapse is mandatory only if salvage treatment (cryosurgery or prostatectomy) is planned.
2.3 Local Recurrence After Cryotherapy
Cryotherapy (CT) of the whole prostate is widely used as primary treatment or salvage treatment for local recurrence after radiation therapy. Focal CT has been considered an investigational procedure in well-selected patient cases as alternative to achieve surveillance or total treatments (surgery or radiation or total CT). Role of imaging to detect local recurrence after total cryoablation is very limited. Absolute PSA levels (>0.5 ng/ml) or PSA kinetic (ASTRO or Phoenix definitions) is widely used and may predict recurrence [62]. B-mode transrectal ultrasound (TRUS) has a low diagnostic accuracy of local recurrence for several pitfalls: (1) isoechoic cancer, (2) posttreatment modifications, and (3) small-volume recurrent cancer. All these pitfalls explain also the limited value of TRUS. The goal of prostate CT is to produce complete necrosis of the prostate glands. Difficulty arises in the evaluation after total CT, because of the large damage zone, created by the treatment. In particular areas located at the margins of the ice ball (anterior zone, far basal zone close to seminal vesicles, distal apical tissue, periprostatic urethra, or subcapsular tissue) may persist a thin rim of untreated tissue (Fig. 27.3) or cancer [61]. These small areas may remain undetected by imaging techniques because of their irregular shape and volume (less than 5 mm) that also could be under the detection limits of MR.
New ultrasound (US) functions and magnetic resonance (MR) applications have the potential to enhance visualization of the residual prostate tissue and local recurrence. Contrast-enhanced US is a useful tool to detect untreated areas but no studies have been reported so far. Elastosonography [63] is not useful after CT since scar tissue and dense fibrosis are seen as hard tissue mimicking tumor. Color Doppler ultrasound may help in the detection of areas with residual vasculature.
There is a strong correlation between magnetic resonance imaging with gadolinium defects and amount of coagulation and necrosis caused by CT. However, gadolinium defects were not seen in areas of viable tissue as determined by histopathologic evaluation [64]. Some investigators reported that findings of postoperative gadolinium enhancement MR were not predictive of six-moth biopsy results or following PSA levels [65].
2.3.1 Biopsy After Cryotherapy
Diagnosis of local recurrence after CT is done by pathological analysis of core-biopsy specimens using end-fire TRUS probe. To date, few studies have assessed long-term pathological findings. After primary CT, biopsy may detect residual carcinoma in 7–23 % of cases and viable benign glands in 45–70 % of patients [66]. After salvage CT, Chin JL et al. reported residual cancer, viable benign prostate glands, and viable stroma in 14 %, 42 %, and 27 %, respectively [67].
Biopsy scheduled per protocol after 6, 12, or 24 months after total cryoablation is rarely performed [68]. Biopsy “for cause” is usually performed in most of the case series reported in literature.
Indication to for-cause biopsy is based on serum PSA level (>0.5 ng/ml) and kinetic (according to ASTRO or Phoenix definition) and digital rectal findings (nodule). In the COLD registry after primary total cryoablation, only 16 % of patients underwent biopsy.
Galosi et al. reported on 80/95 patients who underwent >2 biopsy sessions per protocol after a median follow-up of 70 months: overall disease-free survival was 61.1 %. Cancer in follow-up biopsy was detected in 21.1 % and normal prostatic tissue in 55 % [69].
Biopsy results should be obtained with eight or more core samplings in order to reduce the risk of under detection of residual cancer (Fig. 27.4). Target plus extended random biopsy schemes should be used to evaluate patients with PSA failure after CT in particular in the areas located at the margins of the ice ball.
2.4 Local Recurrences After HIFU Ablation
High-intensity focused ultrasound (HIFU) is a minimally invasive treatment for prostate cancer. Recommendations concerning HIFU in international guidelines are still conflicting [70, 71].
The overall quality of references about local recurrence is generally low because of small series, absence of pre-HIFU study, different timing in the scheduled posttreatment evaluation (1, 3, or 6 months), and generally retrospective evaluation of the results.
TRUS has a limited utility in patients treated with HIFU, since the gland appears diffusely heterogeneous after treatment. Conventional transrectal ultrasound (TRUS) is useless in identifying areas suspected for local recurrence, and even elastography was unable to precisely correlate with biopsy results. Color Doppler can improve recurrent cancer detection by guiding the biopsies towards hypervascular foci, but only 38 % of the sites with recurrent cancer show positive Doppler findings [72]. False-positive results are mostly due to residual benign tissue [73].
MRT2w imaging is difficult to interpret after HIFU ablation, because the gland is heterogeneous and diffusely hypointense. However, recurrent cancer can be visible in some patients as a nodular hypointense lesion [74]. Recurrent cancers are easier to distinguish from post-HIFU fibrosis using DCE imaging [75]. MRSI has been evaluated in few cases of patients, and it has shown to add no additional information than T2w imaging [74]; furthermore, when MRI is used as an indicator or residual viable tissue, it is very difficult to precisely match suspected areas at MRI with ultrasound-guided biopsy [76]. Again, the diagnosis of a local recurrence in this setting is based on the PSA values and PSA kinetics as well as the biopsy of the residual prostate after 12–18 months from treatment.
Conclusions
The diagnosis of local recurrence is highly dependent on the primary treatment used.
After surgery (RP), the role of imaging and biopsy is limited since PSA define very early recurrence as biochemical failure ≥0.2 ng/ml. TRUS and novel imaging have shown limited accuracy at least at early stages and very low PSA values. Choline PET/CT and MR can be recommended for PSA values higher than 1 ng/ml.
After conservative treatments (RT, CT, HIFU), a combined approach using specific imaging, PSA cutoff (>1 ng/ml), and PSA kinetics with image-guided biopsy is necessary to assess the presence of a local recurrence or benign residual tissue. Anatomic (T2w) and functional MR (DCE-MRI, DWI, and MRS) seems particularly promising for differentiating fibrosis from cancer.
Final diagnosis of local recurrence is based on pathological analysis of image-guided core biopsy, and TRUS end-fire remains the most used imaging method to guide biopsy.
Even if different imaging techniques will be extensively used in the future, their accuracy in the detection and localization of prostate cancer local recurrences before salvage treatment remains low, and their clinical utility remains in question.
References
Lightner DJ, Lange PH, Reddy PK, Moore L (1990) Prostate specific antigen and local recurrence after radical prostatectomy. J Urol 144(4):921–926
Abi-Aad AS, Macfarlane MT, Stein A, deKernion JB (1992) Detection of local recurrence after radical prostatectomy by prostate specific antigen and transrectal ultrasound. J Urol 147(3 Pt 2):952–955
Wasserman NF, Kapoor DA, Hildebrandt WC, Zhang G, Born KM, Eppel SM, Reddy PK (1992) Transrectal US in evaluation of patients after radical prostatectomy. Part II. Transrectal US and biopsy findings in the presence of residual and early recurrent prostatic cancer. Radiology 185(2):367–372
Wasserman NF, Kapoor DA, Hildebrandt WC, Zhang G, Born KM, Eppel SM, Reddy PK (1992) Transrectal US in evaluation of patients after radical prostatectomy. Part I. Normal postoperative anatomy. Radiology 185(2):361–366
Salomon CG, Flisak ME, Olson MC, Dudiak CM, Flanigan RC, Waters WB (1993) Radical prostatectomy: transrectal sonographic evaluation to assess for local recurrence. Radiology 189(3):713–719
Foster LS, Jajodia P, Fournier G Jr, Shinohara K, Carroll P, Narayan P (1993) The value of prostate specific antigen and transrectal ultrasound guided biopsy in detecting prostatic fossa recurrences following radical prostatectomy. J Urol 149(5):1024–1028
Koppie TM, Grossfeld GD, Nudell DM, Weinberg VK, Carroll PR (2001) Is anastomotic biopsy necessary before radiotherapy after radical prostatectomy? J Urol 166(1):111–115
Fowler JE Jr, Brooks J, Pandey P, Seaver LE (1995) Variable histology of anastomotic biopsies with detectable prostate specific antigen after radical prostatectomy. J Urol 153(3 Pt 2):1011–1014
Wood DP Jr, Peretsman SJ, Seay TM (1995) Incidence of benign and malignant prostate tissue in biopsies of the bladder neck after a radical prostatectomy. J Urol 154(4):1443–1446
Lepor H, Chan S, Melamed J (1998) The role of bladder neck biopsy in men undergoing radical retropubic prostatectomy with preservation of the bladder neck. J Urol 160(6 Pt 2):2435–2439
Laufer M, Pound CR, Carducci MA, Eisenberger MA (2000) Management of patients with rising prostate-specific antigen after radical prostatectomy. Urology 55(3):309–315
Pound CR, Christens-Barry OW, Gurganus RT, Partin AW, Walsh PC (1999) Digital rectal examination and imaging studies are unnecessary in men with undetectable prostate specific antigen following radical prostatectomy. J Urol 162(4):1337–1340
Ferguson JK, Oesterling JE (1994) Patient evaluation if prostate-specific antigen becomes elevated following radical prostatectomy or radiation therapy. Urol Clin North Am 21(4):677–685
Lange PH, Ercole CJ, Lightner DJ, Fraley EE, Vessella R (1989) The value of serum prostate specific antigen determinations before and after radical prostatectomy. J Urol 141(4):873–879
Partin AW, Pearson JD, Landis PK, Carter HB, Pound CR, Clemens JQ, Epstein JI, Walsh PC (1994) Evaluation of serum prostate-specific antigen velocity after radical prostatectomy to distinguish local recurrence from distant metastases. Urology 43(5):649–659
Cox JD, Gallagher MJ, Hammond EH, Kaplan RS, Schellhammer PF (1999) Consensus statements on radiation therapy of prostate cancer: guidelines for prostate re-biopsy after radiation and for radiation therapy with rising prostate-specific antigen levels after radical prostatectomy. American Society for Therapeutic Radiology and Oncology Consensus Panel. J Clin Oncol 17(4):1155
Kane CJ, Amling CL, Johnstone PA, Pak N, Lance RS, Thrasher JB, Foley JP, Riffenburgh RH, Moul JW (2003) Limited value of bone scintigraphy and computed tomography in assessing biochemical failure after radical prostatectomy. Urology 61(3):607–611
Krämer S, Görich J, Gottfried HW, Riska P, Aschoff AJ, Rilinger N, Brambs HJ, Sokiranski R (1997) Sensitivity of computed tomography in detecting local recurrence of prostatic carcinoma following radical prostatectomy. Br J Radiol 70(838):995–999
Leventis AK, Shariat SF, Slawin KM (2001) Local recurrence after radical prostatectomy: correlation of US features with prostatic fossa biopsy findings. Radiology 219(2):432–439
Parra RO, Wolf RM, Huben RP (1990) The use of transrectal ultrasound in the detection and evaluation of local pelvic recurrences after a radical urological pelvic operation. J Urol 144(3):707–709
Kapoor DA, Wasserman NF, Zhang G, Reddy PK (1993) Value of transrectal ultrasound in identifying local disease after radical prostatectomy. Urology 41(6):594–597
Scattoni V, Roscigno M, Raber M, Montorsi F, Da Pozzo L, Guazzoni G, Freschi M, Rigatti P (2003) Multiple vesico-urethral biopsies following radical prostatectomy: the predictive roles of TRUS, DRE, PSA and the pathological stage. Eur Urol 44(4):407–414
Ornstein DK, Colberg JW, Virgo KS, Chan D, Johnson ET, Oh J, Johnson FE (1998) Evaluation and management of men whose radical prostatectomies failed: results of an international survey. Urology 52(6):1047–1054
Bott SR (2004) Management of recurrent disease after radical prostatectomy. Prostate Cancer Prostatic Dis 7(3):211–216
Shekarriz B, Upadhyay J, Wood DP Jr, Hinman J, Raasch J, Cummings GD, Grignon D, Littrup PJ (1999) Vesicourethral anastomosis biopsy after radical prostatectomy: predictive value of prostate-specific antigen and pathologic stage. Urology 54(6):1044–1048
Saleem MD, Sanders H, Abu El Naser M, El-Galley R (1998) Factors predicting cancer detection in biopsy of the prostatic fossa after radical prostatectomy. Urology 51(2):283–286
Zietman AL, Shipley WU, Willett CG (1993) Residual disease after radical surgery or radiation therapy for prostate cancer. Clinical significance and therapeutic implications. Cancer 71(3 Suppl):959–969
Connolly JA, Shinohara K, Presti JC Jr, Carroll PR (1996) Local recurrence after radical prostatectomy: characteristics in size, location, and relationship to prostate-specific antigen and surgical margins. Urology 47(2):225–231
Naya Y, Okihara K, Evans RB, Babaian RJ (2005) Efficacy of prostatic fossa biopsy in detecting local recurrence after radical prostatectomy. Urology 66(2):350–355
Scattoni V, Roscigno M, Raber M, Montorsi F, Bertini R, Bua L, Da Pozzo L, Rigatti P (2002) Diagnostic value of ultrasound-guided anastomotic biopsies in patients with high PSA (> or = 0,4 ng/ml) after radical prostatectomy. Arch Ital Urol Androl 74(3):129–131
Roscigno M, Cozzarini C, Scattoni V, Bertini R, Da Pozzo L, Pasta A, Montorsi F, Bolognesi A, Fiorino C, Colombo R, Fazio F, Rigatti P (2007) A reappraisal of the role of vesicourethral anastomosis biopsy in patient candidates for salvage radiation therapy after radical prostatectomy. Radiother Oncol 82(1):30–37
Sella T, Schwartz LH, Swindle PW, Onyebuchi CN, Scardino PT, Scher HI, Hricak H (2004) Suspected local recurrence after radical prostatectomy: endorectal coil MR imaging. Radiology 231(2):379–385
Silverman JM, Krebs TL (1997) MR imaging evaluation with a transrectal surface coil of local recurrence of prostatic cancer in men who have undergone radical prostatectomy. AJR Am J Roentgenol 168(2):379–385
Cosciani E, Polettini E, Carmenini E, Floriani I, Masselli G, Bertini L, Gualdi GF (2008) Endorectal and dynamic contrast-enhanced MRI for detection of local recurrence after radical prostatectomy. AJR Am J Roentgenol 190(5):1187–1192
Cirillo S, Petracchini M, Scotti L, Gallo T, Macera A, Bona MC, Ortega C, Gabriele P, Regge D (2009) Endorectal magnetic resonance imaging at 1.5 Tesla to assess local recurrence following radical prostatectomy using T2-weighted and contrast-enhanced imaging. Eur Radiol 19(3):761–769
Yeh SD, Imbriaco M, Larson SM, Garza D, Zhang JJ, Kalaigian H, Finn RD, Reddy D, Horowitz SM, Goldsmith SJ, Scher HI (1996) Detection of bony metastases of androgen-independent prostate cancer by PET-FDG. Nucl Med Biol 23(6):693–697
Schöder H, Herrmann K, Gönen M, Hricak H, Eberhard S, Scardino P, Scher HI, Larson SM (2005) 2-[18F]fluoro-2-deoxyglucose positron emission tomography for the detection of disease in patients with prostate-specific antigen relapse after radical prostatectomy. Clin Cancer Res 11(13):4761–4769
Liu IJ, Zafar MB, Lai YH, Segall GM, Terris MK (2001) Fluorodeoxyglucose positron emission tomography studies in diagnosis and staging of clinically organ-confined prostate cancer. Urology 57(1):108–111
Effert PJ, Bares R, Handt S, Wolff JM, Büll U, Jakse G (1996) Metabolic imaging of untreated prostate cancer by positron emission tomography with 18fluorine-labeled deoxyglucose. J Urol 155(3):994–998
Hofer C, Laubenbacher C, Block T, Breul J, Hartung R, Schwaiger M (1999) Fluorine-18-fluorodeoxyglucose positron emission tomography is useless for the detection of local recurrence after radical prostatectomy. Eur Urol 36(1):31–35
Haseman MK, Reed NL, Rosenthal SA (1996) Monoclonal antibody imaging of occult prostate cancer in patients with elevated prostate-specific antigen. Positron emission tomography and biopsy correlation. Clin Nucl Med 21(9):704–713
Sanz G, Robles JE, Giménez M, Arocena J, Sánchez D, Rodriguez-Rubio F, Rosell D, Richter JA, Berián JM (1999) Positron emission tomography with 18fluorine-labelled deoxyglucose: utility in localized and advanced prostate cancer. BJU Int 84(9):1028–1031
Morris MJ, Akhurst T, Osman I, Nunez R, Macapinlac H, Siedlecki K, Verbel D, Schwartz L, Larson SM, Scher HI (2002) Fluorinated deoxyglucose positron emission tomography imaging in progressive metastatic prostate cancer. Urology 59(6):913–918
de Jong IJ, Pruim J, Elsinga PH, Vaalburg W, Mensink HJ (2003) 11C-choline positron emission tomography for the evaluation after treatment of localized prostate cancer. Eur Urol 44(1):32–38; discussion 38–9
Price DT, Coleman RE, Liao RP, Robertson CN, Polascik TJ, DeGrado TR (2002) Comparison of [18 F]fluorocholine and [18 F]fluorodeoxyglucose for positron emission tomography of androgen dependent and androgen independent prostate cancer. J Urol 168(1):273–280
Oyama N, Miller TR, Dehdashti F, Siegel BA, Fischer KC, Michalski JM, Kibel AS, Andriole GL, Picus J, Welch MJ (2003) 11C-acetate PET imaging of prostate cancer: detection of recurrent disease at PSA relapse. J Nucl Med 44(4):549–555
Kotzerke J, Volkmer BG, Glatting G, van den Hoff J, Gschwend JE, Messer P, Reske SN, Neumaier B (2003) Intraindividual comparison of [11C]acetate and [11C]choline PET for detection of metastases of prostate cancer. Nuklearmedizin 42(1):25–30
Fricke E, Machtens S, Hofmann M, van den Hoff J, Bergh S, Brunkhorst T, Meyer GJ, Karstens JH, Knapp WH, Boerner AR (2003) Positron emission tomography with 11C-acetate and 18F-FDG in prostate cancer patients. Eur J Nucl Med Mol Imaging 30(4):607–611
Reske SN, Blumstein NM, Glatting G (2006) PET and PET/CT in relapsing prostate carcinoma. Urologe A 45(10):1240, 1242–4, 1246–8, 1250
Kotzerke J, Volkmer BG, Neumaier B, Gschwend JE, Hautmann RE, Reske SN (2002) Carbon-11 acetate positron emission tomography can detect local recurrence of prostate cancer. Eur J Nucl Med Mol Imaging 29(10):1380–1384
Picchio M, Briganti A, Fanti S, Heidenreich A, Krause BJ, Messa C, Montorsi F, Reske SN, Thalmann GN (2011) The role of choline positron emission tomography/computed tomography in the management of patients with prostate-specific antigen progression after radical treatment of prostate cancer. Eur Urol 59(1):51–60
Vees H, Buchegger F, Albrecht S, Khan H, Husarik D, Zaidi H, Soloviev D, Hany TF, Miralbell R (2007) 18F-choline and/or 11C-acetate positron emission tomography: detection of residual or progressive subclinical disease at very low prostate-specific antigen values (<1 ng/mL) after radical prostatectomy. BJU Int 99(6):1415–1420. Epub 2007 Apr 8
Heinisch M, Dirisamer A, Loidl W, Stoiber F, Gruy B, Haim S, Langsteger W (2006) Positron emission tomography/computed tomography with F-18-fluorocholine for restaging of prostate cancer patients: meaningful at PSA < 5 ng/ml? Mol Imaging Biol 8(1):43–48
Rinnab L, Mottaghy FM, Blumstein NM, Reske SN, Hautmann RE, Hohl K, Möller P, Wiegel T, Kuefer R, Gschwend JE (2007) Evaluation of [11C]-choline positron-emission/computed tomography in patients with increasing prostate-specific antigen levels after primary treatment for prostate cancer. BJU Int 100(4):786–793
Crook J, Robertson S, Collin G et al (1993) Clinical relevance of trans-rectal ultrasound, biopsy, and serum prostate-specific antigen following external beam radiotherapy for carcinoma of the prostate. Int J Radiat Oncol Biol Phys 27:31–37
Yakar D, Hambrock T, Huisman H, Hulsbergen-van de Kaa CA, van Lin E, Vergunst H, Hoeks CM, van Oort IM, Witjes JA, Barentsz JO, Fütterer JJ (2010) Feasibility of 3T dynamic contrast-enhanced magnetic resonance-guided biopsy in localizing local recurrence of prostate cancer after external beam radiation therapy. Invest Radiol 45(3):121–125
Kim CK, Park BK, Park W, Kim SS (2010) Prostate MR imaging at 3T using a phased-arrayed coil in predicting locally recurrent prostate cancer after radiation therapy: preliminary experience. Abdom Imaging 35(2):246–252
Pickett B, Kurhanewicz J, Coakley F, Shinohara K, Fein B, Roach M 3rd (2004) Use of MRI and spectroscopy in evaluation of external beam radiotherapy for prostate cancer. Int J Radiat Oncol Biol Phys 60:1047–1055
Pucar D, Shukla-Dave A, Hricak H, Moskowitz CS, Kuroiwa K, Olgac S, Ebora LE, Scardino PT, Koutcher JA, Zakian KL (2005) Prostate cancer: correlation of MR imaging and MR spectroscopy with pathologic findings after radiation therapy – initial experience. Radiology 236:545–553
Crook J, et al (2000) Postradiotherapy prostate biopsies: what do they really mean? Results for 498 patients. Int J Radiat Oncol Biol Phys 48(2):355–367
Galosi AB, Lugnani F, Muzzonigro G (2007) Salvage cryosurgery for recurrent prostate carcinoma after radiotherapy. J Endourol 21(1):1–7
Rukstalis DB, Katz A (eds) (2007) Handbook of urologic cryoablation. Informa UK Ldt, London, pp 31–38
Loch T (2007) Urologic imaging for localized prostate cancer in 2007. World J Urol 25(2):121–129
Larson BT, Collins JM, Huidobro C, Corica A, Vallejo S, Bostwick DG (2003) Gadolinium enhanced MRI in the evaluation of the minimally invasive treatment of the prostate: correlation with histopatologic findings. Urology 62:900–904
Donnely SE, Donnely BJ (2004) Prostate cancer: gadolinium enhanced MR imaging at three weeks compared with needle biopsy at six months after cryoablation. Radiology 232:830–833
Shinohara K (2003) Prostate cancer. Cryotherapy. Urol Clin North Am 30:725–736
Chin JL, Touma N, Pautler SE, Guram KS, Bella AJ, Downey DB, Moussa M (2003) Serial histopathology results of salvage cryoablation for prostate cancer after radiation failure. J Urol 170(4 Pt 1):1199–1202
Galosi AB, Muzzonigro G, Polito M Jr, Minardi D, Dellabella M, Lugnani F, Polito M (2000) Role of transrectal ultrasonography in the follow-up of patients treated with prostatic cryosurgery. Arch Ital Urol Androl 72(4):276–281
Galosi AB, Parri G, Montironi R et al (2009) Prostate cryoablation as primary treatment of prostate cancer: oncological results with follow-up biopsy. Poster at the 2nd international workshop focal therapy and imaging in prostate & kidney cancer. Amsterdam, 10–13 June 2009
Heidenreich A, Bolla M, Joniau S et al Guidelines on prostate cancer. European Association of Urology Web site. http://www.uroweb.org/gls/pdf/prostate%20Cancer%202010%20June%2017th.pdf. Retrieved 19 July 2010
Guideline for the management of clinically localized prostate cancer: 2007 update. American Urological Association Web site. http://www.auanet.org/content/guidelines-and-quality-care/clinical-guidelines.cfm?sub=pc. Retrieved 10 Feb 2010
Rouviere O, Mege-Lechevallier F, Chapelon JY, Gelet A, Bouvier R, Boutitie F, Lyonnet D (2006) Evaluation of color doppler in guiding prostate biopsy after HIFU ablation. Eur Urol 50:490–497
Rouvière O, Curiel L, Chapelon JY et al (2004) Can color doppler predict the uniformity of HIFU-induced prostate tissue destruction? Prostate 60:289–297
Cirillo S, Petracchini M, D’Urso L, Dellamonica P, Illing R, Regge D, Muto G (2008) Endorectal magnetic resonance imaging and magnetic resonance spectroscopy to monitor the prostate for residual disease or local cancer recurrence after transrectal high-intensity focused ultrasound. BJU Int 102:452–458
Kim CK, Park BK, Lee HM, Kim SS, Kim E (2008) MRI techniques for prediction of local tumor progression after high-intensity focused ultrasonic ablation of prostate cancer. AJR Am J Roentgenol 190:1180–1186
Warmuth M, Johansson T, Mad P (2010) Systematic review of the efficacy and safety of high-intensity focused ultrasound for the primary and salvage treatment of prostate cancer. Eur Urol 58:803–815
Conflict of Interest
The authors report no conflict interest.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2017 Springer International Publishing Switzerland
About this chapter
Cite this chapter
Martino, P. et al. (2017). Role of Imaging and Biopsy to Assess Local Recurrence After Definitive Treatment for Prostate Carcinoma. In: Martino, P., Galosi, A. (eds) Atlas of Ultrasonography in Urology, Andrology, and Nephrology. Springer, Cham. https://doi.org/10.1007/978-3-319-40782-1_27
Download citation
DOI: https://doi.org/10.1007/978-3-319-40782-1_27
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-40780-7
Online ISBN: 978-3-319-40782-1
eBook Packages: MedicineMedicine (R0)