Abstract
Small multidrug resistance (SMR) transporters confer resistance to a variety of quaternary cation compound antimicrobials. These secondary active transporters are the smallest known transporters and have been demonstrated to function within the membrane. The focus of this chapter explores and updates SMR family diversity and reviews current structural and functional knowledge of these members. This chapter also provides an update of known SMR pump-mediated resistance to antimicrobial substrates (including naturally synthesized quaternary cation compounds) and their clinical significance.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Small multidrug resistance
- Multidrug resistance
- Efflux
- Dual topology
- Antiseptic
- Quaternary ammonium compound
- Quaternary cation compound
- EmrE
- SugE
- Paired SMR
- AbeS
- EbrAB
- YkkCD
- YvdRS
1 Introduction
Small multidrug resistance (SMR) family proteins confer resistance to a diverse assortment of antiseptics and a limited range of antibiotics. They are the smallest known multidrug resistance (MDR) transporters found in prokaryotes and transport toxic quaternary cation compounds (QCCs) (also known as quaternary ammonium compounds [QACs]) using proton motive force [1, 2]. The SMR family is one of the 14 phylogenetically distinct secondary active transporter families that belong to the drug/metabolite transporter (DMT) superfamily [3]. As their name implies, SMR family proteins are short in length (100–170 amino acids) and span the plasma membrane as four hydrophobic α-helical transmembrane strands (TMS). The SMR protein active site of H+/drug binding is centered at a single highly conserved Glu residue located in the first TMS of SMR proteins [2, 4, 5].
SMR family proteins have been studied for many reasons. Their wide distribution within bacterial species [6] and presence on conserved regions of mobile genetic elements [7] make them clinically significant targets to combat the spread of antiseptic resistance [8]. The small size and novel dual-topology dimer orientation also makes the SMR family evolutionarily significant since they are hypothesized to serve as progenitors of larger multidrug transporters [3, 6, 9, 10]. This chapter describes current structural and functional knowledge gathered for this family to explain how these remarkably small proteins are capable of transporting such a diverse array of substrates, with a focus on characterized SMR subclass family members.
2 SMR Family Diversity
SMR family members are encoded chromosomally and/or on mobile genetic elements in conserved 3′ regions of class 1 integrons [7, 8] and on MDR plasmids [11] in archaea and bacteria [6]. Members of this family have been subdivided into three subclasses based on function, isogenicity, and phylogenetic relatedness [6, 12]: small multidrug pumps (SMPs), suppressor of groEL mutation proteins (SUG), and paired small multidrug resistance (PSMR) pumps. A brief summary of experimentally characterized SMR subclass members is provided in the following sections (Table 3.1).
2.1 The SMP Subclass
SMR members belonging to the SMP subclass are characterized by their ability to confer isogenic resistance to a broad range of toxic lipophilic QCC [13–17] and phylogenetic association to the γ-proteobacterial Escherichia coli ethidium MDR protein E (Eco-EmrE) [6, 9]. Eco-EmrE is the archetypical member of both the SMR family and the SMP subclass due to the extensive functional and high-resolution structural characterization of this protein (as reviewed in [12, 65, 66]). These biophysical studies have resulted in cryoelectron microscopy [67–70], X-ray diffraction [71], and nuclear magnetic resonance (NMR) (solution-state [72–74] and solid-state [75, 76]) structures of Eco-EmrE. Although the majority of studies have focused on the Gram-negative Eco-EmrE, experimental structure-function characterization of other closely related SMP subclass members has also been performed on γ-proteobacterial Acinetobacter baumannii AbeS [19, 20] and Pseudomonas aeruginosa EmrE (Pae-EmrE) [21, 77], β-proteobacterial Bordetella pertussis (Bpe-Smr) [21], Gram-positive firmicutes Staphylococcus aureus (Sau-Smr) [27, 28, 78], and actinobacterial Mycobacterium tuberculosis (Mtu-Smr) [21, 24, 79–81]. The consensus from these studies indicates that bacterial SMP members form isogenic functional homooligomers that confer broad polyspecific drug resistance profiles similar to Eco-EmrE despite their variable sequence identity (32–54 % Eco-EmrE). As observed for bacterial SMP members, experimental characterization of the archaeal Halobacterium salinarum (Hsa-Smr) [25] also suggests that archaeal SMP homologs adopt similar structural and functional features, despite their high overall content of Ala and Val residues (40 % total of 112 Hsa-Smr residues) by comparison to Eco-EmrE (13 % of total 110 residues).
Members of the SMP subclass have a diverse distribution within prokaryotes and have been identified from chromosomes, from a variety of MDR plasmids, and within the 3′ conserved gene cassette region of various class 1 integrons and transposons (Table 3.1). SMP members encoded on mobile genetic elements are typically designated as Qac efflux pumps based on their ability to confer resistance to these toxic compounds. The SMP subclass also possesses the greatest diversity of laterally transferred members: QacC, QacE, QacEΔ1, QacF, QacG, QacH, QacJ, and QacZ (Table 3.1 and Fig. 3.1). QacE and the semi-functional QacEΔ1 (which lacks 16 C-terminal residues from the QacE sequence [35]) were identified as conserved genes in the 3′ region of class 1 integrons [7] isolated from Gram-negative and Gram-positive bacteria [35, 36, 83]. The remaining Qac members, QacF, QacG, QacH, QacJ, and QacZ, have been identified from integrons, transposons, and/or MDR plasmids (Table 3.1 and Fig. 3.1). QacF shares a close homology with QacE (68 % identity) and is frequently detected on class 1 integrons and various MDR plasmids in Gram-negatives [39, 40, 84]. QacG, QacH, and QacJ frequently identified on Gram-positive staphylococcal MDR plasmids and share closer homology to Sau-Smr (69–83 % identity) than Eco-EmrE (41–62 %). The most recent addition to this group, QacZ (74 % identity to Sau-Smr), was identified from a Gram-positive enterococcal plasmid (pTEF1) and conferred resistance to benzalkonium chloride but not ethidium or chlorhexidine, indicating that some Qac members may provide selective QCC resistance [47].
A rooted neighbor joining (NJ) phylogenetic tree of experimentally characterized SMR protein family members. NJ distance analysis was performed with a ClustalW [82] multiple sequence alignment of 32 characterized SMR family protein sequences listed in Table 3.1. The outgroup for this analysis was the Archaeoglobus fulgidus QacE (Afu-QacE) based on a previous phylogenetic study [6]. The NJ tree represents a consensus of 100 bootstrap replicates, and nodes with 75 % or more confidence are indicated by black filled circles
2.2 The SUG Subclass
Similar to the SMP subclass, members of the SUG subclass also confer resistance when they are expressed as a single gene but only to a limited range of QCCs [48]. SUG members have been identified on chromosomes and within mobile genetic elements (Table 3.1) indicating that they also share a diverse heritability similar to SMP subclass members. These members are homologous to E. coli suppressor of groEL mutation protein E (Eco-SugE) [6] which was named according to its initial identification in an experiment involving the suppression of groEL chaperonin mutations [49]. Confirmation of Eco-SugE involvement suggests that groEL suppression was caused by a cloning artifact, since Eco-SugE was located adjacent to the groES locus [85]. Despite this study, SugE proteins have been suggested to confer some chaperone-like activity [9, 86, 87] making it uncertain what role this subclass plays in bacterial protein folding processes.
The most well-characterized SUG member is Eco-SugE [48, 50, 88–91], and relatively few studies have examined this protein in comparison to Eco-EmrE, possibly due to its selective resistance profile. Eco-SugE serves as the representative SUG member and confers resistance to a limited range of detergent-like QCCs [48] (Table 3.1). Mutational analysis of Eco-SugE has shown that alteration of specific residues can alter substrate transport from an exporter to an importer [52]. Characterization of other SUG members has focused solely on Gram-negative proteobacterial homologs from Aeromonas molluscorum (Amo-SugE) [51], Citrobacter freundii (Cfr-SugE) [52], and Enterobacter cloacae (Ecl-SugE) [53]. It should be noted that functional analysis of Ecl-SugE [53] and Amo-SugE [51] has demonstrated that other SUG members may confer resistance to a broader range of substrates that include ethidium and tetraphenylphosphonium (Table 3.1). SUG members are most frequently identified from chromosomes and more widely distributed within archaeal and bacterial species than SMP members. In contrast to the SMP subclass, SUG homolog diversity and distribution on mobile genetic elements are low, and many SUG sequences are closely related to or identical to Cfr-SugE [54]. Laterally transferred SUG members are most frequently identified within class 1 integrons and transposons [92] as well as MDR plasmids [37, 54, 55] from Gram-negative proteobacteria and less frequently from Gram-positive species [93] (Table 3.1 and Fig. 3.1).
2.3 PSMR Subclass Members
Unlike the SMP and SUG subclasses, members of PSMR subclass require simultaneous expression of two SMR gene copies located within the same operon/locus to produce drug resistance [57]. PSMR members were originally predicted and identified from sequenced genome surveys [9, 87] and now include a variety of characterized members from both Gram-positive and Gram-negative bacteria (Table 3.1). PSMR diversity within bacteria has been shown to be greater in Gram-positive species (in Bacillus subtilis EbrAB, YkkCD, YvaDE, and YvdRS) as compared to Gram-negative species (E. coli MdtIJ) [6] (Table 3.1 and Fig. 3.1). Phylogenetic analysis of SMR subclass members from taxonomically diverse bacteria has indicated that PSMR members recently evolved from gene duplication events and demonstrated that PSMR members MdtIJ, EbrAB, and YvaDE originated from SMP members, while PSMR members YkkCD and YvdRS evolved from SUG subclass members [6] (Fig. 3.1).
The most well-characterized PSMR members are from B. subtilis EbrAB (Bsu-EbrAB) [57–59, 63, 94, 95] and E. coli MdtIJ/YdgEF (Eco-MdtIJ) [15, 60, 84, 96, 97]. Structural analysis of Bsu-EbrAB has demonstrated that the pair forms a heterooligomer [58, 59, 94]. Studies of Bsu-EbrAB and Eco-MdtIJ demonstrated that PSMR members adopt an opposite insertion orientation from each other in the membrane [94, 97, 98]. It is important to note that drug resistance from the overexpression of a single PSMR gene, specifically Bsu-EbrB [95] and Bsu-YvaE [9], has demonstrated that overexpression of both proteins may not be required to confer resistance in E. coli expression systems. Additionally, one protein of the PSMR pair is generally longer (Bsu-EbrA 105 aa versus Bsu-EbrB 117 aa; Eco-MdtI 109 aa versus Eco-MdtJ 121 aa) which result in loop (loops 1 and 3) and C-terminus lengthening. Mutational analysis of Bsu-EbrA and Bsu-EbrB which removed the loops and C-termini from each protein resulted in a PSMR drug resistant protein when expressed as a single gene [58] indicating that loops and termini enhanced PSMR multimerization. The remaining chromosomally encoded PSMR members YvaDE and YvdRS appear to be the only subclass members with an unknown substrate profile [9]. Studies of the Bsu-YvdRS homolog, PsmrAB from the halophilic Halobacillus dabanensis (Hda-PsmrAB) revealed that this protein functions as a Na+/H+ antiporter [62] suggesting that YvdRS homologs may not confer drug resistance but function solely in osmotic regulation. The identification of PSMR members on mobile genetic elements is relatively low and appears to be present only on plasmids that confer specialized cell functions, as observed for the toxic methylamine efflux pump NepAB (a homolog of EbrAB [6]; Fig. 3.1) from Gram-positive Arthrobacter nicotinovorans [61]. Taken altogether, PSMR subclass distribution and diversity appears to be evolving toward specialized substrate transport that in some cases maintain antimicrobial transport.
3 SMR Transporter Structure Analysis
Structural analysis of SMR family members has primarily focused on Eco-EmrE. Over the past two decades, many high-resolution biophysical techniques, cryoelectron microscopy (EM), X-ray crystal diffraction, and solution-/solid-state NMR, have been performed on Eco-EmrE protein. Early EmrE structural analyses of two-dimensional crystals using cryo-EM [68, 99] provided a low-resolution (7.0–7.5 Å) projection structure (protein database [PDB] code 2I68) [69] that supported an asymmetrically arranged EmrE dimer. The three-dimensional (3D) projection structure also demonstrated tetraphenylphosphonium binding occurring in TMS1–TMS3 regions of each monomer and supported an antiparallel arrangement of each protein monomer within the dimer [69]. Controversy ensued when X-ray crystal structures of EmrE were published that failed to agree with the available biochemical and biophysical data [100, 101] and were later retracted due to software calculation errors [102, 103]. Re-examination of EmrE X-ray diffraction crystals resulted in a 3.8 Å (PDB 3B5D) 3D structure of an EmrE dimer with bound tetraphenylphosphonium [71] (Fig. 3.2b). Analysis of the X-ray structure also resulted in two additional 3D homology structures that provided an apo-EmrE form at 4.5 Å resolution (PDB 3B61) and an EmrE dimer bound to tetraphenylphosphonium at 4.4 Å (PDB 3B62) [71]. The revised EmrE X-ray structures appear to be in greater agreement with previous cryo-EM structures, by confirming an asymmetrical arrangement of each protein monomer in an antiparallel orientation. Other biophysical techniques such as systematic spin-labeling electron paramagnetic resonance (EPR) [105], solution-state NMR [72, 89, 106, 107], and solid-state [75, 76, 90] NMR studies of EmrE in bicelles and liposomes also support an asymmetrical antiparallel EmrE dimer. Altogether, these biophysical structural studies are beginning to support biochemical analyses that indicate EmrE forms a functional antiparallel dimer (as reviewed in [70]).
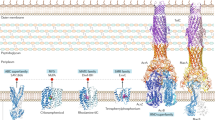
Cartoon diagrams of Eco-EmrE structures. (a) A secondary structure and topology map of the Eco-EmrE protein (PDB P23895) generated using the online Protter program version 1.0 [104]. Amino acid residues are shown as circles, where E14 (blue), W63, Y40, Y60, G90, and G97 (red) are highlighted. The membrane bilayer is represented as horizontal lines. (b) The 3D X-ray diffraction structural model of Eco-EmrE (PDB code 3B5D) bound to the ligand tetraphenylphosphonium [71]. A top down view of all four TMSs (cylinders) and loops (thick lines) in each EmrE monomer (light blue, monomer A; orange, monomer B). The bound ligand tetraphenylphosphonium (green) is shown as a stick chemical diagram where the phosphorous atom is in a circle, and each of the four aromatic rings is shown as hexagons
Currently, high-resolution structural analyses are not available for other SMR subclass members, but attempts have been made to examine other SMP members Mtu-Smr [79–81] and SUG members [89–91] by NMR techniques. Acquiring high-resolution structures of other SMR members would be invaluable for comparing the plasticity, drug selectivity, and structural conservation of Eco-EmrE which can aid the development of efflux pump inhibitors and improve antimicrobial development.
3.1 SMR Transporter Topology
The topological orientation of SMR family members has been a source of considerable controversy over the last decade [100, 101, 108–110]. According to the positive-inside rule, the orientation of a TMS is directed by the number of positively charged (K and R) residues located within loops and termini of the membrane protein [111–113]. The topology or “KR” bias of a membrane protein can be reliably estimated by summing the difference in net positive charges in oppositely facing loops and termini, where loops/termini with the greatest positive charge will orient to face the cell cytoplasm [98]. Interestingly, SMR family members from SMP and SUG subclasses have KR bias values at or close to zero indicating that their insertion orientation is neutral or random [98, 114]. In contrast, heterooligomeric PSMR subclass members are predicted to orient in a fixed but opposite topology from each other to form an antiparallel heterodimer [9, 97]. The topology of SMP member Eco-EmrE has been extensively studied, and evidence supporting antiparallel insertion of EmrE has been demonstrated using reporter tag fusions [98, 114–116], alteration of positively charged residues [98, 116–118], cysteine accessibility and cross-linking [95, 119, 120], tandem genetic fusions [95, 121], NMR [72, 107, 122, 123], and high-resolution X-ray crystal diffraction techniques [71]. It should be noted that experiments have demonstrated that EmrE adopts functional fixed parallel orientations as tandem fusions [95] and as cross-linked monomers [124, 125]; the emerging consensus appears to support a functional antiparallel topology for EmrE due to its reoccurrence in high-resolution structural analyses [70, 72]. Antiparallel PSMR pair insertion orientations have been reported for Bsu-EbrAB [94] and Eco-MdtIJ [97, 98, 114] providing further support that SMR family members can adopt a functional antiparallel topology.
3.2 SMR Transporter Multimerization
All SMR proteins are expected to function as oligomers, where SMP members form homooligomers (as reviewed in [12]) and PSMR proteins form heterooligomers [59]. Multimerization studies of SUG members currently indicate these proteins predominate as monomers in vitro [50, 88], but due to their sequence similarity to SMP members, SUG homologs may also function as multimers. Extensive examination of SMP subclass member, Eco-EmrE, has revealed that the protein can adopt a variety of states: monomeric [89, 126–129], dimeric [66, 67, 71, 73, 99, 120, 128, 130–132], trimeric [5, 17, 128, 133], and higher multimeric [5, 17, 67, 68, 99, 126, 134] states, depending on experimental conditions. The overall consensus from these biochemical and structural approaches shows that the minimal functional unit is a dimer (as reviewed in [135]). Although the arrangement of monomers within the dimer is still contested, growing support for an antiparallel arrangement appears to be emerging [72, 120, 121] (refer to discussion in Sect. 3.3.1). Closer examination of Eco-EmrE and another SMP homolog Hsa-Smr has revealed the importance of the fourth TMS (TMS4) for multimer stability and transport [26, 136], and mutagenesis of moderate to highly conserved Gly residues in Eco-EmrE TMS4 has identified a Gly90-X6-Gly97 motif (Fig. 3.2) [75, 137]. Studies of PSMR Bsu-EbrAB protein variants lacking regions within loops 1 and 3 in addition to the C-terminus resulted in drug resistance from either EbrA or EbrB when expressed individually [58] supporting their involvement in multimerization. Therefore, a variety of SMR regions, TMS4, loops 1–3, and C-terminus, are currently known to participate in SMR multimerization.
The variation in SMR multimerization may be explained by its small size and diverse topology and its plasticity may be linked to modifications and conditions used to isolate these proteins. Most studies of Eco-EmrE have involved the addition of an affinity purification tag and the most commonly used tag adds a C-terminal myc-epitope linker with a hexahistidine tag [5, 138], and this approach has yielded preparations with mixed monomeric and dimeric states [131]. Due to its extreme hydrophobicity, untagged Eco-EmrE purification approaches that involve organic solvent extraction have also been performed and shown to yield predominately monomeric protein with a low occurrence of dimers [128, 139]. The choice of membrane mimetics, such as different detergents (N-dodecyl-β-D-maltoside is the most commonly used as reviewed in [12]), bicelles [72, 73, 76, 78, 106, 107, 123], nanodiscs [91], and liposomes [2, 73, 106, 140–143] used to isolate these proteins, may also influence multimeric diversity and stability.
3.3 SMR Transporter Lipid Dependence
The influence of the membrane mimetic environment on the structure and function of SMR proteins has been gaining interest but has also underscored the importance of the membrane environment used to study these proteins. Membrane composition is known to influence the folding and function of bacterial transporters in vitro [144] and in vivo [145, 146]. The surfactant and membrane disrupting mechanisms of action caused by SMR antimicrobial substrates are also known to significantly alter lipid domain organization in the membrane [147]. Therefore, studies of SMR family members have also highlighted the importance of considering not only the protein and its modifications but also the membrane mimetic systems used for their characterization.
Studies of SMP and SUG protein folding and reconstitution in different detergents have revealed differences in multimerization and ligand binding affinities by proteins [126, 128]. Analysis of Eco-EmrE purified in the detergent N-dodecyl-β-D-maltoside has shown that multimer formation and protein stability alter depending on the concentration of detergent added [128, 131]. Comparisons of Eco-EmrE dimer stability have also been performed in NMR experiments and determined that dimer affinity increased when the protein was reconstituted from N-dodecyl-β-D-maltoside detergent micelles into bicelles composed of dilauroylphosphatidylcholine [73, 106]. These findings indicate that SMR protein multimerization and folding stability vastly improve when membrane mimetics that resemble the native lipid environment of SMR proteins are used for in vitro characterization.
Many in vitro studies examining SMR protein folding and transport activity in liposomes have also been performed on SMP members. The advantage of these self-contained artificial phospholipid bilayer vesicles is the ability to determine transport activities in contrast to using detergent micelles and bicelles/nanodisc systems. Examination of Eco-EmrE transport, folding, and insertion into liposomes composed of derivatized phosphatidylcholine (PC) (a nonnative phospholipid in E. coli) and phosphatidylethanolamine (PE) (the dominant phospholipid [70–75 %] in E. coli membranes [148]) has demonstrated that as the ratio of PE increased, the rate of protein insertion decreased, but the drug transport activity and folding of inserted proteins improved [138, 140, 142]. The addition of derivatized anionic lipids, like phosphatidylglycerol (present at 15–18 % in E. coli membranes [148]), to PE/PC liposomes increased Eco-EmrE drug transport [80]. In the same study, the addition of the derivatized anionic phospholipid, phosphatidylinositol (present at 12.5 % in M. tuberculosis membranes [148]), to PE/PC liposomes containing Mtu-Smr also increased drug transport [80]. Studies of Eco-EmrE protein reconstituted into lipid monolayers also identified that EmrE clustering was significantly altered in the presence of the anionic lipid, cardiolipin, and long unsaturated fatty acid chains [149]. Brewster angle microscopy experiments with Eco-EmrE reconstitution into lipid monolayers have demonstrated preferential lipid domain sorting around EmrE clusters [149, 150]. Bioinformatic analysis of SMR homologs from diverse Gram-positive and Gram-negative bacteria revealed that the conservation and abundance of positively charged residues that determine dual topology were correlated to the total anionic phospholipid abundance [151]. When considered altogether, these findings highlight the importance and influence of anionic phospholipid content on SMR protein structural stability, topology, and transport activity.
3.4 SMR Transporter Ligand Binding
Site-directed mutagenesis studies of SMR members Sau-Smr and Eco-EmrE determined that the antiport of drug and H+ was associated with a single highly conserved and negatively charged glutamate residue (TMS1 Glu14 of Eco-EmrE; TMS1 Glu13 of Sau-Smr) in its membrane-spanning segments [2, 4, 5, 16]. In Eco-EmrE, replacement of Glu14 with Cys or Ala resulted in a complete loss of drug resistance [2, 4, 152] and replacement with Asp resulted in reduced or selective drug resistance compared to wild-type proteins [4, 5]. Analysis of all SMR subclass members indicates that these members possess a glutamate residue within the first TMS [6] and replacement of this conserved residue in mutagenesis studies of PSMR subclass members from B. subtilis Bsu-EbrAB [59, 94], Bsu-YkkCD [63] YvaE [9], and E. coli MdtIJ [60] reduced or eliminated their ability to confer drug resistance.
In addition to Glu14 within TMS1, biochemical and mutagenesis studies targeting residue replacements within the first three TMS domains of Eco-EmrE have identified that a number of aromatic residues, such as conserved residues Tyr40, Tyr60, and Trp63, also contribute to drug binding and resistance within the membrane-spanning domains [153–155]. Charged residue replacement within loop1 (Lys22, Glu24, and Arg29) and loop3 (Arg82 and Asp84) of Eco-EmrE has demonstrated reductions in drug transport [4].
X-ray [71], cryo-EM [69], and NMR [72, 105] structural analyses of the SMR archetype Eco-EmrE all indicate that ligand binding occurs within TMS1–TMS3. Cysteine-scanning mutagenesis of Eco-EmrE determined that the TMS1 residues Ala10, Ile11, and Thr18 all located on the same α-helical face as Glu14 participated in the substrate binding pocket [130, 152, 156]. TMS2 has been implicated as a hydrophobic pathway [156], and alterations of conserved residues in this helix were tolerated to greater extents than other TMSs, suggesting that TMS2 plays a role in determining SMR drug polyspecificity [157]. Eco-EmrE TMS3 flexibility [105] and the structured loop between TMS3 and TMS4 [75] have been shown to alter ligand binding within the dimer. Therefore, a number of key residues located within TMS1–TMS3 and the positioning of these helices relative to TMS4 all appear to influence drug binding interactions.
Due to the chemical diversity of substrates recognized and transported by SMR family proteins, drug binding studies have endeavored to identify additional residues responsible for polyspecificity by these proteins. A recent study exploring SMP protein specificity to methyl viologen identified that Eco-EmrE residue Ser43 was specifically involved in methyl viologen resistance [158]. Alteration of this residue located at the same position in SMP proteins that lack methyl viologen transport ability, Bpe-Smr (Ala43Ser) and Mtu-Smr (Ala42Ser), to Ser conferred resistance to methyl viologen [158]. Arrangement dynamics determined for Eco-EmrE TMS1–TMS3 have also been proposed to contribute to polysubstrate recognition by SMR proteins [105, 157]. A recent study compared the substrate specificity of A. baumannii AbeS with that of EmrE, and several AbeS variants (with Ala16Gly, Tyr3Ala, and/or Ala42Ser substitution) produced a substrate-dependent phenotype, providing the molecular basis of polyspecificity of AbeS pump [20]. Further exploration of conserved and variable residues in SMR family proteins using these approaches will likely identify other residues responsible for specific drug recognition and transport.
The stoichiometry of H+/SMR binding has been demonstrated to be variable, where H+/protein binding was shown to be 1:1 [143, 159], 1:2 [67, 71], and 2:3 [5, 160]. High-resolution structural models currently favor a 1H+/2SMR stoichiometry. Ligand/SMR binding was shown to be much more variable at 1:1 [88, 129], 1:2 [67, 71, 131], 1:3 [5, 67, 160, 161], and 1:5 [67]. The affinity of ligand binding to SMR proteins in these experiments was also shown to range from μM to nM concentrations. These variations may reflect differences in ligand properties, such as differences in cationic charge (methyl viologen +2 versus tetraphenylphosphonium +1), aromatic versus acyl chain composition of the ligand tested, oligomerization, and the membrane mimetics used to reconstitute the protein (as reviewed in [12]). Based on the structural plasticity, dual-topology, and potential lipid dependence of SMR proteins, it is not surprising that SMR/ligand interactions also appear to be dynamic and condition dependent. It is clear that SMR proteins bind and transport a variety of structurally diverse lipophilic cation compounds as well as other potentially lipophilic or transiently charged compounds. It seems likely that the plasticity of SMR proteins is essential to recognize diverse substrates and may be intrinsically tied to their broad substrate recognition [128, 134, 162].
4 Transport Mechanisms of SMR Efflux Pumps
Numerous transport mechanisms have been proposed to explain Eco-EmrE efflux (as reviewed in [12]). Transport mechanisms have been proposed to account for specific multimers such as the trimeric model of EmrE protein [5, 160] or variable multimeric states [139, 163] during H+/drug transport. The remaining mechanisms involve variable H+ binding by EmrE dimers [159] and differ based on the involvement of particular TMS [68, 71, 134] and/or their movements [69, 75]. Recent NMR analyses support the involvement of symmetrical inward and outward conformation transitions of the asymmetric dimer during H+/ligand transport [66, 72]. Based on current studies, further exploration by NMR analysis may provide more detail into EmrE transport dynamics and clarify its transport mechanism. A recent study demonstrated asymmetric protonation of EmrE by focusing on the pKa values of the active-site residue of Glu14 with 1H-15N transverse relaxation optimized spectroscopy-heteronuclear single quantum coherence spectra [164]. Protonation of the membrane-embedded Glu14 was shown to modulate the dynamics of EmrE in an allosteric fashion [165, 166]. This protonation leads to extensive rotation and tilt of TMS1–TMS3 in conjunction with repacking of loops, at this point conformational changes alter the coordination of the bound substrate and modulate its access to the binding site from the lipid bilayer [166]. Additionally, using EmrE as the model transporter, a novel liposome method, termed fluorosomes, was developed to study the interaction of antimicrobial substrates and single efflux transporters [167].
Another question that has concerned SMR transport is how QCCs transported by these proteins are completely expelled from Gram-negative systems. Studies of other MDR transporters such as AcrAB and EmrAB identified the involvement of an outer membrane protein TolC forming a multipartite complex spanning both membranes to completely efflux substrates from the cell (as reviewed in [168, 169]). Studies of Eco-EmrE and other SMP members failed to demonstrate any requirement for TolC [29, 170, 171]. A recent study involving an osmotic growth phenotype and screening of overexpressed Eco-EmrE in outer membrane protein gene deletion mutants identified that OmpW participates with EmrE in drug and osmoprotectant efflux in E. coli [18]. It is uncertain if OmpW forms a multipartite dual membrane-spanning complex with EmrE, but it does support the involvement of outer membrane protein(s), such as OmpW, in substrate efflux by other SMR members and potentially for other TolC-independent MDR transporters in Gram-negative bacteria.
5 SMR Efflux Pumps in Antimicrobial Resistance
SMR proteins confer resistance to a variety of toxic lipophilic QCCs used as industrial surfactants (tetraphenylphosphonium and tetraphenylarsonium), membrane-disrupting detergents (alkylpolyaminoethylglycine cetylpyridinium and cetyltrimethylammonium), antiseptics (benzalkonium chloride, cetrimide, and 8-hydroxyquinoline), DNA-intercalating (acriflavine and ethidium bromide) and toxic dyes (crystal violet, rhodamine 6G, and safranin O), and reactive oxygen-generating compounds (methyl viologen) (Table 3.1). QCCs represent a structurally diverse group of chemicals that possess one or more cationic atoms (most commonly nitrogen and phosphorous) bound to three to four R groups that consist of acyl chain or aromatic hydrocarbons. SMR members have also demonstrated low to moderate resistance to antibiotics such as chloramphenicol, erythromycin, fluoroquinolones, and tetracyclines (Table 3.1) [42, 51, 62, 172] by comparison to other larger MDR transporters [15, 170]. Curiously, reports have also shown that SMR proteins can confer resistance to sodium dodecyl sulfate [15, 42, 53] (Table 3.1). Based on the negative charge of the conserved glutamate residue shown to bind both drugs and protons and the lack of conserved positively charged residues in membrane-spanning segments in SMR proteins [6], it is difficult to understand how anionic sodium dodecyl sulfate can be transported by SMR proteins. It is more likely that tolerance to this detergent is enhanced in bacterial strains overexpressing SMR members due to their affinity for anionic lipids as discussed in Sect. 3.3. In general, it appears that the lipophilicity and cationic properties of a drug determines its potential as an SMR substrate.
Studies of SMR family member drug resistance have also demonstrated SMR members belonging to each subclass appear to differ in their conferred drug resistance profiles suggesting that different SMR subclass members have evolved to accommodate more specific substrates [6]. Supporting evidence of this can be observed when comparing the substrate diversity of SUG and PSMR members to SMP drug resistance profiles as well as comparisons between chromosomally encoded SMR genes and those present on mobile genetic elements (Table 3.1).
5.1 Natural SMR Substrates and Potential Functions
SMR proteins confer resistance to a variety of anthropogenically derived QCC antimicrobials. Naturally synthesized QCCs can also build up in cells as metabolic intermediates that serve as osmoprotectants and/or toxic by-products like polyamines during amino acid catabolism. Recent studies involving the efflux of biologically produced QCCs have identified the involvement of many SMR members (Table 3.1). A study assessing growth phenotype changes in E. coli cells grown in media with high osmolarities identified the Eco-EmrE involvement in osmoprotectant (betaine and choline) export and its participation in cellular osmoregulation [18]. PSMR members Eco-MdtIJ were shown to transport the polyamine spermidine, a toxic metabolite that builds up during amino acid degradation [60]. Mutagenic analysis of Eco-EmrE has demonstrated that a single residue mutation of conserved Trp63Gly converts the protein into a polyamine exporter [173]. These findings agree with evolutionary studies demonstrating that Eco-MdtJI has recently evolved from EmrE homologs in Gram-negative species [60]. The PSMR member Ani-NepAB encoded by a plasmid of a Gram-positive aerobe has shown transport of the toxic nicotinamide degradation intermediate methylamine [61]. Interestingly, the PSMR member Hda-PsmrAB, a homolog of Bsu-YvdRS that fails to confer drug resistance, was recently shown to function as a Na+/H+ antiporter indicating its involvement in cell osmoregulation [62]. Altogether, SMR subclass diversity and their selective transport of natural substrates and ions may also explain why significant differences in drug resistance profiles occur within subclasses and some of driving forces influencing their phylogenetic distinctions [6]. Selective transport of particular biological substrates may also explain the redundancy of SMR family proteins and other MDR transporters that confer resistance to similar drugs (as discussed by [171]).
5.2 Clinical Significance and Pathogenicity
Improving our understanding of the structure, function, and regulation of SMR family proteins is essential to combat the emerging problem of antiseptic resistance. Exposure to QCCs is increasing as these antiseptics are added to commonly used commercial products such as soaps, detergents, mouthwashes, toothpastes, and cosmetics. Large quantities of QCCs are also used in industrial surfactants and in medical/agricultural sterilization resulting in QCC-polluted environments (as reviewed in [8]). SMR family members transmitted via mobile genetic elements and plasmids are frequently associated with QCC-polluted environments [174–176]. The pressure to maintain SMR genes within these mobile elements also appears to be driven by QCC and antibiotic exposure [11, 177] indicating that QCC contamination is a major factor driving SMR-mediated resistance and transmission.
The clinical relevance of SMR-mediated QCC resistance may be associated with bacterial growth states. Enhanced QCC resistance associated with SMR efflux genes has been demonstrated for bacterial cultures grown as sessile surface-attached biofilms [178, 179] and as free-living planktonic cultures [170]. Hence, SMR members may influence the biofilm formation and virulence similar to other MDR transporters [179]. Recent studies identifying SMR family member involvement in the efflux of osmoprotectants, polyamines, and other metabolites (as discussed above) have also shown that similar to other MDR transporters, SMR proteins may confer added benefits and improve cell fitness by removing potentially toxic natural substrates (as reviewed in [180]). Overall, this suggests that the bacterial lifestyle and physiology play an important role in determining the extent of virulence associated with SMR activity and QCC resistance.
Efforts to thwart SMR-mediated QCC resistance have focused on the design and use of TMS-like peptide inhibitors that disrupt multimer formation in the Hsa-Smr complex and its QCC resistance [26, 136]. This inhibition strategy relies on fundamental structural knowledge gained from SMR structural and functional analyses and underscores their importance for novel SMR efflux pump inhibitor design. The initial success of this peptide-based inhibition may provide a future therapeutic strategy that could be applied to selectively inhibit SMR and potentially other MDR efflux systems.
6 Concluding Remarks
After two decades of research examining SMR family protein structure and function, many insights have been gained into how its members confer drug resistance and have provided a number of high-resolution structures. It has also fueled a number of controversies surrounding SMR topology and multimerization which have helped drive and focus structural exploration of Eco-EmrE and other members. Further examination of Eco-EmrE and SMR subclass members, specifically those encoded on mobile genetic elements and SUG subclass members, will reveal more insights into the structure, function, clinical significance, and evolution of these remarkable SMR proteins.
References
Littlejohn TG, Paulsen IT, Gillespie MT, Tennent JM, Midgley M, Jones IG, Purewal AS, Skurray RA (1992) Substrate specificity and energetics of antiseptic and disinfectant resistance in Staphylococcus aureus. FEMS Microbiol Lett 95:259–265. doi:10.1016/0378-1097(92)90439-U
Grinius LL, Goldberg EB (1994) Bacterial multidrug resistance is due to a single membrane protein which functions as a drug pump. J Biol Chem 269:29998–30004
Jack DL, Yang NM, Saier MH Jr (2001) The drug/metabolite transporter superfamily. Eur J Biochem 268:3620–3639. doi:10.1046/j.1432-1327.2001.02265.x
Yerushalmi H, Schuldiner S (2000) An essential glutamyl residue in EmrE, a multidrug antiporter from Escherichia coli. J Biol Chem 275:5264–5269. doi:10.1074/jbc.275.8.5264
Muth TR, Schuldiner S (2000) A membrane-embedded glutamate is required for ligand binding to the multidrug transporter EmrE. EMBO J 19:234–240. doi:10.1093/emboj/19.2.234
Bay DC, Turner RJ (2009) Diversity and evolution of the small multidrug resistance protein family. BMC Evol Biol 9:140. doi:10.1186/1471-2148-9-140
Paulsen IT, Littlejohn TG, Radstrom P, Sundstrom L, Skold O, Swedberg G, Skurray RA (1993) The 3′ conserved segment of integrons contains a gene associated with multidrug resistance to antiseptics and disinfectants. Antimicrob Agents Chemother 37:761–768. doi:10.1128/AAC.37.4.761
Hegstad K, Langsrud S, Lunestad BT, Scheie AA, Sunde M, Yazdankhah SP (2010) Does the wide use of quaternary ammonium compounds enhance the selection and spread of antimicrobial resistance and thus threaten our health? Microb Drug Resist 16:91–104. doi:10.1089/mdr.2009.0120
Chung YJ, Saier MH Jr (2001) SMR-type multidrug resistance pumps. Curr Opin Drug Discov Dev 4:237–245
Saier MH Jr (2001) Evolution of transport proteins. Genet Eng (N Y) 23:1–10. doi:10.1007/0-306-47572-3_1
Bjorland J, Sunde M, Waage S (2001) Plasmid-borne smr gene causes resistance to quaternary ammonium compounds in bovine Staphylococcus aureus. J Clin Microbiol 39:3999–4004. doi:10.1128/JCM.39.11.3999-4004.2001
Bay DC, Rommens KL, Turner RJ (2008) Small multidrug resistance proteins: a multidrug transporter family that continues to grow. Biochim Biophys Acta 1778:1814–1838. doi:10.1016/j.bbamem.2007.08.015
Morimyo M, Hongo E, Hama-Inaba H, Machida I (1992) Cloning and characterization of the mvrC gene of Escherichia coli K-12 which confers resistance against methyl viologen toxicity. Nucleic Acids Res 20:3159–3165. doi:10.1093/nar/20.12.3159
Purewal AS (1991) Nucleotide sequence of the ethidium efflux gene from Escherichia coli. FEMS Microbiol Lett 66:229–231. doi:10.1111/j.1574-6968.1991.tb04870.x
Nishino K, Yamaguchi A (2001) Analysis of a complete library of putative drug transporter genes in Escherichia coli. J Bacteriol 183:5803–5812. doi:10.1128/JB.183.20.5803-5812.2001
Yerushalmi H, Lebendiker M, Schuldiner S (1995) EmrE, an Escherichia coli 12-kDa multidrug transporter, exchanges toxic cations and H+ and is soluble in organic solvents. J Biol Chem 270:6856–6863
Yerushalmi H, Lebendiker M, Schuldiner S (1996) Negative dominance studies demonstrate the oligomeric structure of EmrE, a multidrug antiporter from Escherichia coli. J Biol Chem 271:31044–31048. doi:10.1074/jbc.271.49.31044
Bay DC, Turner RJ (2012) Small multidrug resistance protein EmrE reduces host pH and osmotic tolerance to metabolic quaternary cation osmoprotectants. J Bacteriol 194:5941–5948. doi:10.1128/JB.00666-12
Srinivasan VB, Rajamohan G, Gebreyes WA (2009) Role of AbeS, a novel efflux pump of the SMR family of transporters, in resistance to antimicrobial agents in Acinetobacter baumannii. Antimicrob Agents Chemother 53:5312–5316. doi:10.1128/AAC.00748-09
Lytvynenko I, Brill S, Oswald C, Pos KM (2016) Residues involved in substrate recognition of the small multidrug resistance efflux pump AbeS from Acinetobacter baumannii. J Mol Biol 428:644–657. doi:10.1016/j.jmb.2015.12.006
Ninio S, Rotem D, Schuldiner S (2001) Functional analysis of novel multidrug transporters from human pathogens. J Biol Chem 276:48250–48256. doi:10.1074/jbc.M108231200
Chang LL, Chen HF, Chang CY, Lee TM, Wu WJ (2004) Contribution of integrons, and SmeABC and SmeDEF efflux pumps to multidrug resistance in clinical isolates of Stenotrophomonas maltophilia. J Antimicrob Chemother 53:518–521. doi:10.1093/jac/dkh094
Kovacevic J, Ziegler J, Walecka-Zacharska E, Reimer A, Kitts DD, Gilmour MW (2015) Tolerance of Listeria monocytogenes to quaternary ammonium sanitizers is mediated by a novel efflux pump encoded by emrE. Appl Environ Microbiol 82:939–953. doi:10.1128/AEM.03741-15
De Rossi E, Branzoni M, Cantoni R, Milano A, Riccardi G, Ciferri O (1998) mmr, a Mycobacterium tuberculosis gene conferring resistance to small cationic dyes and inhibitors. J Bacteriol 180:6068–6071
Ninio S, Schuldiner S (2003) Characterization of an archaeal multidrug transporter with a unique amino acid composition. J Biol Chem 278:12000–12005. doi:10.1074/jbc.M213119200
Rath A, Melnyk RA, Deber CM (2006) Evidence for assembly of small multidrug resistance proteins by a “two-faced” transmembrane helix. J Biol Chem 281:15546–15553. doi:10.1074/jbc.M600434200
Grinius L, Dreguniene G, Goldberg EB, Liao CH, Projan SJ (1992) A staphylococcal multidrug resistance gene product is a member of a new protein family. Plasmid 27:119–129. doi:10.1016/0147-619X(92)90012-Y
Paulsen IT, Brown MH, Dunstan SJ, Skurray RA (1995) Molecular characterization of the staphylococcal multidrug resistance export protein QacC. J Bacteriol 177:2827–2833
Fuentes DE, Navarro CA, Tantalean JC, Araya MA, Saavedra CP, Perez JM, Calderon IL, Youderian PA et al (2005) The product of the qacC gene of Staphylococcus epidermidis CH mediates resistance to β-lactam antibiotics in Gram-positive and Gram-negative bacteria. Res Microbiol 156:472–477. doi:10.1016/j.resmic.2005.01.002
Littlejohn TG, DiBerardino D, Messerotti LJ, Spiers SJ, Skurray RA (1991) Structure and evolution of a family of genes encoding antiseptic and disinfectant resistance in Staphylococcus aureus. Gene 101:59–66. doi:10.1016/0378-1119(91)90224-Y
Alam MM, Ishino M, Kobayashi N (2003) Analysis of genomic diversity and evolution of the low-level antiseptic resistance gene smr in Staphylococcus aureus. Microb Drug Resist 9:S1–S7. doi:10.1089/107662903322541838
Heir E, Sundheim G, Holck AL (1999) Identification and characterization of quaternary ammonium compound resistant staphylococci from the food industry. Int J Food Microbiol 48:211–219. doi:10.1016/S0168-1605(99)00044-6
Marchi E, Furi L, Arioli S, Morrissey I, Di Lorenzo V, Mora D, Giovannetti L, Oggioni MR et al (2015) Novel insight into antimicrobial resistance and sensitivity phenotypes associated to qac and norA genotypes in Staphylococcus aureus. Microbiol Res 170:184–194. doi:10.1016/j.micres.2014.07.001
Furi L, Ciusa ML, Knight D, Di Lorenzo V, Tocci N, Cirasola D, Aragones L, Coelho JR et al (2013) Evaluation of reduced susceptibility to quaternary ammonium compounds and bisbiguanides in clinical isolates and laboratory-generated mutants of Staphylococcus aureus. Antimicrob Agents Chemother 57:3488–3497. doi:10.1128/AAC.00498-13
Kazama H, Hamashima H, Sasatsu M, Arai T (1999) Characterization of the antiseptic-resistance gene qacEΔ1 isolated from clinical and environmental isolates of Vibrio parahaemolyticus and Vibrio cholerae non-O1. FEMS Microbiol Lett 174:379–384. doi:10.1111/j.1574-6968.1999.tb13593.x
Kazama H, Hamashima H, Sasatsu M, Arai T (1998) Distribution of the antiseptic-resistance gene qacEΔ1 in Gram-positive bacteria. FEMS Microbiol Lett 165:295–299. doi:10.1111/j.1574-6968.1998.tb13160.x
Chiou CS, Lin JM, Chiu CH, Chu CH, Chen SW, Chang YF, Weng BC, Tsay JG et al (2009) Clonal dissemination of the multi-drug resistant Salmonella enterica serovar Braenderup, but not the serovar Bareilly, of prevalent serogroup C1 Salmonella from Taiwan. BMC Microbiol 9:264. doi:10.1186/1471-2180-9-264
Sandvang D, Aarestrup FM, Jensen LB (1998) Characterisation of integrons and antibiotic resistance genes in Danish multiresistant Salmonella enterica Typhimurium DT104. FEMS Microbiol Lett 160:37–41. doi:10.1111/j.1574-6968.1998.tb12887.x
Ploy MC, Courvalin P, Lambert T (1998) Characterization of In40 of Enterobacter aerogenes BM2688, a class 1 integron with two new gene cassettes, cmlA2 and qacF. Antimicrob Agents Chemother 42:2557–2563
Schluter A, Heuer H, Szczepanowski R, Poler SM, Schneiker S, Puhler A, Top EM (2005) Plasmid pB8 is closely related to the prototype IncP-1β plasmid R751 but transfers poorly to Escherichia coli and carries a new transposon encoding a small multidrug resistance efflux protein. Plasmid 54:135–148. doi:10.1016/j.plasmid.2005.03.001
Mazel D, Dychinco B, Webb VA, Davies J (2000) Antibiotic resistance in the ECOR collection: integrons and identification of a novel aad gene. Antimicrob Agents Chemother 44:1568–1574. doi:10.1128/AAC.44.6.1568-1574.2000
Correa JE, De Paulis A, Predari S, Sordelli DO, Jeric PE (2008) First report of qacG, qacH and qacJ genes in Staphylococcus haemolyticus human clinical isolates. J Antimicrob Chemother 62:956–960. doi:10.1093/jac/dkn327
Bjorland J, Steinum T, Sunde M, Waage S, Heir E (2003) Novel plasmid-borne gene qacJ mediates resistance to quaternary ammonium compounds in equine Staphylococcus aureus, Staphylococcus simulans, and Staphylococcus intermedius. Antimicrob Agents Chemother 47:3046–3052. doi:10.1128/AAC.47.10.3046-3052.2003
Heir E, Sundheim G, Holck AL (1999) The qacG gene on plasmid pST94 confers resistance to quaternary ammonium compounds in staphylococci isolated from the food industry. J Appl Microbiol 86:378–388. doi:10.1046/j.1365-2672.1999.00672.x
Heir E, Sundheim G, Holck AL (1998) The Staphylococcus qacH gene product: a new member of the SMR family encoding multidrug resistance. FEMS Microbiol Lett 163:49–56. doi:10.1111/j.1574-6968.1998.tb13025.x
Muller A, Rychli K, Muhterem-Uyar M, Zaiser A, Stessl B, Guinane CM, Cotter PD, Wagner M et al (2013) Tn6188 – a novel transposon in Listeria monocytogenes responsible for tolerance to benzalkonium chloride. PLoS One 8: e76835. doi:10.1371/journal.pone.0076835
Braga TM, Marujo PE, Pomba C, Lopes MF (2011) Involvement, and dissemination, of the enterococcal small multidrug resistance transporter QacZ in resistance to quaternary ammonium compounds. J Antimicrob Chemother 66:283–286. doi:10.1093/jac/dkq460
Chung YJ, Saier MH Jr (2002) Overexpression of the Escherichia coli sugE gene confers resistance to a narrow range of quaternary ammonium compounds. J Bacteriol 184:2543–2545. doi:10.1128/JB.184.9.2543-2545.2002
Greener T, Govezensky D, Zamir A (1993) A novel multicopy suppressor of a groEL mutation includes two nested open reading frames transcribed from different promoters. EMBO J 12:889–896
Bay DC, Turner RJ (2011) Spectroscopic analysis of the intrinsic chromophores within small multidrug resistance protein SugE. Biochim Biophys Acta 1808:2233–2244. doi:10.1016/j.bbamem.2011.05.005
Cruz A, Micaelo N, Felix V, Song JY, Kitamura S, Suzuki S, Mendo S (2013) sugE: a gene involved in tributyltin (TBT) resistance of Aeromonas molluscorum Av27. J Gen Appl Microbiol 59:39–47. doi:10.2323/jgam.59.47
Son MS, Del Castilho C, Duncalf KA, Carney D, Weiner JH, Turner RJ (2003) Mutagenesis of SugE, a small multidrug resistance protein. Biochem Biophys Res Commun 312:914–921. doi:10.1016/j.bbrc.2003.11.018
He GX, Zhang C, Crow RR, Thorpe C, Chen H, Kumar S, Tsuchiya T, Varela MF (2011) SugE, a new member of the SMR family of transporters, contributes to antimicrobial resistance in Enterobacter cloacae. Antimicrob Agents Chemother 55:3954–3957. doi:10.1128/AAC.00094-11
Wu SW, Dornbusch K, Kronvall G, Norgren M (1999) Characterization and nucleotide sequence of a Klebsiella oxytoca cryptic plasmid encoding a CMY-type β-lactamase: confirmation that the plasmid-mediated cephamycinase originated from the Citrobacter freundii AmpC β-lactamase. Antimicrob Agents Chemother 43:1350–1357
Meunier D, Jouy E, Lazizzera C, Doublet B, Kobisch M, Cloeckaert A, Madec JY (2010) Plasmid-borne florfenicol and ceftiofur resistance encoded by the floR and bla CMY-2 genes in Escherichia coli isolates from diseased cattle in France. J Med Microbiol 59:467–471. doi:10.1099/jmm.0.016162-0
Vourli S, Tzouvelekis LS, Tzelepi E, Lebessi E, Legakis NJ, Miriagou V (2003) Characterization of In111, a class 1 integron that carries the extended-spectrum β-lactamase gene bla IBC-1 . FEMS Microbiol Lett 225:149–153. doi:10.1016/S0378-1097(03)00510-X
Masaoka Y, Ueno Y, Morita Y, Kuroda T, Mizushima T, Tsuchiya T (2000) A two-component multidrug efflux pump, EbrAB, in Bacillus subtilis. J Bacteriol 182:2307–2310. doi:10.1128/JB.182.8.2307-2310.2000
Kikukawa T, Nara T, Araiso T, Miyauchi S, Kamo N (2006) Two-component bacterial multidrug transporter, EbrAB: mutations making each component solely functional. Biochim Biophys Acta 1758:673–679. doi:10.1016/j.bbamem.2006.04.004
Zhang Z, Ma C, Pornillos O, Xiu X, Chang G, Saier MH Jr (2007) Functional characterization of the heterooligomeric EbrAB multidrug efflux transporter of Bacillus subtilis. Biochemistry 46:5218–5225. doi:10.1021/bi7001604
Higashi K, Ishigure H, Demizu R, Uemura T, Nishino K, Yamaguchi A, Kashiwagi K, Igarashi K (2008) Identification of a spermidine excretion protein complex (MdtJI) in Escherichia coli. J Bacteriol 190:872–878. doi:10.1128/JB.01505-07
Ganas P, Mihasan M, Igloi GL, Brandsch R (2007) A two-component small multidrug resistance pump functions as a metabolic valve during nicotine catabolism by Arthrobacter nicotinovorans. Microbiology 153:1546–1555. doi:10.1099/mic.0.2006/004234-0
Jiang J, Wang L, Zhang H, Wu H, Huang H, Yang L (2013) Putative paired small multidrug resistance family proteins PsmrAB, the homolog of YvdSR, actually function as a novel two-component Na+/H+ antiporter. FEMS Microbiol Lett 338:31–38. doi:10.1111/1574-6968.12008
Jack DL, Storms ML, Tchieu JH, Paulsen IT, Saier MH Jr (2000) A broad-specificity multidrug efflux pump requiring a pair of homologous SMR-type proteins. J Bacteriol 182:2311–2313. doi:10.1128/JB.182.8.2311-2313.2000
Yoshida K, Kobayashi K, Miwa Y, Kang CM, Matsunaga M, Yamaguchi H, Tojo S, Yamamoto M et al (2001) Combined transcriptome and proteome analysis as a powerful approach to study genes under glucose repression in Bacillus subtilis. Nucleic Acids Res 29:683–692. doi:10.1093/nar/29.3.683
Schuldiner S, Granot D, Mordoch SS, Ninio S, Rotem D, Soskin M, Tate CG, Yerushalmi H (2001) Small is mighty: EmrE, a multidrug transporter as an experimental paradigm. News Physiol Sci 16:130–134
Henzler-Wildman K (2011) Analyzing conformational changes in the transport cycle of EmrE. Curr Opin Struct Biol 22:38–43. doi:10.1016/j.sbi.2011.10.004
Tate CG, Ubarretxena-Belandia I, Baldwin JM (2003) Conformational changes in the multidrug transporter EmrE associated with substrate binding. J Mol Biol 332:229–242. doi:10.1016/S0022-2836(03)00895-7
Ubarretxena-Belandia I, Baldwin JM, Schuldiner S, Tate CG (2003) Three-dimensional structure of the bacterial multidrug transporter EmrE shows it is an asymmetric homodimer. EMBO J 22:6175–6181. doi:10.1093/emboj/cdg611
Fleishman SJ, Harrington SE, Enosh A, Halperin D, Tate CG, Ben-Tal N (2006) Quasi-symmetry in the cryo-EM structure of EmrE provides the key to modeling its transmembrane domain. J Mol Biol 364:54–67. doi:10.1016/j.jmb.2006.08.072
Korkhov VM, Tate CG (2009) An emerging consensus for the structure of EmrE. Acta Crystallogr D Biol Crystallogr 65:186–192. doi:10.1107/S0907444908036640
Chen YJ, Pornillos O, Lieu S, Ma C, Chen AP, Chang G (2007) X-ray structure of EmrE supports dual topology model. Proc Natl Acad Sci U S A 104:18999–19004. doi:10.1073/pnas.0709387104
Morrison EA, DeKoster GT, Dutta S, Vafabakhsh R, Clarkson MW, Bahl A, Kern D, Ha T et al (2011) Antiparallel EmrE exports drugs by exchanging between asymmetric structures. Nature 481:45–50. doi:10.1038/nature10703
Dutta S, Morrison EA, Henzler-Wildman KA (2014) EmrE dimerization depends on membrane environment. Biochim Biophys Acta 1838:1817–1822. doi:10.1016/j.bbamem.2014.03.013
Morrison EA, Henzler-Wildman KA (2014) Transported substrate determines exchange rate in the multidrug resistance transporter EmrE. J Biol Chem 289:6825–6836. doi:10.1074/jbc.M113.535328
Banigan JR, Gayen A, Cho MK, Traaseth NJ (2015) A structured loop modulates coupling between the substrate-binding and dimerization domains in the multidrug resistance transporter EmrE. J Biol Chem 290:805–814. doi:10.1074/jbc.M114.601963
Cho MK, Gayen A, Banigan JR, Leninger M, Traaseth NJ (2014) Intrinsic conformational plasticity of native EmrE provides a pathway for multidrug resistance. J Am Chem Soc 136:8072–8080. doi:10.1021/ja503145x
Li X-Z, Poole K, Nikaido H (2003) Contributions of MexAB-OprM and an EmrE homolog to intrinsic resistance of Pseudomonas aeruginosa to aminoglycosides and dyes. Antimicrob Agents Chemother 47:27–33. doi:10.1128/AAC.47.1.27-33.2003
Poget SF, Harris R, Cahill SM, Girvin ME (2010) 1H, 13C, 15N backbone NMR assignments of the Staphylococcus aureus small multidrug-resistance pump (Smr) in a functionally active conformation. Biomol NMR Assign 4:139–142. doi:10.1007/s12104-010-9228-7
Basting D, Lorch M, Lehner I, Glaubitz C (2008) Transport cycle intermediate in small multidrug resistance protein is revealed by substrate fluorescence. FASEB J 22:365–373. doi:10.1096/fj.07-9162com
Charalambous K, Miller D, Curnow P, Booth PJ (2008) Lipid bilayer composition influences small multidrug transporters. BMC Biochem 9:31. doi:10.1186/1471-2091-9-31
Mors K, Hellmich UA, Basting D, Marchand P, Wurm JP, Haase W, Glaubitz C (2013) A lipid-dependent link between activity and oligomerization state of the M. tuberculosis SMR protein TBsmr. Biochim Biophys Acta 1828:561–567. doi:10.1016/j.bbamem.2012.10.020
Chenna R, Sugawara H, Koike T, Lopez R, Gibson TJ, Higgins DG, Thompson JD (2003) Multiple sequence alignment with the clustal series of programs. Nucleic Acids Res 31:3497–3500. doi:10.1093/nar/gkg500
Kucken D, Feucht H, Kaulfers P (2000) Association of qacE and qacEΔ1 with multiple resistance to antibiotics and antiseptics in clinical isolates of Gram-negative bacteria. FEMS Microbiol Lett 183:95–98. doi:10.1111/j.1574-6968.2000.tb08939.x
Zou L, Meng J, McDermott PF, Wang F, Yang Q, Cao G, Hoffmann M, Zhao S (2014) Presence of disinfectant resistance genes in Escherichia coli isolated from retail meats in the USA. J Antimicrob Chemother 69:2644–2649. doi:10.1093/jac/dku197
Bishop RE, Penfold SS, Frost LS, Holtje JV, Weiner JH (1995) Stationary phase expression of a novel Escherichia coli outer membrane lipoprotein and its relationship with mammalian apolipoprotein D. Implications for the origin of lipocalins. J Biol Chem 270:23097–23103. doi:10.1074/jbc.270.39.23097
Paulsen IT, Skurray RA, Tam R, Saier MH Jr, Turner RJ, Weiner JH, Goldberg EB, Grinius LL (1996) The SMR family: a novel family of multidrug efflux proteins involved with the efflux of lipophilic drugs. Mol Microbiol 19:1167–1175. doi:10.1111/j.1365-2958.1996.tb02462.x
Saier MH Jr, Paulsen IT (2001) Phylogeny of multidrug transporters. Semin Cell Dev Biol 12:205–213. doi:10.1006/scdb.2000.0246
Sikora CW, Turner RJ (2005) SMR proteins SugE and EmrE bind ligand with similar affinity and stoichiometry. Biochem Biophys Res Commun 335:105–111. doi:10.1016/j.bbrc.2005.07.051
Klammt C, Lohr F, Schafer B, Haase W, Dotsch V, Ruterjans H, Glaubitz C, Bernhard F (2004) High level cell-free expression and specific labeling of integral membrane proteins. Eur J Biochem 271:568–580. doi:10.1111/j.1432-1033.2003.03959.x
Banigan JR, Gayen A, Traaseth NJ (2014) Correlating lipid bilayer fluidity with sensitivity and resolution of polytopic membrane protein spectra by solid-state NMR spectroscopy. Biochim Biophys Acta 1848:334–341. doi:10.1016/j.bbamem.2014.05.003
Roos C, Zocher M, Muller D, Munch D, Schneider T, Sahl HG, Scholz F, Wachtveitl J et al (2012) Characterization of co-translationally formed nanodisc complexes with small multidrug transporters, proteorhodopsin and with the E. coli MraY translocase. Biochim Biophys Acta 1818:3098–3106. doi:10.1016/j.bbamem.2012.08.007
Su LH, Chen HL, Chia JH, Liu SY, Chu C, Wu TL, Chiu CH (2006) Distribution of a transposon-like element carrying bla CMY-2 among Salmonella and other Enterobacteriaceae. J Antimicrob Chemother 57:424–429. doi:10.1093/jac/dki478
Fernandez-Fuentes MA, Abriouel H, Ortega Morente E, Perez Pulido R, Galvez A (2014) Genetic determinants of antimicrobial resistance in Gram positive bacteria from organic foods. Int J Food Microbiol 172:49–56. doi:10.1016/j.ijfoodmicro.2013.11.032
Kikukawa T, Miyauchi S, Araiso T, Kamo N, Nara T (2007) Anti-parallel membrane topology of two components of EbrAB, a multidrug transporter. Biochem Biophys Res Commun 358:1071–1075. doi:10.1016/j.bbrc.2007.05.032
Nasie I, Steiner-Mordoch S, Gold A, Schuldiner S (2010) Topologically random insertion of EmrE supports a pathway for evolution of inverted repeats in ion-coupled transporters. J Biol Chem 285:15234–15244. doi:10.1074/jbc.M110.108746
Kobayashi A, Hirakawa H, Hirata T, Nishino K, Yamaguchi A (2006) Growth phase-dependent expression of drug exporters in Escherichia coli and its contribution to drug tolerance. J Bacteriol 188:5693–5703. doi:10.1128/JB.00217-06
Drew D, Sjostrand D, Nilsson J, Urbig T, Chin CN, de Gier JW, von Heijne G (2002) Rapid topology mapping of Escherichia coli inner-membrane proteins by prediction and PhoA/GFP fusion analysis. Proc Natl Acad Sci U S A 99:2690–2695. doi:10.1073/pnas.052018199
Rapp M, Granseth E, Seppala S, von Heijne G (2006) Identification and evolution of dual-topology membrane proteins. Nat Struct Mol Biol 13:112–116. doi:10.1038/nsmb1057
Tate CG, Kunji ER, Lebendiker M, Schuldiner S (2001) The projection structure of EmrE, a proton-linked multidrug transporter from Escherichia coli, at 7 Å resolution. EMBO J 20:77–81. doi:10.1093/emboj/20.1.77
Schuldiner S (2007) When biochemistry meets structural biology: the cautionary tale of EmrE. Trends Biochem Sci 32:252–258. doi:10.1016/j.tibs.2007.04.002
Schuldiner S (2007) Controversy over EmrE structure. Science 317:748–751. doi:10.1126/science.317.5839.748d
Chang G, Roth CB, Reyes CL, Pornillos O, Chen YJ, Chen AP (2006) Retraction. Science 314:1875. doi:10.1126/science.314.5807.1875b
Miller G (2006) A scientist’s nightmare: software problem leads to five retractions. Science 314:1856–1857. doi:10.1126/science.314.5807.1856
Omasits U, Ahrens CH, Muller S, Wollscheid B (2014) Protter: interactive protein feature visualization and integration with experimental proteomic data. Bioinformatics 30:884–886. doi:10.1093/bioinformatics/btt607
Amadi ST, Koteiche HA, Mishra S, McHaourab HS (2010) Structure, dynamics, and substrate-induced conformational changes of the multidrug transporter EmrE in liposomes. J Biol Chem 285:26710–26718. doi:10.1074/jbc.M110.132621
Morrison EA, Henzler-Wildman KA (2012) Reconstitution of integral membrane proteins into isotropic bicelles with improved sample stability and expanded lipid composition profile. Biochim Biophys Acta 1818:814–820. doi:10.1016/j.bbamem.2011.12.020
Dutta S, Morrison EA, Henzler-Wildman KA (2014) Blocking dynamics of the SMR transporter EmrE impairs efflux activity. Biophys J 107:613–620. doi:10.1016/j.bpj.2014.06.030
Poolman B, Geertsma ER, Slotboom DJ (2007) A missing link in membrane protein evolution. Science 315:1229–1231. doi:10.1126/science.1140073
Schuldiner S (2010) Parallel or antiparallel, who cares? Science 328 (E-letter June 24, 2010). http://science.sciencemag.org/content/328/5986/1698.e-letters
Schuldiner S (2012) Undecided membrane proteins insert in random topologies. Up, down and sideways: it does not really matter. Trends Biochem Sci 37:215–219. doi:10.1016/j.tibs.2012.02.006
von Heijne G (1986) The distribution of positively charged residues in bacterial inner membrane proteins correlates with the trans-membrane topology. EMBO J 5:3021–3027
von Heijne G (1992) Membrane protein structure prediction. Hydrophobicity analysis and the positive-inside rule. J Mol Biol 225:487–494. doi:10.1016/0022-2836(92)90934-C
von Heijne G (2006) Membrane-protein topology. Nat Rev Mol Cell Biol 7:909–918. doi:10.1038/nrm2063
Rapp M, Drew D, Daley DO, Nilsson J, Carvalho T, Melen K, De Gier JW, Von Heijne G (2004) Experimentally based topology models for E. coli inner membrane proteins. Protein Sci 13:937–945. doi:10.1110/ps.03553804
Daley DO, Rapp M, Granseth E, Melen K, Drew D, von Heijne G (2005) Global topology analysis of the Escherichia coli inner membrane proteome. Science 308:1321–1323. doi:10.1126/science.1109730
Kolbusz MA, Slotboom DJ, Lolkema JS (2013) Genomic distribution of the small multidrug resistance protein EmrE over 29 Escherichia coli strains reveals two forms of the protein. FEBS J 280:244–255. doi:10.1111/febs.12065
Seppala S, Slusky JS, Lloris-Garcera P, Rapp M, von Heijne G (2010) Control of membrane protein topology by a single C-terminal residue. Science 328:1698–1700. doi:10.1126/science.1188950
Kolbusz MA, Slotboom DJ, Lolkema JS (2012) Role of individual positive charges in the membrane orientation and activity of transporters of the small multidrug resistance family. Biochemistry 51:8867–8876. doi:10.1021/bi300854c
Nara T, Kouyama T, Kurata Y, Kikukawa T, Miyauchi S, Kamo N (2007) Anti-parallel membrane topology of a homo-dimeric multidrug transporter, EmrE. J Biochem (Tokyo) 142:621–625. doi:10.1093/jb/mvm169
Lloris-Garcera P, Bianchi F, Slusky JS, Seppala S, Daley DO, von Heijne G (2012) Antiparallel dimers of the small multidrug resistance protein EmrE are more stable than parallel dimers. J Biol Chem 287:26052–26059. doi:10.1074/jbc.M112.357590
Lloris-Garcera P, Seppala S, Slusky JS, Rapp M, von Heijne G (2014) Why have small multidrug resistance proteins not evolved into fused, internally duplicated structures? J Mol Biol 426:2246–2254. doi:10.1016/j.jmb.2014.03.012
Lehner I, Basting D, Meyer B, Haase W, Manolikas T, Kaiser C, Karas M, Glaubitz C (2007) The key residue for substrate transport (E14) in the EmrE dimer is asymmetric. J Biol Chem 283:3281–3288. doi:10.1074/jbc.M707899200
Poget SF, Cahill SM, Girvin ME (2007) Isotropic bicelles stabilize the functional form of a small multidrug-resistance pump for NMR structural studies. J Am Chem Soc 129:2432–2433. doi:10.1021/ja0679836
Soskine M, Mark S, Tayer N, Mizrachi R, Schuldiner S (2006) On parallel and antiparallel topology of a homodimeric multidrug transporter. J Biol Chem 281:36205–36212. doi:10.1074/jbc.M607186200
Steiner-Mordoch S, Soskine M, Solomon D, Rotem D, Gold A, Yechieli M, Adam Y, Schuldiner S (2008) Parallel topology of genetically fused EmrE homodimers. EMBO J 27:17–26. doi:10.1038/sj.emboj.7601951
Federkeil SL, Winstone TL, Jickling G, Turner RJ (2003) Examination of EmrE conformational differences in various membrane mimetic environments. Biochem Cell Biol 81:61–70. doi:10.1139/o03-031
Winstone TL, Jidenko M, le Maire M, Ebel C, Duncalf KA, Turner RJ (2005) Organic solvent extracted EmrE solubilized in dodecyl maltoside is monomeric and binds drug ligand. Biochem Biophys Res Commun 327:437–445. doi:10.1016/j.bbrc.2004.11.164
Bay DC, Turner RJ (2012) Spectroscopic analysis of small multidrug resistance protein EmrE in the presence of various quaternary cation compounds. Biochim Biophys Acta 1818:1318–1331. doi:10.1016/j.bbamem.2012.01.022
Sikora CW, Turner RJ (2005) Investigation of ligand binding to the multidrug resistance protein EmrE by isothermal titration calorimetry. Biophys J 88:475–482. doi:10.1529/biophysj.104.049247
Soskine M, Steiner-Mordoch S, Schuldiner S (2002) Crosslinking of membrane-embedded cysteines reveals contact points in the EmrE oligomer. Proc Natl Acad Sci U S A 99:12043–12048. doi:10.1073/pnas.192392899
Butler PJ, Ubarretxena-Belandia I, Warne T, Tate CG (2004) The Escherichia coli multidrug transporter EmrE is a dimer in the detergent-solubilised state. J Mol Biol 340:797–808. doi:10.1016/j.jmb.2004.05.014
Rotem D, Sal-man N, Schuldiner S (2001) In vitro monomer swapping in EmrE, a multidrug transporter from Escherichia coli, reveals that the oligomer is the functional unit. J Biol Chem 276:48243–48249. doi:10.1074/jbc.M108229200
Torres J, Arkin IT (2000) Recursive use of evolutionary conservation data in molecular modeling of membrane proteins a model of the multidrug H+ antiporter EmrE. Eur J Biochem 267:3422–3431. doi:10.1046/j.1432-1327.2000.01324.x
Ubarretxena-Belandia I, Tate CG (2004) New insights into the structure and oligomeric state of the bacterial multidrug transporter EmrE: an unusual asymmetric homo-dimer. FEBS Lett 564:234–238. doi:10.1016/S0014-5793(04)00228-5
Schuldiner S (2009) EmrE, a model for studying evolution and mechanism of ion-coupled transporters. Biochim Biophys Acta 1794:748–762. doi:10.1016/j.bbapap.2008.12.018
Bellmann-Sickert K, Stone TA, Poulsen BE, Deber CM (2015) Efflux by small multidrug resistance proteins is inhibited by membrane-interactive helix-stapled peptides. J Biol Chem 290:1752–1759. doi:10.1074/jbc.M114.616185
Elbaz Y, Salomon T, Schuldiner S (2008) Identification of a glycine motif required for packing in EmrE, a multidrug transporter from Escherichia coli. J Biol Chem 283:12276–12283. doi:10.1074/jbc.M710338200
Miller D, Charalambous K, Rotem D, Schuldiner S, Curnow P, Booth PJ (2009) In vitro unfolding and refolding of the small multidrug transporter EmrE. J Mol Biol 393:815–832. doi:10.1016/j.jmb.2009.08.039
Winstone TL, Duncalf KA, Turner RJ (2002) Optimization of expression and the purification by organic extraction of the integral membrane protein EmrE. Protein Expr Purif 26:111–121. doi:10.1016/S1046-5928(02)00525-9
Curnow P, Lorch M, Charalambous K, Booth PJ (2004) The reconstitution and activity of the small multidrug transporter EmrE is modulated by non-bilayer lipid composition. J Mol Biol 343:213–222. doi:10.1016/j.jmb.2004.08.032
Elbaz Y, Steiner-Mordoch S, Danieli T, Schuldiner S (2004) In vitro synthesis of fully functional EmrE, a multidrug transporter, and study of its oligomeric state. Proc Natl Acad Sci U S A 101:1519–1524. doi:10.1073/pnas.0306533101
Miller D, Booth PJ (2009) The use of isothermal titration calorimetry to study multidrug transport proteins in liposomes. Methods Mol Biol 606:233–245. doi:10.1007/978-1-60761-447-0_17
Rotem D, Schuldiner S (2004) EmrE, a multidrug transporter from Escherichia coli, transports monovalent and divalent substrates with the same stoichiometry. J Biol Chem 279:48787–48793. doi:10.1074/jbc.M408187200
Vitrac H, Bogdanov M, Dowhan W (2013) In vitro reconstitution of lipid-dependent dual topology and postassembly topological switching of a membrane protein. Proc Natl Acad Sci U S A 110:9338–9343. doi:10.1073/pnas.1304375110
Dowhan W, Mileykovskaya E, Bogdanov M (2004) Diversity and versatility of lipid-protein interactions revealed by molecular genetic approaches. Biochim Biophys Acta 1666:19–39. doi:10.1016/j.bbamem.2004.04.010
Bogdanov M, Mileykovskaya E, Dowhan W (2008) Lipids in the assembly of membrane proteins and organization of protein supercomplexes: implications for lipid-linked disorders. Subcell Biochem 49:197–239. doi:10.1007/978-1-4020-8831-5_8
Epand RM, Epand RF (2009) Lipid domains in bacterial membranes and the action of antimicrobial agents. Biochim Biophys Acta 1788:289–294. doi:10.1016/j.bbamem.2008.08.023
Bay DC, Booth SC, Turner RJ (2015) Respiration and ecological niche influence bacterial membrane lipid compositions. Environ Microbiol 17:1777–1793. doi:10.1111/1462-2920.12637
Nathoo S, Litzenberger JK, Bay DC, Turner RJ, Prenner EJ (2013) Visualizing a multidrug resistance protein, EmrE, with major bacterial lipids using Brewster angle microscopy. Chem Phys Lipids 167–168:33–42. doi:10.1016/j.chemphyslip.2013.01.007
Groger T, Nathoo S, Ku T, Sikora C, Turner RJ, Prenner EJ (2012) Real-time imaging of lipid domains and distinct coexisting membrane protein clusters. Chem Phys Lipids 165:216–224. doi:10.1016/j.chemphyslip.2011.12.012
Bay DC, Turner RJ (2013) Membrane composition influences the topology bias of bacterial integral membrane proteins. Biochim Biophys Acta 1828:260–270. doi:10.1016/j.bbamem.2012.09.003
Gutman N, Steiner-Mordoch S, Schuldiner S (2003) An amino acid cluster around the essential Glu-14 is part of the substrate- and proton-binding domain of EmrE, a multidrug transporter from Escherichia coli. J Biol Chem 278:16082–16087. doi:10.1074/jbc.M213120200
Elbaz Y, Tayer N, Steinfels E, Steiner-Mordoch S, Schuldiner S (2005) Substrate-induced tryptophan fluorescence changes in EmrE, the smallest ion-coupled multidrug transporter. Biochemistry 44:7369–7377. doi:10.1021/bi050356t
Rotem D, Steiner-Mordoch S, Schuldiner S (2006) Identification of tyrosine residues critical for the function of an ion-coupled multidrug transporter. J Biol Chem 281:18715–18722. doi:10.1074/jbc.M602088200
Sharoni M, Steiner-Mordoch S, Schuldiner S (2005) Exploring the binding domain of EmrE, the smallest multidrug transporter. J Biol Chem 280:32849–32855. doi:10.1074/jbc.M504910200
Mordoch SS, Granot D, Lebendiker M, Schuldiner S (1999) Scanning cysteine accessibility of EmrE, an H+-coupled multidrug transporter from Escherichia coli, reveals a hydrophobic pathway for solutes. J Biol Chem 274:19480–19486. doi:10.1074/jbc.274.27.19480
Wang J, Rath A, Deber CM (2014) Functional response of the small multidrug resistance protein EmrE to mutations in transmembrane helix 2. FEBS Lett 588:3720–3725. doi:10.1016/j.febslet.2014.08.018
Brill S, Sade-Falk O, Elbaz-Alon Y, Schuldiner S (2015) Specificity determinants in small multidrug transporters. J Mol Biol 427:468–477. doi:10.1016/j.jmb.2014.11.015
Soskine M, Adam Y, Schuldiner S (2004) Direct evidence for substrate-induced proton release in detergent-solubilized EmrE, a multidrug transporter. J Biol Chem 279:9951–9955. doi:10.1074/jbc.M312853200
Yerushalmi H, Schuldiner S (2000) A model for coupling of H+ and substrate fluxes based on “time-sharing” of a common binding site. Biochemistry 39:14711–14719. doi:10.1021/bi001892i
Yerushalmi H, Mordoch SS, Schuldiner S (2001) A single carboxyl mutant of the multidrug transporter EmrE is fully functional. J Biol Chem 276:12744–12748. doi:10.1074/jbc.M010979200
Bay DC, Budiman RA, Nieh MP, Turner RJ (2010) Multimeric forms of the small multidrug resistance protein EmrE in anionic detergent. Biochim Biophys Acta 1798:526–535. doi:10.1016/j.bbamem.2009.12.017
Weinglass AB, Soskine M, Vazquez-Ibar JL, Whitelegge JP, Faull KF, Kaback HR, Schuldiner S (2005) Exploring the role of a unique carboxyl residue in EmrE by mass spectrometry. J Biol Chem 280:7487–7492. doi:10.1074/jbc.M413555200
Morrison EA, Robinson AE, Liu Y, Henzler-Wildman KA (2015) Asymmetric protonation of EmrE. J Gen Physiol 146:445–461. doi:10.1085/jgp.201511404
Gayen A, Leninger M, Traaseth NJ (2016) Protonation of a glutamate residue modulates the dynamics of the drug transporter EmrE. Nat Chem Biol 12:141–145. doi:10.1038/nchembio.1999
Dastvan R, Fischer AW, Mishra S, Meiler J, McHaourab HS (2016) Protonation-dependent conformational dynamics of the multidrug transporter EmrE. Proc Natl Acad Sci U S A 113:1220–1225. doi:10.1073/pnas.1520431113
Melchior DL, Brill S, Wright GE, Schuldiner S (2015) A liposomal method for evaluation of inhibitors of H+-coupled multidrug transporters. J Pharmacol Toxicol Methods 77:53–57. doi:10.1016/j.vascn.2015.09.007
Nikaido H, Takatsuka Y (2009) Mechanisms of RND multidrug efflux pumps. Biochim Biophys Acta 1794:769–781. doi:10.1016/j.bbapap.2008.10.004
Yan N (2013) Structural advances for the major facilitator superfamily (MFS) transporters. Trends Biochem Sci 38:151–159. doi:10.1016/j.tibs.2013.01.003
Sulavik MC, Houseweart C, Cramer C, Jiwani N, Murgolo N, Greene J, DiDomenico B, Shaw KJ et al (2001) Antibiotic susceptibility profiles of Escherichia coli strains lacking multidrug efflux pump genes. Antimicrob Agents Chemother 45:1126–1136. doi:10.1128/AAC.45.4.1126-1136.2001
Tal N, Schuldiner S (2009) A coordinated network of transporters with overlapping specificities provides a robust survival strategy. Proc Natl Acad Sci U S A 106:9051–9056. doi:10.1073/pnas.0902400106
Li X-Z, Zhang L, Nikaido H (2004) Efflux pump-mediated intrinsic drug resistance in Mycobacterium smegmatis. Antimicrob Agents Chemother 48:2415–2423. doi:10.1128/AAC.48.7.2415-2423.2004
Brill S, Falk OS, Schuldiner S (2012) Transforming a drug/H+ antiporter into a polyamine importer by a single mutation. Proc Natl Acad Sci U S A 109:16894–16899. doi:10.1073/pnas.1211831109
Gaze WH, Abdouslam N, Hawkey PM, Wellington EM (2005) Incidence of class 1 integrons in a quaternary ammonium compound-polluted environment. Antimicrob Agents Chemother 49:1802–1807. doi:10.1128/AAC.49.5.1802-1807.2005
Wright MS, Baker-Austin C, Lindell AH, Stepanauskas R, Stokes HW, McArthur JV (2008) Influence of industrial contamination on mobile genetic elements: class 1 integron abundance and gene cassette structure in aquatic bacterial communities. ISME J 2:417–428. doi:10.1038/ismej.2008.8
Nandi S, Maurer JJ, Hofacre C, Summers AO (2004) Gram-positive bacteria are a major reservoir of class 1 antibiotic resistance integrons in poultry litter. Proc Natl Acad Sci U S A 101:7118–7122. doi:10.1073/pnas.0306466101
Rosser SJ, Young HK (1999) Identification and characterization of class 1 integrons in bacteria from an aquatic environment. J Antimicrob Chemother 44:11–18. doi:10.1093/jac/44.1.11
Matsumura K, Furukawa S, Ogihara H, Morinaga Y (2011) Roles of multidrug efflux pumps on the biofilm formation of Escherichia coli K-12. Biocontrol Sci 16:69–72. doi:10.4265/bio.16.69
Kvist M, Hancock V, Klemm P (2008) Inactivation of efflux pumps abolishes bacterial biofilm formation. Appl Environ Microbiol 74:7376–7382. doi:10.1128/AEM.01310-08
Piddock LJ (2006) Multidrug-resistance efflux pumps – not just for resistance. Nat Rev Microbiol 4:629–636. doi:10.1038/nrmicro1464
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2016 Springer International Publishing Switzerland
About this chapter
Cite this chapter
Bay, D.C., Turner, R.J. (2016). Small Multidrug Resistance Efflux Pumps. In: Li, XZ., Elkins, C., Zgurskaya, H. (eds) Efflux-Mediated Antimicrobial Resistance in Bacteria. Adis, Cham. https://doi.org/10.1007/978-3-319-39658-3_3
Download citation
DOI: https://doi.org/10.1007/978-3-319-39658-3_3
Published:
Publisher Name: Adis, Cham
Print ISBN: 978-3-319-39656-9
Online ISBN: 978-3-319-39658-3
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)