Abstract
This chapter deals with the structure-function relationships, genetics, gene regulation systems, and clinical/biologic significance of efflux pumps expressed by the sexually transmitted human pathogen Neisseria gonorrhoeae. The overarching theme emphasized herein is that bacterial efflux pumps contribute not only to the ability of N. gonorrhoeae to evade many antibiotics in current or past treatment regimens for gonorrhea but they also help this pathogen to evade antimicrobials that contribute to innate host defense during infection. Accordingly, gonococcal drug efflux pumps should be viewed not only in the context of their capacity to negatively impact antimicrobial therapies but in the larger picture as virulence factors that promote survival of N. gonorrhoeae during infection. Based on this hypothesis, we posit that strategies that cripple gonococcal efflux pump activities may prove useful in the design of new therapies, which is of special importance in this era when antibiotic-based treatment options for gonorrhea are dwindling due to mechanisms of bacterial resistance that include the action of drug efflux pumps.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Neisseria gonorrhoeae
- Antimicrobial resistance
- Efflux pumps
- Regulation
- Structure
- Virulence
- Vaccine
- MtrCDE
- MtrF
- FarAB
- MacAB
- NorM
- MtrA
- MtrR
- MpeR
1 Introduction
Neisseria gonorrhoeae is a strictly human, Gram-negative pathogen that is typically transmitted by sexual contact and causes the disease termed gonorrhea also known as “the clap,” which is a slang term thought to be derived from the name of the district Les Clapiers in Paris, France, that housed prostitutes in the late fourteenth century [1]. Gonorrhea typically presents as urethritis in men and cervicitis in women, but rectal and pharyngeal infections also occur in both sexes; the spectrum of clinical manifestations of gonorrhea has been reviewed recently [1]. If gonorrhea is left undetected, untreated, and inappropriately treated or fails to respond to conventional antibiotic treatment due to resistance (an important contemporary concern), the disease can ascend to the upper genital tract where it can cause severe reproductive complications (especially for women) such as endometritis, pelvic inflammatory disease, penile edema, and epididymitis. These complications can have a significant, devastating impact on general and reproductive health, including infertility, of infected individuals. Moreover, involuntary loss of life due to gonorrhea-associated ectopic pregnancy is not uncommon especially in the developing world. On rare occasions, gonococci can enter the bloodstream causing disseminated gonococcal infection and triggering damage to organs and joints distant from the site of infection. Conjunctivitis, more frequent in newborns (ophthalmia neonatorum) than adults, can result in blindness; transmission to the newborn can occur during vaginal delivery when the mother has cervical gonorrhea. It is also important to note that gonorrhea can increase transmission and host susceptibility to other sexually transmitted infections (STIs) including HIV, which emphasizes the importance of effective antimicrobial treatment regimens for gonorrhea. Thus, to prevent these medical issues and complications, it is imperative that effective and accessible antibiotic treatment and prevention regimens exist.
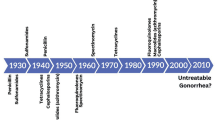
Historical writings suggest that gonorrhea has afflicted humans for thousands of years [1]. In this respect, a passage from the Book of Leviticus of the Old Testament warning women to avoid men with discharges and the writings of the second-century Greek physician Galen that described purulent exudates observed often in men with what is now considered as gonorrhea (“flow of seed”) suggest that this is an ancient disease; a more in-depth and recent historical review of gonorrhea can be found in a recent review by Unemo and Shafer [1]. Presently, gonorrhea is without question a major public health problem as emphasized by the estimated 78 million infections worldwide per year and the emergence of strains resistant to first-line antibiotics [1–3]. The looming public health disaster of gonococcal resistance to current first-line antibiotics, combined with retained resistance to previous first-line antibiotics, and the lack of new, effective antibiotics that can serve as replacement therapies has been recently emphasized [3]. In this context, it is important to emphasize that in the absence of a vaccine, effective antibiotic therapy is crucial for reducing spread of gonorrhea in the community.
As a strict human pathogen, gonococci have over the millennia found ways to resist the multitude of innate and adaptive immune responses that occur during infection (summarized in [4]). Pathogens, like gonococci, encounter numerous host biocides (e.g., antimicrobial peptides, toxic-free fatty acids, bile salts, and progesterone), and their capacity to resist these antimicrobials likely promotes their survival and proliferation during infection. It is now evident that many of these antimicrobials are substrates for gonococcal multidrug transporters (see Sect. 17.7 below). Moreover, strong evidence exists that implicates gonococcal efflux pumps in exporting important antibiotics (e.g., β-lactams and macrolides) that are currently used in the clinic (see Sect. 17.6 [5]). In sum, studies on efflux pumps produced by gonococci provide a unique opportunity to understand the role of bacterial drug efflux pumps in the overall pathogenic mechanisms of bacteria during infection and how they also contribute to resistance to antibiotics used to treat infection. This review concentrates on the genetics, structure-function relationships, gene regulation systems, and overall significance of gonococcal drug efflux pumps.
2 Types and Substrate Profiles of Gonococcal Drug Efflux Pumps
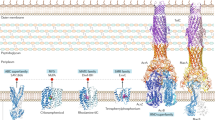
In contrast to other bacteria that express a wide number and type of drug efflux pumps (the reader is directed to other chapters in this book for the vast repertoire of efflux pumps produced by bacteria), gonococci produce much fewer drug efflux pumps (Fig. 17.1; summarized in [6]). These pumps belong to the resistance-nodulation-cell division (RND) superfamily (MtrCDE), the major facilitator superfamily (MFS) (FarAB-MtrE), the ATP-binding cassette (ABC) transporter superfamily (MacAB-MtrE), and the multidrug toxic compound extrusion (MATE) family (NorM); see other reviews [1, 6] for a description of the discovery of these efflux pumps. Recently, a fifth gonococcal efflux pump, the MtrF protein first described by Veal and Shafer [7], which belongs to the AbgT family of transporters that typically behave as importers, was designated as an efflux pump by virtue of its ability to export sulfonamides [8]. Finally, some (rare) clinical isolates have been reported to harbor the mef gene [9], which encodes a pump that exports macrolides and appears to have been acquired from Acinetobacter junii.
Membrane organization of drug efflux pumps in gonococci. The proposed membrane organization and class of the known drug efflux pumps common to gonococcal strains (NorM, FarAB-MtrE, MacAB-MtrE, MtrF, and MtrCDE) as well as their substrates are depicted. MtrE most likely acts as an outer membrane channel protein for three of the pumps. MtrF possibly also acts as an accessory protein for the MtrCDE efflux pump. The crystallized NorM (PDB code: 5C6P), MtrF (4R1I), MtrD (2DHH), and MtrE (4MT0) from gonococci are shown. For MacA, MacB, FarA, FarB, and MtrC, the crystallized homologues MacA (3FPP), MacB (2HYD), EmrD (2GFP), EmrA (4TKO), and MexA (1VF7) are shown. OM outer membrane, PS periplasmic space, IM inner membrane. The ratio of monomeric unit number for the MtrC, MtrD, and MtrE proteins are 2:1:1, respectively, as defined earlier [25]
With respect to efflux of antibiotics, it is now recognized that the MtrCDE efflux pump can export structurally diverse hydrophobic antibiotics, β-lactams (including penicillin, nafcillin, and extended-spectrum cephalosporins) and tetracycline [5, 10–12], while the NorM pump exports quinolones [13] and the MacAB-MtrE pump exports macrolides [14]. The newly described MtrF pump recognizes sulfonamides and is proposed to be used by gonococci to export antimetabolites that accumulate within the cytosol [8]. The MtrCDE and FarAB-MtrE efflux pumps also recognize host (human and mouse)-derived antimicrobials, including cationic antimicrobial peptides (such as human cathelicidin LL-37 and murine cathelin-related antimicrobial peptide CRAMP), bile salts and progesterone (MtrCDE) [15, 16], and long-chain fatty acids (FarAB-MtrE) [17] that often bathe mucosal surfaces infected by gonococci. A functionally intact MtrCDE pump, but not FarAB-MtrE pump, is required for long-term colonization by gonococci of the lower genital tract of female mice [18], suggesting that its capacity to export host antimicrobials that pass the outer membrane helps this pathogen to establish an infection. Importantly, overproduction of the MtrCDE pump due to cis- or trans-acting regulatory mutations (see below) cannot only increase the resistance to antimicrobials but can also increase gonococcal fitness during experimental infection of the lower genital tract of female mice [16, 19]. In total, the cumulative evidence supports the notion that the MtrCDE efflux pump is a virulence factor. Interestingly, this pump also recognizes nonionic detergents such as the spermicide nonoxynol-9, and its use over the past six decades may have inadvertently selected for gonococcal mutants that overproduce this pump [20]. The MacAB-MtrE efflux pump exports macrolides (e.g., azithromycin and erythromycin) [14]. The NorM efflux pump behaves as a Na+-dependent transporter [21] and exports compounds harboring a quaternary ammonium on an aromatic ring (e.g., acriflavine, berberine, ethidium bromide, and 2-N-methylellipticinium) as well as ciprofloxacin and norfloxacin to the periplasmic space [13]. It is unknown if these latter two efflux pumps recognize and export host-derived antimicrobials.
3 Structural Features of Gonococcal Drug Efflux Pumps
Structural data on proteins that function as drug transporters or critical components of efflux pumps contribute to basic knowledge that can facilitate or advance studies on new drug development (e.g., efflux pump inhibitors (EPIs)) or alternative therapies and prevention strategies (e.g., vaccine development). With respect to gonococci, critical structural information is now available regarding two proteins (MtrD and MtrE) [22, 23] of the MtrCDE efflux pump as well as the MtrF inner membrane protein; as described above, MtrF appears to be an independent efflux pump that recognizes sulfonamides [8] and, possibly, acts as an accessory protein of the MtrCDE pump [7]. It is important to stress that, while structures have been solved for proteins that are highly similar to MtrD, MtrE, and MtrF, the information for the gonococcal proteins emphasizes that “one structure does not fit all” and that critical differences can exist between homologous proteins. These differences, as well as the potential ramifications of such, are discussed below.
3.1 Crystal Structure of the MtrD Inner Membrane Efflux Pump Transporter
The gonococcal MtrD inner membrane transporter protein of strain PID332, which is 11 amino acids shorter at the C-terminus in comparison with FA19 MtrD, was crystallized using vapor diffusion [24]. This membrane protein exists as a trimer linked to a hexamer of the periplasmic adapter MtrC, which is in turn linked to a trimer of the MtrE outer membrane channel protein [25]. At the amino acid sequence and substrate recognition levels, MtrD shares many, but not all, features of other transporters in the RND efflux pump family produced by Gram-negative bacteria.
The crystal structure of the full-length PID 332 MtrD multidrug efflux pump transporter protein (114 kDa) was determined to a resolution of 3.53 Å (Protein Data Bank [PDB] code: 4MT1) [22]. The final model included 97 % of the amino acids (residues 2–493 and 508–1,040) (Fig. 17.2a). The structure of MtrD is closer to the conformation of the “access” protomer of AcrB of Escherichia coli [26]. However, superimposition of these two structures results in a high root-mean-square deviation (RMSD) of 7.6 Å over 1,000 Cα atoms, suggesting that there are significant differences between these two transporters. MtrD assembles as a 125-Å-long and 95-Å-wide homotrimer (Fig. 17.2b). Each protomer comprises 12 transmembrane helices (TM1-TM12). Like other RND transporters, the N-terminal (TM1-TM6) and C-terminal (TM7-TM12) halves of MtrD are related by a pseudo-twofold symmetry. A large periplasmic domain is created by two extensive periplasmic loops connecting TM1 with TM2 and TM7 with TM8, respectively. As in AcrB [26–30] and MexB of Pseudomonas aeruginosa [31], this periplasmic domain can be divided into six sub-domains: PN1, PN2, PC1, PC2, DN, and DC. Sub-domains PN1, PN2, PC1, and PC2 form the pore domain, with PN1 making up the central pore and stabilizing the trimeric organization. However, sub-domains DN and DC contribute to form the docking domain, presumably interacting with the outer membrane channel MtrE (structural information for MtrE is described below). The trimeric MtrD structure suggests that sub-domains PN2, PC1, and PC2 are located at the outermost core of the periplasmic domain, facing the periplasm. Sub-domains PC1 and PC2 also form an external cleft, and this cleft is open in the MtrD structure (Fig. 17.2). Based on the co-crystal structure of CusBA [32, 33] of the CusCBA tripartite efflux complex of E. coli [34–39], the upper regions of PN2, PC1, PC2, and sub-domains DN and DC should directly interact with the MtrC membrane fusion protein to form a functional adaptor-transporter complex.
Structure of the MtrD inner membrane transporter. (a) Ribbon diagram of a protomer of MtrD viewed in the membrane plane. The molecule is colored using a rainbow gradient from the N-terminus (blue) to the C-terminus (red). Sub-domains DN, DC, PN2, PC1, and PC2 are labeled. The location of PN1 is behind PN2, PC1, and PC2. (b) Ribbon diagram of the MtrD trimer viewed in the membrane plane. Each subunit of MtrD is labeled with a different color. Residues 917–927 (only found in MtrD) forming the upper portion of TM9 and the loop connecting TM9 and TM10 are in blue color (This figure has been reproduced from [22])
Structures of AcrB in complex with a variety of substrates [26, 29, 30] have identified that the periplasmic cleft of the pump forms several mini-binding pockets within the extensive, large periplasmic multidrug binding site. This site is supposed to play a predominant role in the selection of drugs for export. Protein sequence alignment reveals that many of the amino acids forming the large periplasmic binding site of AcrB are conserved with MexB and MtrD, indicating that these three multidrug efflux pumps may have a similar substrate binding profile for drug recognition. These conserved amino acids in MtrD are made up of several charged and polar residues, such as Ser79, Ser134, Arg174, Asp272, Glu669, and Arg714, and aromatic residues, such as phenylalanine at 136, 176, 610, 612, and 623. In addition, a flexible loop is found inside the large periplasmic cleft, which forms the multidrug binding site of the pump. This flexible loop is located deep inside the cleft between sub-domains PC1 and PC2, composed of residues 608–619, and should correspond to the Phe-617 loop [29] in AcrB. The loop is highly conserved among MtrD, AcrB, and MexB. It is expected that this flexible loop is important for drug recognition and extrusion. There is a chance that this loop may shift positions during the course of the extrusion process to facilitate drug export.
Perhaps the most interesting secondary structural feature appears in TM9 of the MtrD pump [22]. In contrast to other known structures of the RND transporters, MtrD contains an extended region that protrudes into the periplasm and contributes part of the periplasmic domain (Fig. 17.2). This region (residues 917–927) comprises an α-helix extending from the upper portion of TM9 and also to the loop connecting TM9 and TM10. Protein sequence alignment suggests that these extra residues are only found in MtrD, but not in other homologous RND proteins. Therefore, this fragment should represent a unique feature of this pump that cannot be found in other RND pumps. TM9 is distinct in that it is not vertically oriented. Instead, it is inclined from the horizontal membrane plane by 54°. The spatial arrangement between the extra elongated helix and loop (upper portion of TM9) and the periplasmic cleft formed between PC1 and PC2 suggests that these extra structural features may help the pump to transport its substrates more effectively from the outer leaflet of the inner membrane to the multidrug binding site at the periplasmic domain.
Drug export by RND multidrug transporters is proton motive force dependent [40]. Based on the crystal structure of MtrD, it is expected that the charged residues Asp405 and Asp406 of TM4 and Lys948 of TM10 are important for forming the proton-relay network of the pump. These residues are supposed to undergo protonation and deprotonation within the transport cycle. The involvement of these charged amino acids in proton translocation was supported by a previous study showing mutations of these residues inhibit proton translocation [41]. In turn, the MtrE outer membrane channel protein is blocked and unable to dissociate from the MtrCDE tripartite efflux complex [41].
3.2 Crystal Structure of the MtrE Outer Membrane Channel Protein
The gonococcal MtrE protein serves as the outer membrane protein channel for the MtrCDE efflux pump, and most likely also for the MacAB and FarAB efflux pumps, that facilitates exit of antimicrobial pump substrates from the bacterial cell (Fig. 17.1; [42]). In this respect, its function in the trimer state resembles that of other outer membrane channel proteins (e.g., OprM of P. aeruginosa and TolC of E. coli) of RND efflux pumps possessed by Gram-negative bacteria. However, important differences between MtrE vs. OprM/TolC exist, and these were illuminated by structural work [23] and are summarized below.
The gonococcal MtrE protein of strain FA136 was used for crystallization using vapor diffusion. Protein sequence alignment suggests that FA136 MtrE and FA19 MtrE are nearly identical, except for the amino acid residues between 178 and 197. In FA136 MtrE, these residues are NAVRIAVQGRRDFRRRPAPA. The corresponding residues in FA19 MtrE are KLSELRYKAGVISAVALRQQ. As mentioned in the following paragraph, these residues form a loop region between the periplasmic helices H3 and H4 of FA136 MtrE. It was later found that the protein sequence of FA136 MtrE was in error, possibly due to a DNA sequencing error when the sequence of FA136 mtrE gene was determined two decades ago. Nonetheless, the crystal structure of the FA136 MtrE outer membrane channel protein (prepared from expression of a synthetic gene based on the FA136 sequence) was determined to a resolution of 3.29 Å (PDB code: 4MT0) [23]. The final model comprises 99 % of the total amino acids (residues 1–445) (Fig. 17.3). The MtrE and OprM channels share 40 % protein sequence identity. However, superimposition of the structure of MtrE with that of OprM [41] results in an RMSD of 18.2 Å over 445 Cα atoms, suggesting a highly significant difference in the overall tertiary structures between these two channel proteins. An important insight gained from the MtrE structural data is the recognition of the presence of two, short surface-exposed domains (6 per trimer) that are antigenic and now under investigation as part of the newly rejuvenated vaccine effort (see below); these surface-exposed domains represent residues 93–98 and 300–310 [23].
Structure of the MtrE outer membrane channel protein. (a) Ribbon diagram of a protomer of MtrE viewed in the membrane plane. The molecule is colored using a rainbow gradient from the N-terminus (blue) to the C-terminus (red). (b) Ribbon diagram of the MtrE trimer viewed in the membrane plane. Each subunit of MtrE is labeled with a different color (This figure has been reproduced from [23])
Like OprM [43], TolC [44], CusC [36, 37], and CmeC [45], MtrE exists as a homotrimer that forms a ~130-Å-long α/β barrel (Fig. 17.3). Each subunit of MtrE contains four β-strands (contributing to the 12-stranded outer membrane β-barrel) and eight α-helices (forming the elongated periplasmic α-barrel): H1 (41–53), H2 (55–78), S1 (85–92), S2 (99–114), H3 (119–182), H4 (188–226), H5 (254–260), H6 (262–284), S3 (288–299), S4 (311–320), H7 (328–395), and H8 (400–435). These four β-strands (S1, S2, S3, and S4) constitute the β-barrel domain and are organized in an antiparallel fashion, spanning the outer membrane. In contrast, the elongated periplasmic tunnel of MtrE contains six α-helices. Similar to the structure of TolC [44], two long α-helices (H3 and H7) are found to extend across the entire length of the periplasmic α-helical tunnel. The α-helical tunnel of MtrE also includes two pairs of shorter α-helices (H2 and H4) and (H6 and H8). These two pairs of shorter α-helices stack end to end to form pseudo-continuous helices, which contribute coiled-coil interactions with the two long helices. The equatorial domain of MtrE is composed of two α-helices (H1, H5), and the remaining elements at this domain are mostly unstructured. The periplasmic tunnel of MtrE is ~100 Å long with an outermost diameter of ~35 Å at the tip of the tunnel.
To date, all available structures of outer membrane channel proteins, such as TolC [44], OprM [43], CusC [36, 37], and CmeC [45], indicate that the interior surfaces of these channels are highly electronegative. However, MtrE is distinct in that its internal surface does not have extensive positively or negatively charged patches. On the contrary, the charge distribution of the outside surface of MtrE is very similar to other outer membrane channels, in which the outside surfaces of all these channels have no extensive charged patches. In view of the crystal structure of MtrE, the internal surface of the protein forms a continuous channel. This channel is completely open and fully accessible through both the periplasmic end and outer membrane surface, suggesting that the MtrE channel is at its open conformational state. Most of the available structures of outer membrane channels, including TolC, OprM, CusC, and CmeC, are closed at one or both sides [42–45]. The widest section of the channel is located at the surface of the outer membrane, with the internal diameter of ~22 Å. The volume of the continuous channel formed by the internal surface of the MtrE trimer is ~45,000 Å3 [23].
The architecture of the interior of the MtrE channel is quite similar to that of TolC [42, 46]. An aspartate ring is found at the periplasmic entrance of the interior of the MtrE channel. Each protomer of MtrE contributes Asp402 and Asp405 to form two concentric circles of negative charges in the inner cavity of the trimeric MtrE channel [23]. Thus, this interior aspartate ring is composed of six aspartate residues. In TolC, the corresponding aspartate ring creates a selectivity gate for this channel and this ring can be blocked by large cations. The internal diameter of the MtrE aspartate ring is ~12 Å, which creates the narrowest region of the tunnel. It is likely that this aspartate ring is responsible for the selectivity of the channel, similar to the case of TolC [46]. Indeed, it has been demonstrated that the aspartate ring of MtrE can be blocked by the large positively charged hexamminecobalt (III) complex [41]. During the course of substrate import or export, the aspartate ring may still need to dilate more and increase its internal diameter to allow for the passage of substrates through the channel. Although the structure indicates that MtrE is capable of opening this channel by itself, it has been suggested that the dilation and constriction of the aspartate ring may be controlled by the MtrC periplasmic membrane fusion protein [25]. In addition, it has been observed that the MtrE channel is able to allow the large vancomycin molecule to enter the cell but only does so in response to the binding of the membrane fusion protein MtrC adaptor [25], presumably enhancing the degree of dilation of the MtrE channel. It appears that the opening and closing of the MtrE channel may be induced by the change in conformation of the MtrC protein, which propagates the progressive motion of the MtrD multidrug efflux pump within the transport cycle to the MtrE channel. As MtrD is a proton motive force-dependent pump, this may imply that active proton translocation within the MtrD efflux pump provides the energy to open and close the MtrE channel.
As mentioned earlier, it is likely that the sequence of the reported FA136 mtrE gene (and the originally predicted MtrE amino acid sequence) was incorrect due to a DNA sequencing error; we thank V. Bavro (University of Birmingham, United Kingdom) for bringing this to our attention. We have started crystallizing the FA19 MtrE channel protein. Hopefully, its structure will be determined and reported in the near future so that a more complete understanding of MtrE can be obtained.
3.3 Crystal Structure of MtrF: A Novel Drug Efflux Pump Protein
The MtrF transporter protein was recently implicated as a novel efflux pump protein possessed by gonococci that has the capacity to export antimetabolites and sulfonamide drugs [8]. The capacity of MtrF to export sulfonamide drugs was first realized when recombinant MtrF expressed in E. coli reduced levels of bacterial susceptibility to sulfonamides, which was verified by biochemical and genetic studies using mutant versions of the mtrF gene. At the amino acid level, the 522-amino acid MtrF protein belongs to the p-aminobenzoyl-glutamate transporter (AbgT) family that typically serve as importers [7]. MtrF shares 38 % identity with E. coli AbgT. Interestingly, AbgT has been shown to enable uptake of the folate catabolite p-aminobenzoyl-glutamate, but work with MtrF indicates that it can export the folate metabolite paraminobenzoic acid (PABA). Circumstantial genetic evidence also suggests that MtrF can serve as an accessory protein for the MtrCDE efflux system when the latter is overexpressed in strains of gonococci harboring regulatory mutations ([7]; see below).
We have determined the crystal structure of gonococcal MtrF (strain FA19) to a resolution of 3.95 Å (PDB code: 4R1I) (Fig. 17.4) [8]. Crystals of MtrF belong to space group P65. Two molecules of MtrF, which assemble as a dimer, are found in the asymmetric unit. Superimposition of these two MtrF molecules gives an RMSD of 0.5 Å over 506 Cα atoms, indicating that their conformations are nearly identical to each other. The crystal structure of the MtrF dimer reveals a bowl-shaped concave aqueous basin, approximately 75 Å tall, 80 Å wide, and 50 Å thick. The rim of the basin is as large as 45 Å in diameter and penetrates into the inner leaflet of the cytoplasmic membrane by approximately 25 Å. This deep basin probably allows aqueous solution to reach until the midpoint of the membrane bilayer.
Structure of the MtrF efflux pump. (a) Ribbon diagram of a protomer of MtrF viewed in the inner membrane. The molecule is colored using a rainbow gradient from the N-terminus (blue) to the C-terminus (red). (b) Ribbon diagram of a dimer of MtrF viewed in the inner membrane. The right subunit of the dimer is colored red, whereas the left subunit is colored green. The MtrF dimer forms a bowl-shaped structure with a concave aqueous basin facing the intracellular solution (This figure has been reproduced from [8, 48] (with permission from the publisher))
Overall, the secondary structure of the MtrF dimer is very similar to that of Alcanivorax borkumensis YdaH [47], which also belongs to the AbgT family of transporters. A pairwise superimposition of the MtrF dimer onto YdaH results in overall RMSD of approximately 4 Å. Each molecule of MtrF comprises nine transmembrane α-helices and two helical hairpins: TM1 (a (12–22) and b (26–47)), TM2 (a (78–92), b (94–112), and c (114–125)), HP1 (a (128–145) and b (147–164)), TM3 (a (168–182) and b (191–205)), TM4 (218–240), TM5 (269–292), TM6 (310–334), TM7 (a (341–353), b (356–374), and c (376–391)), HP2 (a (396–413) and b (417–434)), TM8 (a (438–451) and b (462–471)), and TM9 (480–506). In addition to HP1 and HP2, which are only long enough to span half of the membrane, the transmembrane helices TM1, TM2, TM3, TM7, and TM8 are broken into multiple segments within the membrane. These segmented loops allow the transporter to form an internal cavity within the membrane [8, 48].
Each protomer of MtrF contains a relatively small periplasmic domain. This domain is made up of two long loops formed between TMs 1 and 2 and TMs 5 and 6, respectively. Below the inner leaflet of the membrane, a small cytoplasmic domain links TMs 4 and 5 together. This domain is comprised by a relatively long random loop and helix (α1). The MtrF dimer can also be divided into inner and outer core regions. The inner core, comprising TM1, TM2, TM5, TM6, and TM7, creates a frame-like housing for the inner core and contributes to the dimerization domain. Involved in this dimerization interface are TM1b, TM2a, TM2b, TM6, TM7a, and TM7b, as well as the corresponding segments from the next subunit.
The outer core comprises TM3, TM4, TM8, TM9, HP1, and HP2. Like YdaH [47], the helices and hairpins of the outer core of MtrF form a tunnel spanning approximately from the middle of the inner membrane up to the periplasm. Interestingly, this tunnel is connected to the cytoplasm via an opening in the basin formed by the loop regions of HP1, HP2, TM3, and TM8. Importantly, several conserved residues, including Asp193, Trp420, Pro438, and Asp449, line the wall of the tunnel. It is expected that these residues may play an important role for the function of this transporter. It has also been shown that MtrF is able to export PABA and sulfonamide drugs from the bacterial cell and that the conserved residues Asp193, Trp420, Pro438, and Asp449 are important for this function [8, 48]. Accordingly, these conserved residues are critical for the function of the N. gonorrhoeae MtrF efflux pump and strongly support the idea that MtrF act as efflux pumps and participate in exporting the metabolite PABA and sulfonamide antimetabolites from the cell.
4 Gonococcal Efflux Pump Genes
Of the five efflux pumps associated with all gonococci strains thus far examined [1, 6, 8], participating proteins of three are encoded by genes organized in an operon (i.e., mtrCDE, farAB, and macAB). The multiple transferable resistance (mtr) locus, which includes mtrF, was first identified by Pan and Spratt [49] and then further elucidated by Hagman et al. [10, 24], Delahay et al. [42], and Veal and Shafer [7] by conventional cloning and sequencing technologies. The other gonococcal efflux pump systems [13, 14, 17] were identified by mining the FA1090 genome sequence that was made available online by D. Dyer and colleagues in 1998 (http://www.genome.ou.edu).
4.1 The mtrCDE Efflux Pump Operon
The genetics of the mtr system have been extensively studied for the past two decades, and it is the best understood gonococcal efflux pump system to date. Mtr has its origin from a phenotype identified by Maness and Sparling in 1973 [50] when they isolated a spontaneous gonococcal mutant that exhibited increased resistance to multiple, structurally diverse antimicrobial hydrophobic compounds. It was originally thought that the associated, but then unknown, mutation decreased cell envelope permeability by overproducing a 52-kDa outer membrane protein and increasing the degree of peptidoglycan cross-linking [51]. However, subsequent cloning/sequencing experiments in the mid-1990s [10, 49] showed that the Mtr phenotype was due to mutations within the coding region or in a promoter upstream of a gene encoding a transcriptional repressor (MtrR) in the TetR/QacR family [52]. The mtrR gene is positioned 250 base pairs (bp) upstream and transcriptionally divergent from the mtrCDE operon that encodes the tripartite MtrCDE efflux pump similar to other RND-type pumps (e.g., AcrAB-TolC) of Gram-negative bacteria [10, 24, 42, 49]. As described above, like other RND efflux pumps, the three proteins that form the pump are a cytoplasmic (inner) membrane transporter (MtrD), a membrane fusion protein (also called periplasmic adapter protein; MtrC), and an outer membrane channel protein (MtrE) (Fig. 17.1); as mentioned above, it is most likely that MtrE also serves as the outer membrane channel protein for the FarAB and MacAB efflux pumps (Fig. 17.1; [14, 17] and W. M. Shafer et al. unpublished).
Directly or indirectly, other proteins or outer membrane components also participate in efflux mediated by the MtrCDE pump. In this respect, Veal and Shafer [7] suggested that MtrF could serve as an accessory protein for high levels of antimicrobial resistance mediated by the MtrCDE efflux system. Additionally, energy supplied by the TonB-ExbBD system is needed for inducible antimicrobial resistance mediated by MtrCDE [53] and this induction process requires the participation of a transcriptional activator termed MtrA (see below). Lipooligosaccharide structure is also important in the function of the MtrCDE efflux pump in strains overexpressing mtrCDE because a deep rough lipooligosaccharide mutant expressing a core oligosaccharide that was severely truncated was unable to express high-level resistance to substrates of the pump [54].
While the mtr locus is highly conserved in gonococci, clinical isolates frequently contain mutations within the mtrR structural gene or the associated promoter that increase mtrCDE expression and resistance to antimicrobial substrates (see below). The cis- or trans-acting regulatory mutations are of clinical and biological significance since they appear to be important in the ability of gonococci to resist certain clinically useful antibiotics as well as provide a fitness advantage over wild-type gonococci (see below). Interestingly, a minority of strains, some of which also have mtrR mutations, contain small deletions in the mtrC or mtrD genes that increase gonococcal susceptibility to antimicrobials recognized by the MtrCDE efflux pump [55]. These so-called env (envelope) mutants were originally identified by the Sparling laboratory in the mid-1970s [56]. The advantage such strains might have during infection is unclear, but their ability to donate antimicrobial resistance due to mtrR mutations is of interest since such genetic exchange may help in the emergence of natural gonococcal variants with decreased susceptibility to efflux pump substrates during infection.
4.2 The mtrF Gene
The mtrF gene was discovered by Veal and Shafer [7] during a study of a gonococcal strain that displayed an env phenotype (hypersusceptibility to antimicrobials recognized by the MtrCDE efflux pump) yet contained a wild-type mtrCDE operon. Subsequent cloning and sequencing efforts showed that the clinical isolate contained a small deletion in an open reading frame downstream of mtrR that would encode a protein within the AbgT family of transporters [8]; the structural aspects of MtrF and AbgT proteins are described above. Interestingly, loss of mtrF seemed to have an impact on gonococcal resistance to antimicrobials recognized by the MtrCDE efflux pump only when mtrCDE was overexpressed due to cis-acting mutations (described below) and the gonococcal strain had a mutant version of the PorB1b porin as opposed to wild-type PorB1b or PorB1a [7]; the latter phenomenon highlights the interplay between membrane components for gonococcal resistance to antimicrobials [57]. In this context, amino acid replacements in PorB1b at position 120 alone (Gly120Lys) or positions 120 and 121 (Gly120Asp/Ala121Asp) are frequently observed in gonococcal strains expressing decreased susceptibility to β-lactam antibiotics [1, 57]. Thus, it was proposed that MtrF serves as an accessory protein used by the MtrCDE efflux pump when gonococci are confronted by high levels of antimicrobials [7]. While this original hypothesis may still be valid, more recent work [8] implicates MtrF as an independent efflux pump that exports sulfonamide antimetabolite drugs. As a member of the AbgT family of proteins that normally import substrates, the illumination of MtrF efflux activity defines a new family of bacterial efflux pumps; continued work is necessary to better understand its roles in gonococcal physiology and metabolism.
4.3 The farAB Efflux Pump Operon
The farAB operon in gonococci was identified by mining the FA1090 genome sequence (http://www.genome.ou.edu) for open reading frames that would encode drug efflux pumps similar to those possessed by other Gram-negative bacteria [17]; the farAB gene products are similar to the emrAB-encoding efflux pump system of E. coli. Expression of farAB is regulated by both cis- and trans-acting systems (see below). As mentioned above, the FarAB efflux pump in gonococci most likely uses MtrE as its outer membrane protein channel (Fig. 17.1) to export long-chain fatty acids such as palmitic and oleic acid. Fecal-derived fatty acids can have potent anti-gonococcal activity under laboratory conditions [58], and their efflux by the FarAB-MtrE pump may help gonococci survive during rectal infections, which are frequently asymptomatic [1].
4.4 The macAB Efflux Pump Operon
The macA and macB genes are organized in an operon, and their predicted products are similar to the MacA and MacB proteins produced by other Gram-negative bacteria [14]. The MacAB efflux pump most likely uses MtrE as its outer membrane protein channel, belongs to the ABC transporter superfamily, and exports macrolides and, at least to some extent, possibly also β-lactams and the fluoroketolide solithromycin (Fig. 17.1; [5]). The promoter that drives expression of macAB in strain FA19 has a point mutation in the –10 hexamer sequence that dampens expression of this operon [14]; this mutation has been observed in other gonococcal strains (Reimche et al. unpublished observations). Recent evidence (Kandler et al. manuscript in preparation) suggests that macAB expression is enhanced by a response regulator (MisR) of a two-component regulatory system (MisRS).
4.5 The norM Efflux Pump Gene
The norM gene encodes the NorM efflux pump [13], which belongs to the MATE family and exports compounds harboring a quaternary ammonium on an aromatic ring, quinolones, and, at least to some extent, possibly also β-lactams and solithromycin [5]. The norM gene is located upstream of open reading frames that have been provisionally annotated (http://www.genome.ou.edu) as encoding the MurB enzyme involved in N-acetylmuramic acid biosynthesis and a TetR-like repressor. Additional work is needed, however, to confirm these functions and if the three genes in this region are co-transcribed by a common promoter. Point mutations within the norM ribosome binding site and a putative −35 promoter hexamer sequence have been identified, and these likely modulate expression of norM and, as a consequence, levels of NorM [13].
5 Transcriptional Regulation of Gonococcal Efflux Pump Genes
Discoveries of bacterial efflux pumps were often made prior to the availability of whole genome sequences and were facilitated by the isolation of mutants that expressed decreased susceptibility to antimicrobials [52, 59]. These resistance-conferring mutations frequently mapped to a gene that encoded a DNA-binding protein that would normally dampen expression of a closely linked gene or operon encoding efflux pump proteins. As an example of this and with respect to gonococci, Pan and Spratt [49] discovered the mtr locus in strain CH95 by identifying a mutation in mtrR, which encodes a transcriptional repressor (MtrR) of the mtrCDE efflux pump-encoding operon (see below). It is now recognized that, in addition to mutations that impact the activity of DNA-binding proteins, cis-acting mutations located within promoter sequences can have stronger influences on efflux pump gene expression and levels of bacterial susceptibility to antimicrobials recognized by the cognate efflux pump. Against this background, the complexities of cis- and trans-acting transcriptional factors that modulate gonococcal efflux pump gene expression and bacterial susceptibility to antimicrobials are described below.
5.1 Cis-Acting Factors that Regulate Efflux Pump Genes
Cis-acting regulatory mutations can have a profound impact on the expression of gonococcal genes encoding efflux pumps and can be responsible for levels of antimicrobial resistance due to export mechanisms; the most comprehensively studied cis-acting regulatory system involves expression of the mtrCDE-encoded pump (Fig. 17.5). In this respect, point mutations, deletions, or insertions in the nucleotide sequences between mtrR and mtrCDE can provide gonococci with higher levels of antimicrobial resistance than mutations within the mtrR-coding region. For instance, a single-bp deletion within the 13-bp inverted repeat element localized in the mtrR promoter (Fig. 17.5) can significantly enhance transcription of mtrCDE [10, 60]. While not all macrolide-resistant clinical gonococcal isolates have this bp deletion [61], it was frequently observed in a panel of strains isolated in Seattle, USA, obtained from men who have sex with men during an outbreak of macrolide-resistant gonorrhea [62]. Some clinical isolates have a dinucleotide insertion within this inverted repeat [63]. In either case, the optimal 17-bp spacing between the −10 and −35 elements is disrupted, and this significantly reduces mtrR transcription to nearly undetectable levels. This mechanism, however, cannot be the sole reason why such strains express high-level antimicrobial resistance since their level of mtrCDE expression is greater than strains with mtrR loss-of-function or null mutations [10]. Instead, because the promoters for mtrR and mtrCDE transcription overlap and are divergent, it is more likely that the mutations enhance RNA polymerase interactions with the mtrCDE promoter.
Regulatory features of the mtr locus. Shown are the cis- and trans-acting regulatory elements that control expression of the mtrCDE operon as well as “off-target” genes (see text for details). Negative regulation of genes by DNA-binding proteins is shown by the barred lines, while transcriptional activation of genes by proteins is shown with arrows. Sites of binding by MtrR, MtrA, and MpeR are shown. The main mutations impacting promoters are the single-base pair (bp) deletion in the mtrR promoter region, which is designated by the larger font T nucleotide as is the mutation that generates the novel mtr 120 promoter. The nucleotide sequence shown represents the mtrCDE coding strand; the translational start codons for mtrR and mtrC are shown at the ends of the sequence. The 13-bp inverted sequence within the mtrR promoter is shown in italics (This figure has been modified from [1])
A limited number of gonococcal strains (e.g., MS11 and WHO L) have a point mutation (C→T) located 120 nucleotides upstream of the mtrC translational start codon (mtr 120), changing the sequence at this region from TATAAC to TATAAT [19, 64] and thereby generating a consensus −10 element (Fig. 17.5) [65]. This new −10 element acts as a stronger promoter (mtr 120) for mtrCDE transcription than the wild-type promoter, and its use results in high levels of mtrCDE expression and resistance to hydrophobic agents, as well as enhanced in vivo fitness [19]; importantly, this new promoter is outside of the control by MtrR, which exerts repressive activity on the wild-type promoter. The identification of this novel promoter that enhanced transcription of mtrCDE independently of MtrR repression allowed Ohneck et al. [66] to study the genome-wide transcriptional influence that might result when the MtrCDE pump is produced at elevated levels. Such a study had not been possible previously since all other mutants that overexpressed mtrCDE did so in a background where MtrR would be absent making it difficult to know if any observed transcriptional changes were influenced by this transcriptional regulator. In brief, use of the mtr 120 promoter (Fig. 17.5) in an otherwise wild-type background resulted in increased expression of 13 genes outside of the mtr locus. Included in this set of genes was ccp, and its overexpression due to elevated expression of mtrCDE was correlated with decreased susceptibility of gonococci to peroxides. Thus, it is important to realize that overexpression of bacterial efflux pumps can have unexpected consequences on gene expression that could influence metabolism and resistance to antimicrobials that are not pump substrates.
Some gonococcal strains, notably those from an outbreak of azithromycin-resistant gonorrhea in Kansas City, USA, in 1999 [67], contain a 153-bp insertion in the mtrR-mtrCDE intervening region. These isolates expressed resistance to MtrCDE efflux pump substrates. Interestingly, this (or a closely related) insertion, identified as a Correia element (CE) [68], can be found within this region of the mtr locus in many, but not all, strains of Neisseria meningitidis (meningococci) [69, 70]. Some serogroup Y meningococcal strains also have a tandemly linked IS1301 sequence [69]. In meningococci, the presence of the Correia element dampened mtrCDE expression as a result of providing a binding site for integration host factor (IHF) and a new site for posttranscriptional processing of the mtrC transcript [71]; IHF is also important in regulating the farAB efflux pump operon (see below). It is unclear how the Correia element and perhaps more importantly the IHF-binding site and IHF function in gonococci since hydrophobic agent resistance is elevated in strains bearing the Correia element sequence. Nevertheless, the presence (albeit rare) of the Correia element at this site in gonococci suggests that horizontal gene transfer occurred between meningococci and gonococci. If true, this event emphasizes the importance of recombination in generating diversity in clinical strains of both pathogens.
Expression of norM and macAB genes is also modified by cis-acting control elements that were defined by mutations in gonococcal clinical isolates. The presence of point mutations in the −35 hexamer of the norM promoter (C to T) or in a putative ribosome binding site (A to G; reference 70 for consensus sequence) can enhance gonococcal resistance to all substrates of the NorM pump [13]. A point mutation in the −10 hexamer of the macAB promoter (G to T) has been identified that increases expression of macAB and levels of macrolide resistance in gonococci [14]. Together with other mutations that impact antimicrobial susceptibility levels in gonococci, the presence of these cis-acting mutations could influence the efficacy of antimicrobial therapy, particularly for strains with susceptibilities near the minimal inhibitory concentration (MIC) breakpoint for the relevant antimicrobials. Like the mtr 120 mutation for mtrCDE, the point mutations found to regulate norM and macAB alter the sequence of their respective promoter elements to be closer to consensus [65]. Thus, it appears that point mutations within bacterial promoters can upregulate gene expression by enhancing promoter recognition by RNA polymerase outside of the control of transcriptional regulators or, in the case of norM, may also increase translation through improved ribosomal recognition of the norM transcript.
5.2 Trans-Acting Factors that Regulate Efflux Pump Genes
A number of DNA-binding proteins directly or indirectly control expression of genes encoding structural proteins of bacterial drug efflux pumps [51, 58], including those of gonococci [12]. Their function proved crucial in early studies dealing with the identification of cognate efflux pump-encoding genes [52]. For gonococci, the DNA-binding proteins that control mtrCDE, mtrF, and farAB expression are especially understood and are the subject of discussion below.
DNA-binding proteins that control mtrCDE and mtrF gene expression
Expression of genes within the mtr locus is directly or indirectly controlled negatively or positively by a number of DNA-binding proteins (summarized in Fig. 17.5 and references [1, 6, 12]). Central to this regulation is MtrR, which was first described by Pan and Spratt [49] that acts to repress mtrCDE expression by binding as two homodimers to the mtrCDE promoter region [72, 73]. Studies on antimicrobial-resistant clinical isolates have shown that such strains often contain mutations that cause radical amino acid replacements in the helix-turn-helix (HTH) motif (residues 32–53) of MtrR that decrease its binding to target DNA sequences [72] while other mutations map outside of the HTH region that might alter dimer formation or drug interactions. All of these loss-of-function mutations can enhance mtrCDE transcription two- to threefold and, depending on the substrate, increase resistance to antimicrobials by four- to tenfold [11, 74]. It is important to emphasize that, because loss-of-function mutations of mtrR have been observed in gonococcal clinical isolates, their occurrence is relevant for considering antibiotic treatment regimens used worldwide.
In addition to its prominent regulatory action on the mtrCDE promoter, MtrR can directly or indirectly control expression of a number (>65) of other genes at different phases of growth [75]. Cumulative results from genetic and transcriptional profiling studies identified MtrR as a global regulatory protein. Briefly, MtrR has been shown to control genes involved in the generalized stress response (rpoH) [75], peptidoglycan biosynthesis (ponA) [76], amino acid biosynthesis (glnA and glnE) [77, 78], polyamine uptake (potF and potH) [75], and regulation of a regulator (farR) of the farAB efflux pump operon [79]; the capacity of MtrR to activate or repress expression of a non-mtr related genes is shown in Fig. 17.5. We have termed these genes as being “off-target,” since MtrR is acting as a trans-regulator to distinguish them from the adjacent mtrCDE genes [77].
Expression of mtrR is negatively controlled by the product of the mpeR gene [80], which in turn is negatively controlled by Fur (ferric uptake regulator) + iron [81] and the MtrA transcriptional activator of mtrCDE (Fig. 17.5; [1]). This result suggests that levels of antimicrobial resistance and in vivo fitness due to the MtrCDE efflux pump could be influenced by the availability of free iron during infection or inducing agents that work through MtrA (see below). Accordingly, it is important to realize that MIC values obtained for gonococci grown on iron-rich laboratory media may not reflect absolute susceptibility of the test strain during infection and these MICs might accordingly be higher in vivo. MpeR was discovered during a search of the FA1090 genome sequence for regulators [81], in addition to MtrR, that might control expression of mtrF [82]. The capacity of Fur + iron to repress mpeR and the ability of MpeR to repress mtrR likely explain why mtrCDE is maximally expressed late in growth when levels of free iron would be low [81]. It has been shown that MpeR activates expression of fetA [83], which encodes a single-component TonB-dependent receptor that allows the gonococcus to acquire iron from enterobactin-like siderophores produced by enteric bacteria [84]. Taken together, these findings show that MpeR plays important roles in both drug efflux and iron acquisition by gonococci, emphasizing the need to consider regulators of efflux genes in a larger context that includes general concepts of bacterial physiology and pathogenesis.
Gonococci, like other bacteria, can activate transcription of drug efflux pump-encoding genes when confronted with antimicrobial substrates. In this instance, transcriptional activators typically bind “inducers” (pump substrates) and then more effectively interact with DNA sequences that are usually located near the efflux pump-encoding genes. The best example of this phenomenon in gonococci is the action of MtrA [20], a member of the AraC family of transcriptional regulators such as Rob, which is critical in activating transcription of efflux pump-encoding genes ([85]; see [1, 86] for summary reviews). MtrA was discovered during experiments that evaluated whether growth of the gonococci in sublethal level of substrates recognized by the MtrC-MtrD-MtrE efflux pump could enhance bacterial resistance to such antimicrobials. A panel of strains was tested and some were found to increase their resistance to Triton X-100 by an MtrA-dependent manner when incubated overnight in sublethal levels of Triton X-100 [20]. Interestingly, many gonococcal isolates [20, 87], including strain FA1090 [20], contain an 11-bp deletion within mtrA and are unable to transcriptionally activate mtrCDE in the presence of an inducer. This MtrA-dependent inducible resistance property requires energy delivered from the TonB-ExbBD system. MtrA has been purified, and its DNA-binding action upstream of the mtrCDE promoter has been defined [88]). Consistent with Triton X-100 being an inducer, its presence increased MtrA binding to its target DNA and even outcompeted MtrR in the latter’s binding to the mtrCDE promoter (Fig. 17.5; [88]). This result provides a mechanism for upregulation of mtrCDE expression in the presence of an inducer. MtrA, like MtrR and MpeR, has global regulatory action and can modulate expression of a number of genes outside of the mtr locus (W. M. Shafer et al. unpublished) including direct or indirect repression of mpeR (Fig. 17.5; [1]).
DNA-binding proteins that regulate the farAB operon
The farAB operon in gonococci is subject to both direct and indirect regulation by DNA-binding proteins. First, it is subject to direct repression by FarR, a member of the MarR family of transcriptional regulators, by its interaction with the farAB promoter [79]. This binding is enhanced by the DNA-binding/DNA-bending action of IHF [89]. Second, but indirectly, transcription of farAB is controlled by MtrR as this protein binds to the farR promoter resulting in dampening of farR transcription [79]. Thus, with opposing consequences, MtrR can directly and indirectly regulate levels of two gonococcal efflux pump operon systems, which results in repression of one operon (mtrCDE) and activation of another (farAB).
6 Efflux Pumps as Contributors to Gonococcal Antimicrobial Resistance
From a historical perspective (summarized in [1, 6]), the first line of evidence that an efflux pump could impact levels of gonococcal susceptibility to antimicrobials can be gleaned from the early work of the Sparling laboratory conducted in the 1970s [50, 90]. Their studies on antimicrobial resistance showed that the then undefined mtr mutation, now known to be the single-bp deletion in the inverted repeat sequence of the mtrR promoter region (Fig. 17.5), could increase gonococcal resistance (two- to eightfold) to relatively hydrophobic antibiotics such as erythromycin, rifampicin, chloramphenicol, and tetracycline, as well as benzylpenicillin. This increased antimicrobial resistance afforded to gonococci by overexpression of the MtrCDE efflux pump was not by itself clinically significant. However, subsequent work by the Sparling group, and later by the Nicholas laboratory, showed that coresident mutations that altered the structure of the β-lactam targets penicillin-binding protein 2 (PBP2) and PBP1 [91, 92], as well as the porin PorB1b [57, 93], which decrease the influx of antimicrobials, would act with the mtr mutation to provide full penicillin resistance.
In hindsight, the findings of Sparling and coworkers were remarkable in that we now can better appreciate that, while overexpression of bacterial drug efflux pumps may not provide bacteria with clinical resistance to antimicrobials, it can in conjunction with other mutations be of clinical importance. Work with the mtr system in gonococcal clinical isolates such as strain FA6140, which caused the penicillin-resistant (non-β-lactamase) outbreak of gonorrhea in North Carolina, USA, in 1983 [94], and others is an example of such cooperativity of mutations in providing clinically relevant levels of resistance to antimicrobial drugs. Veal et al. [11] showed that loss of the MtrCDE efflux pump rendered this isolate clinically sensitive to penicillin. FA6140 contains the single-bp deletion in the mtrR promoter as well as mutations in ponA (encoding PBP1), penA (encoding PBP2), and porB1b (encoding PorB1b). Zarantonelli et al. [63] further showed that mtrR promoter mutations that increased expression of mtrCDE provided gonococci with low, but clinically relevant, levels of azithromycin resistance and that this could be reversed by genetic inactivation of the mtrCDE efflux pump operon. More recently, Golparian et al. [5] showed that loss of the mtrCDE efflux pump system in clinical isolates, including the infamous HO41 strain from Japan [95], which caused the first reported case of high-level ceftriaxone-resistant gonorrhea, reversed both penicillin and azithromycin resistance; resistance to extended-spectrum cephalosporins (cefixime and ceftriaxone) was reduced by up to twofold and solithromycin by fourfold (Fig. 17.6; [5]). In addition to the contributions of the MtrCDE pump, the NorM and MacAB-MtrE efflux pumps can contribute to gonococcal resistance levels to certain antibiotics. Based on results from MIC determination assays that compared wild-type and efflux pump-deficient mutant strains, NorM antimicrobial substrates seem to include quinolones, possibly β-lactams, and solithromycin, while MacAB-MtrE substrates include macrolides and solithromycin (Fig. 17.6; [5, 13, 14]). Taken together, it is now clear that the presence and overexpression of gonococcal efflux pumps can contribute significantly to bacterial resistance to antimicrobial drugs used previously (e.g., penicillin), presently (azithromycin and cephalosporins) or in the future (solithromycin) in gonorrhea treatment regimens. As is discussed below, efforts that target these pumps in drug therapy or vaccines may help in future treatment protocols or prevention strategies.
Loss of gonococcal efflux pumps MtrCDE, MacAB-MtrE, and NorM increases bacterial susceptibility to antimicrobials. The impact of loss of individual pumps on gonococcal (strain HO41) susceptibility to selected antimicrobials is shown as fold decrease in MIC relative to parental strain HO41 (as designated by the numeral over each bar). A onefold difference represents a one dilution change in the susceptibility of the mutant compared to parent strain HO41, and the absence of a bar signifies that loss of the respective pump had no impact on the MIC value (The figure was adapted from data reported by Golparian et al. [5])
7 Gonococcal Efflux Pumps as Virulence Factors
In the absence of classical antibiotics, does expression of an efflux pump provide an advantage for gonococci (or any bacteria) during infection? This is a critical question because contributions of efflux pumps to bacterial survival prior to appearance of symptoms may influence proliferation and dissemination of the invading pathogen before antibiotic therapy begins. Results from studies on the MtrCDE efflux pump suggest that this is the case since it was required for gonococcal survival during experimental infection of the lower genital tract of female mice [18]. Loss of this efflux pump increases gonococcal susceptibility to intracellular killing by mouse and human polymorphonuclear leukocytes (A. E. Jerse et al. unpublished) and mouse macrophages, but not human ME180 cervical epithelial cells (D’ Andrea and Shafer, unpublished). Importantly, differential expression of the mtrCDE operon by the transcriptional regulators MtrA and MtrR can modulate fitness levels of gonococci in the murine infection model [16, 19]. Briefly, loss of MtrA in strain FA19 decreased in vivo fitness of gonococci by ca. 1,000-fold, while null mutations in mtrR or promoter mutations (e.g., single-bp deletion in the mtrR promoter or the novel mtr 120 promoter) increased in vivo fitness by ca. 100- and 1,000-fold, respectively. In total, these results with the murine experimental infection model suggest that the MtrCDE efflux pump is important for gonococci to survive in vivo when challenged by mediators of innate host defense [16, 19]. This capacity to resist mediators of the innate host defense may be important during human infection because gonococci elicit a strong TH17 pro-inflammatory response [96], and such a response is often characterized by enhanced production of antimicrobial peptides. The capacity of gonococci to export free long-chained fatty acids (e.g., palmitic and oleic acid) via the FarAB-MtrE efflux pump may help it survive in the rectum where concentrations of such fatty acids are elevated [58, 74]; however, direct experimental evidence is lacking since FarAB-MtrE was not required for in vivo survival in the genital tract of female mice, and currently, there is no animal model of rectal infection by gonococci [18].
Based on the above observations, one might ask: why did gonococci not dispense with MtrR since its loss increases antimicrobial resistance and in vivo fitness? It is possible that so-called “off-target” genes activated by MtrR (see above) hold the key. Thus, in the mouse infection experiments performed by Warner et al. [16], loss of MtrR afforded a fitness advantage for the first 5 days, but this advantage waned, suggesting that possession of MtrR may be advantageous at later stages of infection. One of these MtrR-activated genes that may be important in vivo is glnE [75, 78], which is of importance in Salmonella enterica serovar Typhimurium growth and fitness in vivo [97]. GlnE encodes the enzymatic regulator of glutamine synthetase and can activate or deactivate the enzyme. Since levels of glutamine are low at mucosal surfaces and within phagocytes, the ability to synthesize glutamine in vivo may be important for survival and maximal fitness of gonococci. A possible advantage for MtrR-producing gonococci is also suggested by work of Kunz et al. [98], which concentrated on the impact of gyrA and parC mutations on gonococcal fitness during infection; these mutations confer gonococcal resistance to fluoroquinolones. In relationship to mtrR, a spontaneous mutant recovered from mice infected with a strain bearing gyrA and parC mutations as well as a single-bp deletion in the mtrR promoter displayed increased fitness. This mutant had, surprisingly, repaired the deletion such that a wild-type mtrR promoter would exist, but the reason why this in vivo evolved mutant had a competitive advantage over the parental strain remains unclear [98]. Additional work that uses transcriptional profiling and whole genome sequencing to identify additional mutations that might contribute to this phenotype and construction of mutant strains in this genetic background is needed to pinpoint the mechanism(s) by which this mutant strain has a competitive advantage in vivo.
8 Targeting Gonococcal Efflux Pumps for Drug or Vaccine Development
Given the importance of the MtrCDE efflux pump in resistance to classical antibiotics and host-derived antimicrobials, would it be beneficial to target this pump for future drug development (e.g., EPIs) or vaccine development? Using EPIs, coadministered with antimicrobials, could allow for the return of previously used and relatively inexpensive antibiotics (e.g., penicillin [5]) and/or render gonococci more susceptible to host-derived antimicrobials, thereby promoting natural clearance by the host. Furthermore, if the EPI has the identical binding site on the efflux pump as the antimicrobial administered, a target modification would be disadvantageous for the bacteria, and this would likely also suppress the resistance development. Below, two recent approaches that target the MtrCDE efflux pump are described.
Efforts to develop safe and effective EPIs have to date failed largely due to toxicity issues [99]. It is important to emphasize, however, that these EPIs targeted inner membrane transporters (e.g., MtrD-like proteins) and cross-recognition by eukaryotic importers/exporters may explain the observed toxicity. A proposed alternative strategy [100] would be to target the membrane fusion/adapter component (MtrC-like proteins) of the RND pumps as their structures do not have eukaryotic homologues. Indeed, one of our laboratories (E. W. Yu) is developing peptides that bind MtrC and prevent assembly of the Mtr pump in vitro. Follow-up studies are in progress to determine if they can sensitize gonococci to antimicrobial drugs such as β-lactams and macrolides.
After decades of relative futility in developing a vaccine to prevent gonorrhea, renewed efforts are now underway by several international groups to develop a gonorrhea vaccine [101]. To a large extent, these efforts have been driven by the emerging problem of antimicrobial resistance and the fear of untreatable infections in the future. One of us (A. E. Jerse) is targeting MtrE as it has two short immunogenic peptide sequences exposed on the surface (see Sect. 17.3.2 above; [23]). The value of targeting MtrE is that it is highly conserved among gonococci, stably produced, and most likely used by at least three efflux pumps (MtrCDE, FarAB-MtrE, and MacAB-MtrE) to export antimicrobials (Fig. 17.1). In preliminary experiments, female mice immunized with recombinant MtrE lacking the first two N-terminal amino acids in conjunction with CpG adjuvant were found to clear gonococci faster than mice immunized with MtrE + cholera toxin adjuvant or CpG adjuvant alone. An important consideration herein is that the CpG adjuvant would drive a TH1 response thought to be important in generating protective immunity against gonococci [96, 101]. Although these studies are in their infancy, the initial results give hope that MtrE could be part of a future vaccine to at least protect at-risk populations from gonorrhea. It should also be noted that anti-MtrE antibodies might also sensitize gonococci to antimicrobials (e.g., β-lactams and macrolides) recognized by the MtrCDE efflux pump system, which would in theory counteract efflux-dependent resistance mechanisms.
9 Concluding Remarks
As has been recently emphasized [1, 3], the public health problem of multidrug-resistant gonococcal strains is becoming more severe, and strains expressing clinical resistance to extended-spectrum cephalosporins would significantly impact therapeutic options in controlling gonorrhea worldwide. In the absence of new antimicrobials that recognize novel targets, this problem will only worsen, and new efforts in the drug development field are required. Based on the considerable progress made in the past 20 years on gonococcal efflux pumps with respect to their identification, structure, function, gene regulation, and important contributions to antimicrobial resistance and virulence, which have been the major topics covered herein, we posit that targeting efflux pumps for drug/vaccine development has merit and should be part of the current anti-infective pipeline to combat gonorrhea. Finally, given the importance of efflux pumps in antimicrobial resistance in gonococci, these basic research efforts over the past decades have helped to contribute to a better understanding of the genetics, gene regulation, structure, and biologic functions of bacterial efflux pumps in general.
References
Unemo M, Shafer WM (2014) Antimicrobial resistance in Neisseria gonorrhoeae in the 21st century: past, evolution, and future. Clin Microbiol Rev 27:587–613. doi:10.1128/CMR.00010-14
Newman LM, Rowley J, Vander Hoorn S, Wijesooriya NS, Unemo M, Low N, Stevens G, Gottlieb S et al (2015) Global estimates of the prevalence and incidence of four curable sexually transmitted infections in 2012 based on systematic review and global reporting. PLoS One 10:e0143304. doi:10.1371/journal.pone.0143304
Bolan GA, Sparling PF, Wasserheit JN (2012) The emerging threat of untreatable gonococcal infection. N Engl J Med 366:485–487. doi:10.1056/NEJMp1112456
Bauer ME, Shafer WM (2015) On the in vivo significance of bacterial resistance to antimicrobial peptides. Biochim Biophys Acta 1848:3101–3111. doi:10.1016/j.bbamem.2015.02.012
Golparian D, Shafer WM, Ohnishi M, Unemo M (2014) Importance of multidrug efflux pumps in the antimicrobial resistance property of clinical multidrug-resistant isolates of Neisseria gonorrhoeae. Antimicrob Agents Chemother 58:3556–3559. doi:10.1128/AAC.00038-14
Unemo M, Nicholas RA, Jerse AE, Davies C, Shafer WM (2014) Molecular mechanisms of antibiotic resistance expressed by the pathogenic Neisseria. In: Davies JK, Kahler CM (eds) Pathogenic Neisseria: genomics, molecular biology and disease intervention. Caister Academic Press, Norfolk, UK. pp 161–192
Veal WL, Shafer WM (2003) Identification of a cell envelope protein (MtrF) involved in hydrophobic antimicrobial resistance in Neisseria gonorrhoeae. J Antimicrob Chemother 51:27–37. doi:10.1093/jac/dkg031
Su CC, Bolla JR, Kumar N, Radhakrishnan A, Long F, Delmar JA, Chou TH, Rajashankar KR et al (2015) Structure and function of Neisseria gonorrhoeae MtrF illuminates a class of antimetabolite efflux pumps. Cell Rep 11:61–70. doi:10.1016/j.celrep.2015.03.003
Luna VA, Cousin S Jr, Whittington WL, Roberts MC (2000) Identification of the conjugative mef gene in clinical Acinetobacter junii and Neisseria gonorrhoeae isolates. Antimicrob Agents Chemother 44:2503–2506. doi:10.1128/AAC.44.9.2503-2506.2000
Hagman KE, Pan W, Spratt BG, Balthazar JT, Judd RC, Shafer WM (1995) Resistance of Neisseria gonorrhoeae to antimicrobial hydrophobic agents is modulated by the mtrRCDE efflux system. Microbiology 141:611–622. doi:10.1099/13500872-141-3-611
Veal WL, Nicholas RA, Shafer WM (2002) Overexpression of the MtrC-MtrD-MtrE efflux pump due to an mtrR mutation is required for chromosomally mediated penicillin resistance in Neisseria gonorrhoeae. J Bacteriol 184:5619–5624. doi:10.1128/JB.184.20.5619-5624.2002
Shafer WM, Folster JP, Nicholas RA (2010) Molecular mechanisms of antibiotic resistance expressed by the pathogenic Neisseria. In: Genco CA, Wetzler L (eds) Neisseria: molecular mechanisms of pathogenesis. Caister Academic Press, Norfolk, pp 245–268
Rouquette-Loughlin C, Dunham SA, Kuhn M, Balthazar JT, Shafer WM (2003) The NorM efflux pump of Neisseria gonorrhoeae and Neisseria meningitidis recognizes antimicrobial cationic compounds. J Bacteriol 185:1101–1106. doi:10.1128/JB.185.3.1101-1106.2003
Rouquette-Loughlin CE, Balthazar JT, Shafer WM (2005) Characterization of the MacA-MacB efflux system in Neisseria gonorrhoeae. J Antimicrob Chemother 56:856–860. doi:10.1093/jac/dki333
Shafer WM, Qu X, Waring AJ, Lehrer RI (1998) Modulation of Neisseria gonorrhoeae susceptibility to vertebrate antibacterial peptides due to a member of the resistance/nodulation/division efflux pump family. Proc Natl Acad Sci U S A 95:1829–1833
Warner DM, Folster JP, Shafer WM, Jerse AE (2007) Regulation of the MtrC-MtrD-MtrE efflux-pump system modulates the in vivo fitness of Neisseria gonorrhoeae. J Infect Dis 196:1804–1812. doi:10.1086/522964
Lee EH, Shafer WM (1999) The farAB-encoded efflux pump mediates resistance of gonococci to long-chained antibacterial fatty acids. Mol Microbiol 33:839–845. doi:10.1046/j.1365-2958.1999.01530.x
Jerse AE, Sharma ND, Simms AN, Crow ET, Snyder LA, Shafer WM (2003) A gonococcal efflux pump system enhances bacterial survival in a female mouse model of genital tract infection. Infect Immun 71:5576–5582. doi:10.1128/IAI.71.10.5576-5582.2003
Warner DM, Shafer WM, Jerse AE (2008) Clinically relevant mutations that cause derepression of the Neisseria gonorrhoeae MtrC-MtrD-MtrE Efflux pump system confer different levels of antimicrobial resistance and in vivo fitness. Mol Microbiol 70:462–478. doi:10.1111/j.1365-2958.2008.06424.x
Rouquette C, Harmon JB, Shafer WM (1999) Induction of the mtrCDE-encoded efflux pump system of Neisseria gonorrhoeae requires MtrA, an AraC-like protein. Mol Microbiol 33:651–658. doi:10.1046/j.1365-2958.1999.01517.x
Long F, Rouquette-Loughlin C, Shafer WM, Yu EW (2008) Functional cloning and characterization of the multidrug efflux pumps NorM from Neisseria gonorrhoeae and YdhE from Escherichia coli. Antimicrob Agents Chemother 52:3052–3060. doi:10.1128/AAC.00475-08
Bolla JR, Su CC, Do SV, Radhakrishnan A, Kumar N, Long F, Chou TH, Delmar JA et al (2014) Crystal structure of the Neisseria gonorrhoeae MtrD inner membrane multidrug efflux pump. PLoS One 9:e97903. doi:10.1371/journal.pone.0097903
Lei HT, Chou TH, Su CC, Bolla JR, Kumar N, Radhakrishnan A, Long F, Delmar JA et al (2014) Crystal structure of the open state of the Neisseria gonorrhoeae MtrE outer membrane channel. PLoS One 9:e97475. doi:10.1371/journal.pone.0097475
Hagman KE, Lucas CE, Balthazar JT, Snyder L, Nilles M, Judd RC, Shafer WM (1997) The MtrD protein of Neisseria gonorrhoeae is a member of the resistance/nodulation/division protein family constituting part of an efflux system. Microbiology 143:2117–2125. doi:10.1099/00221287-143-7-2117
Janganan TK, Zhang L, Bavro VN, Matak-Vinkovic D, Barrera NP, Burton MF, Steel PG, Robinson CV et al (2011) Opening of the outer membrane protein channel in tripartite efflux pumps is induced by interaction with the membrane fusion partner. J Biol Chem 286:5484–5493. doi:10.1074/jbc.M110.187658
Murakami S, Nakashima R, Yamashita E, Matsumoto T, Yamaguchi A (2006) Crystal structures of a multidrug transporter reveal a functionally rotating mechanism. Nature 443:173–179. doi:10.1038/nature05076
Murakami S, Nakashima R, Yamashita E, Yamaguchi A (2002) Crystal structure of bacterial multidrug efflux transporter AcrB. Nature 419:587–593. doi:10.1038/nature01050
Seeger MA, Schiefner A, Eicher T, Verrey F, Diederichs K, Pos KM (2006) Structural asymmetry of AcrB trimer suggests a peristaltic pump mechanism. Science 313:1295–1298. doi:10.1126/science.1131542
Nakashima R, Sakurai K, Yamasaki S, Nishino K, Yamaguchi A (2011) Structures of the multidrug exporter AcrB reveal a proximal multisite drug-binding pocket. Nature 480:565–569. doi:10.1038/nature10641
Nakashima R, Sakurai K, Yamasaki S, Hayashi K, Nagata C, Hoshino K, Onodera Y, Nishino K et al (2013) Structural basis for the inhibition of bacterial multidrug exporters. Nature 500:102–106. doi:10.1038/nature12300
Sennhauser G, Bukowska MA, Briand C, Grutter MG (2009) Crystal structure of the multidrug exporter MexB from Pseudomonas aeruginosa. J Mol Biol 389:134–145. doi:10.1016/j.jmb.2009.04.001
Su CC, Long F, Zimmermann MT, Rajashankar KR, Jernigan RL, Yu EW (2011) Crystal structure of the CusBA heavy-metal efflux complex of Escherichia coli. Nature 470:558–562. doi:10.1038/nature09743
Su CC, Long F, Lei HT, Bolla JR, Do SV, Rajashankar KR, Yu EW (2012) Charged amino acids (R83, E567, D617, E625, R669, and K678) of CusA are required for metal ion transport in the Cus efflux system. J Mol Biol 422:429–441. doi:10.1016/j.jmb.2012.05.038
Franke S, Grass G, Nies DH (2001) The product of the ybdE gene of the Escherichia coli chromosome is involved in detoxification of silver ions. Microbiology 147:965–972. doi:10.1099/00221287-147-4-965
Franke S, Grass G, Rensing C, Nies DH (2003) Molecular analysis of the copper-transporting efflux system CusCFBA of Escherichia coli. J Bacteriol 185:3804–3812. doi:10.1128/JB.185.13.3804-3812.2003
Kulathila R, Kulathila R, Indic M, van den Berg B (2011) Crystal structure of Escherichia coli CusC, the outer membrane component of a heavy metal efflux pump. PLoS One 6:e15610. doi:10.1371/journal.pone.0015610
Lei HT, Bolla JR, Bishop NR, Su CC, Yu EW (2014) Crystal structures of CusC review conformational changes accompanying folding and transmembrane channel formation. J Mol Biol 426:403–411. doi:10.1016/j.jmb.2013.09.042
Long F, Su CC, Zimmermann MT, Boyken SE, Rajashankar KR, Jernigan RL, Yu EW (2010) Crystal structures of the CusA efflux pump suggest methionine-mediated metal transport. Nature 467:484–488. doi:10.1038/nature09395
Su CC, Yang F, Long F, Reyon D, Routh MD, Kuo DW, Mokhtari AK, Van Ornam JD et al (2009) Crystal structure of the membrane fusion protein CusB from Escherichia coli. J Mol Biol 393:342–355. doi:10.1016/j.jmb.2009.08.029
Tseng TT, Gratwick KS, Kollman J, Park D, Nies DH, Goffeau A, Saier MH Jr (1999) The RND permease superfamily: an ancient, ubiquitous and diverse family that includes human disease and development proteins. J Mol Microbiol Biotechnol 1:107–125. doi:10.1007/s13205-013-0155-z
Janganan TK, Bavro VN, Zhang L, Borges-Walmsley MI, Walmsley AR (2013) Tripartite efflux pumps: energy is required for dissociation, but not assembly or opening of the outer membrane channel of the pump. Mol Microbiol 88:590–602. doi:10.1111/mmi.12211
Delahay RM, Robertson BD, Balthazar JT, Shafer WM, Ison CA (1997) Involvement of the gonococcal MtrE protein in the resistance of Neisseria gonorrhoeae to toxic hydrophobic agents. Microbiology 143:2127–2133. doi:10.1099/00221287-143-7-2127
Akama H, Kanemaki M, Yoshimura M, Tsukihara T, Kashiwagi T, Yoneyama H, Narita S-i, Nakagawa A et al (2004) Crystal structure of the drug discharge outer membrane protein, OprM, of Pseudomonas aeruginosa: dual modes of membrane anchoring and occluded cavity end. J Biol Chem 279:52816–52819. doi:10.1074/jbc.C400445200
Koronakis V, Sharff A, Koronakis E, Luisi B, Hughes C (2000) Crystal structure of the bacterial membrane protein TolC central to multidrug efflux and protein export. Nature 405:914–919. doi:10.1038/35016007
Su CC, Radhakrishnan A, Kumar N, Long F, Bolla JR, Lei HT, Delmar JA, Do SV et al (2014) Crystal structure of the Campylobacter jejuni CmeC outer membrane channel. Protein Sci 23:954–961. doi:10.1002/pro.2478
Andersen C, Koronakis E, Hughes C, Koronakis V (2002) An aspartate ring at the TolC tunnel entrance determines ion selectivity and presents a target for blocking by large cations. Mol Microbiol 44:1131–1139. doi:10.1046/j.1365-2958.2002.02898.x
Bolla JR, Su CC, Delmar JA, Radhakrishnan A, Kumar N, Chou TH, Long F, Rajashankar KR et al (2015) Crystal structure of the Alcanivorax borkumensis YdaH transporter reveals an unusual topology. Nat Commun 6:6874. doi:10.1038/ncomms7874
Delmar JA, Yu EW (2016) The AbgT family: a novel class of antimetabolite transporters. Protein Sci 25:322–337. doi:10.1002/pro.2820
Pan W, Spratt BG (1994) Regulation of the permeability of the gonococcal cell envelope by the mtr system. Mol Microbiol 11:769–775. doi:10.1111/j.1365-2958.1994.tb00354.x
Maness MJ, Sparling PF (1973) Multiple antibiotic resistance due to a single mutation in Neisseria gonorrhoeae. J Infect Dis 128:321–330. doi:10.1093/infdis/128.3.321
Guymon LF, Walstad DL, Sparling PF (1978) Cell envelope alterations in antibiotic-sensitive and-resistant strains of Neisseria gonorrhoeae. J Bacteriol 136:391–401
Grkovic S, Brown MH, Skurray RA (2002) Regulation of bacterial drug export systems. Microbiol Mol Biol Rev 66:671–701. doi:10.1128/MMBR.66.4.671-701.2002
Rouquette-Loughlin C, Stojiljkovic I, Hrobowski T, Balthazar JT, Shafer WM (2002) Inducible, but not constitutive, resistance of gonococci to hydrophobic agents due to the MtrC-MtrD-MtrE efflux pump requires TonB-ExbB-ExbD proteins. Antimicrob Agents Chemother 46:561–565. doi:10.1128/AAC.46.2.561-565.2002
Lucas CE, Hagman KE, Levin JC, Stein DC, Shafer WM (1995) Importance of lipooligosaccharide structure in determining gonococcal resistance to hydrophobic antimicrobial agents resulting from the mtr efflux system. Mol Microbiol 16:1001–1009. doi:10.1111/j.1365-2958.1995.tb02325.x
Veal WL, Yellen A, Balthazar JT, Pan W, Spratt BG, Shafer WM (1998) Loss-of-function mutations in the mtr efflux system of Neisseria gonorrhoeae. Microbiology 144:621–627. doi:10.1099/00221287-144-3-621
Sarubbi FA Jr, Sparling PF, Blackman E, Lewis E (1975) Loss of low-level antibiotic resistance in Neisseria gonorrhoeae due to env mutations. J Bacteriol 124:750–756
Olesky M, Zhao S, Rosenberg RL, Nicholas RA (2006) Porin-mediated antibiotic resistance in Neisseria gonorrhoeae: ion, solute, and antibiotic permeation through PIB proteins with penB mutations. J Bacteriol 188:2300–2308. doi:10.1128/JB.188.7.2300-2308.2006
Morse SA, Lysko PG, McFarland L, Knapp JS, Sandstrom E, Critchlow C, Holmes KK (1982) Gonococcal strains from homosexual men have outer membranes with reduced permeability to hydrophobic molecules. Infect Immun 37:432–438
Nikaido H (1996) Multidrug efflux pumps of Gram-negative bacteria. J Bacteriol 178:5853–5859
Hagman KE, Shafer WM (1995) Transcriptional control of the mtr efflux system of Neisseria gonorrhoeae. J Bacteriol 177:4162–4165
Cousin SL Jr, Whittington WL, Roberts MC (2003) Acquired macrolide resistance genes and the 1 bp deletion in the mtrR promoter in Neisseria gonorrhoeae. J Antimicrob Chemother 51:131–133. doi:10.1093/jac/dkg040
Xia M, Whittington WL, Shafer WM, Holmes KK (2000) Gonorrhea among men who have sex with men: outbreak caused by a single genotype of erythromycin-resistant Neisseria gonorrhoeae with a single-base pair deletion in the mtrR promoter region. J Infect Dis 181:2080–2082. doi:10.1086/315510
Zarantonelli L, Borthagaray G, Lee EH, Veal W, Shafer WM (2001) Decreased susceptibility to azithromycin and erythromycin mediated by a novel mtr(R) promoter mutation in Neisseria gonorrhoeae. J Antimicrob Chemother 47:651–654. doi:10.1093/jac/47.5.651
Ohneck EA, Zalucki YM, Johnson PJ, Dhulipala V, Golparian D, Unemo M, Jerse AE, Shafer WM (2011) A novel mechanism of high-level, broad-spectrum antibiotic resistance caused by a single base pair change in Neisseria gonorrhoeae. mBio 2:e00187–11. doi:10.1128/mBio.00187-11
Browning DF, Busby SJ (2004) The regulation of bacterial transcription initiation. Nat Rev Microbiol 2:57–65. doi:10.1038/nrmicro787
Ohneck EA, Goytia M, Rouquette-Loughlin CE, Joseph SJ, Read TD, Jerse AE, Shafer WM (2015) Over-production of the MtrCDE efflux pump in Neisseria gonorrhoeae produces unexpected changes in cellular transcription patterns. Antimicrob Agents Chemother 59:724–726. doi:10.1128/AAC.04148-14
Johnson SR, Sandul AL, Parekh M, Wang SA, Knapp JS, Trees DL (2003) Mutations causing in vitro resistance to azithromycin in Neisseria gonorrhoeae. Int J Antimicrob Agents 21:414–419. doi:10.1016/S0924-8579(03)00039-6
Correia FF, Inouye S, Inouye M (1988) A family of small repeated elements with some transposon-like properties in the genome of Neisseria gonorrhoeae. J Biol Chem 263:12194–12198
Rouquette-Loughlin CE, Balthazar JT, Hill SA, Shafer WM (2004) Modulation of the mtrCDE-encoded efflux pump gene complex of Neisseria meningitidis due to a Correia element insertion sequence. Mol Microbiol 54:731–741. doi:10.1111/j.1365-2958.2004.04299.x
Enriquez R, Abad R, Chanto G, Corso A, Cruces R, Gabastou JM, Gorla MC, Maldonado A et al (2010) Deletion of the Correia element in the mtr gene complex of Neisseria meningitidis. J Med Microbiol 59:1055–1060. doi:10.1099/jmm.0.021220-0
Shine J, Dalgarno L (1975) Determinant of cistron specificity in bacterial ribosomes. Nature 254:34–38. doi:10.1038/254034a0
Lucas CE, Balthazar JT, Hagman KE, Shafer WM (1997) The MtrR repressor binds the DNA sequence between the mtrR and mtrC genes of Neisseria gonorrhoeae. J Bacteriol 179:4123–4128
Hoffmann KM, Williams D, Shafer WM, Brennan RG (2005) Characterization of the multiple transferable resistance repressor, MtrR, from Neisseria gonorrhoeae. J Bacteriol 187:5008–5012. doi:10.1128/JB.187.14.5008-5012.2005
Shafer WM, Balthazar JT, Hagman KE, Morse SA (1995) Missense mutations that alter the DNA-binding domain of the MtrR protein occur frequently in rectal isolates of Neisseria gonorrhoeae that are resistant to faecal lipids. Microbiology 141:907–911. doi:10.1099/13500872-141-4-907
Folster JP, Johnson PJ, Jackson L, Dhulipali V, Dyer DW, Shafer WM (2009) MtrR modulates rpoH expression and levels of antimicrobial resistance in Neisseria gonorrhoeae. J Bacteriol 191:287–297. doi:10.1128/JB.01165-08
Folster JP, Dhulipala V, Nicholas RA, Shafer WM (2007) Differential regulation of ponA and pilMNOPQ expression by the MtrR transcriptional regulatory protein in Neisseria gonorrhoeae. J Bacteriol 189:4569–4577. doi:10.1128/JB.00286-07
Johnson PJT, Stringer VA, Shafer WM (2011) Off-target gene regulation mediated by transcriptional repressors of antimicrobial efflux pump genes in Neisseria gonorrhoeae. Antimicrob Agents Chemother 55:2559–2565. doi:10.1128/AAC.00010-11
Johnson PJT, Shafer WM (2015) The transcriptional repressor, MtrR, of the efflux pump operon of Neisseria gonorrhoeae can also serve as an activator of “off target” gene (glnE) expression. Antibiotics 4:188–197. doi:10.3390/antibiotics4020188
Lee EH, Rouquette-Loughlin C, Folster JP, Shafer WM (2003) FarR regulates the farAB-encoded efflux pump of Neisseria gonorrhoeae via an MtrR regulatory mechanism. J Bacteriol 185:7145–7152. doi:10.1128/JB.185.24.7145-7152.2003
Mercante AD, Jackson L, Johnson PJ, Stringer VA, Dyer DW, Shafer WM (2012) MpeR regulates the mtr efflux locus in Neisseria gonorrhoeae and modulates antimicrobial resistance by an iron-responsive mechanism. Antimicrob Agents Chemother 56:1491–1501. doi:10.1128/AAC.06112-11
Jackson LA, Ducey TF, Day MW, Zaitshik JB, Orvis J, Dyer DW (2010) Transcriptional and functional analysis of the Neisseria gonorrhoeae Fur regulon. J Bacteriol 192:77–85. doi:10.1128/JB.00741-09
Folster JP, Shafer WM (2005) Regulation of mtrF expression in Neisseria gonorrhoeae and its role in high-level antimicrobial resistance. J Bacteriol 187:3713–3720. doi:10.1128/JB.187.11.3713-3720.2005
Hollander A, Mercante AD, Shafer WM, Cornelissen CN (2011) The iron-repressed, AraC-like regulator MpeR activates expression of fetA in Neisseria gonorrhoeae. Infect Immun 79:4764–4776. doi:10.1128/IAI.05806-11
Carson SD, Klebba PE, Newton SM, Sparling PF (1999) Ferric enterobactin binding and utilization by Neisseria gonorrhoeae. J Bacteriol 181:2895–2901
Ariza RR, Li Z, Ringstad N, Demple B (1995) Activation of multiple antibiotic resistance and binding of stress-inducible promoters by Escherichia coli Rob protein. J Bacteriol 177:1655–1661
Zalucki YM, Mercante AD, Cloward JM, Ohneck EA, Kandler JL, Goytia M, Johnson PJT, Shafer WM (2013) Function and regulation of Neisseria gonorrhoeae efflux pumps. In: Yu EW, Zhang Q, Brown MH (eds) Microbial efflux pumps: current research. Caister Academic Press, Poole, pp 207–221
Ezewudo MN, Joseph SJ, Castillo-Ramirez S, Dean D, Del Rio C, Didelot X, Dillon JA, Selden RF et al (2015) Population structure of Neisseria gonorrhoeae based on whole genome data and its relationship with antibiotic resistance. Peer J 3:e806. doi:10.7717/peerj.806
Zalucki YM, Dhulipala V, Shafer WM (2012) Dueling regulatory properties of a transcriptional activator (MtrA) and repressor (MtrR) that control efflux pump gene expression in Neisseria gonorrhoeae. mBio 3:e00446–12. doi:10.1128/mBio.00446-12
Lee EH, Hill SA, Napier R, Shafer WM (2006) Integration host factor is required for FarR repression of the farAB-encoded efflux pump of Neisseria gonorrhoeae. Mol Microbiol 60:1381–1400. doi:10.1111/j.1365-2958.2006.05185.x
Sparling PF, Sarubbi FA Jr, Blackman E (1975) Inheritance of low-level resistance to penicillin, tetracycline, and chloramphenicol in Neisseria gonorrhoeae. J Bacteriol 124:740–749
Tomberg J, Unemo M, Davies C, Nicholas RA (2010) Molecular and structural analysis of mosaic variants of penicillin-binding protein 2 conferring decreased susceptibility to expanded-spectrum cephalosporins in Neisseria gonorrhoeae: role of epistatic mutations. Biochemistry 49:8062–8070. doi:10.1021/bi101167x
Ropp PA, Hu M, Olesky M, Nicholas RA (2002) Mutations in ponA, the gene encoding penicillin-binding protein 1, and a novel locus, penC, are required for high-level chromosomally mediated penicillin resistance in Neisseria gonorrhoeae. Antimicrob Agents Chemother 46:769–777. doi:10.1128/AAC.46.3.769-777.2002
Olesky M, Hobbs M, Nicholas RA (2002) Identification and analysis of amino acid mutations in porin IB that mediate intermediate-level resistance to penicillin and tetracycline in Neisseria gonorrhoeae. Antimicrob Agents Chemother 46:2811–2820. doi:10.1128/AAC.46.9.2811-2820.2002
Faruki H, Kohmescher RN, McKinney WP, Sparling PF (1985) A community-based outbreak of infection with penicillin-resistant Neisseria gonorrhoeae not producing penicillinase (chromosomally mediated resistance). N Engl J Med 313:607–611. doi:10.1056/NEJM198509053131004
Ohnishi M, Golparian D, Shimuta K, Saika T, Hoshina S, Iwasaku K, Nakayama S, Kitawaki J et al (2011) Is Neisseria gonorrhoeae initiating a future era of untreatable gonorrhea?: Detailed characterization of the first strain with high-level resistance to ceftriaxone. Antimicrob Agents Chemother 55:3538–3545. doi:10.1128/AAC.00325-11
Liu Y, Feinen B, Russell MW (2011) New concepts in immunity to Neisseria gonorrhoeae: innate responses and suppression of adaptive immunity favor the pathogen, not the host. Front Microbiol 2:52. doi:10.3389/fmicb.2011.00052
Klose KE, Mekalanos JJ (1997) Simultaneous prevention of glutamine synthesis and high-affinity transport attenuates Salmonella typhimurium virulence. Infect Immun 65:587–596
Kunz AN, Begum AA, Wu H, D’Ambrozio JA, Robinson JM, Shafer WM, Bash MC, Jerse AE (2012) Impact of fluoroquinolone resistance mutations on gonococcal fitness and in vivo selection for compensatory mutations. J Infect Dis 205:1821–1829. doi:10.1093/infdis/jis277
Lomovskaya O, Bostian KA (2006) Practical applications and feasibility of efflux pump inhibitors in the clinic – a vision for applied use. Biochem Pharmacol 71:910–918. doi:10.1016/j.bcp.2005.12.008
Blair JM, Richmond GE, Piddock LJ (2014) Multidrug efflux pumps in Gram-negative bacteria and their role in antibiotic resistance. Future Microbiol 9:1165–1177. doi:10.2217/fmb.14.66
Jerse AE, Deal CD (2013) Vaccine research for gonococcal infections: where are we? Sex Transm Infect 89(Suppl 4):v63–v68. doi:10.1136/sextrans-2013-051225
Acknowledgments
We thank all past and present members of our laboratories and our collaborators who have contributed significantly to the work reviewed herein. We are grateful to V. Bavro for communicating information regarding the mtrE sequence of N. gonorrhoeae. This work was supported by NIH grants R37 AI021150 (W.M.S.), R01 AI114629 (E.W.Y.), RO1 AI42053 (A.E.J.), and U19 AI113170 (A.E.J.) and funds from the Örebro County Council Research Committee and the Foundation for Medical Research at Örebro University Hospital, Sweden (M.U.). W.M.S. is the recipient of a Senior Research Career Scientist Award from the Biomedical Laboratory Research and Development Service of the Department of Veterans Affairs. The contents of this chapter do not represent the views of the Department of Veterans Affairs, the National Institutes of Health, or the US government.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2016 Springer International Publishing Switzerland
About this chapter
Cite this chapter
Shafer, W.M., Yu, E.W., Rouquette-Loughlin, C., Golparian, D., Jerse, A.E., Unemo, M. (2016). Efflux Pumps in Neisseria gonorrhoeae: Contributions to Antimicrobial Resistance and Virulence. In: Li, XZ., Elkins, C., Zgurskaya, H. (eds) Efflux-Mediated Antimicrobial Resistance in Bacteria. Adis, Cham. https://doi.org/10.1007/978-3-319-39658-3_17
Download citation
DOI: https://doi.org/10.1007/978-3-319-39658-3_17
Published:
Publisher Name: Adis, Cham
Print ISBN: 978-3-319-39656-9
Online ISBN: 978-3-319-39658-3
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)