Abstract
The noninvasive diagnosis of hepatocellular carcinoma (HCC) relies heavily on imaging-based primarily on sequential changes in the intranodular blood supply during the process of hepatocarcinogenesis.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
1 Introduction
The noninvasive diagnosis of hepatocellular carcinoma (HCC) relies heavily on imaging-based primarily on sequential changes in the intranodular blood supply during the process of hepatocarcinogenesis [1]; regenerative nodules (RN) show similar blood supply to normal liver, borderline lesions show wide variations of blood supply [2] and typical HCC are supplied by abnormal neoplastic arteries alone. Once a focal hepatic nodule is detected during HCC surveillance typically with ultrasound (US), a diagnostic imaging test is performed. While contrast-enhanced CT or MRI is most commonly selected as the diagnostic test, contrast-enhanced ultrasound (CEUS) using a microbubble contrast is an excellent choice that has several advantages over CT or MRI including a real-time demonstration of continuous hemodynamic changes of liver tumors, a purely intravascular contrast material, availability in patients with renal failure, excellent patient compliance, and repeatability in short intervals.
Management strategy for HCC is often decided in multidisciplinary consensus meetings including physicians from several different specialties. The role of imaging in the diagnosis and staging for HCC is crucial to determine the management plan. Recent practice guidelines for HCC provide recommendations for the diagnostic algorithm for newly detected nodules at HCC surveillance [3–5]. The application of the imaging test varies depending on the size of the nodules. For very small lesions (<1 cm in size), follow-up with US scan is usually recommended in 3 months as further imaging tests may not be reliable for the diagnosis. For lesions of 1 cm or larger, multiphasic contrast-enhanced CT, MRI or CEUS is usually performed as a diagnostic test. As the imaging diagnosis of small nodules of 1–2 cm in size can be particularly challenging, a multimodality approach is often needed [6]. Borderline lesions, i.e., high-grade dysplastic nodule (DN) and well-differentiated HCC, often show indeterminate imaging findings and imaging may not be reliable to differentiate between the two [2]. Biopsy is performed only when imaging findings are indeterminate.
A large number of CEUS examinations are also performed to characterize small indeterminate focal liver lesions seen on CT or MR scans, producing satisfactory results [7]. CEUS is particularly useful for detecting arterial-phase hypervascularity of HCC utilizing the real-time evaluation of the lesion perfusion. CEUS is an excellent modality to assess post-ablative therapy for HCC. CEUS is also useful to differentiate between malignant and benign venous thrombosis in patients with HCC [8], which is often critical to determine the management plan.
In this chapter, we review the CEUS techniques and typical CEUS imaging features of HCC and other cirrhosis-related nodules. We also discuss the role of CEUS in the algorithms for the diagnosis and staging of HCC and in monitoring therapeutic responses to local ablation therapy.
2 Contrast-Enhanced Ultrasound Techniques
US contrast agents consist of microbubbles of perfluorocarbon gas stabilized by a protein, lipid, or polymer shell. The microbubbles are sufficiently small and stable to traverse the pulmonary and cardiac circulations following peripheral venous injection. The microbubbles disappear as the gas diffuses through the thin shell, with a typical half-life of a few minutes in blood and there is no renal excretion. There are a few different types of microbubble contrast agents that are commercially available. Presently, Definity (Lantheus Medical Imaging, Billerica MA) and SonuVue (Bracco, Milan, Italy) are most widely used. Microbubbles are approximately the same size as red blood cells and cannot move through the vascular endothelium into the interstitium; therefore, they are true blood pool agents [9]. Sonazoid (Daiichi), which is most actively used in Japan, shows similar vascular enhancement but is taken up by Kupffer cells in the late phase [10]. In our experience of using Definity for over 12 years, patient acceptance has been very high and there have been no serious adverse events. A large retrospective study from Europe using SonoVue reported 0.0086 % incidence of serious adverse events without any fatality among 23,188 examinations [11]. Microbubble contrast agents are approved for radiologic use in more than 50 countries, including the European Union, Canada, and many Asian countries.
Definity and Sonovue are both approved for cardiac use in the United States, and on April 2016, the FDA approved Sonovue for liver mass characterization for adults and children. Sonovue is marketed as Lumason in the USA. CEUS requires a contrast imaging mode that is available on most high-end commercially available ultrasound systems. Low mechanical index (MI) contrast-specific mode is used to visualize the microbubbles continuously while suppressing signals from tissue. A dual-imaging mode (Fig. 24.1), which enables simultaneous real-time display of contrast-specific mode and the gray-scale mode, is essential for scanning small liver lesions. Typically, the contrast agent is injected manually through a three-way stopcock, followed by a 5-mL saline solution flush. Continuous scan with video acquisition is performed in the arterial phase (usually <30 s after saline flush) to evaluate the real-time enhancement pattern of the liver lesion. Then the liver lesion is intermittently scanned typically every 30 s for 4–5 min to minimize inadvertent microbubble destruction. Sweeping of the entire liver can be performed in the late phase to detect any additional washout lesions. Slightly higher MI along with a larger amount of microbubbles can be used for deep seated lesions or lesions within an attenuating fatty liver.
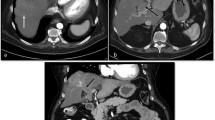
Well-differentiated HCC in a 73-year-old man with hepatitis C. a A dual-imaging mode CEUS displays contrast-specific mode on the left and gray-scale mode on the right simultaneously. There are two hypoechoic masses (short arrows) in the liver on gray-scale mode that are slightly hypoechoic (hypovascular) relative to the liver (long arrows) on contrast-specific mode in the arterial phase. b In the portal venous phase, the hypoechoic masses (short arrows) on gray-scale mode are not seen on contrast-specific mode as they are isoechoic to the liver at 2 min
The first injection usually includes a stationary field of view to include the lesion of interest and the adjacent liver, both observed for 4–5 min. Subsequent injections concentrate on arterial phase vessel morphology and enhancement as well as sweeps of the entire liver in the portal phase to look for any further abnormalities. Injections are typically repeated 2–3 times to obtain images of the same lesion or to evaluate a different lesion. Each injection is separated by 3–5 min. High MI frames can be used to disrupt microbubbles and evaluate the pattern of refilling of the microbubbles in the scanning plane. This may be optimized by using bubble tracking technology which is called maximum-intensity projection, most optimally used to show the filling pattern and vascular morphology of hypervascular liver tumors (Fig. 24.2) [12].
HCC in a 83-year-old woman with hepatitis B. a CEUS scan in the arterial phase shows a large hypervascular mass in the liver with heterogeneous enhancement and non-enhancing areas representing necrosis. b, c Two maximum-intensity projection CEUS images after microbubble disruption by using high MI frames demonstrate irregular, dysmorphic, neoplastic arteries within the mass that are not seen on regular CEUS image (a)
3 Differential Diagnosis of Nodules in Liver Cirrhosis
Typical HCC are supplied by abnormal neoplastic arteries alone and show hyperenhancement relative to the liver in the hepatic arterial phase (hypervascularity) and hypoenhancement in the late phase (washout) (Fig. 24.3) [13–15]. There are irregular dysmorphic arteries within the tumor often visualized in large HCC at the early arterial filling phase (Fig. 24.2). Arterial-phase enhancement pattern of HCC is usually homogeneous in small lesions (Fig. 24.3) and tends to be heterogeneous in large lesions with or without non-enhancing areas representing necrosis (Fig. 24.2). Peripheral rim-like enhancement is uncommon in HCC. A nodule-in-nodule pattern is occasionally seen when there is a hypervascular HCC focus developing within an underlying DN or well-differentiated HCC (Fig. 24.4) [2, 16]. The hypervascular focus in HCC usually shows washout and should not be confused with a nodular enhancement in hemangioma, which progresses centrally over time and shows sustained enhancement without washout.
Typical hypervascular HCC with late, mild washout in a 70-year-old man with hepatitis C. a US scan shows a hypoechoic, solid mass in the liver. The liver is cirrhotic with a nodular surface and there is a large amount of ascites. b CEUS scan in the arterial phase at 20 s shows homogeneous hypervascularity of the mass (arrow). c The mass (arrow) is isoechoic to the liver CEUS scan at 3 min. d The mass (arrow) shows mild washout at 5 min
HCC with nodule-in-nodule pattern in a 60-year-old man with hepatitis B. a US scan shows a hypoechoic mass (short arrows) with a slightly hyperechoic focus (long arrow) in the liver. b CEUS scan in the arterial phase at 8 s shows hypovascularity of the mass (short arrows) with a hypervascular focus (long arrow), a nodule-in-nodule pattern. c The mass is not seen because of isoechogenicity at 90 s. d Focal washout (arrow) is only seen at 3 min where a hypervascular focus was seen in the arterial phase (a)
Detection of arterial-phase hypervascularity is crucial to make a noninvasive diagnosis of HCC. CEUS allows a real-time assessment of arterial-phase enhancement, eliminating the issue of inappropriate arterial-phase timing. CEUS often detects arterial-phase hypervascularity when CT or MRI fails to show this because of incorrect arterial-phase timing [17]. One of the most common indications of CEUS is to evaluate small, indeterminate, non-hypervascular nodules seen on CT or MRI. CEUS is often able to diagnose HCC by detecting hypervascularity in some of these lesions (Fig. 24.5), preventing an invasive biopsy [17–19]. It is often difficult to assess arterial-phase hypervascularity in markedly hyperintense nodules on unenhanced T1-weighted MR images, especially when there is an iron overload in the underlying liver with marked hypointensity (Fig. 24.6). CEUS can be used as a problem-solving method as the nodules are completely anechoic on contrast-specific mode before microbubble injection.
HCC in a 65-year-old woman with hepatitis C. a CT scan in the arterial phase shows a subtle hyperattenuating lesion (arrow) in the left lobe of the liver. b The lesion is not seen in the delayed phase. CT findings of the liver lesion are indeterminate. c CEUS scan in the arterial phase at 15 s shows a hypervascular mass (arrows) in the liver. d The mass is not seen due to isoechogenicity to the liver at 150 s. e The mass (arrows) shows clear washout at 270 s. CEUS findings are diagnostic of HCC
HCC in a 43-year-old man with thalassemia and secondary hemochromatosis. a Unenhanced T1-weighted MR scan shows a brightly hyperintense nodule (arrow) in the liver. Underlying liver is diffusely hypointense due to hemochromatosis. b, c The nodule (arrow) is hyperintense in the arterial-phase (b) and delayed phase (c) MR images. The findings are indeterminate as the evaluation of arterial-phase hypervascularity and washout is challenging. d US scan shows a slightly hyperechoic nodule with thin hypoechoic halo (arrow). e The nodule (arrow) is hypervascular in the arterial phase at 7 s on CEUS. f The nodule (arrow) shows slight washout at 135 s
Hypoenhancement or “washout” in the late phase is also an essential imaging feature for diagnosing HCC as typical HCC lack portal venous supply. Washout is more consistently seen on CEUS than CT or MRI due to the differing characteristics of the contrast material. CT or MRI may not show washout in malignant tumors with large extracellular space and high vascular permeability as the contrast material leaks and accumulates into the tumor interstitium, whereas microbubbles in CEUS are purely intravascular and show washout (Fig. 24.7) [17]. The intensity of enhancement of HCC in the late phase, however, generally decreases more slowly than cholangiocarcinoma or metastasis. Washout in HCC often begins later than 90 s after injection (Figs. 24.3 and 24.5) whereas metastases or intrahepatic cholangiocarcinomas consistently show rapid washout beginning before s 60 s (Fig. 24.8) [20–22]. In our study of 115 hypervascular HCC [23], only 50 % showed washout by 90 s. Extended evaluation over 4–5 min is important to characterize HCC by demonstrating “eventual” washout (Figs. 24.3 and 24.5). Washout timing is related to the pathologic differentiation of HCC: well-differentiated HCC tends to show later washout or no washout, whereas poorly differentiated HCC tends to show more rapid washout [23]. Therefore, no washout for 4–5 min should not be considered for a diagnostic finding of a benign lesion (Fig. 24.9). In fact, most new hypervascular nodules on CEUS detected during HCC surveillance are HCC regardless of washout if the nodules do not show the appearance of hemangioma [24]. However, a biopsy is needed to confirm HCC for hypervascular nodules without washout.
HCC in 56-year-old man with hepatitis B. a MR scan in the arterial phase shows an exophytic hypervascular nodule (arrow) in the liver. b The nodule (arrow) is isointense to the liver in the delayed phase without washout. c The nodule (arrow) is hypervascular in the arterial phase of CEUS. d The nodule (arrow) is isoechoic at 3 min. e The nodule (arrow) shows washout at 5 min, confirming the diagnosis of HCC
Intrahepatic CC in a 58-year-old man with hepatitis B. a CEUS scan in the arterial phase at 17 s shows a mass (arrow) with diffuse arterial-phase hypervascularity. b CEUS scan at 28 s still in the arterial-phase time frame shows washout (arrow). c CEUS scan at 280 s shows marked washout with a punched-out appearance (arrow)
HCC with no washout in a 53-year-old woman with hepatitis C. a CT scan in the arterial phase shows a hypervascular nodule (arrow) in the liver. b The nodule is not seen due to isoattenuation to the liver in the delayed phase. c CEUS scan in the arterial phase at 15 s shows a hypervascular nodule (arrows) in the liver. d. The nodule (arrow) remains hyperechoic on CEUS scan at 250 s. No washout is seen in either CT or CEUS
There is a small subset of HCC with no arterial-phase hypervascularity, including particularly those that are well differentiated. In our study of 112, HCC that were evaluated with CEUS, 23/112 (21 %) were well-differentiated and 9/23 (39 %) were not hypervascular [23]. These lesions occasionally show a transient hypoenhancement in the arterial phase followed by gradual enhancement and the lesions become isoechoic relative to the normal liver in the late phase (Fig. 24.1). These hypovascular HCC cannot be reliably differentiated from DN by imaging findings alone, requiring biopsy for confirmation.
Liver cirrhosis related to viral hepatitis is also identified as a risk factor for development of intrahepatic cholangiocarcinoma (CC) although the incidence of CC is much lower than that of HCC. Therefore, small CC is infrequently detected during HCC surveillance. Accurate imaging differentiation between CC and HCC, however, is important because the treatments for the two conditions are different. On CEUS, small CC usually shows arterial-phase hypervascularity and washout similar to HCC [25]. However, the diagnosis of intrahepatic CC can be suggested by CEUS in most cases, by demonstrating rim-like arterial-phase enhancement (Fig. 24.10) and/or rapid washout (<60 s) and/or a punched-out appearance of the washout at its first observation (Fig. 24.8) [20–22, 26]. Punched-out washout is not commonly seen in HCC and, if observed, follows an initial observation of weak washout. Combined CC and HCC in a single liver mass is rare and the clinical and imaging findings are determined by the dominant proportion of the histological component [27]. CEUS findings can be similar to CC when the CC component is dominant. Hepatic capsular retraction near the liver mass (Fig. 24.11) is a suggestive finding of CC [28] as it is rarely seen in HCC. Biopsy should be performed when these unusual enhancement patterns for HCC are observed on CEUS.
Intrahepatic CC in an 80-year-old man with liver cirrhosis related to primary sclerosing cholangitis. a CEUS scan in the arterial phase at 14 s shows a mass (arrows) with rim-like hyperenhancement in the periphery. b The enhancing rim (arrows) becomes thicker and slightly more hyperechoic compared to adjacent normal liver at 21 s. c, d Washout (arrows) is seen at 40 s (c) and progresses to a punched-out lesion (arrows) at 3 min (d)
Combined HCC and CC in a 36-year-old man with hepatitis B. a CT scan in the arterial phase shows a hypervascular mass (arrows) in the left lobe of the liver. b The mass (arrows) shows washout in the delayed phase. Note hepatic capsular retraction (short arrow) near the mass. c CEUS scan in the arterial phase at 10 s shows heterogeneous hypervascularity in the mass (arrows). d The mass (arrows) shows rapid washout at 40 s, which is unusual for HCC. e Washout progresses and the mass (arrows) is markedly hypoechoic at 180 s
RN form the essential component of a cirrhotic liver and are small and usually do not stand out on imaging. On grayscale US, numerous RN in cirrhotic livers are typically seen as coarse and heterogeneous liver with a nodular surface. Most RN are isoechoic to the parenchyma during all phases on CEUS. As DN have more histological atypia, abnormal arteries increase while normal arterial and portal supply decrease. The arterial and portal supplies to DN, therefore, are variable and inconsistent [29] (Fig. 24.12). As there is significant overlap of vascular supply between high-grade DN and well-differentiated HCC, imaging differentiation between the two is challenging and often unreliable [2]. Biopsy is often performed for the differentiation; however, the differential diagnosis in small needle biopsy specimens can be also challenging due to histological heterogeneity within the nodules. In the setting of a competing potentially fatal disease (i.e., cirrhosis), imaging follow-up instead of invasive biopsy is often applied for evaluating small borderline liver lesions [30].
Dysplastic nodule in a 39-year-old man with hepatitis B. a US scan shows a slightly hyperechoic nodule (arrows) in the liver. b CEUS scan in the arterial phase at 13 s shows decreased arterial-phase vascularity (hypovascular) within the nodule (arrows) relative to the liver. c The nodule is not seen due to isoechogenicity at 120 s
Hemangiomas are frequently detected during HCC surveillance. In our study [31], 43/184 (23 %) of newly detected nodules at HCC surveillance were hemangiomas. Diffuse hyperechogenicity on gray-scale US is a well-known typical finding of hemangioma. However, diffuse hyperechoic nodules are not specific for hemangioma in the setting of liver cirrhosis as DN or HCC with fatty metamorphosis can show similar findings (Fig. 24.13) and further evaluation should be performed [32]. Immediate performance of CEUS at the time of detection of such a nodule can achieve a diagnosis of hemangioma by demonstrating the characteristic enhancement pattern that includes peripheral nodular enhancement, gradual central fill-in, and sustained enhancement. This can avoid further imaging tests such as CT or MRI and reduces patient’s additional hospital visits and anxiety as well as medical cost [7, 33]. CEUS is also useful to demonstrate the characteristic enhancement pattern in fast filling hemangiomas which often show a nonspecific homogeneous enhancement in the arterial phase of CT or MRI (Fig. 24.14). Slow filling hemangiomas, on the other hand, can be seen as nonspecific hypoattenuating masses on multiphasic CT scan. CEUS can diagnose a hemangioma in those cases by utilizing highly sensitive detection of contrast enhancement and prolonged observation (Fig. 24.15) [34].
HCC in a 75-year-old man with hepatitis B. a US scan shows a homogeneously hyperechoic nodule (arrow) in the liver, which mimics the gray-scale appearance of hemangioma. b CEUS scan in the arterial phase at 27 s shows homogeneous hypervascularity within the nodule (arrow). c The nodule is not seen due to isoechogenicity at 125 s. d The nodule (arrow) shows washout at 240 s, confirming the diagnosis of HCC
Hemangioma in a 60-year-old woman with hepatitis B. a Contrast-enhanced T1-weighted MR scan in the arterial phase shows a slightly heterogeneous hyperenhancing nodule (arrow) in the liver. b The nodule (arrow) is homogenously hyperintense in the delayed phase. MR findings are indeterminate as the arterial-phase enhancement pattern is nonspecific and there is no washout. c–e CEUS scans at 9 (b), 10 (c), and 35 (d) seconds after injection of the contrast material show peripheral nodular enhancement with subsequent central fill-in in the nodule (arrows), which is diagnostic of hemangioma
Hemangioma in a 41-year-old woman with non-alcoholic steatohepatitis. a CT scan in the arterial phase shows a subtle hypoattenuating mass (arrow) in the liver. b The mass (arrow) is hypoattenuating in the delayed phase. CT findings are indeterminate. c, d CEUS scans at 15 (c) and 100 (d) seconds after injection of the contrast material show peripheral nodular enhancement with subsequent central fill-in in the nodule (arrows), which is diagnostic of hemangioma
Nontumorous arterioportal shunting is a common mimicker of malignancy in a cirrhotic liver and is frequently seen on multiphasic CT or MRI [35, 36]. It is typically wedge-shaped, peripherally located, and homogeneously hypervascular in the arterial phase. The lesion becomes isointense to the liver in the late phase and never shows washout. This potentially creates a pseudolesion as the differentiation of arterioportal shunting from HCC without washout is difficult. Nontumorous arterioportal shunting is not seen on gray-scale US as it is not a real parenchymal liver lesion. Therefore, CEUS is excellent to resolve this dilemma showing no abnormality in the presence of shunting. By comparison, if an HCC is present, ultrasound will show a nodule with appropriate CEUS characteristics [22].
4 Role of CEUS in HCC Diagnosis and Staging
Surveillance for HCC in high-risk patients is widely practiced particularly in endemic regions of hepatitis B and C, such as East Asia. Surveillance generally includes US at 6 month intervals. Further contrast-enhanced diagnostic imaging tests are performed when there is any new liver nodule 1 cm or larger found at surveillance US. The diagnosis of HCC can be made without biopsy when the nodules show typical findings on diagnostic imaging tests. Recent practice guidelines define a typical enhancement pattern of HCC as hypervascularity of the lesion in the arterial phase and negative enhancement (washout) of the lesion relative to the hepatic parenchyma in the portal venous or delayed phase [3–5].
There has been a controversy on the use of CEUS in international guidelines with exclusion in the most recent AASLD guidelines because of the claim that intrahepatic CC can mimic HCC with resultant misdiagnosis [3, 25]. However, subsequent rebuttal suggests that intrahepatic CC is relatively rare in liver cirrhosis and CEUS can depict typical findings of CC including arterial-phase rim enhancement (Fig. 24.10), rapid washout (<60 s), and/or a punched-out appearance in the late phase (Fig. 24.8) [20–22, 26, 37]. In fact, CEUS is still actively used as one of the diagnostic tests for HCC in other jurisdictions (for example, Italy, Japan, and Canada) and in large academic institutions where CEUS is available. CEUS is very well accepted by clinicians as it often plays a crucial role in diagnosing indeterminate nodules on CT or MRI and in diagnosing liver nodules in patients with renal failure (Fig. 24.16) [33]. Recently a CEUS working group has been formed in Liver Imaging Reporting and Data System (LI-RADS) by the American College of Radiology. LI-RADS aims to reduce imaging interpretation variability and errors to optimize diagnosis of HCC [38].
HCC in a 52-year-old man with hepatitis C cirrhosis and renal failure. a US scan shows a slightly hypoechoic nodule (arrow) in the liver. Contrast-enhanced CT or MRI could not be performed due to renal failure. b CEUS scan in the arterial phase at 24 s shows homogeneous hypervascularity within the nodule (arrow). c The nodule (arrow) shows washout at 90 s, confirming the diagnosis of HCC
Multiphasic CT or MRI are proper staging techniques for HCC and should be performed once the diagnosis of HCC is made. There are occasional cases, however, where critical staging information such as tumor thrombosis within the portal or hepatic vein is unclear on CT or MRI. The presence of malignant thrombus of portal or hepatic veins in patients with HCC is a critical determinant of tumor staging and prognosis as it directly influences treatment strategy [39, 40]. Bland thrombus can be found in 4.5–26 % of patients with chronic liver disease and up to 42 % of patients with HCC [41]. Moreover, malignant venous thrombus can occur in the absence of primary parenchymal HCC, either as an intravascular growth of this neoplasm [42] or after treatments such as ablation or chemoembolization, as a first indicator of recurrence.
CEUS is excellent in the differentiation of tumor thrombosis and benign thrombosis in the portal or hepatic veins. Tumor thrombi invariably show heterogeneous enhancement and linear, irregular feeding vessels after injection of the microbubbles in the arterial phase (Fig. 24.17), whereas benign thrombi are avascular (Fig. 24.18). In our study of 50 HCC patients with 38 malignant and 13 benign venous thrombosis, the area under the curve (AUC) at receiver operating characteristic (ROC) analysis was 0.947 and 0.958 by two independent blind readers. Demonstration of arterial flow within the thrombi is specific for malignant thrombosis; however, it is important to be aware that recanalized benign thrombosis may show enhancement in the portal venous phase [8].
Tumor thrombosis in the portal vein in a 70-year-old man with HCC and hepatitis C. a US scan shows a hypoechoic tubular lesion (arrows) in the liver, representing thrombosis within the portal vein. b CEUS scan in the arterial phase at 15 s shows strong, homogeneous enhancement within the portal venous thrombi (arrows). c There is mild washout (arrows) at 200 s, confirming the diagnosis of tumor thrombosis in the portal vein
Benign thrombosis in the portal vein in a 66-year-old man with hepatitis C and history of liver transplantation for HCC. a US scan shows a hyperechoic thrombosis (arrow) within the portal vein near the hilar hilum in the liver. b, c There is no contrast enhancement within the thrombus (arrow) on CEUS scans at 10 s (b) and 30 s (c), confirming the diagnosis of benign thrombosis in the portal vein
5 Post-treatment Monitoring of HCC
Local ablative therapy such as radiofrequency (RFA) or microwave ablation has become one of the main treatment modalities for patients with small HCC. RFA is also frequently performed as a bridge therapy for patients on the waiting list for liver transplantation. Real-time gray-scale US scan is most frequently used for the guidance of RFA procedures; however, there are uncommon cases with poor visibility on US scan. CEUS can be extremely helpful in these situations to localize the lesion by demonstrating the arterial-phase hypervascularity and washout. The use of a dual-imaging mode, which displays gray-scale imaging and contrast-specific imaging side-by-side, is critical to visualize the lesion and the needle simultaneously [43]. A routine use of pre-procedure CEUS can reduce the number of incomplete or erroneous RFA significantly. Fusion imaging techniques that can coordinate the CEUS images with CT/MRI images are also helpful to localize difficult lesions and reduce the overall procedure time [44]. One of the unique advantages of CEUS in RFA is that the microbubbles can be repeatedly injected over short intervals as necessary. For example, CEUS can be performed just before the placement of the ablation needle and repeated after the needle placement to ensure its proper location. CEUS can be also performed after ablation to determine the completeness of the therapy. Repeat ablation can be immediately performed if there is any residual enhancing tumor [45, 46].
Multiphasic CT or MRI is typically performed in 1 month after ablative therapy for HCC in our institution. CT or MRI is an appropriate restaging modality as it provides information on the rest of the liver, vascular invasion, lymph nodes, and any extrahepatic metastasis better than CEUS. CEUS is a useful alternative modality when the patient has renal failure with contraindication for CT or MRI contrast agent. One of the limitations of CEUS is that it is not possible to scan the whole liver in the arterial phase. Repeated sweepings through the entire liver in the late phase should be routinely performed to detect any unexpected recurrent HCC which is not detected on gray-scale US. While CT or MRI is useful for restaging HCC after ablation, there are occasional challenging cases with difficulty of indeterminate imaging findings. CEUS is an excellent problem-solving method in these cases [43]. Subsequent follow-up after therapy is variable and may include CEUS and/or MRI.
Hypervascular abnormalities adjacent to the ablation zone are common and can be residual HCC or benign perfusion abnormalities related to the ablation procedures [47]. These benign perfusion abnormalities adjacent to the ablation zone are frequently seen and may persist several months after the RFA procedure. The differentiation between benign perfusion abnormalities and recurrent HCC can be difficult when washout is not clearly seen on CT or MRI. Benign perfusion abnormalities are not seen on gray-scale US as they are not real lesions. Marginal recurrence of HCC is usually seen as a focal gray-scale abnormality adjacent to the ablation zone on unenhanced ultrasound. Subsequent CEUS shows hypervascularity followed by washout, confirming the presence of recurrent HCC and its exact location on grayscale US, which is extremely helpful for repeat ablation therapy (Fig. 24.19).
Marginal tumor recurrence in a 63-year-old man who underwent radiofrequency ablation for HCC. a US scan shows a hyperechoic ablation zone (asterisk) and an adjacent mixed-echo lesion (arrows) in the liver. b CEUS scan in the arterial phase shows hypervascularity (arrows) within the mixed-echo lesion adjacent to the ablation zone (asterisk). c CEUS scan at 70 s shows washout (arrows), confirming the presence of marginal tumor recurrence adjacent to the ablation zone (asterisk). Repeat radiofrequency ablation was performed under CEUS guidance (not shown)
Recurrent HCC adjacent to the ablation zone is occasionally non-hypervascular on CT or MRI due to mistiming of the arterial phase. Subtle hypervascularity of recurrent HCC can also be obscured by adjacent perfusion changes. CEUS is useful to further assess hypoattenuating/hypointense abnormalities adjacent to the ablation zone on CT or MRI. CEUS can often show the presence of hypervascularity of the lesion, utilizing the advantage of real-time assessment of lesion perfusion (Fig. 24.20) [43].
Recurrent HCC in a 68-year-old man who underwent radiofrequency ablation for HCC. a CT scan in the arterial phase shows a subtle hypoattenuating lesion (arrow) medical to the ablation zone (asterisk). b The lesion (arrow) is hypoattenuating in the delayed phase. CT findings are indeterminate. c CEUS scans in the arterial phase at 12 s shows a focal hypervascular lesion (arrow). D The lesion (arrow) shows washout at 125 s, confirming the diagnosis of recurrent HCC
6 Conclusion
CEUS is an excellent imaging technique with several unique advantages over CT or MRI for the imaging of nodules in a cirrhotic liver. These advantages in the arterial phase include the real-time depiction of specific features of benign hepatic nodules, resolution of arterioportal shunts, resolution of absent enhancement on mistimed CT and MR scan, and sensitive demonstration of hypervascularity in HCC. Absence of washout of suspect HCC on CT or MR scan may also be resolved by CEUS. Therefore, CEUS can be effectively used as one of the diagnostic tests for HCC, differentiation between benign and malignant venous thrombosis, immediate diagnosis of hemangioma, and pre- or post-RFA evaluation for HCC. Added to this is the absence of nephrotoxicity of CEUS as well as the standard benefits of US including absence of ionizing radiation, and excellent patient compliance. Nonetheless, CEUS is operator-dependent and the performance of liver CEUS requires extensive hands-on experience.
References
Matsui O. Detection and characterization of hepatocellular carcinoma by imaging. Clin Gastroenterol Hepatol. 2005;3:S136–40.
Choi BI, Lee JM, Kim TK, et al. Diagnosing borderline hepatic nodules in hepatocarcinogenesis: imaging performance. AJR Am J Roentgenol. 2015;205:10–21.
Bruix J, Sherman M, American Association for the Study of Liver D. Management of hepatocellular carcinoma: an update. Hepatology. 2011;53:1020–22.
European Association for the Study of the L, European Organisation for R and Treatment of C. EASL-EORTC clinical practice guidelines: management of hepatocellular carcinoma. J Hepatol. 2012;56:908–43.
Omata M, Lesmana LA, Tateishi R, et al. Asian Pacific Association for the study of the liver consensus recommendations on hepatocellular carcinoma. Hepatol Int. 2010;4:439–74.
Khalili K, Kim TK, Jang HJ, et al. Optimization of imaging diagnosis of 1–2 cm hepatocellular carcinoma: an analysis of diagnostic performance and resource utilization. J Hepatol. 2011;54:723–8.
Lanka B, Jang HJ, Kim TK, et al. Impact of contrast-enhanced ultrasonography in a tertiary clinical practice. J Ultrasound Med. 2007;26:1703–14.
Raza SA, Jang HJ, Kim TK. Differentiating malignant from benign thrombosis in hepatocellular carcinoma: contrast-enhanced ultrasound. Abdom Imaging. 2014;39:153–61.
Brannigan M, Burns PN, Wilson SR. Blood flow patterns in focal liver lesions at microbubble-enhanced US. Radiographics. 2004;24:921–35.
Korenaga K, Korenaga M, Furukawa M, et al. Usefulness of sonazoid contrast-enhanced ultrasonography for hepatocellular carcinoma: comparison with pathological diagnosis and superparamagnetic iron oxide magnetic resonance images. J Gastroenterol. 2009;44:733–41.
Piscaglia F, Bolondi L. The safety of sonovue in abdominal applications: retrospective analysis of 23188 investigations. Ultrasound Med Biol. 2006;32:1369–75.
Wilson SR, Jang HJ, Kim TK, et al. Real-time temporal maximum-intensity-projection imaging of hepatic lesions with contrast-enhanced sonography. AJR Am J Roentgenol. 2008;190:691–5.
Nicolau C, Catala V, Vilana R, et al. Evaluation of hepatocellular carcinoma using SonoVue, a second generation ultrasound contrast agent: correlation with cellular differentiation. Eur Radiol. 2004;14:1092–9.
Quaia E, Calliada F, Bertolotto M, et al. Characterization of focal liver lesions with contrast-specific US modes and a sulfur hexafluoride-filled microbubble contrast agent: diagnostic performance and confidence. Radiology. 2004;232:420–30.
Wilson SR, Burns PN. An algorithm for the diagnosis of focal liver masses using microbubble contrast-enhanced pulse-inversion sonography. AJR Am J Roentgenol. 2006;186:1401–12.
Numata K, Fukuda H, Nihonmatsu H, et al. Use of vessel patterns on contrast-enhanced ultrasonography using a perflubutane-based contrast agent for the differential diagnosis of regenerative nodules from early hepatocellular carcinoma or high-grade dysplastic nodules in patients with chronic liver disease. Abdom Imaging. 2015;40:2372–83.
Wilson SR, Kim TK, Jang HJ, et al. Enhancement patterns of focal liver masses: discordance between contrast-enhanced sonography and contrast-enhanced CT and MRI. AJR Am J Roentgenol. 2007;189:W7–12.
Maruyama H, Takahashi M, Ishibashi H, et al. Contrast-enhanced ultrasound for characterisation of hepatic lesions appearing non-hypervascular on CT in chronic liver diseases. Br J Radiol. 2012;85:351–7.
Takahashi M, Maruyama H, Shimada T, et al. Characterization of hepatic lesions (</=30 mm) with liver-specific contrast agents: a comparison between ultrasound and magnetic resonance imaging. Eur J Radiol. 2013;82:75–84.
Bhayana D, Kim TK, Jang HJ, et al. Hypervascular liver masses on contrast-enhanced ultrasound: the importance of washout. AJR Am J Roentgenol. 2010;194:977–83.
Han J, Liu Y, Han F, et al. The degree of contrast washout on contrast-enhanced ultrasound in distinguishing intrahepatic cholangiocarcinoma from hepatocellular carcinoma. Ultrasound Med. Biol. 2015;41(12):3088–95.
Jang HJ, Kim TK, Burns PN, et al. CEUS: an essential component in a multimodality approach to small nodules in patients at high-risk for hepatocellular carcinoma. Eur J Radiol. 2015;84:1623–35.
Jang HJ, Kim TK, Burns PN, et al. Enhancement patterns of hepatocellular carcinoma at contrast-enhanced US: comparison with histologic differentiation. Radiology. 2007;244:898–906.
Jang HJ, Kim TK, Wilson SR. Small nodules (1–2 cm) in liver cirrhosis: characterization with contrast-enhanced ultrasound. Eur J Radiol. 2009;72:418–24.
Vilana R, Forner A, Bianchi L, et al. Intrahepatic peripheral cholangiocarcinoma in cirrhosis patients may display a vascular pattern similar to hepatocellular carcinoma on contrast-enhanced ultrasound. Hepatology. 2010;51:2020–9.
Li R, Yuan MX, Ma KS, et al. Detailed analysis of temporal features on contrast enhanced ultrasound may help differentiate intrahepatic cholangiocarcinoma from hepatocellular carcinoma in cirrhosis. PLoS ONE. 2014;9:e98612.
Yin X, Zhang BH, Qiu SJ, et al. Combined hepatocellular carcinoma and cholangiocarcinoma: clinical features, treatment modalities, and prognosis. Ann Surg Oncol. 2012;19:2869–76.
Kim TK, Choi BI, Han JK, et al. Peripheral cholangiocarcinoma of the liver: two-phase spiral CT findings. Radiology. 1997;204:539–43.
Lim JH, Cho JM, Kim EY, et al. Dysplastic nodules in liver cirrhosis: evaluation of hemodynamics with CT during arterial portography and CT hepatic arteriography. Radiology. 2000;214:869–74.
Khalili K, Kim TK, Jang HJ, et al. Indeterminate 1–2-cm nodules found on hepatocellular carcinoma surveillance: biopsy for all, some, or none? Hepatology. 2011;54:2048–54.
Kim TK, Lee KH, Jang HJ, et al. Analysis of gadobenate dimeglumine-enhanced MR findings for characterizing small (1–2-cm) hepatic nodules in patients at high risk for hepatocellular carcinoma. Radiology. 2011;259:730–8.
Caturelli E, Pompili M, Bartolucci F, et al. Hemangioma-like lesions in chronic liver disease: diagnostic evaluation in patients. Radiology. 2001;220:337–42.
Kim TK, Jang HJ. Contrast-enhanced ultrasound in the diagnosis of nodules in liver cirrhosis. World J Gastroenterol. 2014;20:3590–6.
Kim TK, Jang HJ, Wilson SR. Benign liver masses: imaging with microbubble contrast agents. Ultrasound Q. 2006;22:31–9.
Kim TK, Choi BI, Han JK, et al. Nontumorous arterioportal shunt mimicking hypervascular tumor in cirrhotic liver: two-phase spiral CT findings. Radiology. 1998;208:597–603.
Yu JS, Kim KW, Jeong MG, et al. Nontumorous hepatic arterial-portal venous shunts: MR imaging findings. Radiology. 2000;217:750–6.
Barreiros AP, Piscaglia F, Dietrich CF. Contrast enhanced ultrasound for the diagnosis of hepatocellular carcinoma (HCC): comments on AASLD guidelines. J Hepatol. 2012;57:930–2.
Mitchell DG, Bruix J, Sherman M, et al. LI-RADS (liver imaging reporting and data system): summary, discussion, and consensus of the LI-RADS management working group and future directions. Hepatology. 2015;61:1056–65.
Sakata J, Shirai Y, Wakai T, et al. Preoperative predictors of vascular invasion in hepatocellular carcinoma. Eur J Surg Oncol (EJSO). 2008;34:900–5.
Takizawa D, Kakizaki S, Sohara N, et al. Hepatocellular carcinoma with portal vein tumor thrombosis: clinical characteristics, prognosis, and patient survival analysis. Dig Dis Sci. 2007;52:3290–5.
Ogren M, Bergqvist D, Bjorck M, et al. Portal vein thrombosis: prevalence, patient characteristics and lifetime risk: a population study based on 23,796 consecutive autopsies. World J Gastroenterol. 2006;12:2115–9.
Lim JH, Auh YH. Hepatocellular carcinoma presenting only as portal venous tumor thrombosis: CT demonstration. J Comput Assist Tomogr. 1992;16:103–6.
Kim TK, Khalili K, Jang HJ. Local ablation therapy with contrast-enhanced ultrasonography for hepatocellular carcinoma: a practical review. Ultrasonography. 2015;34:235–45.
Lee MW. Fusion imaging of real-time ultrasonography with CT or MRI for hepatic intervention. Ultrasonography. 2014;33:227–39.
Dill-Macky MJ, Asch M, Burns P, et al. Radiofrequency ablation of hepatocellular carcinoma: predicting success using contrast-enhanced sonography. AJR Am J Roentgenol. 2006;186:S287–95.
Solbiati L, Tonolini M, Cova L. Monitoring RF ablation. Eur Radiol. 2004;14(Suppl 8):P34–42.
Catalano O, Esposito M, Nunziata A, et al. Multiphase helical CT findings after percutaneous ablation procedures for hepatocellular carcinoma. Abdom Imaging. 2000;25:607–14.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2016 Springer International Publishing Switzerland
About this chapter
Cite this chapter
Kim, T.K., Jang, HJ., Wilson, S.R. (2016). Ultrasound of Hepatocellular Carcinoma: The Important Contribution of Contrast Enhancement. In: Carr, B. (eds) Hepatocellular Carcinoma. Current Clinical Oncology. Springer, Cham. https://doi.org/10.1007/978-3-319-34214-6_24
Download citation
DOI: https://doi.org/10.1007/978-3-319-34214-6_24
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-34212-2
Online ISBN: 978-3-319-34214-6
eBook Packages: MedicineMedicine (R0)