Abstract
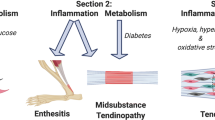
Painful and non-healing musculoskeletal disorders, eg. tendinopathy, pose a tremendous burden on society and the quality of life for patients. New advances in the understanding of connective tissue disorders such as tendinopathy reveal that common health problems such as obesity, atherosclerosis, hormonal dysfunctions and diabetes mellitus are closely linked to the metabolism of components of the musculoskeletal system, particularly tendons. As tendons function as multi-component “organ systems” (Muscle-TMJ-Tendon-Enthesis to Bone), tendons can be influenced directly, or indirectly via, for instance, alterations to muscle. However, this volume/set of chapters focus mainly on the tendon.
Emerging findings in musculoskeletal research have established important new links in our understanding of tendon metabolism. Thereby, the function of the neuroendocrine/-immune axis, as well as supply of neuro-vascular factors, can be directly linked to the quality of tendon metabolism.
Since some conditions, eg. atherosclerosis and diabetes mellitus, are more common in individuals as they age, and aging can also affect pain and tissue repair, convergence of such complications will potentially exert an increasingly significant impact on tendons as the demographics of many societies change with expanding percentages of the populations >60–65 years of age.
Comorbidities related to metabolic dysfunction have to be identified early in patients with musculoskeletal disorders, such as acute tendon injuries or chronic tendinopathy, for therapeutic considerations regarding both operative and non-operative treatment protocols. Necessary interactions between researchers and clinicians with different subspecialties have to be initiated in order to optimize tissue metabolism for improved healing potentials.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
Introduction
Musculoskeletal conditions affect more
than 1.7 billion people worldwide and are the second greatest cause of disability, and as such have the 4th greatest impact on the overall health of the world population.
Work-related musculoskeletal disorders (MSDs) in the US are estimated at $20 billion a year in direct costs, and up to five times more in indirect costs for MSD-related workers’ compensation. In addition, there is the substantial toll on affected workers who develop significant difficulties in performing simple upper extremity tasks, according to US Occupational Safety and Health Administration (OSHA 2014).
However, when it comes to current available treatment alternatives for chronic MSD, such as tendinopathy, there is a lack of proven efficient therapies partly due to poor understanding of the mechanisms leading to the development and progression of connective tissue disorders. The purpose of this integration chapter is to summarize some of the emerging new knowledge on how metabolic disturbances and associated disorders can influence tendon connective tissue homeostasis.
Our aim is to address how this new integrative knowledge will lead to novel therapeutic approaches targeting underlying metabolic deficits, and result in optimized connective tissue healing and reductions in the chronic pain of these conditions. MSD, especially with regard to tendons, will have an expanding significant impact on our society considering our more active lifestyles during aging, as well as the detrimental effects of aging on tendon connective tissue homeostasis (see Chap. 24).
The emerging knowledge in this book, and integrated in this chapter, is essential for all clinicians, researchers and those interested in musculoskeletal disorders. To advance the care of people with unmet needs, we must collaborate on all levels to address the underlying tissue pathophysiology in a targeted manner.
Basic Tendon Biology and Anatomy
Tendon anatomy and structure are optimized to fulfil their functional role in differing environments, with highly aligned and abundant collagen fibers providing the highest tensile strength required for efficient force transfer. However, in order to fully understand tendon structural relationships, the specific functional roles of each component of the tendon matrix must be determined (see Chap. 1). Presently, little is still known about the metabolically very active sheaths around the tendon, which work in concert with the tendons to provide targeted gliding, and the interfascicular matrix .
Although the metabolic activity of the tendon is generally low as compared to other tissues, the major metabolic regulatory factor is mechanical loading. Subsequent to loading or unloading, mechanotransduction and molecular anabolic/catabolic signalling result in tissue adaptations in the tendon, which particularly during youth and adolescence (growth and maturation), produce tendon size adjustments (see Chap. 2). Adaptive responses may vary in different tendon compartments or environments, and still more knowledge is needed regarding loading responses in the tendon and in surrounding structures.
Tendons, like nearly all other connective tissues, subscribe to the “use it or lose it” paradigm. Thus, tendons require consistent loading to maintain their functioning set point. Unlike ligaments that are passive structures, tendons are active connective tissues, which in many environments, work near their mechanical limit and thus are perhaps exposed to higher risks for damage (see Chaps. 1 and 2). Such risks can lead to compromised function, pain, and impaired repair mechanisms when impacted by metabolic derangements such as discussed in specific chapters. That is, metabolic disorders can exacerbate risk for tendon compromise and development and progression of certain types of MSD. The impact of metabolic disorders can be on the actual tendon proper, or even at the level of tendon regulatory elements (e.g. nerves and blood supply).
Innervation and blood supply of intact healthy tendons are localized in the surrounding interfascicular matrix of the tendon proper, i.e. paratenon, endotenon and epitenon, whereas the tendon proper is practically devoid of a neuronal supply. This anatomical finding reflects that tendon metabolism is primarily regulated from the tendon envelope or surrounding tissues (see Chaps. 4 and 5). In tendon healing and tendinopathy, however, extensive blood vessel and nerve ingrowth occur into the tendon proper, followed by a time-dependent expression of different neuronal mediators. The integrated action of the neuro-vascular system in tendon metabolism is an emerging research field, which may provide both molecular and clinical targets for future therapies. Given this scenario, disruption of tendon regulation via the “envelope” or the tendon proper by metabolic disorders could dramatically impact tendon function and development of symptoms such as pain via neuropeptides and sensory nerves.
Specific recent knowledge demonstrates how neuronal factors, such as substance P can recruit tendon stem/progenitor cells (TSCs) to sites of repair or tendinopathy. The discovery of tendon TSCs in itself is a remarkable advancement in the tendon research field (see Chap. 6). TSCs play a critical role in tendon physiology and metabolism as well as pathology such as tendinopathy. Additionally, TSCs could potentially be used for tendon tissue engineering in vitro, and serve as a promising source of cell-based therapies.
To better elucidate the role of TSCs, as a potential source for enhancing tendon regeneration and to engineer new tendon, tenogenic differentiation and neotissue formation has to be better understood. Thus, the structure-property relationships of embryonic tendon as well as tendon progenitor cell function during development has been studied in detail (see Chap. 6). The potential to guide tenogenic differentiation of adult mesenchymal stem cells with factors that play integral roles in tenogenic differentiation of embryonic tendon progenitor cells during normal development has been demonstrated.
Further studies are also needed to better elucidate the role of TSCs, as a potential source for enhancing endogenous repair. Emerging knowledge has shed light on fundamental tenocyte signalling pathways (see Chap. 7). Future work must also reveal pathways that can be manipulated to prevent matrix degradation, and even support functional matrix replacement with the use of TSCs to promote proper tendon metabolism (see Chap. 8).
Tendon Disorders Associated with Metabolism and Metabolic Disorders
Still today, there is a big gap in our knowledge of how basic research translates into the clinical settings dealing with lifestyle diseases, e.g. hypercholesterolemia, affecting tendon metabolism. If we aim to “solve” the riddle of musculoskeletal disorders, especially tendon problems, we need first to understand via which pathways lifestyle affects tendon metabolism in order to develop targeted means to address the connective tissue pathophysiology on a molecular level.
Let us start to look at genetic disorders by which small genetic variations in many individuals contribute to an increased susceptibility of sustaining a tendon injury. The future is already out there, online, with genetic testing, although the clinical knowledge and implications are sparse. To date, 18 genetic intervals and 32 polymorphisms have been associated with risk of tendon pathologies, which relate to collagen isoforms and variants of structural matrix homeostasis (see Chap. 9). However, these associations cannot be viewed independently and have in the future to be verified by other scientific approaches.
Other genetic disorders involve defects in genes that code for enzymes involved in metabolic pathways (see Chap. 10). The most well-known diseases are familial hypercholesterolemia leading to tendon xanthomas, alkaptonuria resulting in acid accumulation, whereby tendons get a typical ochre/yellow pigmentation (ochronosis), with ensuing inflammation, calcification and rupture, and hypophosphatasia associated with tendon deposition of hydroxyapatite crystals. However, there are likely many more subclinical diseases that may never get diagnosed, but which may provide an increased susceptibility or risk to develop subsequent tendon disorders.
One such important metabolic condition, which may not be readily diagnosed, but cause repeated tendon problems, is hyperuricemia (see Chap. 11). Hyperuricemia and monosodium urate crystal deposition can challenge tendon homeostasis because of their potential to induce inflammation in the host. Today, there is little information available regarding hyperuricemia-mediated adjuvancity in tendinopathy. More knowledge about the interactions of eg. urate with both innate immune and local cells, may help researchers and clinicians to determine if hyperuricemia is a potential target for effective treatments for a subset of tendon problems.
Other metabolic disturbances associated with specific substances that can affect tendon homeostasis include the thyroid hormones (see Chap. 12). Autoimmune thyroid diseases can lead to connective tissue disorders, as thyroid hormones can alter tendon metabolism. Other disorders of thyroid function have so far been investigated only for rotator cuff calcific tendinopathy and tears. Further research is needed to clarify the role of thyroid hormones in the onset and progression of tendinopathies.
The endocrine system holds a strong control on tendon metabolism not only via thyroid hormones, but also via sex hormones (see Chap. 13). Thus, in active young female athletes, physiological high concentrations of estrogen may lead to increased risk for injuries due to reduced fibrillar crosslinking and enhanced joint laxity. Testosterone, on the other hand, augments tendon stiffness due to an enhanced tendon collagen turnover and collagen content. Testosterone steroid injections, among many other side-effects, often result in tendon ruptures. However, the specific effects of individual hormones on tendon metabolism are not yet fully elucidated and still need further study. As well, since the natural environment contains multiple hormones and their respective receptors, analysis of individual hormone effects do not reflect the in vivo situation.
Hypercholesterolemia can exist both as hereditary dyslipidemias and as a result of lifestyle. However, associations between elevated total cholesterol and tendon problems exist in all these patients. High cholesterol environments have been demonstrated to alter tendon biomechanical properties with a few underlying mechanisms explored, showing eg.: altered protein synthesis; dysfunctional local extracellular matrix composition and turnover; and inflammatory gene expression (see Chap. 14). Future research within this area would also benefit from incorporation of additional clinically better translatable study elements.
In addition to hypercholesterolemia , obesity has been demonstrated to exert harmful effects on tendons (see Chap. 15). The pathogenesis being multi-factorial including overload, attributable to the increased body weight, and systemic factors such as bioactive peptides (e.g. chemerin, leptin, adiponectin) that contribute to a chronic, sub-clinic, low-grade inflammation (e.g. metabolic syndrome). Therefore, personalized training programs with regular check-ups are important components for the effective treatment of tendon pathology in such individuals.
Adiposity is also clearly linked to pre-diabetes and diabetes mellitus, conditions which have well documented detrimental effects on both tendon homeostasis, as well as tendon healing. Furthermore, one of the first sites of insulin resistance in obesity is in muscles, which in turn drive tendon activity. The regenerative capability of tendons is compromised in diabetes with corresponding expressional changes in collagens, matrix metalloproteinases and various inflammatory and growth mediators and their receptors (see Chap. 16 and 17). Another specific factor associated with diabetes that affects the mechanical properties of the tendon is the formation of advanced glycation end-products that lead to cross-links with collagen extracellular matrix (see Chap. 18).
So how do we treat patients with diabetes mellitus and tendon problems? Exercise is and likely will become the most important early and non-invasive intervention for these patients. However, the prescribed exercise has to be individually managed by an experienced, trained physiotherapist who is aware of all different musculoskeletal interactions associated with diabetes mellitus (see Chap. 19). A holistic approach should be used to optimize musculotendinous function, including a comprehensive exercise prescription addressing strength, flexibility, and aerobic fitness.
An important question remaining is related to whether the above mentioned metabolic disorders partly may act via inflammatory pathways to affect tendon homeostasis. Advances in our understanding of the basic science of inflammation have provided further insight into its potential role in specific forms of tendon disease, and the motive powers such as excessive mechanical stresses and systemic inflammatory diseases (see Chap. 20).
Inflammatory pathways are additionally involved in the pathogenesis of deep venous thrombosis (DVT), which may occur in up to 50 % of the patients with Achilles tendon rupture – a late stage complication of tendinopathy. DVT has recently been demonstrated as an independent predictive factor for impaired patient outcome at 1 year after Achilles tendon rupture, possibly via negative influences on tendon healing by impaired blood circulation (see Chap. 21). These findings suggest that specific interventions are warranted to prevent DVT. Thus, recently adjuvant treatments with intermittent pneumatic compression applied during lower limb immobilization was demonstrated to reduce the incidence of DVT.
An often-underestimated risk factor for tendon disorders is the influence of intake of different drugs used as prescribed medications (see Chap. 22). Thus, there can be detrimental side-effects of drugs on the tendon, including both tendinopathy and the risk for tendon ruptures. Four main drug classes have been reported to be associated with disturbed tendon metabolism: (1) Corticosteroids, (2) Chinolon antibiotics, (3) Aromatase inhbitors, and (4) Statins (HMG-CoA-reductase inhibitors). The intake of these drugs may increase tendon risk for compromise directly, or indirectly via a concurrent factor such as tendon loading, leading to detrimental effects on tendon integrity.
Corticosteroids (Glucocorticoids) are still widely used to relieve a wide variety of musculoskeletal disorders is. However, the negative influences of glucocorticoids on tendon metabolism are compelling (see Chap. 23). Glucocorticoids reduce tendon derived cell proliferation, reduce extracellular matrix synthesis, contribute to collagen disorganisation and inflammatory cell infiltration and negatively affect the mechanical properties of tendons. Therefore, glucocorticoids should be used with extreme caution in treating tendon problems.
Consider the aging population, which at the same time of life has increasing demands for an active lifestyle. This combination will lead to an increased pressure on the healthcaresystem to provide help for musculoskeletal and tendon disorders. Recent evidence clearly demonstrate increased susceptibility of tendons to injury with advancing age (see Chap. 24). These challenges suggest that we need a much better understanding of common pathways of aging and alterations to tendon homeostasis.
Novel Therapies That May Affect Tendon Metabolism
A recent highly popularized therapy for tendon disorders are injections with platelet-rich plasma (PRP). The background for PRP therapy is that platelet degranulation leads to a release of various growth factors and cytokines, which in experimental studies have shown positive effects on tendon metabolic activity. However, when it comes to clinical studies the evidence from the literature demonstrates that PRP does not lead to improved patient-reported outcomes (see Chap. 25). Therefore, currently PRP should only be used in experimental and clinical studies as to further explore if PRP has a role for treating tendon disorders.
Another increasingly used treatment for tendon disorders are shockwave treatments , which have accumulating clinical evidence for their clinical effectiveness (see Chap. 26). The few underlying mechanisms that have been explored relate to destruction of calcifications, pain relief, mechanotransduction-initiated tissue regeneration, and remodeling of the tendon. However, the heterogeneity of shockwave systems, treatment protocols and study populations, and the fact that there seems to be responders and non-responders, warrants further basic and clinical research.
Since obesity, hyperlipidemia, hyperuricemia, diabetes mellitus, and likely also inflammation in general, affect tendon metabolism negatively, it would be logical to assume that the diet could influence, at least indirectly, several tendon disorders (see Chap. 27). Today, however, the direct effects of diet on tendon metabolism, as well as long-term indirect effects on tendon disorders, are for the most part, completely unknown.
Conclusion
Today there are still no effective drug therapies for many painful and non-healing musculoskeletal disorders, eg. tendinopathy, which negatively affect the quality and functionality of life for millions of people. There is an immense need for a more holistic understanding of the underlying causes of such connective tissue problems. Recent advances in musculoskeletal research have established important new links in our understanding of how tendon disorders are linked to diseases and conditions associated with metabolic disturbances such as obesity, atherosclerosis, hormonal dysfunctions, inflammation and diabetes mellitus. However, there is still a great need for further mapping of the molecular pathways involved, as well as to characterise the extent of metabolically associated disorders in patients. Further challenging tasks are to identify biomarkers of disease and biomarkers predictive of treatment response. A prerequisite for the success of research in this area is a close interaction between researchers and clinicians with various subspecialties in order to identify underlying targets for new therapies.
The individual chapters in this book address many of the individual elements discussed here in more detail. The key to success going forward will be to both understand the individual factors and their interplay to impact subsets of people to increase risk for tendinopathies and to contribute to unique and/or common pathways for progression and resolution of these tendon disorders.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2016 Springer International Publishing Switzerland
About this chapter
Cite this chapter
Ackermann, P.W., Hart, D.A. (2016). General Overview and Summary of Concepts Regarding Tendon Disease Topics Addressed Related to Metabolic Disorders. In: Ackermann, P., Hart, D. (eds) Metabolic Influences on Risk for Tendon Disorders. Advances in Experimental Medicine and Biology, vol 920. Springer, Cham. https://doi.org/10.1007/978-3-319-33943-6_28
Download citation
DOI: https://doi.org/10.1007/978-3-319-33943-6_28
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-33941-2
Online ISBN: 978-3-319-33943-6
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)