Abstract
Citrate regional anticoagulation in continuous renal replacement therapy (CRRT) is a relatively recent technique, which was developed to reduce the complications of anticoagulation in critically ill patients at high risk of bleeding. Citrate anticoagulation was demonstrated to reduce the bleeding and the overall complication rate in critically ill patients on CRRT, without shortening circuit survival. Citrate anticoagulation is the only anticoagulation technique that demonstrated to reduce mortality in critically ill patients with acute kidney injury, when compared with other techniques. With technological improvement and development of more reliable protocols and management algorithms, citrate could become the first choice for anticoagulation during CRRT in critically ill patients.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Acute Kidney Injury
- Unfractionated Heparin
- Continuous Renal Replacement Therapy
- Trisodium Citrate
- Citrate Regional Anticoagulation
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
1 General Principles
Acute kidney injury (AKI) is an independent predictor of mortality in critically ill patients [1]. Continuous renal replacement therapy (CRRT) developed as a treatment for renal failure in patients who are unable to undergo standard dialytic treatment due to hemodynamic instability.
Several different renal replacement therapies are now available, including continuous or intermittent techniques. These strategies need some form of anticoagulation to increase circuit survival and to reduce the complications associated with circuit clotting such as thrombocytopenia. Continuous intravenous administration of unfractionated heparin (UH) is the most common approach. However, systemic anticoagulation with UH is associated with potentially serious adverse effects such as bleeding and heparin-induced thrombocytopenia (HIT) [2]. Regional anticoagulation with citrate has been proposed as an effective and safe mean for anticoagulation during CRRT. Recently, the first international web-based consensus conference on mortality reduction in patients with or at risk for AKI [3] included citrate anticoagulation for continuous venovenous hemofiltration (CVVH) among drugs and techniques which may increase survival in critically ill patients with AKI.
2 Main Evidence
Oudemans-van Straaten et al. [4] conducted a non-blinded randomized controlled trial to compare the effect of nadroparin, a low molecular weight heparin (LMWH), and citrate regional anticoagulation in critically ill patients on CVVH from a single-center teaching hospital. A total of 215 patients were randomized. Adverse events, including bleeding complications, were more frequent in the nadroparin than in the citrate group (p < 0.001). In this study, hospital mortality and mortality at 3 months were unexpectedly reduced in the citrate group in both per-protocol and intention-to-treat analyses. This is a strong finding, as mortality is rarely modified by a single technique in intensive care unit (ICU) patients, and this is the only technique that proved superior among those adopted for CRRT. As survival is rarely modified by interventions in these studies, other surrogate outcomes are commonly used as proxy of safety and performance, including circuit survival time, bleeding and major adverse events, platelet count, and discontinuation of CRRT for bleeding.
Several other strategies have been developed to minimize filter clotting during CRRT. One is predilution, without circuit anticoagulation that prolongs circuit survival and is a safe approach in critically ill patients at high risk of bleeding [5]. This technique has the advantage of avoiding any form of anticoagulation, reducing bleeding complications. However, so far it is not clear which category of patients can truly benefit from predilution without anticoagulation, as most critically ill patients are non-bleeding or not at high risk of bleeding. This technique can indeed reduce circuit lifespan, consequently increasing costs and reducing CVVH efficacy. Moreover, predilution reduces CRRT efficacy, as uremic toxins and other substances are diluted in blood before CRRT filter, and it is not the best available option when higher CRRT doses are needed.
As mentioned, systemic anticoagulation with unfractionated heparin (UH) is the most common strategy employed during CVVH. Several randomized controlled trials have compared UH and regional citrate anticoagulation [2, 4, 6, 7]. A meta-analysis of randomized trials, published in 2012, found a decreased risk of bleeding in patients treated with citrate, but no difference in survival [8]. Other studies found that a reduced bleeding risk with citrate anticoagulation as compared with systemic heparin is achieved without reducing or even increasing clotting-free circuit survival time [9, 10]. However, despite a lower complication rate, no difference in mortality was identified when comparing UH and citrate anticoagulation [9].
Several studies compared the efficacy and safety of UH and LMWH. A similar rate of bleeding, circuit survival, and platelet consumption was found. No significant difference in mortality was identified for these treatments [11, 12].
In conclusion, citrate has proven to be superior to LMWH on mortality, while no definitive conclusion can be drawn from studies comparing citrate and UH. However, considering that UH was not proven superior to LMWH in terms of mortality in several studies, and that citrate showed a reduced complication rate when compared with UH, citrate may be considered at least as safe, and probably safer, than UH in terms of mortality. Further randomized studies with larger sample size are needed for a definitive answer.
3 Pathophysiological Principles
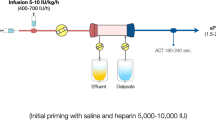
Blood flow through the extracorporeal circuit directly triggers coagulation, due to the contact with artificial surfaces and with air in the bubble trap, to turbulent and low flow, and to hemoconcentration. Citrate is a well-known anticoagulant. It has been used for decades as an anticoagulant to preserve stored blood products. Indeed, citrate chelates calcium ions, a necessary cofactor in the coagulation cascade, thus reducing calcium levels. The reduced calcium concentration hampers thrombin generation, the fundamental final step of intrinsic and extrinsic pathways in the coagulation cascade.
The citrate-calcium complex is removed from the circuit through hemofiltration and dialysis, while normal calcium levels are restored through a post-filter calcium infusion. This normally grants regional anticoagulation within the extracorporeal circuit, with low risk of bleeding in most patients.
The local extracorporeal effect of citrate anticoagulation is the main principle behind its safety. All other anticoagulants are administered and exert their effects systemically, causing complications in every organ. In patients at high risk of bleeding, anticoagulants are used at a lower dose to reduce complications. However, this strategy may reduce CRRT circuit lifespan. Conversely, during citrate regional anticoagulation blood clotting is impaired only in the CRRT circuit, as citrate is infused and removed (for the largest part) before blood reinfusion to the patient. Metabolic and ionic derangements due to the small amount of citrate that enters systemic circulation are easily monitored and reversible using point of care analyzers.
4 Therapeutic Use
The main indication for citrate regional anticoagulation is CRRT in critically ill patients at high risk of bleeding.
The number needed to treat to prevent one bleeding event with citrate regional anticoagulation was calculated to be 6.87 [8, 13]. While citrate CRRT presents higher direct costs than other standard dialytic techniques, citrate anticoagulation was demonstrated to be eventually cheaper than systemic UH, due to increased circuit survival and due to a reduced transfusion and complication rate [14, 15].
The advantages and the indications for the different anticoagulation strategies are summarized in Table 7.1.
Clinicians should consider, during citrate anticoagulation, that despite the removal of most citrate-calcium complex within the dialytic circuit, a small amount of citrate may be delivered to the patient. This can have profound consequences on systemic acid-base balance. Citrate is normally cleared by the liver, almost independently from renal function. Citrate is metabolized in the hepatocytes through Krebs cycle, liberating three molecules of carbon dioxide that are then converted to bicarbonate. Thus, citrate may act as a buffer in the systemic circulation, possibly leading to metabolic alkalosis.
Although trisodium citrate is the primary citrate form in commercial solutions for CRRT, in some cases a small amount of citric acid is added in the preparation. This enhances the anticoagulation effect and reduces the risk of metabolic alkalosis as citric acid is not metabolized to bicarbonate. Moreover, the risk of hypernatremia due to trisodium citrate is reduced by citric acid use.
Citrate may accumulate in patients with hepatic dysfunction, resulting in metabolic acidosis and hypocalcemia. This is the direct consequence of an impairment in citrate metabolism due to liver failure. Acidosis may also occur due to continuous loss of bicarbonate and calcium/citrate complex in the filtrate fluid. The most frequent and dangerous complication is the development of systemic calcium derangements that may be life threatening. Moreover, other electrolytes are chelated by citrate, including phosphorus and magnesium. Therefore, calcium and electrolyte levels should be monitored closely in clinical practice to reduce citrate toxicity.
Liver failure is a relative contraindication for citrate regional anticoagulation, as it implies a higher risk of citrate toxicity. However, citrate anticoagulation can be used even in patients with liver failure if needed, with closer metabolic monitoring [16–18]. Citrate metabolism may be impaired in other conditions with systemic hypoperfusion causing reduced liver blood flow and reduced citrate clearance, such as cardiogenic shock or septic shock. To increase the safety of this technique, standardized local protocols should be employed [13]. Moreover, new commercial solutions and more accurate algorithms for citrate management are being developed to simplify citrate anticoagulation, to reduce the risk of metabolic derangements, and to widen its use in clinical practice [19].
In conclusion, considering the lower risk of complications and some evidence of survival advantage for citrate anticoagulation, the addition of new technological improvements and the evidence of similar total costs, citrate use for CRRT in critically ill patients should probably be increased in the next future. New clinical trials are warranted to definitively assess the effect of citrate anticoagulation in terms of survival benefits, complication rate, and cost-effectiveness.
Clinical Summary
Technique | Indications | Cautions | Side effects | Dose | Notes |
|---|---|---|---|---|---|
Citrate in CRRT | Need for renal replacement therapy in critically ill patients at risk of bleeding | Liver failure, shock | Citrate intoxication, especially in liver failure, leading to hypocalcemia and/or other electrolyte disturbances Acid bases disturbances, including metabolic alkalosis and acidosis | Citrate solution is infused in the arterial line, at 102 mmol/L The rate of calcium chloride reinfused through venous line (starting at 0.5 mL/min) is modified according to calcium levels monitoring | Calcium and other electrolytes levels should be closely monitored to avoid the risk of hypocalcemia Acid-base status should be monitored to avoid acidosis and alkalosis |
References
Rimes-Stigare C, Frumento P, Bottai M et al (2015) Evolution of chronic renal impairment and long-term mortality after de novo acute kidney injury in the critically ill; a Swedish multi-centre cohort study. Crit Care Lond Engl 19:221
Hetzel GR, Schmitz M, Wissing H et al (2011) Regional citrate versus systemic heparin for anticoagulation in critically ill patients on continuous venovenous haemofiltration: a prospective randomized multicentre trial. Nephrol Dial Transplant 26(1):232–239
Landoni G, Bove T, Székely A et al (2013) Reducing mortality in acute kidney injury patients: systematic review and international web-based survey. J Cardiothorac Vasc Anesth 27(6):1384–1398
Oudemans-van Straaten HM, Bosman RJ, Koopmans M et al (2009) Citrate anticoagulation for continuous venovenous hemofiltration. Crit Care Med 37(2):545–552
Tan HK, Baldwin I, Bellomo R (2000) Continuous veno-venous hemofiltration without anticoagulation in high-risk patients. Intensive Care Med 26(11):1652–1657
Kutsogiannis DJ, Gibney RTN, Stollery D, Gao J (2005) Regional citrate versus systemic heparin anticoagulation for continuous renal replacement in critically ill patients. Kidney Int 67(6):2361–2367
Monchi M, Berghmans D, Ledoux D et al (2004) Citrate vs. heparin for anticoagulation in continuous venovenous hemofiltration: a prospective randomized study. Intensive Care Med 30(2):260–265
Wu MY, Hsu YH, Bai CH et al (2012) Regional citrate versus heparin anticoagulation for continuous renal replacement therapy: a meta-analysis of randomized controlled trials. Am J Kidney Dis Off J Natl Kidney Found 59(6):810–818
Betjes MGH, van Oosterom D, van Agteren M, van de Wetering J (2007) Regional citrate versus heparin anticoagulation during venovenous hemofiltration in patients at low risk for bleeding: similar hemofilter survival but significantly less bleeding. J Nephrol 20(5):602–608
Gattas DJ, Rajbhandari D, Bradford C et al (2015) A randomized controlled trial of regional citrate versus regional heparin anticoagulation for continuous renal replacement therapy in critically Ill adults. Crit Care Med 43(8):1622–1629
Joannidis M, Kountchev J, Rauchenzauner M et al (2007) Enoxaparin vs. unfractionated heparin for anticoagulation during continuous veno-venous hemofiltration: a randomized controlled crossover study. Intensive Care Med 33(9):1571–1579
Reeves JH, Cumming AR, Gallagher L et al (1999) A controlled trial of low-molecular-weight heparin (dalteparin) versus unfractionated heparin as anticoagulant during continuous venovenous hemodialysis with filtration. Crit Care Med 27(10):2224–2228
Tolwani A, Wille KM (2012) Regional citrate anticoagulation for continuous renal replacement therapy: the better alternative? Am J Kidney Dis 59(6):745–747
Oudemans-van Straaten HM (2014) Citrate for continuous renal replacement therapy: safer, better and cheaper. Crit Care Lond Engl 18(6):661
Schilder L, Nurmohamed SA, Bosch FH et al (2014) Citrate anticoagulation versus systemic heparinisation in continuous venovenous hemofiltration in critically ill patients with acute kidney injury: a multi-center randomized clinical trial. Crit Care Lond Engl 18(4):472
De Vico P, Messino V, Tartaglione A et al (2015) Safety and efficacy of citrate anti-coagulation continuous renal replacement therapies in post-cardiac surgery patients with liver dysfunction. Ther Apher Dial 19(3):272–278
Slowinski T, Morgera S, Joannidis M et al (2015) Safety and efficacy of regional citrate anticoagulation in continuous venovenous hemodialysis in the presence of liver failure: the Liver Citrate Anticoagulation Threshold (L-CAT) observational study. Crit Care 19:349
Lahmer T, Messer M, Rasch S et al (2015) Sustained low-efficiency dialysis with regional citrate anticoagulation in medical intensive care unit patients with liver failure: a prospective study. Crit Care 30(5):1096–1100
Broman M, Klarin B, Sandin K et al (2015) Simplified citrate anticoagulation for CRRT without calcium replacement. ASAIO J 61(4):437–442
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2016 Springer International Publishing Switzerland
About this chapter
Cite this chapter
Greco, M., Monti, G., Cabrini, L. (2016). Citrate Anticoagulation to Reduce Mortality in Patients Needing Continuous Renal Replacement Therapy. In: Landoni, G., Pisano, A., Zangrillo, A., Bellomo, R. (eds) Reducing Mortality in Acute Kidney Injury. Springer, Cham. https://doi.org/10.1007/978-3-319-33429-5_7
Download citation
DOI: https://doi.org/10.1007/978-3-319-33429-5_7
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-33427-1
Online ISBN: 978-3-319-33429-5
eBook Packages: MedicineMedicine (R0)