Abstract
Renal replacement therapy (RRT) is currently the only reasonable approach in the clinical management of patients with severe acute kidney injury (AKI). However, there is scarce literature evidence to clearly identify the most efficacious RRT approach. High-intensity RRT may be suggested for fluid and solute control in critically ill patients. Indeed, this strategy has been shown to provide a survival benefit in patients with AKI. Yet, more recent large randomized trials did not confirm these findings. Ultimately, the adequacy of treatment should take into consideration not only the prescribed “dose” but also the actually delivered dose through continuous monitoring of treatment. Anticoagulation has a key role in reducing downtime and membrane fouling and, accordingly, discrepancies between the prescribed and the actually delivered dose.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Renal Replacement Therapy
- Acute Kidney Injury
- Continuous Renal Replacement Therapy
- Uremic Toxin
- Renal Replacement Therapy Modality
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
1 General Principles
Because initial studies showed a direct relationship between the intensity of renal replacement therapy (RRT) and survival, both for intermittent and continuous techniques [1–3], great attention has been paid to identify the optimal “dose” of RRT in the last 10 years.
Dose of RRT may be represented by the efficiency of the treatment, which can in turn be expressed as clearance (K) that is the amount of blood cleared of toxins and waste products by the extracorporeal circuit during a given period of time [4]. The concept of clearance needs to be referred to a particular solute. Urea is widely adopted as uremic toxin marker in clinical practice, and its clearance is most commonly used to quantify RRT efficiency and, accordingly, dose. Given that RRT is usually performed over several days or weeks, it is important to provide information about the total time during which the treatment clearance is delivered. The intensity of treatment (Fig. 6.1) is thus expressed as the product of clearance and the effective time (t) of treatment (Kt) [4]. Including the downtime (i.e., the amount of time in which the treatment is interrupted), a significant difference could be found between the prescribed and the actually delivered doses. Finally, considering the entire pool of solutes that needs to be cleared, the efficacy of treatment (Fig. 6.1) can be expressed as the ratio between the intensity and the volume of distribution (V) of the marker solute (Kt/V) [4]. Considering all these concepts during the prescription phase of RRT, it has seemed reasonable, since the birth of critical care nephrology [5], that an adequate treatment should have to be delivered to critically ill patients. In a few words, the idea was to provide “intense” blood purification, generally proportional to the severity of critical illness. However, back in the late 1980s, RRT machines used in the intensive care units (ICUs) were mostly adapted from the chronic hemodialysis ward, or in any case lacked several of current automatisms which are routinely applied to third- and fourth-generation RRT machines, and were probably unsuited for providing accurate and targeted treatments to critically ill patients with acute kidney injury (AKI) [6]. At that time, accordingly, RRT was certainly mostly underdosed. In this chapter, the available literature is analyzed with the aim to identify the current “optimal” dose of RRT in ICU patients with AKI, as well as to clarify whether and to what extent an increased treatment dose/intensity might provide a survival benefit in these patients.
2 Main Evidence
Several efforts have been made in the literature in order to define the most adequate RRT dose in AKI: the underlying idea is that RRT delivery may imply a dose-dependent range, where treatment efficiency correlates with outcomes, and a dose-independent range in which further dose increases will not result in additional benefits for the patients. Accordingly, during the last decade, the dose that was first shown to be associated to better patient outcome (≥35 mL kg−1 h−1) has been considered a milestone of critical care nephrology [1]. In particular, in 2000 Ronco et al. [1] randomized 425 ICU patients with acute renal failure (ARF) to receive continuous venovenous hemofiltration (CVVH) at 20, 35, or 45 mL kg−1 h−1 and found a significantly higher mortality in the 20 mL kg−1 h−1 group, as compared with the other two groups (which had similar survival rates). This study also suggested that post-dilution hemofiltration at higher does (45 mL kg−1 h−1) may be indicated in specific conditions such as sepsis. The hypothesis that an increased intensity of RRT may improve survival was apparently confirmed also in patients receiving intermittent hemodialysis (IHD). In 2002, in fact, Schiffl et al. [2] randomized 160 ARF patients to either daily or conventional (i.e., on alternate days) IHD and showed a significant reduction in mortality in the daily dialysis group (28 % vs. 46 %, p = 0.01). Interestingly, a few years later, Saudan et al. [3] evaluated the effect of additional RRT dose, delivered by adding a continuous diffusive technique to a purely convective treatment, in 206 ICU patients with ARF: again, patients receiving continuous venovenous hemodiafiltration (CVVHDF) showed a significant improvement in 90-day survival as compared with patients receiving CVVH (59 % vs. 34 %, p = 0.0005).
More recently, however, two large multicenter randomized clinical trials examined the issue of the optimal RRT dose in ICU patients with AKI and the effect of increased intensity of RRT on mortality: the Randomized Evaluation of Normal versus Augmented Level of RRT (RENAL) study [7] and the Veterans Affairs/National institutes of Health Acute Renal Failure Trial Network (ATN) study [8].
In the RENAL trial [7], 1,508 patients were randomized to receive post-dilution CVVHDF with an effluent flow of either 25 or 40 mL kg−1 h−1. The ATN study [8] included 1,124 patients who were randomly assigned to either 20 mL kg−1 h−1 CVVHDF or thrice-weekly IHD. Both studies failed to demonstrate that higher RRT doses were associated with better outcomes, except for a septic subgroup of the RENAL study with a reduced mortality when a higher dose was applied (odds ratio [OR] 0.84, 95 % confidence interval [CI] 0.62–1.12). However, under-dialysis should be always avoided in critically ill patients with AKI, and great attention should be paid in order to minimize the discrepancy between the prescribed and the actually delivered dose.
In light of this major issue, RRT downtime (defined as the overall time of RRT “standstill” over 24 h) was specifically explored in the “DOse REsponse Multicenter International collaborative initiative” (DO-RE-MI) [9]. Membrane clotting, vascular access issues (inducing physicians to modify the setting), and prescription errors (due to lack of knowledge) were the main contributors to continuous RRT (CRRT) stop. Therefore, if a “minimal” dose of 20–25 mL kg−1 h−1 (according to RENAL and ATN studies) should be prescribed, physicians should be advised to overprescribe the dose of at least 25 % (targeting 30–35 mL kg−1 h−1), in order to limit the downtime effect.
Two important post hoc analyses of the RENAL trial were performed [10, 11]. The first suggested that fluid balance, rather than RRT dose, may actually affect patients’ outcomes (see Chap. 19) [10]. In fact, the authors found that mean daily fluid balance among survivors was −234 mL/day compared with +560 mL/day among non-survivors (p < 0.0001) and that a negative fluid balance was independently associated with favorable outcomes, including survival, RRT days, mechanical ventilation days, and both ICU and hospital length of stay. The second post hoc analysis examined acid-base balance and vasopressor utilization in the subgroup of patients with metabolic acidosis [11]. This study showed that the high-intensity group had a greater increase in mean arterial pressure from baseline to 24 h (7 ± 3 vs. 0 ± 3 mmHg, p < 0.01) and a greater decrease in norepinephrine dose (from 12.5 to 3.5 vs. 5 to 2.5 μg/min, p < 0.05). Despite a similar improvement in acid-base balance was observed in both groups, strong ion gap seemed to be better corrected by high-dose RRT. Although the authors acknowledged that a mechanistic analysis of the physiological effects induced by high-intensity RRT cannot be provided, they suggested that a more efficient removal of biologic mediators which are responsible for hypotension or vasodilation might be the potential mechanism of the observed hemodynamic improvement. Indeed, the changes in strong ion gap may indicate the removal of some of these mediators [11].
Finally, Uchino et al. [12] analyzed data from two multicenter investigations, the Beginning and Ending Supportive Therapy (BEST) study [13] and the Japanese Society for physician and trainees Intensive Care (JSEPTIC) trial [14], including 1,006 patients from 54 ICUs around the world and 343 patients from 12 Japanese ICUs, respectively. They found that AKI patients receiving low-dose CRRT (14.3 mL kg−1 h−1) had not a worse short-term outcome as compared with patients receiving CRRT at doses closer to those currently considered as standard (20.4 mL kg−1 h−1).
3 Pathophysiological Principles
One of the key issues of the modern concept of RRT is the clinical target: deriving from nephrology considerations, urea is the main solute that has been referred as the biomarker indicating how efficiently solutes are removed. However, urea is not the only solute which accumulates due to kidney injury and its kinetic of removal and volume of distribution differ by the other uremic toxins [15]. Considering urea as a target solute could result particularly useless in ICU patients. In fact, unlike patients with chronic kidney disease (CKD), uremic symptoms are rarely observed in the ICU, and they usually do not affect the clinical decision about CRRT in these patients. Other target solutes rather than urea should be considered in ICU patients. In particular, CRRT should be addressed to specific targets in specific clinical conditions (e.g., myoglobin in patients with compartment syndrome, interleukins during sepsis, novel biomarkers in case of early AKI, fluid balance in case of fluid overload). This concept would also redefine the concept of adequacy itself, which should probably include not only the amount of RRT to provide but also the exact circuits, filters, machines, and timing to be applied.
4 Therapeutic Use
A specific treatment that can be defined as “adequate” for all ICU patients in all conditions does not exist but, like mechanical ventilation, CRRT should be continuously tailored on patients’ characteristics and their actual clinical needs.
Advantages and disadvantages of the different RRT modalities are summarized in Table 6.1 (see also Chap. 4).
Although three studies suggested a survival advantage with higher effluent dose [1], more frequent (daily) IHD [2], and the adjunct of continuous dialysis to CVVH [3], respectively, subsequent studies failed to confirm these findings. Accordingly, an increase in RRT intensity in order to reduce mortality cannot be recommended [16].
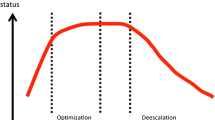
Currently, a CRRT dose prescription below 20 mL kg−1 h−1 and over 35 mL kg−1 h−1 may be definitely identified as the dose-dependent range [17] (Fig. 6.2). In fact, reducing RRT intensity below 20 mL kg−1 h−1 is likely to negatively affect outcomes due to under-dialysis, while increasing it above 35 mL kg−1 h−1 might lead to electrolyte disorders and removal of nutrients and drugs, also potentially reducing survival. Conversely, prescriptions between 20 and 35 mL kg−1 h−1 can be considered as practice dependent: within this range, variables such as timing, patients’ characteristics, comorbidities, or concomitant supportive pharmacological therapies may have a significant role in affecting patients’ outcome and should trigger a careful prescription and a close monitoring of dose delivery.
Relationship between delivered dose and patient’s survival. Below 20 mL kg−1 h−1 (panel a), the higher the dose the higher patients’ survival (dose-dependent region). Above this limit, further increase in dose prescription (up to 35 mL kg−1 h−1) may not influence patients’ survival (panel b). In this range, other variables (e.g., time of treatment, optimization of blood perfusion, drug adjustments) may influence the outcome (practice-dependent region). With further increase of prescribed dose (over 35 mL kg−1 h−1), electrolyte disorders and removal of nutrients and drugs (e.g., antibiotics) may occur, potentially reducing survival (panel c)
Nowadays, a delivered dose (without downtime) between 20 and 25 mL kg−1 h−1 may be considered as clinically acceptable [17]. From a practical standpoint, considering that average downtime reduces delivered dose by 10–20 %, it might be recommended to prescribe 25–35 mL kg−1 h−1 in order to achieve an actual dose of at least 20–25 mL kg−1 h−1. A dose prescription above 35 mL kg−1 h−1, which is also associated to increased costs [18], is currently not recommended in any clinical condition.
Clinical Summary
Strategy | Side effects | Dose | Notes |
|---|---|---|---|
Increased intensity of renal replacement therapy | Possible electrolyte disorders and removal of nutrients and drugs (e.g., antibiotics) with effluent dose >35 mL kg−1 h−1 Increased costs | Increased intensity intended as: Increased effluent dose (≥35 mL kg−1 h−1) Daily (rather than alternate-day) dialysis | None recommended in order to reduce mortality A targeted approach depending on the clinical condition may be rather advisable |
References
Ronco C, Bellomo R, Homel P et al (2000) Effects of different doses in continuous venovenous hemofiltration on outcomes of acute renal failure: a prospective randomised trial. Lancet 356:26–30
Schiffl H, Lang SM, Fischer R (2002) Daily hemodialysis and the outcome of acute renal failure. N Engl J Med 346:305–310
Saudan P, Niederberger M, De Seigneux S et al (2006) Adding a dialysis dose to continuous hemofiltration increases survival in patients with acute renal failure. Kidney Int 70:1312–1317
Ricci Z, Bellomo R, Ronco C (2006) Dose of dialysis in acute renal failure. Clin J Am Soc Nephrol 1:380–388
Ronco C, Bellomo R, Feriani M et al (1998) Critical care nephrology: the time has come. Kidney Int Suppl 66:S1–S2
Ronco C, Ricci Z (2015) Pediatric continuous renal replacement: 20 years later. Intensive Care Med 41:985–993
RENAL Replacement Therapy Study Investigators, Bellomo R, Cass A, Cole L et al (2009) Intensity of continuous renal-replacement therapy in critically ill patients. N Engl J Med 361:1627–1638
VA/NIH Acute Renal Failure Trial Network, Palevsky PM, Zhang JH, O’Connor TZ et al (2008) Intensity of renal support in critically ill patients with acute kidney injury. N Engl J Med 359:7–20
Monti G, Herrera M, Kindgen-Milles D et al (2007) The DOse REsponse Multicentre International Collaborative Initiative (DO-RE-MI). Contrib Nephrol 156:434–443
RENAL Replacement Therapy Study Investigators, Bellomo R, Cass A, Cole L et al (2012) An observational study fluid balance and patient outcomes in the randomized evaluation of normal vs. augmented level of replacement therapy trial. Crit Care Med 40:1753–1760
Bellomo R, Lipcsey M, Calzavacca P et al (2013) Early acid-base and blood pressure effects of continuous renal replacement therapy intensity in patients with metabolic acidosis. Intensive Care Med 39:429–436
Uchino S, Toki N, Takeda K et al (2013) Validity of low-intensity continuous renal replacement therapy. Crit Care Med 41:2584–2591
Uchino S, Kellum J, Bellomo R et al (2005) Acute renal failure in critically ill patients: a multinational, multicenter study. JAMA 294:813–818
Yasuda H, Uchino S, Uji M, Japanese Society for Physicians and Trainees in Intensive Care Clinical Trial Group et al (2014) The lower limit of intensity to control uremia during continuous renal replacement therapy. Crit Care 18:539
Ricci Z, Romagnoli S, Ronco C (2011) Renal support. Minerva Anestesiol 77:1204–1215
Landoni G, Bove T, Székely A et al (2013) Reducing mortality in acute kidney injury patients: systematic review and international web-based survey. J Cardiothorac Vasc Anesth 27(6):1384–1398
Kidney Disease: Improving Global Outcomes (KDIGO) Acute Kidney Injury Work Group (2012) KDIGO clinical practice guideline for acute kidney injury. Kidney Int Suppl 2:1–138
Srisawat N, Lawsin L, Uchino S, BEST Kidney Investigators et al (2010) Cost of acute renal replacement therapy in the intensive care unit: results from The Beginning and Ending Supportive Therapy for the Kidney (BEST Kidney) study. Crit Care 14:R46
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2016 Springer International Publishing Switzerland
About this chapter
Cite this chapter
Ricci, Z., Romagnoli, S. (2016). Increased Intensity of Renal Replacement Therapy to Reduce Mortality in Patients with Acute Kidney Injury. In: Landoni, G., Pisano, A., Zangrillo, A., Bellomo, R. (eds) Reducing Mortality in Acute Kidney Injury. Springer, Cham. https://doi.org/10.1007/978-3-319-33429-5_6
Download citation
DOI: https://doi.org/10.1007/978-3-319-33429-5_6
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-33427-1
Online ISBN: 978-3-319-33429-5
eBook Packages: MedicineMedicine (R0)