Abstract
Although not infectious, acute kidney injury (AKI) is pandemic. Interestingly, like infection by Yersinia pestis, AKI has “spread” to both high- and low-income countries (even if likely secondary to significantly different pathogenetic pathways), and its outcomes are bad worldwide [1]: the deadly burden of AKI affects up to 5,000 cases per million people per year and kills up to 50 % of patients requiring renal replacement therapy (RRT) secondary to AKI [2]. Again, similarly to the Black Death (Atra Mors, in Latin) pandemics which broke out between the fourteenth and the nineteenth century, we are fighting against a barely known enemy without a specific therapy to administer. Very differently from the plague, AKI is a syndrome and is caused by multiple etiologies, frequently occurring simultaneously. However, the exact damage occurring to kidneys’ structure and function, through multiple and complex pathophysiologic mechanisms, is largely unknown. This uncertainty led the medical community (only recently, about 10 years ago) to search for a standard AKI definition [3] which is able to conventionally describe that the abrupt decrease of kidney function is not an “on-off” disease, but it has a spectrum of phenotypes (currently known as “AKI stages”; see Chap. 2). The standard definition is unable to identify and differentiate AKI etiologies and somehow causes a “one-fits-all” issue: detractors of “consensus-based” definitions argue that, for example, a stage II septic AKI might not be clinically comparable to a stage II postabdominal surgery AKI [3]. At least, however, some light has been shed on the obscure epidemiology of AKI, and it is now clear that AKI occurs with a different incidence in different clinical settings [4], inevitably leading, regardless of etiology, to significantly worse outcomes as compared to non-affected (plagued) patients. Exactly as it happened before the availability of antibiotics during plague pandemics, prevention of AKI might represent today the most significant way to improve outcomes in those populations at risk of developing an acute renal dysfunction.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Chronic Kidney Disease
- Renal Replacement Therapy
- Acute Kidney Injury
- Multiple Organ Failure
- Fluid Overload
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
1 The “Atra Mors”
Although not infectious, acute kidney injury (AKI) is pandemic. Interestingly, like infection by Yersinia pestis, AKI has “spread” to both high- and low-income countries (even if likely secondary to significantly different pathogenetic pathways), and its outcomes are bad worldwide [1]: the deadly burden of AKI affects up to 5,000 cases per million people per year and kills up to 50 % of patients requiring renal replacement therapy (RRT) secondary to AKI [2]. Again, similarly to the Black Death (Atra Mors, in Latin) pandemics which broke out between the fourteenth and the nineteenth century, we are fighting against a barely known enemy without a specific therapy to administer. Very differently from the plague, AKI is a syndrome and is caused by multiple etiologies, frequently occurring simultaneously. However, the exact damage occurring to kidneys’ structure and function, through multiple and complex pathophysiologic mechanisms, is largely unknown. This uncertainty led the medical community (only recently, about 10 years ago) to search for a standard AKI definition [3] which is able to conventionally describe that the abrupt decrease of kidney function is not an “on-off” disease, but it has a spectrum of phenotypes (currently known as “AKI stages”; see Chap. 2). The standard definition is unable to identify and differentiate AKI etiologies and somehow causes a “one-fits-all” issue: detractors of “consensus-based” definitions argue that, for example, a stage II septic AKI might not be clinically comparable to a stage II postabdominal surgery AKI [3]. At least, however, some light has been shed on the obscure epidemiology of AKI, and it is now clear that AKI occurs with a different incidence in different clinical settings [4], inevitably leading, regardless of etiology, to significantly worse outcomes as compared to non-affected (plagued) patients. Exactly as it happened before the availability of antibiotics during plague pandemics, prevention of AKI might represent today the most significant way to improve outcomes in those populations at risk of developing an acute renal dysfunction.
2 Why AKI Kills
From the milestone paper by Meitnitz, back in 2002 [5], clinicians understood two fundamental concepts: (1) if two critically ill patients with the same severity of disease (assessed through common metrics such as APACHE score) are admitted to the same intensive care unit (ICU), the one with AKI has an independently higher risk of dying: the “only” fact the kidneys are not working, regardless how good is medical treatment in your ward, how early, intense, and optimal is your RRT, and how appropriate is your antibiotic therapy, your patient has AKI and, as such, his chances of surviving decrease; (2) this frustrating scenario (again similar to that of Indian fellows staring powerlessly at hundreds of patients suffering from Yersinia’s lesions) taught us that the commonly used “severity scores” have overlooked for years the actual impact of renal function on patient outcomes: a novel and specific AKI risk stratification was absolutely needed [3]. Interestingly, the impact of isolated AKI (e.g., in case of glomerulonephritis in a previously healthy patient) on patients’ outcome is significantly different compared to AKI occurring in patients with multiple comorbidities (e.g., cardiorenal or hepatorenal syndrome) or multiple organ failure (MOF). As a matter of fact, it is currently unknown if this harmful disease affects critically ill patients in association with the most severe clinical pictures, already hampered by a worst outcome, or is itself the cause of increased death rate. It is possible that the truth is in the middle: kidneys are victims and culprits in the course of MOF, being most frequently injured by systemic diseases (e.g., sepsis) and causing themselves, in a sort of vicious circle, damage to other organs. AKI is a “pan-metabolic, pan-endocrine, and pan-organ” problem [6]. Vaara and coauthors [7] elegantly described the “population-attributable mortality” of AKI by attempting to compare AKI and non-AKI patients through a most complex system of propensity matching in a large database from several Finnish ICUs that included more than 60 variables. These authors concluded that almost 20 % of mortality in the ICU population is caused by AKI. In particular, AKI seems to affect and enhance inflammatory processes and to cause a profound depression of immunocompetence. This is associated with the release of cytokines and inflammatory mediators, increase in oxidative stress, activation of white line cells, neutrophil extravasation, generalized endothelial injury, increased vascular permeability, and tissue edema formation [8]. The alteration of the delicate equilibrium in multiple immuno-homeostatic mechanisms further justifies the role of “injured” kidneys as “activators” of MOF: the lungs, heart, liver, and brain are all equally exposed to this largely unexplored syndrome [9].
The alteration of fluid management is another key issue in patients suffering from AKI [10]: critically ill patients are necessarily administered with large amounts of fluids (fluid challenges, transfusions, antibiotics, parenteral nutrition, vasoactive drugs, etc.). Fluid overload (the percentage of cumulative fluid balance over patients’ body weight) may result from overzealous fluid administration or oliguria or a combination of the two (see Chap. 19). It has been speculated that these two aspects may combine, again, into a vicious circle: it is possible that the largest fluid replacement is needed in most severe patients who are those at highest risk for AKI. Furthermore, infused fluids for volume replacement have been recently claimed to be in cause, per se, for nephrotoxicity and renal damage [11, 12]. Third, fluid overload and AKI share endothelial dysfunction due to inflammation or ischemia/reperfusion with glycocalyx alteration and subsequent capillary leakage [13]. As a matter of fact, organ edema (affecting the lungs, heart, liver, brain, and kidneys themselves) impairs organ function, and it is considered a fundamental constituent of MOF. It is actually difficult to understand who comes first (AKI or fluid overload) but it is clear that in case of severe AKI, the only way to manage fluid balance is aggressive ultrafiltration through RRT [14].
3 The Mark of AKI
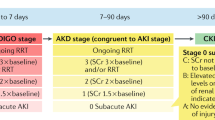
Differently from plague infection, patients who survive AKI carry the signs of the disease in the following years. Recently, Heung and coworkers on behalf of the Centers for Disease Control and Prevention CKD Surveillance Team [15] showed that, in a cohort of about 100,000 hospitalized patients, the majority (70.8 %) had fast recovery (within 2 days), 12.2 % had intermediate recovery (3–10 days), 11.0 % had slow or no recovery (above 10 days), and the remaining 6.0 % were lost to follow-up: one patient over ten (maybe more) does not recover an intra-hospital AKI episode and is destined to chronic kidney disease (CKD) thereafter. Impressively, the authors remarked that, at 1-year follow-up, the presence of any AKI episode was strongly associated with the development of CKD, with a relative risk of 1.43 (95 % confidence interval [CI] 1.39–1.48), 2.00 (95 % CI 1.88–2.12), and 2.65 (95 % CI 2.51–2.80) for fast, intermediate, and slow recovery, respectively. Thus, even a transient AKI episode, lasting less than 2 days, leaves a scar in patients’ kidneys that subsequently increases the risk of further renal damage. Follow-up should be warranted to all AKI patients.
4 How to Reduce AKI Mortality
Dr. Alexandre Yersin, from the Pasteur Institute, significantly contributed to plague therapy by isolating the bacterium in 1894 and was thereafter honored by giving his name to the etiologic agent. Today, the therapeutic solution of AKI is far from being identified, and we possibly will never see a single name on such treatment. However, several approaches can be currently suggested.
Primum non nocere: the avoidance of useless and not effective treatments may certainly help clinicians to focus on more consistent approaches [16].
In the same light, the earliest diagnosis of AKI is currently considered a fundamental aspect of plague’s management: the identification of renal dysfunction from its milder forms [17] or, better, before the manifest sings are apparent [18] is useful in order to promote preventive measures (e.g., administer antibiotics targeting serum levels, reduce contrast media, avoid starches administration, etc.) and to keep clinicians aware about kidney’s health in the eventual attempt of precluding the worsening of AKI severity. Great expectations are currently trusted on renal biomarkers for early detection of AKI (see Chap. 2) [19] and “acute kidney stress” [20].
Third, act upon disease pathogenesis. Sepsis, fluid overload, surgery, cardiac dysfunction, and trauma: they all have partially different clinical pictures and deserve tailored attention. Possibly, a surgical patient will benefit from an accurate and aggressive goal-directed fluid replacement (see Chap. 10), whereas a septic one should be “fluid restricted,” mostly avoiding starch infusion (see Chaps. 19 and 20). Research is ongoing in every single setting, and scientific updating is certainly an important part of clinicians’ efforts: we should attempt to administer the most appropriate therapy according to the most recent evidences.
Then, do not delay RRT (see Chap. 5) and treat fluid accumulation. Importantly, RRT dose should be closely monitored during the entire ICU stay and changed basing on clinical needs (see Chap. 6) [21].
Finally, read this book carefully: the most updated therapeutic approaches are described in the next chapters in order to increase clinician’s awareness and good clinical practice against AKI, the plague of critically ill patients.
References
Lameire NH, Bagga A, Cruz D et al (2013) Acute kidney injury: an increasing global concern. Lancet 382:170–179
Bellomo R, Kellum JA, Ronco C (2012) Acute kidney injury. Lancet 380:756–766
Cruz DN, Ricci Z, Ronco C (2009) Clinical review: RIFLE and AKIN – time for reappraisal. Crit Care 13:211
Hoste EA, Bagshaw SM, Bellomo R et al (2015) Epidemiology of acute kidney injury in critically ill patients: the multinational AKI-EPI study. Intensive Care Med 41:1411–1423
Metnitz PGH, Krenn CG, Steltzer H et al (2002) Effect of acute renal failure requiring renal replacement therapy on outcome in critically ill patients. Crit Care Med 30:2051–2058
Druml W, Lenz K, Laggner AN (2015) Our paper 20 years later: from acute renal failure to acute kidney injury—the metamorphosis of a syndrome. Intensive Care Med 41(11):1941–1949
Vaara ST, Kaukonen K, Bendel S et al (2014) The attributable mortality of acute kidney injury: a sequentially matched analysis. Crit Care Med 42:1–8
Druml W (2014) Systemic consequences of acute kidney injury. Curr Opin Crit Care 20:613–619
Feltes CM, Van Eyk J, Rabb H (2008) Distant-organ changes after acute kidney injury. Nephron Physiol 109(4):p80–p84
Ostermann M, Straaten HMO, Forni LG (2015) Fluid overload and acute kidney injury: cause or consequence? Crit Care 19:443
Young P, Bailey M, Beasley R et al (2015) Effect of a buffered crystalloid solution vs saline on acute kidney injury among patients in the intensive care unit. JAMA 314(16):1701–1710
Myburgh JA, Mythen MG (2013) Resuscitation fluids. N Engl J Med 369:1243–1251
Ricci Z, Romagnoli S, Ronco C (2012) Perioperative intravascular volume replacement and kidney insufficiency. Best Pract Res Clin Anaesthesiol 26:463–474
RENAL replacement therapy study Investigators (2012) An observational study fluid balance and patient outcomes in the randomized evaluation of normal vs. augmented level of replacement therapy trial. Crit Care Med 40:1753–1760
Heung M, Steffick DE, Zivin K et al (2016) Acute kidney injury recovery pattern and subsequent risk of CKD: an analysis of veterans health administration data. Am J Kidney Dis 67:742–752
Landoni G, Bove T, Székely A et al (2013) Reducing mortality in acute kidney injury patients: systematic review and international web-based survey. J Cardiothorac Vasc Anesth 27:1384–1398
Kellum JA, Lameire N, Aspelin P et al (2012) KDIGO clinical practice guideline for acute kidney injury. Kidney Int Suppl 2:1–138
Chawla LS, Goldstein SL, Kellum JA, Ronco C (2015) Renal angina: concept and development of pretest probability assessment in acute kidney injury. Crit Care 19:93
Kellum JA (2015) Diagnostic criteria for acute kidney injury: present and future. Crit Care Clin 31:621–632
Katz N, Ronco C (2015) Acute kidney stress—a useful term based on evolution in the understanding of acute kidney injury. Crit Care 20:23
Villa G, Ricci Z, Ronco C (2015) Renal replacement therapy. Crit Care Clin 31:839–848
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2016 Springer International Publishing Switzerland
About this chapter
Cite this chapter
Ricci, Z., Ronco, C. (2016). Acute Kidney Injury: The Plague of the New Millennium. In: Landoni, G., Pisano, A., Zangrillo, A., Bellomo, R. (eds) Reducing Mortality in Acute Kidney Injury. Springer, Cham. https://doi.org/10.1007/978-3-319-33429-5_1
Download citation
DOI: https://doi.org/10.1007/978-3-319-33429-5_1
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-33427-1
Online ISBN: 978-3-319-33429-5
eBook Packages: MedicineMedicine (R0)