Abstract
Chronic kidney disease (CKD) is a major worldwide public health problem associated with significant morbidity and mortality. The limitations of existing therapeutic options for patients with end-stage renal disease (ESRD) emphasize the need for innovative approaches to the treatment of CKD, such as stem cell-based regenerative strategies aimed at restoring or replacing lost kidney function. Human pluripotent stem cells (hPSCs), including embryonic stem cells (ESCs) and induced pluripotent stem cells (iPSCs), represent an ideal starting substrate to generate functional kidney cells and tissues. Within the last few years, significant advances have been made in the effort to direct the differentiation of hPSCs into cells of the kidney lineage. Current methods are now capable of efficiently differentiating hPSCs into nephron progenitor cell populations that can give rise to multi-segmented nephron structures within kidney organoids in two- and three-dimensional culture. hPSC-derived kidney cells can now be used for a number of translational applications, including drug nephrotoxicity testing, in vitro kidney disease modeling, and tissue bioengineering. While further studies still need to be done to validate the functionality of these kidney cells and tissues, these recent advances highlight the rapid progress of the field of kidney regenerative medicine and provide hope for novel therapies for patients with kidney disease.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Pluripotent stem cells
- Embryonic stem cells
- Induced pluripotent stem cells
- Regenerative medicine
- Kidney regeneration
- Directed differentiation
- Kidney on a chip
4.1 Introduction
There is a growing need for innovative approaches to the treatment of chronic kidney disease (CKD) , as end-stage renal disease (ESRD) has reached epidemic proportions. In the United States alone, more than 600,000 individuals require renal replacement therapy. Annual Medicare expenditures for treatment of ESRD patients exceeded $500 billion as early as 2012 [1]. Meanwhile, current projections indicate that the US population of patients with ESRD may reach more than two million by 2030 [2]. Currently, approximately 70 % of patients receive dialysis-based therapy while 30 % have a functioning renal transplant. Although both treatment modalities prolong survival, each has significant limitations.
Dialysis imperfectly filters blood. Uremic retention products are known to induce premature atherosclerosis [3] and retained beta-2 microglobulin has been linked to the development of amyloidosis [4]. For dialysis patients, the relative risk of mortality has been reported to be as high as 8.2, compared to matched individuals in the general population [5]. Additionally, dialysis does not recapitulate the endocrine functions of the kidney, necessitating erythropoietin and activated vitamin D supplementation. Lastly, dialysis therapy greatly reduces the health-related quality of life of patients of all ages, both genders, and multiple ethnicities [6].
Transplantation, although having both a survival and cost benefit compared to dialysis, suffers from the paucity of transplantable organs. The limited kidney supply, coupled with increasing demand, has resulted in an average transplant-list wait time of >3.8 years for adults in the USA. While 12 people die each day while waiting for a kidney transplant, every 14 min a patient is added to the kidney transplant list (OPTN/UNOS 2015). Despite the development of potent immunosuppressive agents, kidney transplant recipients have a nearly 10 % risk of acute rejection in the first year after transplantation [7]. Additionally, the majority of those patients who receive a kidney transplant require lifelong immunosuppression, which is associated with increased infection risk, morbidity, and mortality.
Given these limitations, stem cell-based regenerative medicine represents an innovative approach to the treatment of CKD and ESRD. By virtue of their intrinsic properties of self-renewal and ability to differentiate into cells of all three germ layers, pluripotent stem cells (PSCs) provide an optimal and scalable cell source for tissue and organ regeneration [8]. Induced pluripotent stem cells (iPSCs) have the added advantage of being theoretically immunocompatible with the host from which they were derived. The implications are that patient-specific, functional kidney tissue may one day be possible.
4.2 Pluripotent Stem Cells
PSCs represent populations of early embryonic progenitor cells, which are believed to correspond to the blastocyst or epiblast stage of mammalian embryonic development [9]. These early cell types arise 5–9 days following human conception and are defined by two intrinsic properties: self-renewal and pluripotency. PSCs have the ability to self-renew theoretically indefinitely in culture, without transformation or differentiation. In addition, PSCs have the capacity to give rise to all cell types derived from the three embryonic germ layers, namely the mesoderm, endoderm, and ectoderm [10]. Perhaps the greatest demonstration for both the differentiation capacity and the ability to generate complex functional tissue from PSCs is cloned mice developed from tetraploid complementation methods [11, 12].
PSCs comprise embryonic stem cells (ESCs) and iPSCs. ESCs are derived from the isolation and culture of cells from the inner cell mass of the embryonic blastocyst [13–15]. In contrast, iPSCs are derived by the retroviral transduction of four key transcription factors (Oct4, Sox2, Klf4, and c-Myc) into adult skin fibroblasts, which directly reprograms them into cells that appear morphologically and behave almost identically to ESCs (Fig. 4.1) [16, 17].
Reprogramming adult somatic cells into induced pluripotent stem (iPS) cells . The retroviral transduction of four pluripotency transcription factors (Oct4, Sox2, c-Myc, and Klf4) converts somatic cells into iPSCs, which are capable of differentiating into cells of the three germ layers of the embryo (mesoderm, ectoderm, endoderm)
While human ESCs still remain the gold standard for human PSCs and differences do exist between ESCs and iPSCs, human iPSCs have a number of distinct advantages. First, unlike ESCs, iPSC derivation does not involve the use of human embryos, a limitation that has previously led to ethical concerns over the use of human ESCs [18]. Protocols now exist to derive human iPSCs from a variety of different somatic cell types, including peripheral blood mononuclear cells, keratinocytes, hepatocytes, neural stem cells, and kidney mesangial and tubular epithelial cells using both viral and nonviral reprogramming methods [19–25]. Secondly, because human iPSCs can be generated from essentially any human individual—adult or child, healthy or diseased—and retain the individual’s genotype, they represent a starting substrate to generate tissue that is theoretically immunocompatible with the individual from which the iPSCs were originally derived. Thirdly, human iPSCs, which can be generated from patients with specific diseases, can be used to develop in vitro models to better study disease pathogenesis. For diseases that are particularly rare or do not have relevant animal models, iPSCs offer a novel strategy to study pathogenetic mechanisms.
4.3 PSC Differentiation Methods
PSCs can be differentiated into a wide variety of differentiated cell types from multiple organs, including the heart, lungs, liver, pancreas, intestines, kidneys, and nervous system [26]. The withdrawal of growth factors that are required for the maintenance of pluripotency in PSCs results in spontaneous and stochastic differentiation. In the absence of specific exogenous growth factors or chemicals to influence cell fate, PSCs undergo stochastic differentiation into embryoid bodies (EBs) in vitro and teratomas in vivo [15–17]. Both EBs and teratomas are heterogeneous tissues that contain the three embryonic germ layers, confirming pluripotency. However, the efficiency of differentiation into any one particular cell type is low. The efficient generation of specific differentiated cell types with greater purity requires a more directed approach to force PSCs to adopt a particular cell fate. Directed differentiation refers to the process by which PSCs are sequentially treated with growth factors and chemicals to efficiently induce a particular cell or tissue type. Most often, directed differentiation protocols use embryonic organ development as a paradigm for differentiation, subdividing the process into a series of discrete intermediate stages that can be chemically induced and monitored by the expression of key stage-specific markers [26]. Differentiation can be carried out in two-dimensional (2D) monolayer culture, in three-dimensional (3D) EB culture, or a combination of these two approaches. While 2D monolayer culture offers the advantages of better control and monitoring of differentiation, the successful generation of certain organized tissue structures and architecture may require 3D culture environments for realization.
4.4 Mammalian Nephrogenesis
Current strategies to direct the differentiation of PSCs into cells of the kidney lineage have been based on vertebrate animal kidney development as a model. The kidneys are derived from the mesoderm germ layer, specifically from the intermediate mesoderm (IM). During kidney organogenesis, the IM sequentially gives rise to the pronephros, mesonephros, and metanephros (Fig. 4.2). In humans, the pronephros is nonfunctional and regresses by the fourth week of gestation, but for certain primitive jawless fish such as the lamprey and hagfish, it is the primary kidney. The mesonephros forms just prior to degeneration of the pronephros in humans and serves as the primary excretory organ from the fourth to the eighth week of gestation. In females, the mesonephros degenerates, whereas in males it gives rise to portions of the reproductive organs. The metanephros, which begins to form caudal to the mesonephros in the fifth week of gestation, becomes the definitive adult kidney in humans.
Stages of mammalian kidney development . The pronephros is the initial nephric stage in mammals and degenerates by the fourth week of embryonic life in humans. From the fourth to the eighth week of human embryogenesis, the mesonephros is the primary excretory organ. Thereafter, the developing metanephros gives rise to the mature kidney in humans
The metanephric kidney forms through the reciprocally inductive interactions between two distinct IM tissues, the metanephric mesenchyme (MM) and ureteric bud (UB). The MM arises from the posterior IM and contains a population of multipotent nephron progenitor cells (NPCs) that expresses the transcription factors Six2, Cited1, Pax2, Sall1, and WT1 [27–29]. These Six2+ NPCs are present in the MM that surrounds each UB tip (cap mesenchyme) and, upon receiving inductive Wnt signals from the UB, will undergo mesenchymal-to-epithelial transition and give rise to nearly all the epithelial cells of the nephron except for those of the collecting duct [27, 28]. The UB develops as an epithelial outpouching from the caudal region of the nephric (or Wolffian) duct and, upon receiving inductive signals from the MM, undergoes iterative branching to form the collecting system. Nephrogenesis in humans is completed between 32 and 36 weeks of gestation and results in the formation of approximately one million nephrons in each kidney. After birth, no new nephrons are formed, even under circumstances of kidney injury and repair.
Recent work from Taguchi and colleagues has provided important insight into the embryonic origins of the NPCs in the MM [29]. Employing lineage tracing techniques in mice, the authors showed that the embryonic origin of NPCs could be traced back to a population of T+ cells in the primitive streak that persists to give rise to T + Tbx6+ posterior nascent mesoderm and then WT1 + Osr1+ posterior IM. In contrast, the UB originates from anterior IM, which is incapable of giving rise to MM. Thus, careful consideration of these diverging developmental pathways is critical for the efficient differentiation of PSCs into cells of these two different lineages.
4.5 Differentiation of Mouse PSCs to Kidney Lineage Cells
Early studies attempting to generate kidney cells from PSCs were performed using mouse ESCs (mESCs). Labeled mESCs microinjected into E12 to E13 mouse metanephroi resulted in the integration of these cells into tubular structures, some of which expressed Lotus tetragonolobus lectin (LTL)+ and Na+/K+-ATPase+ [30]. EBs generated from the stochastic differentiation of mESCs expressed a number of kidney developmental genes, including Pax2, WT1, Lhx1, Emx2, c-ret, and Sall1. Transplantation of these cells into the mouse retroperitoneum produced teratoma-like growths with regions co-expressing the renal epithelial markers Dolichos biflorus agglutinin (DBA) and Pax2 [31]. These early experiments served as proof-of-concept that mammalian PSCs have the potential to generate cells of the kidney lineage in vivo. Stochastic differentiation gave way to directed differentiation in an effort to improve the efficiency of renal epithelial cell induction. Developmental studies identified activin, retinoic acid (RA), and bone morphogenetic proteins (BMPs) as signaling molecules involved in mesoderm and IM differentiation [32]. Utilizing a combination of activin, RA, and BMP7, Kim and Dressler differentiated mouse EBs into cells expressing the IM markers Pax2, WT1, and Lhx1 [33]. Vigneau and colleagues treated a Brachyury (T)-GFP reporter mESC line with activin and generated Brachyury+ cells with 50 % efficiency [34]. In both of the aforementioned studies, transplantation of the differentiated cells into mouse embryonic kidneys resulted in incorporation of the cells into forming tubular structures. Similarly, other studies have shown that activin, RA, and BMPs are potential nephrogenic factors [35–38].
Taguchi and colleagues used a developmental approach, first determining combinations of growth factors and small molecules required to induce differentiation of isolated mouse T+ posterior mesoderm cells into MM cells [29]. Based on these findings, they established multistep protocols to differentiate mESCs and hiPSCs into EBs that expressed multiple markers of NPCs of the MM, including Pax2, Six2, Sall1, and WT1. Co-culture of the EBs containing NPCs with mouse embryonic spinal cord, a tissue known to induce kidney tubulogenesis, resulted in the generation of 3D tubular structures expressing markers of kidney tubules and glomeruli. The protocols for mESCs and hiPSCs were similar, though MM induction required 14 days with hiPSCs compared to 8.5 days with mESCs.
4.6 Differentiation of Human PSCs to Kidney Lineage Cells
The initial approach to kidney differentiation with hPSCs was based on the studies with mPSCs. The work of Batchelder and Lin was the earliest to demonstrate that hESCs could be differentiated into cells expressing developmental kidney markers such as PAX2 and WT1 [39, 40]. Song and colleagues devised a protocol combining EB and monolayer culture methods to generate podocyte-like cells from hiPSCs. Using a combination of activin, BMP7, and RA, the authors generated EBs that incorporated cells bearing the podocyte markers podocin, synaptopodin, and PAX2. Moreover, through a similar but extended protocol, they developed a monolayer culture of these podocyte-like cells that integrated into WT1+ glomerular structures when combined with dissociated-reaggregated E13.5 kidneys [41]. Using a combination of BMP2 and BMP7 in renal epithelial growth medium (REGM), Narayanan and colleagues induced hESCs to differentiate into aquaporin-1 (AQP1)+ proximal tubule-like cells. Flow-sorted AQP1+ cells integrated into tubular compartments of ex vivo newborn mouse kidneys and spontaneously formed cord-like structures when cultured on Matrigel. In addition, AQP1+ cells, increased cAMP production in response to stimulation with parathyroid hormone, demonstrated GGT activity, and produced ammonia [42].
Recent studies have focused on generating populations of kidney progenitor cells, particularly cells of the IM and MM. Mae and colleagues sequentially treated an OSR1-GFP reporter hiPSC line with the glycogen synthase kinase-3β inhibitor CHIR99021 (CHIR) and activin followed by BMP7 and generated OSR1+ cells with 90 % efficiency within 11–18 days of differentiation. OSR1-GFP+ cells were capable of differentiating in vitro into cells expressing markers of mature kidneys, adrenal glands, and gonads and could integrate into dissociated-reaggregated E11.5 mouse embryonic kidneys, albeit with low efficiency [43]. The same group of investigators subsequently demonstrated in a follow-up study that substitution of activin and BMP7 with either of the retinoic acid receptor agonists, AM580 and TTNPB, could reduce the time of the original protocol to 6 days [44].
Lam and colleagues sequentially treated hESCs and hiPSCs with CHIR followed by FGF2 and RA and generated PAX2+LHX1+ IM-like cells within 3 days with 70–80 % efficiency [45]. Upon growth factor withdrawal, PAX2+LHX1+ cells stochastically differentiated to form polarized, ciliated, tubular structures that expressed the proximal tubule markers LTL, N-cadherin, and kidney-specific protein. Treatment of PAX2+LHX1+ cells with FGF9 and activin generated cells co-expressing markers of MM including SIX2, SALL1, and WT1. Similar findings were reported by Takasato and colleagues, who treated hESCs with CHIR and FGF9 and induced PAX2+LHX1+ IM cells within 6 days [46]. By maintaining FGF9 treatment of these cells, the authors could generate SIX2+ cells within 14 days with 10–20 % efficiency. Clusters of cells co-expressing PAX2 and E-cadherin were also observed in the same cultures with SIX2+ cells, suggesting that the cultures were heterogeneous and comprised cells of both MM and UB lineages. Mixing these cells with dissociated-reaggregated mouse embryonic kidneys resulted in the incorporation of a small proportion of human cells within mouse tubular structures. Three-dimensional aggregates of SIX2+ cells contained tubular structures expressing markers such as AQP1, AQP2, JAG1, E-cadherin, WT1, and PAX2.
While considerable work has been done to differentiate hPSCs into MM, efforts to differentiate hPSCs into cells of the UB lineage have been limited by comparison. Xia and colleagues treated hESCs and hiPSCs with BMP4 and FGF2, followed by RA, activin, and BMP2 to generate PAX2+, OSR1+, WT1+, LHX1+ IM-like cells that spontaneously upregulated transcripts of the UB markers HOXB7, RET, and GFRA1 within 2 days. Upon co-culture with dissociated-reaggregated E11.5 mouse embryonic kidneys, these putative UB progenitor-like cells partially integrated into mouse UB tips and trunks [47].
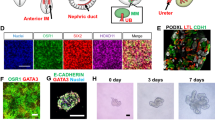
Most recently, two groups demonstrated the ability to differentiate hPSCs into 3D kidney organoids containing complex, multi-segmented nephron-like structures [48, 49]. Takasato and colleagues treated hPSCs with CHIR for 4 days, followed by FGF9 for 3 days, and transferred the cells into 3D suspension culture for up to 20 days to generate kidney organoids. Resultant organoids consisted of nephron-like formations with segmentation into proximal and distal tubules, early loops of Henle, and podocyte-like cells. In addition, organoids contained tubular structures expressing markers of collecting ducts, stromal cells expressing markers of the renal interstitium, and endothelial cells. A pulse of CHIR for 1 h after transferring the cells to suspension culture was optimal for the generation of nephron-like formations. Concurrently, Morizane and colleagues devised a protocol to robustly differentiate hPSCs into SIX2+SALL1+PAX2+WT1+ NPCs that could be induced to form nephron organoids in both 2D and 3D culture (Fig. 4.3) [49]. The authors were able to recapitulate the critical stages of MM development by first efficiently differentiating hPSCs into T+TBX6+ primitive streak cells with high-dose CHIR for 4 days, inducing WT1+HOXD11+ posterior IM cells with activin, then inducing SIX2+SALL1+PAX2+WT1+ NPCs with 90 % efficiency using low-dose FGF9. NPCs could be induced with FGF9 and transient CHIR treatment to form PAX8+LHX1+ renal vesicles that spontaneously formed nephron-like structures in 2D culture. Transfer of the NPCs into 3D suspension culture resulted in the formation of organoids containing multi-segmented nephron-like formations expressing markers of glomerular podocytes (NPHS1+PODXL+), proximal tubules (LTL+CDH2+), loops of Henle (CDH1+UMOD+), and distal tubules (CHD1+UMOD−) in a contiguous arrangement mimicking the in vivo nephron. The authors then demonstrated that these nephron organoids could be applied to study mechanisms of kidney development and drug toxicity.
Directed differentiation of hPSCs into 3D kidney organoids. Stepwise induction of hPSCs into late-stage primitive streak (T + TBX6+), posterior intermediate mesoderm (WT1 + HOXD11+), and SIX2 + SALL1 + PAX2 + WT1+ NPCs. NPCs transferred to suspension culture and treated with FGF9 and a transient CHIR pulse self-assemble into 3D organoids that contain multi-segmented, contiguous nephron structures expressing markers of glomerular podocytes, proximal tubules, loops of Henle, and distal tubules
The establishment of efficient protocols for directing the differentiation of hPSCs into NPCs and kidney organoids marks a significant advance in the ongoing effort to apply human stem cells to the regeneration of kidney tissue, modeling of human kidney disease, and drug testing for therapeutic efficacy and toxicity. However, the development of definitive functional assays and the establishment of reliable genetic markers will be required to verify whether induced hPSC-derived kidney cells and tissues are identical to their in vivo complements (Fig. 4.4).
Nephron segment-specific marker expression. Known biomarkers of nephron segments have been used to assess directed differentiation protocols and to determine the efficiency of inducing a cell type of interest. Certain biomarkers are found in multiple distinct nephron segments. It is important to note that traditionally used biomarkers have variable specificity for kidney tissue
4.7 Pluripotent Stem Cells for Nephrotoxicity Testing
Toxic effects of drugs and their metabolites often manifest as nephrotoxicity. The kidneys are highly vascular, receiving ~20 % of the cardiac output, and can accumulate toxins in the vascular, interstitial, tubular, and glomerular spaces. A retrospective multinational and multicenter study revealed that 17–26 % of in-hospital AKI was attributed to the administration of a nephrotoxic agent [50]. During drug development, 19 % of failures in Phase III clinical trials are due to nephrotoxicity [51]. The cost to bring a drug to market is currently ~2.6 billion dollars [52]. The availability of high-throughput systems for screening nephrotoxicity during drug development would potentially save considerable time and expenditure.
Recent reports have demonstrated that hPSC-derived kidney cells and tissues may respond to nephrotoxic drugs in a manner that mimics in vivo kidney injury [48, 49, 53]. In the study by Takasato and colleagues, hPSC-derived kidney organoids subjected to the chemotherapeutic agent cisplatin demonstrated histologic evidence of proximal tubular injury as evidenced by the expression of cleaved caspase-3, consistent with known effects of cisplatin-induced AKI [48]. Similar findings were reported by Morizane and colleagues, who subjected nephron organoids to two different nephrotoxic agents. Organoids treated with cisplatin showed upregulation of kidney injury molecule-1 (KIM-1) and γH2AX in injured proximal tubules and a reduction in E-cadherin+ distal tubules, which the authors interpreted as evidence of both proximal and distal tubular toxicity [48]. Treatment of organoids with the antibiotic gentamicin also upregulated KIM-1 in injured proximal tubules without any discernible effect on the distal tubules.
Given that the proximal tubule is a common site of drug-induced nephrotoxicity, Kandasamy and colleagues developed a toxicity assay using hiPSC-derived proximal tubule-like cells. The nephrotoxic response to 30 compounds was determined using a machine learning algorithm called random forest. Human proximal tubular toxicity could be predicted with >87 % accuracy, with hiPSC results congruent with human and animal data [54].
4.8 Pluripotent Stem Cells for Modeling of Kidney Diseases
Patient-derived hiPSCs represent a valuable resource for studying human pathophysiology and the development of novel therapeutics. As hiPSCs carry the genome of the patients from which they are derived, they provide a means of human genetic disease modeling. Additionally, many genetic diseases are rare enough to preclude enrollment in clinical trials. This fact, coupled with the lack of incentive for drug companies to develop pharmaceuticals for the treatment of rare diseases, fuels the hope that hiPSC-based assays can be a scalable and reliable option at low cost. Once established, reliable human disease models may allow for clinical trials-in-a-dish. Human stem cell-based systems may ultimately replace animal testing that is known to be poorly predictive of the human response [55]. To date, human iPSC lines have been generated for autosomal dominant polycystic kidney disease (ADPKD) [47, 53, 56, 57], autosomal recessive polycystic kidney disease (ARPKD) [56], and systemic lupus erythematosus [57, 58].
ADPKD hiPSC lines are particularly noteworthy, owing to the frequency of the disease and uncertainties regarding existing animal models. ADPKD is the most common potentially lethal single gene disorder, affecting 1 in 600–1 in 1000 live births [59]. Approximately 50 % of individuals with ADPKD develop ESRD by age 60. The traditionally used mouse models are homozygous carriers of ADPKD mutations while afflicted humans are heterozygotes, as heterozygote mice manifest only mild cystic disease [60]. Freedman and colleagues established hiPSC lines of three ADPKD and two ARPKD patients via fibroblast reprogramming. ADPKD iPSCs with mutations in the PKD1 gene, which encodes the protein polycystin-1, exhibited reduced polycystin-2 expression at the primary cilia. Similar results were observed in ADPKD iPSC-derived hepatoblasts, precursors to the biliary cholangiocytes that are the origin of liver cysts in ADPKD patients. The ectopic expression of wild-type polycystin-1 in these hepatoblasts rescued ciliary expression of polycystin-2 [56]. A subsequent study from the same group used gene-editing with the clustered regularly interspaced short palindromic repeats (CRISPR)/Cas9 system to generate knockout PKD1 or PKD2 human ESC lines. Two-dimensional kidney organoids derived from these gene-edited hESCs developed cystic structures from kidney tubules, suggesting that this model could potentially serve as a novel means to study cystogenesis in ADPKD [53].
4.9 Pluripotent Stem Cells for Bioengineering Kidney Tissue
The rising prevalence of ESRD, coupled with the shortage of transplantable organs, has led researchers to apply regenerative medicine techniques towards kidney bioengineering. Human iPSCs serve as a theoretically immunocompatible and scalable cell source, with therapeutic applications for both CKD and AKI.
The kidney comprises >50 distinct cell types, arranged in a complex 3D structure that facilitates exocrine, endocrine, and metabolic functions. The primary elements of a bioengineered kidney would include multiple hiPSC-derived cell types and a scaffolding to provide cellular support, segregation, and compartmentalization. Two scaffolding approaches have been undertaken: kidney decellularization and a 3D printed framework.
Decellularized kidney approaches have the benefit of preserving the intricate extracellular matrix (ECM) of distinct kidney compartments, retaining matrix-associated signals and growth factors of specific regions, and conserving the arterial, capillary, and venous vascular tree. Using the detergent sodium dodecyl sulfate (SDS) and the cell membrane toxicant Triton X-100, Nakayama and colleagues decellularized adult rhesus monkey kidney sections. Hematoxylin and eosin (H&E) staining confirmed the removal of cellular material and immunohistochemistry demonstrated the preservation of native ECM [61]. Orlando and colleagues successfully decellularized porcine kidneys and surgically implanted unseeded scaffolds into pigs. Although the decellularized kidneys were easily reperfused, sustained blood pressure, and demonstrated a lack of blood extravasation, the vascular tree was completely thrombosed due to denuded ECM [62]. Song and colleagues seeded decellularized rat kidney scaffolds with rat fetal kidney cells via the ureter and endothelial cells via the renal artery and performed an orthotopic transplantation in a rat. The graft was perfused by the recipient’s circulation and produced urine through a ureteral conduit. However, urinary excretion was not substantial and histopathology of the recipient’s graft demonstrated vascular thromboses [63]. Similarly, Ross and colleagues seeded murine ESCs into decellularized rat kidneys via the renal artery and ureter. Resultantly, cells lost their pluripotent phenotype and expressed kidney immunohistochemical markers when contacting ECM, while cells not in contact became apoptotic [64]. However, this approach was also limited by small vessel thrombosis. To overcome thrombosis in the small vessel conduits utilized in decellularized scaffolds, Wertheim and colleagues developed a biocompatible polymer that binds denuded ECM. Decellularized rat aortas were lined with poly(1,8-octanediol citrate), functionalized with heparin, and perfused with whole blood. The polymer-ECM reduced platelet adhesion, inhibited whole blood clotting, and supported endothelial cell-adhesion [65]. Given their advantage of maintained architecture, decellularized kidneys represent a valuable resource in the efforts to create a bioengineered kidney. However, it remains to be seen whether proper localization of seeded renal epithelial cell types to their appropriate compartment within the kidney can be achieved. Furthermore, such grafts must also retain significant functionality upon transplantation.
The biologic application of 3D printing has gained both notoriety and credibility with the organ-on-a-chip series, including the lung-on-a-chip, the gut-on-a-chip, the proximal tubule-on-a-chip, and bone marrow-on-a-chip [66–69]. Such devices employ a soft lithography method for the creation of microfluidic chambers, first conceived by Duffy and Whitesides in 1998 [70]. Recent advances in 3D printing have enabled faithful manufacturing of micrometer scale, multicomponent structures. Current precision allows for the biomimicry of multiple physical aspects of native kidneys, such as multicellular architecture, submillimeter tubular diameter, and high surface area to volume ratio. Additionally, applying a perfusate can simulate physiologic levels of shear stress in epithelial and vascular channels. Vascular channels are imperative, as bioengineered tissue structures develop necrotic regions without vasculature within <2 mm of tissue depth [71]. Of note, current commercially available 3D printing resins for both stereolithography and multijet modeling demonstrate poor biocompatibility [72, 73]. To overcome obstacles of resin cytotoxicity and the need for a vascular network in tissue engineering, Kolesky and colleagues used ECM-containing bioink resins to develop a method of printing directly in cells. HUVEC cells, embedded in a Pluronic F127 fugitive hydrogel, were printed in channels and surrounded by gelatin methacrylate (GelMA). Removal of the fugitive ink yielded tubular channels consisting of a confluent monolayer of HUVECs [74].
Fabrication of a bioengineered nephron, the individual functioning unit of the kidney, is a potential intermediate step in the development of a bioengineered kidney. The glomerular, tubular, and collecting duct compartments of the nephron could be modeled separately and connected in series, replete with vasculature, overcoming the compartmentalization difficulties of decellularized kidney methods. Additionally, complex microscale printing provides a means of maximizing absorptive and secretory functions by increased surface area to volume ratio. Homogenous populations of varying types of kidney epithelia may be obtained through cell-sorting of dissociated organoids developed from patient-specific iPSCs. Integration of these cells into 3D printed scaffolds may allow for the generation of an immunocompatible bioengineered nephron, which can be scaled up to restore in vivo kidney filtration.
4.10 Conclusion
Significant advances over the past few years alone have clearly demonstrated that human PSCs represent a powerful tool to study kidney regeneration, disease, and injury. Recently established methods are now capable of directing the differentiation of hESCs and hiPSCs into human kidney organoids in vitro. These kidney organoids can mimic the in vivo pathophysiologic response when subject to nephrotoxic agents, providing novel nephrotoxicity models that may facilitate the identification of lead candidates, reduce developmental costs, and reduce future rates of drug-induced AKI. Patient-derived hiPSCs, bearing naturally occurring human mutations, can recapitulate human disease phenotypes. While disease modeling using hiPSCs may someday supplant animal testing, currently it provides a means of studying rare genetic diseases and allows for clinical trials-in-a-dish. hiPSCs are a theoretically immunocompatible and scalable cell source for kidney regeneration. With the development of advanced bioengineering techniques such as decellularized kidney scaffolds and 3D printing, the integration of stem cell biology with bioengineering may someday contribute to the development of transplantable human kidney tissue.
References
Monda KL, et al. Comparative changes in treatment practices and clinical outcomes following implementation of a prospective payment system: the STEPPS study. BMC Nephrol. 2015;16:67.
Szczech LA, Lazar IL. Projecting the United States ESRD population: issues regarding treatment of patients with ESRD. Kidney Int Suppl. 2004;90:S3–7.
Depner T, Himmelfarb J. Uremic retention solutes: the free and the bound. J Am Soc Nephrol. 2007;18(3):675–6.
Floege J, Ketteler M. [beta]2-Microglobulin-derived amyloidosis: an update. Kidney Int. 2001;59(S78):S164–71.
Chandrashekar A, Ramakrishnan S, Rangarajan D. Survival analysis of patients on maintenance hemodialysis. Indian J Nephrol. 2014;24(4):206–13.
Fukuhara S, et al. Health-related quality of life among dialysis patients on three continents: the Dialysis Outcomes and Practice Patterns Study. Kidney Int. 2003;64(5):1903–10.
Stegall MD, Chedid MF, Cornell LD. The role of complement in antibody-mediated rejection in kidney transplantation. Nat Rev Nephrol. 2012;8(11):670–8.
Baumann K. Stem cells: multiple routes to pluripotency. Nat Rev Mol Cell Biol. 2015;16(1):1.
Lam AQ, Freedman BS, Bonventre JV. Directed differentiation of pluripotent stem cells to kidney cells. Semin Nephrol. 2014;34(4):445–61.
Reijo Pera RA, et al. Gene expression profiles of human inner cell mass cells and embryonic stem cells. Differentiation. 2009;78(1):18–23.
Boland MJ, et al. Adult mice generated from induced pluripotent stem cells. Nature. 2009;461(7260):91–4.
Zhao XY, et al. iPS cells produce viable mice through tetraploid complementation. Nature. 2009;461(7260):86–90.
Martin GR. Isolation of a pluripotent cell line from early mouse embryos cultured in medium conditioned by teratocarcinoma stem cells. Proc Natl Acad Sci U S A. 1981;78(12):7634–8.
Evans MJ, Kaufman MH. Establishment in culture of pluripotential cells from mouse embryos. Nature. 1981;292(5819):154–6.
Thomson JA, et al. Embryonic stem cell lines derived from human blastocysts. Science. 1998;282(5391):1145–7.
Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126(4):663–76.
Takahashi K, et al. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007;131(5):861–72.
Giacomini M, Baylis F, Robert J. Banking on it: public policy and the ethics of stem cell research and development. Soc Sci Med. 2007;65(7):1490–500.
Montserrat N, et al. Generation of induced pluripotent stem cells from human renal proximal tubular cells with only two transcription factors, Oct4 and Sox2. J Biol Chem. 2012;287(29):24131–8.
Song B, et al. Generation of induced pluripotent stem cells from human kidney mesangial cells. J Am Soc Nephrol. 2011;22(7):1213–20.
Zhou T, et al. Generation of human induced pluripotent stem cells from urine samples. Nat Protoc. 2012;7(12):2080–9.
Seki T, Yuasa S, Fukuda K. Generation of induced pluripotent stem cells from a small amount of human peripheral blood using a combination of activated T cells and Sendai virus. Nat Protoc. 2012;7(4):718–28.
Aasen T, et al. Efficient and rapid generation of induced pluripotent stem cells from human keratinocytes. Nat Biotechnol. 2008;26(11):1276–84.
Liu H, et al. Generation of endoderm-derived human induced pluripotent stem cells from primary hepatocytes. Hepatology. 2010;51(5):1810–9.
Kim JB, et al. Pluripotent stem cells induced from adult neural stem cells by reprogramming with two factors. Nature. 2008;454(7204):646–50.
Cohen DE, Melton D. Turning straw into gold: directing cell fate for regenerative medicine. Nat Rev Genet. 2011;12(4):243–52.
Kobayashi A, et al. Six2 defines and regulates a multipotent self-renewing nephron progenitor population throughout mammalian kidney development. Cell Stem Cell. 2008;3(2):169–81.
Self M, et al. Six2 is required for suppression of nephrogenesis and progenitor renewal in the developing kidney. EMBO J. 2006;25(21):5214–28.
Taguchi A, et al. Redefining the in vivo origin of metanephric nephron progenitors enables generation of complex kidney structures from pluripotent stem cells. Cell Stem Cell. 2014;14(1):53–67.
Steenhard BM, et al. Integration of embryonic stem cells in metanephric kidney organ culture. J Am Soc Nephrol. 2005;16(6):1623–31.
Yamamoto M, et al. Branching ducts similar to mesonephric ducts or ureteric buds in teratomas originating from mouse embryonic stem cells. Am J Physiol Renal Physiol. 2006;290(1):F52–60.
Dressler GR. Advances in early kidney specification, development and patterning. Development. 2009;136(23):3863–74.
Kim D, Dressler GR. Nephrogenic factors promote differentiation of mouse embryonic stem cells into renal epithelia. J Am Soc Nephrol. 2005;16(12):3527–34.
Vigneau C, et al. Mouse embryonic stem cell-derived embryoid bodies generate progenitors that integrate long term into renal proximal tubules in vivo. J Am Soc Nephrol. 2007;18(6):1709–20.
Bruce SJ, et al. In vitro differentiation of murine embryonic stem cells toward a renal lineage. Differentiation. 2007;75(5):337–49.
Morizane R, Monkawa T, Itoh H. Differentiation of murine embryonic stem and induced pluripotent stem cells to renal lineage in vitro. Biochem Biophys Res Commun. 2009;390(4):1334–9.
Nishikawa M, et al. Stepwise renal lineage differentiation of mouse embryonic stem cells tracing in vivo development. Biochem Biophys Res Commun. 2012;417(2):897–902.
Morizane R, et al. Kidney specific protein-positive cells derived from embryonic stem cells reproduce tubular structures in vitro and differentiate into renal tubular cells. PLoS One. 2014;8(6):e64843.
Batchelder CA, et al. Renal ontogeny in the rhesus monkey (Macaca mulatta) and directed differentiation of human embryonic stem cells towards kidney precursors. Differentiation. 2009;78(1):45–56.
Lin SA, et al. Subfractionation of differentiating human embryonic stem cell populations allows the isolation of a mesodermal population enriched for intermediate mesoderm and putative renal progenitors. Stem Cells Dev. 2010;19(10):1637–48.
Song B, et al. The directed differentiation of human iPS cells into kidney podocytes. PLoS One. 2012;7(9):e46453.
Narayanan K, et al. Human embryonic stem cells differentiate into functional renal proximal tubular-like cells. Kidney Int. 2013;83(4):593–603.
Mae S, et al. Monitoring and robust induction of nephrogenic intermediate mesoderm from human pluripotent stem cells. Nat Commun. 2013;4:1367.
Araoka T, et al. Efficient and rapid induction of human iPSCs/ESCs into nephrogenic intermediate mesoderm using small molecule-based differentiation methods. PLoS One. 2014;9(1):e84881.
Lam AQ, et al. Rapid and efficient differentiation of human pluripotent stem cells into intermediate mesoderm that forms tubules expressing kidney proximal tubular markers. J Am Soc Nephrol. 2014;25(6):1211–25.
Takasato M, et al. Directing human embryonic stem cell differentiation towards a renal lineage generates a self-organizing kidney. Nat Cell Biol. 2014;16(1):118–26.
Xia Y, et al. Directed differentiation of human pluripotent cells to ureteric bud kidney progenitor-like cells. Nat Cell Biol. 2013;15(12):1507–15.
Takasato M, et al. Kidney organoids from human iPS cells contain multiple lineages and model human nephrogenesis. Nature. 2015;526:564–8.
Morizane R, et al. Nephron organoids derived from human pluripotent stem cells model kidney development and injury. Nat Biotechnol. 2015;33(11):1193–200.
Pazhayattil GS, Shirali AC. Drug-induced impairment of renal function. Int J Nephrol Renov Dis. 2014;7:457–68.
Su R, et al. Supervised prediction of drug-induced nephrotoxicity based on interleukin-6 and -8 expression levels. BMC Bioinf. 2014;15 Suppl 16:S16.
Bertolini F, Sukhatme VP, Bouche G. Drug repurposing in oncology[mdash]patient and health systems opportunities. Nat Rev Clin Oncol. 2015;12(12):732–42.
Freedman BS, et al. Modelling kidney disease with CRISPR-mutant kidney organoids derived from human pluripotent epiblast spheroids. Nat Commun. 2015;6.
Kandasamy K, et al. Prediction of drug-induced nephrotoxicity and injury mechanisms with human induced pluripotent stem cell-derived cells and machine learning methods. Sci Rep. 2015;5:12337.
Olson H, et al. Concordance of the toxicity of pharmaceuticals in humans and in animals. Regul Toxicol Pharmacol. 2000;32(1):56–67.
Freedman BS, et al. Reduced ciliary polycystin-2 in induced pluripotent stem cells from polycystic kidney disease patients with PKD1 mutations. J Am Soc Nephrol. 2013;24(10):1571–86.
Thatava T, et al. Successful disease-specific induced pluripotent stem cell generation from patients with kidney transplantation. Stem Cell Res Ther. 2011;2(6):48.
Chen Y, et al. Generation of systemic lupus erythematosus-specific induced pluripotent stem cells from urine. Rheumatol Int. 2013;33(8):2127–34.
Iglesias CG, et al. Epidemiology of adult polycystic kidney disease, Olmsted County, Minnesota: 1935–1980. Am J Kidney Dis. 1983;2(6):630–9.
Wilson PD. Mouse models of polycystic kidney disease. Curr Top Dev Biol. 2008;84:311–50.
Nakayama KH, et al. Decellularized rhesus monkey kidney as a three-dimensional scaffold for renal tissue engineering. Tissue Eng Part A. 2010;16(7):2207–16.
Orlando G, et al. Production and implantation of renal extracellular matrix scaffolds from porcine kidneys as a platform for renal bioengineering investigations. Ann Surg. 2012;256(2):363–70.
Song JJ, et al. Regeneration and experimental orthotopic transplantation of a bioengineered kidney. Nat Med. 2013;19(5):646–51.
Ross EA, et al. Embryonic stem cells proliferate and differentiate when seeded into kidney scaffolds. J Am Soc Nephrol. 2009;20(11):2338–47.
Jiang B, et al. A polymer-extracellular matrix composite with improved thromboresistance and recellularization properties. Acta Biomater. 2015;18:50–8.
Huh D, et al. A human disease model of drug toxicity-induced pulmonary edema in a lung-on-a-chip microdevice. Sci Transl Med. 2012;4(159):159ra147.
Kim HJ, et al. Human gut-on-a-chip inhabited by microbial flora that experiences intestinal peristalsis-like motions and flow. Lab Chip. 2012;12(12):2165–74.
Jang KJ, et al. Human kidney proximal tubule-on-a-chip for drug transport and nephrotoxicity assessment. Integr Biol (Camb). 2013;5(9):1119–29.
Torisawa YS, et al. Bone marrow-on-a-chip replicates hematopoietic niche physiology in vitro. Nat Methods. 2014;11(6):663–9.
Duffy DC, et al. Rapid prototyping of microfluidic systems in poly(dimethylsiloxane). Anal Chem. 1998;70(23):4974–84.
Griffith CK, et al. Diffusion limits of an in vitro thick prevascularized tissue. Tissue Eng. 2005;11(1–2):257–66.
Chia HN, Wu BM. Recent advances in 3D printing of biomaterials. J Biol Eng. 2015;9:4.
Macdonald NP, et al. Assessment of biocompatibility of 3D printed photopolymers using zebrafish embryo toxicity assays. Lab Chip. 2016;16(2):291–7.
Kolesky DB, et al. 3D bioprinting of vascularized, heterogeneous cell-laden tissue constructs. Adv Mater. 2014;26(19):3124–30.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2016 Springer International Publishing Switzerland
About this chapter
Cite this chapter
Gupta, N.R., Lam, A.Q. (2016). Pluripotent Stem Cells for Kidney Diseases. In: Abdelalim, E. (eds) Recent Advances in Stem Cells. Stem Cell Biology and Regenerative Medicine. Humana Press, Cham. https://doi.org/10.1007/978-3-319-33270-3_4
Download citation
DOI: https://doi.org/10.1007/978-3-319-33270-3_4
Published:
Publisher Name: Humana Press, Cham
Print ISBN: 978-3-319-33268-0
Online ISBN: 978-3-319-33270-3
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)