Abstract
Patients with active Systemic Lupus Erythematosus (SLE) experience a large variation of symptoms and manifestations. The symptoms and manifestations of SLE impact the patients’ health-related quality of life (HRQoL). Many of the patients’ SLE symptoms are not always adequately captured by objective clinical outcome measures or laboratory assessments alone. Therefore in addition to well-established clinical outcome measures used in clinical practice, the patient perspective should be captured through patient reported outcome measures (PROMs). Key concepts/domains derived from a recently developed conceptual model published by Holloway et al. (Qual Life Outcomes. 2014;12:116) are the basis for the recommendations of PROMs. Recommendations for PROMs to apply in clinical practice are based on the face and content validity as well as psychometric properties of each of the PROMs. In the conceptual model, fatigue, pain, and emotional well-being/depression were identified as key domains impacting the lives of patients with SLE. Appropriate PROMs to use in clinical practice capturing the key domains are the Functional Assessment of Chronic Illness Therapy—Fatigue scale (FACIT-Fatigue), Brief Pain Inventory (BPI-SF), and Hospital Anxiety and Depression Scale (HADS). In addition the generic HRQoL measure Short Form (36 item) Health Survey version 2 (SF-36v2) and the disease-specific HRQoL measure LupusQoL can be applied in clinical practice. It might be favorable in standard clinical practice to consider including one cohesive PROM for the assessment of patient reported key symptoms and impacts in SLE, however, further research and validation studies are needed. PROMs capturing key symptoms of SLE such as fatigue, pain, and HRQoL supplement objective clinical outcome measures and laboratory assessments providing clinicians with a valuable patient perspective on the impact of SLE.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Systemic lupus erythematosus
- Patient reported outcomes
- Conceptual model
- Fatigue
- Pain
- Emotional well-being
- Health-related quality of life
- Clinical practice
Introduction and Background
Systemic Lupus Erythematosus (SLE) is a heterogeneous, inflammatory, multisystem autoimmune disease. Symptoms include joint pain and swelling, skin rash, and fatigue [1]. These symptoms impact daily and leisure activities, work productivity, emotional well-being, relationships, physical functioning, and social functioning. The symptoms of SLE appear to occur in “flares.” Subsequently, the impact of SLE can vary over time, depending on whether symptoms are present and/or more intense in severity. In addition to joint inflammation, SLE often impacts the heart, skin, lungs, blood vessels, liver, kidneys, and nervous system of patients [1]. The symptoms of SLE contribute to a substantially reduced health-related quality of life (HRQoL) [2]. A number of patient reported outcome measures (PROMs) have been used to assess the burden of SLE on patients, including measurements of fatigue, pain, emotional/psychological well-being, and work productivity. Furthermore, both SLE-specific and generic PROMs measuring HRQoL have been used.
Treatment of the more severe cases of SLE involves a balance between suppressing the signs and symptoms of the disease and minimizing the toxicities of the drugs used. With treatment, disease activity indices might improve but the patient might feel potentially worse due to the side effects of the medication. In the evaluation of patients with SLE, it is important to measure the patients’ perspective because the disease is likely to have a significant impact on physical, social, and psychological aspects impacting the patients’ HRQoL. Improvements in clinical outcome measures (e.g., lab tests, clinical evaluation) in patients with SLE may not always translate to improvements in how patients feel or function. PROMs can be used to measure all relevant and important SLE symptoms and patient-perceived abilities to function and perform daily activities.
Conceptual Model for SLE
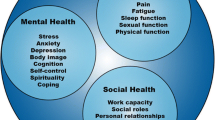
A conceptual model can be used to illustrate the humanistic and economic burden of key symptoms and their impact. Such models are valuable in terms of identifying key measurement concepts, which can be used to demonstrate treatment benefit, providing insight into how best to measure particular concepts, and providing a contextual basis for interpreting patient reported findings. The conceptual model (Fig. 6.1) published by Holloway et al. (2014) [3] is based on a structured literature review of qualitative and quantitative articles and can be used to assess whether available disease-specific PROMs target key symptoms and impacts of SLE. The resulting conceptual model shows the symptoms and impacts identified as key concepts related to SLE (Fig. 6.1) [3].
Fatigue and pain are two of the most important and frequent symptoms for patients with SLE [4–10]. Specifically, patients describe mental and physical symptoms of fatigue including impacts on social life [4], emotional well-being [4, 11], physical functioning [4, 12], sleep [9, 13–15], and the ability to complete daily tasks and leisure activities [16, 17]. Important cognitive symptoms include being “unable to think clearly” and memory loss [12]. Other SLE symptoms include skin rash [16, 17], weight gain [4, 16], and hair loss [5, 16]. Symptoms impact all areas of HRQoL, with detrimental consequences observed in the physical, emotional, and social functioning of SLE patients, as well as in their working life. In terms of the impact on emotional well-being, patients with SLE frequently feel sad, depressed, angry, and demoralized [4, 5, 8, 12, 18, 19]. In particular, patients feel embarrassed [4–6] or self-conscious, or they lack self-esteem, primarily because of the changes in their appearance (such as hair loss and skin manifestations) [6, 12]. Patients fear their disease worsening, and experience anxiety or stress related to the symptoms and the unpredictability of SLE [8, 16, 18, 19]. Many also experience feelings of frustration and a lack of: (1) confidence, (2) independence, (3) control over one’s life, and (4) belonging [20]. SLE has a significant negative impact on patients’ physical functioning, such as walking difficulty and other mobility problems [2, 12, 21, 22]. This affects various daily activities including opening jars and moving heavy objects [22], shopping [12], doing laundry [6], getting dressed [6], and caring for their children [4, 6]. Wider impacts on social functioning and working life are also reported [7, 20]. Specifically, patients have difficulty maintaining family and sexual relationships [4, 6, 18]. SLE also impacts negatively on patients’ career progression [5], absence from work [12], difficulty concentrating at work or study [6, 10, 12], and their choice of work [6, 16].
The conceptual model presented suggests that patients use various coping mechanisms for the unpredictability of flares, including: (1) seeking and using information, (2) seeking emotional and practical help via the Internet, (3) receiving support from hospital meetings, (4) receiving support from family, (5) attending lupus support groups, and (6) religious practice [4, 6, 16]. The conceptual model also includes concepts such as treatment satisfaction, adherence, and the impact of flares in a “future considerations” box. There was a lack of evidence pertaining to these concepts in the currently available literature.
The conceptual model also demonstrates the economic burden of disease, in particular the high medical costs associated with SLE compared to other chronic diseases [23]. Substantial levels of inpatient care, medication/prescriptions, and visits to healthcare professionals (HCP), which are all increased by “flares,” are the main drivers of direct costs in the treatment of SLE [24]. The conceptual model also shows that SLE is associated with high indirect costs due to lost productivity [25] resulting from unemployment and absenteeism [26], with “in-flare” patients with SLE having increased frequency and duration of time off work [27, 28].
Patient Reported Outcome Measures
Fatigue
Fatigue is one of the most important and frequent symptoms for patients with SLE. For many patients it is the most enduring complaint [15, 18]. Fatigue is described in various ways including tiredness, reduced energy, and mental fatigue, and it often impacts the HRQoL in patients with SLE [9, 20]. The lack of a clear definition of fatigue is evident in the literature and reflects the complex nature of the concept. Furthermore, there is a lack of consistent definition from patients and clinicians in terms of what fatigue really means to patients and how it differs from other related concepts such as “normal tiredness” and “energy.” As a result, there is a notable variety and disparity in the content of the various PROMs developed to measure fatigue.
Several PROMs measuring fatigue exist. Some of the most frequently used are the Multidimensional Fatigue Inventory (MFI), Multidimensional Assessment of Fatigue (MAF), Fatigue Severity Scale (FSS), and the Functional Assessment of Chronic Illness Therapy—Fatigue scale (FACIT-Fatigue). For none of the listed fatigue PROMs the content and face validity have been established in patients with SLE using qualitative and cognitive debriefing methodologies in the development process. Of the fatigue measures, FACIT-Fatigue (Appendix 1) is currently one of the most frequently applied in recent clinical trials of belimumab [29, 30], and has been extensively validated within rheumatic diseases [31–33]. In a qualitative research study, patients with SLE perceived FACIT-Fatigue as a relevant and appropriate measure of fatigue in SLE [17].
FACIT-Fatigue is a one-dimensional 13-item PROM assessing self-reported fatigue and its impact upon functioning and daily activities. It asks patients to indicate how true each statement is on a 5-point Likert scale from 0 (Not at all) to 4 (Very much) with a 7-day recall period (see Table 6.1 and Appendix 1). The estimated completion time for the patient is 3–5 min, which limits the burden to both patient and medical staff at the clinic. The written instructions to the patient appear clear and no complex clinical terminology is included. In general the item-wording is written in a simple and understandable language for most patients.
FACIT-Fatigue has demonstrated the strong psychometric properties in terms of evidence of internal consistency, reliability, known-groups validity, concurrent validity, and ability to detect change in patients with SLE (Box 6.1) [31]. Further, test–retest reliability has been demonstrated in patients with psoriatic arthritis [32]. A minimal clinically important difference (MCID) has not been established in patients with SLE; however, in patients with rheumatoid arthritis the MCID has been estimated to be a 3–4 point change from a baseline in the score [33].
Box 6.1: Fatigue
Fatigue is one of the most frequent symptoms reported by patients with SLE.
The Functional Assessment for Chronic Illness Therapy — Fatigue scale (FACIT- Fatigue) is a well-established fatigue measure in SLE, and its psychometric properties in SLE has been established. It consists of 13 items written in a simple language without complex clinical terminology.
Pain
Pain is one of the most common complaints for patients with SLE and is described as “pain,” “hurt,” or “ache” and some patients speak specifically of “joint pain” [4–6]. Due to the subjective and variable nature of pain, it is best evaluated using patient-reported assessments.
In a review of previous studies involving SLE patients, it was reported that amongst a mean of 460 patients per study, 71–89 % of patients reported experiencing pain [7]. Many publications suggest there is an association of pain with fatigue [13–15, 34] and between pain and poor sleep quality [15]. PROMs specific to pain include the McGill Pain Questionnaire (MPQ) and the Brief Pain Inventory (BPI) (Table 6.2).
The MPQ exists as both a standard form (20 items) [35] and a short form (15 items) [36]. The standard form is more comprehensive. The MPQ is a multidimensional instrument designed to measure the physical and emotional components of pain. The MPQ was developed with minimal patient input (n = 10) and the patient group or inclusion/exclusion criteria was not specified. The instrument can be administered in any mode (e.g., self-administered or by a clinician), but the selected mode of administration should be consistent. The item and response wording is very clinical and patients with a low reading ability are likely to not understand the terminology. The recall period for assessment is “currently” or “presently.” The MPQ focuses on pain, primarily assessing descriptors of pain. Some impacts of pain are assessed including pain-related fatigue and emotional impacts. However, in the literature review for the conceptual model, it was found that SLE patients tended to discuss SLE-related pain in terms of its location—for example, muscle pain, joint pain, or headaches—rather than how it feels (i.e., aches or discomfort), which could be problematic as the MPQ does not assess where pain occurs. The recall period of current/present pain may not be appropriate for SLE, given that symptoms may arise at any time and, unless the patient is experiencing symptoms during completion, such episodes could be missed. The Brief Pain Inventory (Appendix 2) is a PROM designed to assess the intensity of pain and the extent to which pain interferes with normal function [37]. The BPI is available as a standard form and a short form. The shorter version (BPI-SF) has become the standard for use in clinical and research applications [38] and is the focus for this review (Box 6.2). The BPI-SF focuses on pain and assesses various aspects of pain including the location, severity, and the impact of pain on patients’ HRQoL. In line with the conceptual model (Fig. 6.1), the impact concepts assessed include daily activities, emotional/psychological impacts, physical functioning, relationships, and sleep problems. With a focus on pain, the BPI-SF has good concept coverage, assessing not only descriptors of pain, but also the location of pain and the impact on patients’ HRQoL. Most items have an 11-point rating scale; for severity, 0 = no pain and 10 = pain as bad as you can imagine; and for interference, 0 = does not interfere and 10 = completely interferes. One item has a binary yes/no response and another asks patients to shade a diagram to show where they have pain. One item has a 0–100 % scale increasing in 10 % increments. The format of the questionnaire is clear and simple to follow, and thus does not appear to pose any problems for comprehension or accurate completion. BPI-SF has demonstrated strong psychometric properties in terms of internal consistency [39], test–retest reliability [37], construct [39–41] and discriminant [37, 42] validity and responsiveness [42], and a recent study confirmed the findings in an SLE population [43]. The BPI-SF appears to be the strongest measure of pain of the 2 reviewed.
Box 6.2: Pain
Pain is one of the most common complaints for patients with SLE in qualitative research and is associated with fatigue and poor sleep quality.
The Brief Pain Inventory (BPI-SF) can be recommended for use in patients with SLE to assess the intensity of pain and the extent to which pain interferes with normal function.
Further, qualitative research and validation of the psychometric properties of BPI are recommended to be explored in patients with SLE.
Emotional Well-Being and Depression
SLE has been shown to impact patient’s emotional well-being. Changes in appearance due to the disease and side effects of treatment affect the patient’s perception of their body image and sexuality, which in turn impacts their emotional well-being [8]. Patients with SLE frequently feel sad, depressed, angry, embarrassed, and have lack of self-esteem [4–6, 12]. Emotional well-being is a very broad term, and the focus of this discussion will be on anxiety and depression as it arose most frequently in the qualitative literature of patients with SLE.
Two frequently used PROMs assessing anxiety and depression are Beck’s Depression Inventory (BDI) and the Hospital Anxiety and Depression Scale (HADS). Neither BDI nor HADS have been validated in patients with SLE. However, both instruments are suitable to use in clinical practice in patients with SLE who experience an impact on anxiety and depression. However, HADS could be considered over BDI, as the instructions are more detailed and straightforward and the item wording is clearer. Further, the response options in the HADS are worded simply and clearly defined, and thus should not pose any problems for patients with SLE.
HADS is a 14-item PROM assessing self-reported anxiety and depression (Box 6.3). Patients should indicate to which degree each of the 14 statements applies on a 4-point Likert-scale with a recall period of a week [44, 45] (Table 6.3) . It consists of two domains (anxiety and depression) with seven items each. The estimated completion time is 2–5 min, which provides a limited burden to both patient and medical staff at clinic.
No evidence of validation of the psychometric properties of HADS has been published in patients with SLE [3]. The HADS has demonstrated strong psychometric properties in a general population and in patients with psychiatric disorders. Evidence of the ability to detect change in response to an intervention has been established in various diseases such as depression, neurotic disorder, and cancer [46].
Box 6.3: Anxiety and Depression
Anxiety and depression is frequently expressed by patients with SLE in qualitative research.
The Hospital Anxiety and Depression Scale (HADS) can be recommended for use in patients with SLE where the medical staff suspects that the patient’s emotional well-being is impacted by anxiety or depression.
Further, qualitative research and validation of the psychometric properties of HADS are recommended to be explored in patients with SLE.
Health-Related Quality of Life
HRQoL in patients with SLE is influenced by treatment, disease activity, and symptoms of fatigue, depression, pain, sleep disturbances, and cognitive dysfunction [47]. Due to the radical nature of the disease, HRQoL is an important outcome measure in patients with SLE. HRQoL can be accessed through generic or disease-specific PROMs.
Generic Assessment of HRQoL
The generic HRQoL measure selected for review is the 36-item Short Form Health Survey version 2 (SF-36v2) (Table 6.4) . SF-36v2 has been validated in many different health conditions and is a widely used and accepted measure of HRQoL [40, 48]. This PROM covers many domains of importance to patients including physical function, social function, pain, vitality (fatigue and energy), and mental health, and distinguishes limitation on activities by physical and emotional factors. This is crucial in a chronic disease such as SLE where the disease, as well as the therapies used, may cause physical and emotional effects; SF-36v2 makes it possible to assess these different aspects of health status and quality of life separately.
The SF-36v2 has 36-items; 26 are rated on a 5-point scale and 10 are rated on a 3-point scale. These items and response options are generally clear and easy to understand, and the instructions are simple and straightforward to follow. In terms of the recall period of the questionnaire, both a 4-week recall and an acute 1-week recall version exist. A recall period of the past 7 days may be more appropriate, given the fluctuating nature of the condition—patient’s symptoms and limitations may vary significantly from day to day. SF-36v2 has demonstrated good psychometric properties in terms of internal consistency, reliability, and test–retest reliability, construct validity, and concurrent validity in the general population [48, 49]. More importantly, in an SLE population, the SF-36v2 has demonstrated evidence of internal consistency reliability, concurrent validity, and known groups validity [50]. Of note, the SF36v2 is able to detect change in many conditions [48, 51] and distribution and anchor-based estimates suggest Minimal Clinically Important Differences (MCIDs) of approximately 3–6 points in an SLE population [50]. SF-36v2 is able to discriminate between levels of disease severity, which is important for assessing change. Patients were not involved in the initial development, but the SF-36v2 has been widely used in general health populations since its development.
SLE-Specific Assessment of HRQoL
Several disease-specific instruments have been designed to assess HRQoL in SLE: Lupus Quality of Life (LupusQoL), L-QoL, SLE-QoL, and Lupus-PRO. The LupusQoL is the strongest of the disease-specific HRQoL measures in terms of development, conceptual coverage, and validation and will be the focus for this review. The LupusQoL (Appendix 3, Table 6.4) is a 34-item questionnaire designed to assess SLE patients’ HRQoL (Box 6.4). Concept elicitation interviews were conducted with 30 SLE patients to gather information regarding concepts that are relevant to patients [52]. The LupusQoL comprises 8 domains: physical health, pain, planning, intimate relationships, burden to others, emotional health, body image, and fatigue [52]. It emphasizes areas such as sleep, body image, and sexual health, which are not specifically queried in SF-36v2. LupusQoL has demonstrated good internal consistency, test–retest reliability, and concurrent validity with the generic SF-36v2 [52].
The response options are clearly worded and appear to be easy for patients to understand. The item wording is clear and simple to understand, however the response options may be somewhat skewed toward the higher end of the severity spectrum and some options could be difficult to differentiate between. Patients are required to think over the past 4 weeks. This is a fairly long period and may elicit inaccurate responses, as some patients may forget the impact that their illness had over this time. LupusQoL has good psychometric properties in terms of reliability, construct validity, discriminant validity, and concurrent/convergent validity [52]. No evidence is available on ability to detect change.
Box 6.4: Health-Related Quality of Life
Health-Related Quality of Life (HRQoL) in patients with SLE is influenced by treatment, disease activity, and symptoms of fatigue, depression, pain, sleep disturbances, and cognitive dysfunction.
The Short Form Health Survey (SF-36v2) can be recommended to assess different aspects of general health status and quality of life.
The LupusQoL can be used to assess the impact that SLE has upon patients’ HRQoL and it emphasizes areas such as sleep, body image, and sexual health, which are not specifically queried in SF-36v2.
Reflections and Considerations for the Future
To understand the value of therapies for SLE from the patient perspective, PROMs should be included in clinical practice in conjunction with well-established clinical assessments. The selection of suitable measures to assess SLE-related symptoms and impacts in clinical practice requires careful consideration [53, 54]. This chapter therefore presented a conceptual model of the key symptoms and impacts associated with SLE. The key patient-reported concepts identified within the model were fatigue, pain, cognition, daily activities, emotional well-being, physical/social functioning, and work productivity. The subjective nature of many SLE symptoms and impacts requires accurate and reliable measurement of these symptoms based on patient self-report. In light of this, it is important to also review and evaluate the content validity and psychometric properties of PROMs that may be appropriate for use in an SLE population.
The FACIT-Fatigue, LupusQoL, BPI, SF-36v2, and LupusQoL appear to be the strongest PROMs as measures of the key concepts identified in the conceptual model and all had evidence of the psychometric validity. In addition, the generic SF-36v2 is widely used in randomized clinical trials with patients with SLE and is recognized and accepted by clinical, patient, regulatory, reimbursement, and academic communities. FACIT-Fatigue has proven to be a valid measure of fatigue through a qualitative study [17] and the psychometric properties in an SLE population are well documented [31]. Of the PROMs reviewed, only the LupusQoL has documented evidence of qualitative input from patients with SLE in the development process.
In clinical standard practice it could be an advantageous if all of the key symptoms and impacts were covered in one single PROM. Some PROMs have recently been developed for this purpose such as the Multi-Dimensional Questionnaire for Patient Reported Outcome Measures-SLE (MDPROMs SLE) [55] and Lupus Impact Tracker (LIT) [56]. Further research and experience with the use of multidimensional measures in clinical practice are needed.
It is important to acknowledge that patients with SLE may experience many symptom-free days, followed by a severe flare. Flares are likely to impact patients’ HRQoL [2, 57]. Therefore, further research in developing PROMs that capture the impact of flares should be considered in the future. SLE often involves day-to-day symptom fluctuations due to these flares, thus the recall period of the measurement instrument is also an important consideration. PROs with shorter recall periods may underestimate symptom burden and may place undue demand on patients; however, longer recall period may not allow for reliable symptom and impact reporting.
The recommended PROMs in this chapter have been selected on the basis of identification of key SLE symptoms and impacts in the conceptual model. PROMs of other symptoms of SLE not reported in the conceptual model were thus de-prioritized and therefore not included. Appropriate and validated PROMs for some key concepts identified in the model (e.g., skin manifestations of the disease, impact of flares, and treatment satisfaction) were not identified, or no PROMs have been used to measure these concepts in patients with SLE. This represents a gap in knowledge that may benefit from further research. PROMs are in this context considered complementary to more objective measures and should be incorporated into clinical practice.
Conclusion
SLE is a condition associated with high unmet need and considerable burden to patients, as demonstrated by the conceptual model presented in this chapter. This review highlights some of the existing PROMs of SLE signs and symptoms and HRQoL that demonstrate appropriate content validity and are psychometrically adequate for a population of patients with SLE, and as a result such measures may be suitable for use in clinical practice for patients with SLE.
Both generic and disease-specific PROMs were reviewed. Those PROMs included HRQoL, measures of fatigue, pain, and depression/anxiety. The Functional Assessment for Chronic Illness Therapy Fatigue scale (FACIT-fatigue) is the strongest fatigue measure in terms of psychometric properties and conceptual coverage. The Brief Pain Inventory (BPI-SF) is the strongest pain instrument in terms of content validity. However, qualitative research in patients with SLE is needed to ensure the applicability of the items and the appropriateness of the recall period. The Hospital Anxiety and Depression Scale (HADS) is the recommended PROM for measurement of anxiety and depression as the instructions and response options are straightforward and clearly defined. The LupusQoL is the strongest HRQoL measure in terms of the development, conceptual coverage, and validation. It might be favorable in standard clinical practice to consider including 1 cohesive PROM for the assessment of patient reported key symptoms and impacts in SLE. However, further research and validation studies as well as experience with the use of these “all-in-one” PROMs in clinical practice are needed.
References
Manson JJ, Rahman A. Systemic lupus erythematosus. Orphanet J Rare Dis. 2006;1:6.
Jolly M, Pickard AS, Wilke C, Mikolaitis RA, Teh LS, McElhone K, Fogg L, Block J. Lupus-specific health outcome measure for US patients: the LupusQoL-US version. Ann Rheum Dis. 2010;69:29–33.
Holloway L, Humphrey L, Heron L, Pilling C, Kitchen H, Højbjerre L, Strandberg-Larsen M, Bekker Hansen B. Patient-reported outcome measures for systemic lupus erythematosus clinical trials: a review of content validity, face validity and psychometric performance. Health Qual Life Outcomes. 2014;12:116.
Beckerman NL. Living with lupus: a qualitative report. Soc Work Health Care. 2011;50:330–43.
McElhone K, Abbott J, Gray J, Williams A, Teh LS. Patient perspective of systemic lupus erythematosus in relation to health-related quality of life concepts: a qualitative study. Lupus. 2010;19:1640–7.
Robinson J, Aguilar D, Schoenwetter M, Dubois R, Russak S, Ramsey-Goldman R, Navarra S, Hsu B, Revicki D, Cella D, Rapaport MH, Renahan K, Ress R, Wallace D, Weisman M. Impact of systemic lupus erythematosus on health, family, and work: the patient perspective. Arthritis Care Res. 2010;62:266–73.
Schneider M, Schmeding A, Carnarius H, Ager M, McWade V. Systemic lupus erythematosus (SLE): understanding the burden. Value Health. 2010;13:A470.
Yee CS, McElhone K, Teh LS, Gordon C. Assessment of disease activity and quality of life in systemic lupus erythematosus—new aspects. Best Pract Res Clin Rheumatol. 2009;23:457–67.
Aberer E. Epidemiologic, socioeconomic and psychosocial aspects in lupus erythematosus. Lupus. 2010;19:1118–24.
Strand V, Galateanu C, Pushparajah DS, Nikai E, Sayers J, Wood R, van Vollenhoven RF. Limitations of current treatments for systemic lupus erythematosus: a patient and physician survey. Lupus. 2013;22:819–26.
Pettersson S, Lovgren M, Eriksson LE, Moberg C, Svenungsson E, Gunnarsson I, Henriksson EW. An exploration of patient-reported symptoms in systemic lupus erythematosus and the relationship to health-related quality of life. Scand J Rheumatol. 2012;41:383–90.
Gallop K, Nixon A, Swinburn P, Sterling KL, Naegeli AN, Silk ME. Development of a conceptual model of health-related quality of life for systemic lupus erythematosus (SLE) from the patients’ perspective. Lupus. 2012;21:934–43.
Ad Hoc Committee on Systemic Lupus Erythematosus Response Criteria for Fatigue. Measurement of fatigue in systemic lupus erythematosus: a systematic review. Arthritis Care Res. 2007;57:1348–57.
Cleanthous S, Tyagi M, Isenberg DA, Newman SP. What do we know about self-reported fatigue in systemic lupus erythematosus? Lupus. 2012;21:465–76.
Ramsey-Goldman R, Rothrock N. Fatigue in systemic lupus erythematosus and rheumatoid arthritis. PM R. 2010;2:384–92.
Mattsson M, Moller B, Stamm T, Gard G, Bostrom C. Uncertainty and opportunities in patients with established systemic Lupus Erythematosus: a qualitative study. Musculoskeletal Care. 2012;10:1–12.
Kosinski M, Gajria K, Fernandes AW, Cella D. Qualitative validation of the FACIT-fatigue scale in systemic lupus erythematosus. Lupus. 2013;22:422–30.
Danoff-Burg S, Friedberg F. Unmet needs of patients with systemic lupus erythematosus. Behav Med. 2009;35:5–13.
Yazdany J, Yelin E. Health-related quality of life and employment among persons with systemic Lupus Erythematosus. Rheum Dis Clin North Am. 2010;36:15–32.
Doward LC, McKenna SP, Whalley D, Tennant A, Griffiths B, Emery P, Veale DJ. The development of the L-QoL: a quality-of-life instrument specific to systemic lupus erythematosus. Ann Rheum Dis. 2009;68:196–200.
Robinson M, Sheets CS, Currie LM. Systemic lupus erythematosus: a genetic review for advanced practice nurses. J Am Acad Nurse Pract. 2011;23:629–37.
Johnsson PM, Sandqvist G, Bengtsson A, Nived O. Hand function and performance of daily activities in systemic lupus erythematosus. Arthritis Rheum. 2008;59:1432–8.
Carls G, Li T, Panopalis P, Wang S, Mell AG, Gibson TB, Goetzel RZ. Direct and indirect costs to employers of patients with systemic lupus erythematosus with and without nephritis. J Occup Environ Med. 2009;51:66–79.
Zhu TY, Tam LS, Li EK. The socioeconomic burden of systemic lupus erythematosus: state-of-the-art and prospects. Expert Rev Pharmacoecon Outcomes Res. 2012;12:53–69.
Zhu TY, Tam LS, Li EK. Cost-of-illness studies in systemic lupus erythematosus: a systematic review. Arthritis Care Res (Hoboken). 2011;63:751–60.
Yelin E, Katz P. Introduction to special section: cost and social and psychological impact of rheumatic diseases. Arthritis Rheum. 2008;59:457.
Zhu TY, Tam LS, Lee VWY, Lee KKC, Li EK. The impact of flare on disease costs of patients with systemic lupus erythematosus. Arthritis Rheum. 2009;61:1159–67.
Aghdassi E, Zhang W, St. Pierre Y, Clarke AE, Morrison S, Peeva V, Landolt-Marticorena C, Su J, Reich H, Scholey J, Herzenberg A, Pope JE, Peschken C, Lunnet Canios I, Wither JE, Fortin PR. Healthcare cost and loss of productivity in a Canadian population of patients with and without lupus nephritis. J Rheumatol. 2011;38:658–66.
Strand V, Chu AD. Measuring outcomes in systemic lupus erythematosus clinical trials. Expert Rev Pharmacoecon Outcomes Res. 2011;11:455–68.
Furie R, Petri MA, Strand V, Gladman DD, Zhong ZJ, Freimuth WW. Clinical, laboratory and health-related quality of life correlates of Systemic Lupus Erythematosus Responder Index response: a post hoc analysis of the phase 3 belimumab trials. Lupus Sci Med. 2014;1, e000031.
Lai JS, Beaumont JL, Ogale S, Brunetta P, Cella D. Validation of the functional assessment of chronic illness therapy-fatigue scale in patients with moderately to severely active systemic lupus erythematosus, participating in a clinical trial. J Rheumatol. 2011;38:672–9.
Chandran V, Bhella S, Schentag C, Gladman DD. Functional assessment of chronic illness therapy-fatigue scale is valid in patients with psoriatic arthritis. Ann Rheum Dis. 2007;66:936–9.
Cella D, Yount S, Sorensen M, Chartash E, Sengupta N, Grober J. Validation of the Functional Assessment of Chronic Illness Therapy Fatigue Scale relative to other instrumentation in patients with rheumatoid arthritis. J Rheumatol. 2005;32:811–9.
Ozel F, Argon G. The effects of fatigue and pain on daily life activities in systemic lupus erythematosus. European J Internal Med. 2011;22:S70.
Melzack R. The McGill Pain Questionnaire: major properties and scoring methods. Pain. 1975;1(3):277–99.
Melzack R. The short-form McGill Pain Questionnaire. Pain. 1987;30(2):191–7.
Cleeland CS, Ryan KM. Pain assessment: global use of the Brief Pain Inventory. Ann Acad Med Singapore. 1994;23(2):129–38.
Cleeland CS. The brief pain inventory: user guide. 2009. 63 p. http://www.mdanderson.org/education-and-research/departments-programs-andlabs/departments-and-divisions/symptom-research/symptom-assessmenttools/BPI_UserGuide.pdf.
Cleeland CS, Gonin R, Hatfield AK, Edmonson JH, Blum RH, Stewart JA, Pandya KJ. Pain and its treatment in outpatients with metastatic cancer. N Engl J Med. 1994;330(9):592–6.
Food and Drug Administration. Guidance for industry: patient-reported outcome measures: use in medical product development to support labelling claims. Silver Spring, MD: Food and Drug Administration; 2009.
Atkinson TM, Rosenfeld BD, Sit L, Mendoza TR, Fruscione M, Lavene D, Shaw M, Li Y, Hay J, Cleeland CS, Scher HI, Breitbart WS, Basch E. Using confirmatory factor analysis to evaluate construct validity of the Brief Pain Inventory (BPI). J Pain Symptom Manage. 2011;41(3):558–65.
Daut RL, Cleeland CS, Flannery RC. Development of the Wisconsin Brief Pain Questionnaire to assess pain in cancer and other diseases. Pain. 1983;17(2):197–210.
Naegeli AN, Tomaszewski EL, Al Sawah S. Psychometric validation of the Brief Pain Inventory-Short Form in patients with systemic lupus erythematosus in the United States. Lupus. 2015;24:1377–83.
Zigmond AS, Snaith RP. The hospital anxiety and depression scale. Acta Psychiatr Scand. 1983;67:361–70.
Snaith RP. The hospital anxiety and depression scale. Health Qual Life Outcomes. 2002;1:29.
Herrmann C. International experiences with the Hospital Anxiety and Depression Scale-A review of validation data and clinical results. J Psychosom Res. 1997;42:17–41.
Garris CP, Oglesby AK. Assessment of health care resource utilization by level of flare severity in patients with systemic lupus erythematosus (SLE) in the managed care setting. J Manag Care Pharm. 2011;17:552.
QualityMetric Inc. User’s manual for the SF-36v2 health survey. 3rd ed. 2011. http://www.qualitymetric.com/WhatWeDo/ManualsUserGuides/UsersManualfortheSF36v2HealthSurvey/tabid/328/Default.aspx.
Brazier JE, Harper R, Jones NM, O'Cathain A, Thomas KJ, Usherwood T, Westlake L. Validating the SF-36 health survey questionnaire: new outcome measure for primary care. BMJ. 1992;305(6846):160–4.
Beaumont JLLJ, Cella D, Brunetta P, Ogale S. Validation of the SF-36 in patients with systemic lupus erythematosus (SLE). Arthritis Rheum. 2009;60:296.
Busija L, Pausenberger E, Haines TP, Haymes S, Buchbinder R, Osborne RH. Adult measures of general health and health-related quality of life: Medical Outcomes Study Short Form 36-Item (SF-36) and Short Form 12-Item (SF-12) Health Surveys, Nottingham Health Profile (NHP), Sickness Impact Profile (SIP), Medical Outcomes Study Short Form 6D (SF-6D), Health Utilities Index Mark 3 (HUI3), Quality of Well-Being Scale (QWB), and Assessment of Quality of Life (AQOL) [abstract]. Arthritis Care Res. 2011;63:S383–412.
McElhone K, Abbott J, Shelmerdine J, Bruce IN, Ahmad Y, Gordon C, Peers K, Isenberg D, Ferenkeh-Koroma A, Griffiths B, Akil M, Maddison P, Teh L-S. Development and validation of a disease-specific health-related quality of life measure, the LupusQoL, for adults with systemic lupus erythematosus. Arthritis Care Res. 2007;57:972–9.
Ware Jr JE, Sherbourne CD. The MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selection. Med Care. 1992;30(6):473–83.
Food and Drug Administration. Guidance for industry: systemic lupus erythematosus—developing medical products for treatment. 2010. http://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/ucm072063.pdf.
European Medicines Agency. Guideline on clinical investigation of medicinal products for the treatment of systemic lupus erythematosus, cutaneous lupus and lupus nephritis (draft). 2013. http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2013/03/WC500139615.pdf.
El Miedany Y, El Gaafary M, El Yassaki A, Ahmed I, Youssef S, Hegazi MO, Palmer D. Incorporating patient reported outcome measures in clinical practice: development and validation of a PROMs questionnaire for SLE. Ann Rheum Dis. 2013;72 Suppl 3:484.
Jolly M, Garris CP, Mikolaitis RA, Jhingran PM, Dennis G, Wallace DJ, Clarke A, Dooley MA, Parke A, Strand V, Alárcon GS, Kosinski M. Development and validation of the Lupus Impact Tracker: a patient-completed tool for clinical practice to assess and monitor the impact of systemic lupus erythematosus. Arthritis Care Res (Hoboken). 2014;66(10):1542–50.
Ruperto N, Hanrahan LM, Alarcon GS, Belmont HM, Brey RL, Brunetta P, Buyon JP, Costner MI, Cronin ME, Dooley MA, Filocamo G, Fiorentino D, Fortin PR, Franks Jr AG, Gilkeson G, Ginzler E, Gordon C, Grossman J, Hahn B, Isenberg DA, Kalunian KC, Petri M, Sammaritano L, Sánchez-Guerrero J, Sontheimer RD, Strand V, Urowitz M, von Feldt JM, Werth VP, Merrill JT. International consensus for a definition of disease flare in lupus. Lupus. 2011;20:453–62.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Appendices
Appendix 1: FACIT-Fatigue is presented with permission from the copyright holder. Potential users
Potential users should go to http://www.facit.org/FACITOrg and contact copyright holder for permission before using FACIT-Fatigue in studies and clinical practice.
FACIT Fatigue Scale (Version 4)
Below is a list of statements that other people with your illness have said are important. Please circle or mark one number per line to indicate your response as it applies to the past 7 days.
Not at all | A little bit | Some-what | Quite a bit | Very much | ||
HI7 | I feel fatigued | 0 | 1 | 2 | 3 | 4 |
HI12 | I feel weak all over | 0 | 1 | 2 | 3 | 4 |
An1 | I feel listless (washed out) | 0 | 1 | 2 | 3 | 4 |
An2 | I feel tired | 0 | 1 | 2 | 3 | 4 |
An3 | I have trouble starting things because I am tired | 0 | 1 | 2 | 3 | 4 |
An4 | I have trouble finishing things because I am tired | 0 | 1 | 2 | 3 | 4 |
An5 | I have energy | 0 | 1 | 2 | 3 | 4 |
An7 | I am able to do my usual activities | 0 | 1 | 2 | 3 | 4 |
An8 | I need to sleep during the day | 0 | 1 | 2 | 3 | 4 |
An12 | I am too tired to eat | 0 | 1 | 2 | 3 | 4 |
An14 | I need help doing my usual activities | 0 | 1 | 2 | 3 | 4 |
An15 | I am frustrated by being too tired to do the things I want to do | 0 | 1 | 2 | 3 | 4 |
An16 | I have to limit my social activity because I am tired | 0 | 1 | 2 | 3 | 4 |
Appendix 2: Brief Pain Inventory—Short Form
BPI-SF is presented with permission from the copyright holder. Potential users should go to www.mdanderson.org/departments/prg and contact copyright holder for permission before using BPI-SF in studies and clinical practice.


Appendix 3: LupusQoL
LupusQoL is presented with permission from the copyright holders. Anyone running a commercially funded study must obtain a license for the LupusQoL and pay the license fee. Use is free for noncommercially funded studies but copyright holders requires that researchers contact the licensors for permission before using to ensure that researchers use the professionally developed and validated translations only.
Potential users should go to www.lupusqol.com for more information on using LupusQoL in studies and clinical practice.




Appendix 4: Multidimensional Questionnaire for Patient Reported Outcome Measures—SLE


Rights and permissions
Copyright information
© 2016 Springer International Publishing Switzerland
About this chapter
Cite this chapter
Hansen, B.B., Højbjerre, L. (2016). PROMs for Systemic Lupus Erythematosus. In: El Miedany, Y. (eds) Patient Reported Outcome Measures in Rheumatic Diseases. Springer, Cham. https://doi.org/10.1007/978-3-319-32851-5_6
Download citation
DOI: https://doi.org/10.1007/978-3-319-32851-5_6
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-32849-2
Online ISBN: 978-3-319-32851-5
eBook Packages: MedicineMedicine (R0)