Abstract
Ciliates have available most of the intracellular signaling mechanisms known from metazoans. Long-range signals are represented by firmly installed microtubules serving as gliding rails aiming at specific targets. Many components are distinctly arranged to guarantee locally restricted effects. Short-range signals include Ca2+, provided from different sources, and proteins for membrane recognition and fusion, such as SNAREs, GTPases and high affinity Ca2+-binding proteins (still to be defined). A battery of ion conductances serves for electric coupling from the outside medium to specific responses, notably ciliary activity, which also underlies gravitaxis responses. Eventually cyclic nucleotides are involved, e.g. in ciliary signaling. Furthermore, an elaborate system of protein kinases and phosphatases exerts signaling mechanisms in widely different processes.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
2.1 Introduction—Basic Aspects of Signaling in Ciliates
As for every eukaryotic cell one may ask also for ciliates which cellular processes require signaling, how signaling is executed and over which distances, whether principles are shared with metazoans and plants, whether mechanisms are maintained during evolution, abolished or newly invented. Together with Dictyostelium, the ciliates Paramecium and Tetrahymena represent the protozoa which, at this time, are best analyzed with regard to signaling. It is useful to differentiate between long- and short-range signaling, e.g. by microtubules or electrical signals and by molecular interactions or spatially restricted Ca2+ signals (Plattner and Klauke 2001), respectively.
2.1.1 Basic Phenomena Applicable to Ciliates
Signaling pertinent to ciliary activity in ciliated protozoa is as elaborate, or even more than in metazoan (Machemer 1988a) as these cells are highly mobile and capable of reacting to various environmental stimuli (Machemer 1988b; Bell et al. 2007). To achieve this, mechanical, electrical, biochemical and molecular signals, i.e. long range and short-range signals, can be combined in some variation to the basic theme.
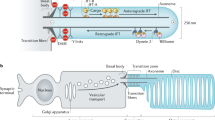
Ciliates have at their disposal a highly sophisticated vesicle trafficking system, as illustrated (http://www5.pbrc.hawaii.edu/allen/) and summarized (Allen and Fok 2000) for Paramecium and Tetrahymena (Frankel 2000). The routes have to be addressed here. (i) Endoplasmic reticulum (ER) → Golgi apparatus → lysosomes + dense core-secretory organelles (trichocysts in Paramecium and mucocysts in Tetrahymena). (ii) Constitutive exocytosis of surface coat materials (Flötenmeyer et al. 1999) and dense core-secretory organelle exocytosis (Plattner et al. 1985; Plattner and Kissmehl 2003a). (iii) Phagocytosis, from cytostome → phagosome → endosomal and lysosomal input → phagolysosome (called “food [digesting] vacuole”) → discharge of spent vacuoles at the cytoproct. (iv) Endocytosis via early endosomes → links to phagosomes + lysosomes. (v) Vesicle recycling from the cytoproct to the nascent phagosome. (vi) In addition, the contractile vacuole complex impresses not only by its dynamic activity (Allen and Naitoh 2002) in the context of ongoing osmoregulation (Allen et al. 2009), but it also represents a site endowed with the machinery typical of vesicle trafficking (Plattner 2015b) although vesicle trafficking within the organelle is less obvious. Steps (iii) to (v) have been documented in detail for Paramecium (Allen and Fok 2000) as well as for Tetrahymena (Frankel 2000). Beyond short-range signaling, steps (i), (iii) and (v) include long-range signaling. All these pathways serve for proper delivery and positioning of signaling elements so that they can execute their signaling function at distinct sites of the cell.
2.1.2 Molecular Key Players
Recent availability of a macronuclear genome database for the most frequently used species, P. tetraurelia and T. thermophila, has enabled the identification, localization and assessment of the functional relevance of key players. In Paramecium such work has included mainly SNARE (soluble N-ethylmaleimide sensitive factor [NSF] attachment protein receptors) proteins, actin and H+-ATPase, as summarized previously (Plattner 2010) as well as Ca2+-release channels (CRC) of the type inositol 1,4,5-trisphosphate receptors (InsP3R) and ryanodine receptor-like proteins (RyR-LP) (Ladenburger and Plattner 2011; Ladenburger et al. 2006, 2009; Plattner 2015a), as summarized recently (Plattner and Verkhratsky 2015). This is complemented by monomeric GTP (guanosine trisphosphate) binding proteins (G-proteins), the GTPases, not only in higher eukaryotes (Zhen and Stenmark 2015) but also in ciliates (Bright et al. 2010). Isoforms, i.e. paralogs or ohnologs (in case of diversification following whole genome duplications, particularly in P. tetraurelia), can be assigned to different steps and routes of vesicle trafficking and, thus, mirror the high complexity of the ciliate cell.
2.1.3 Long- and Short-Range Signals
The distinction between short-range and long-range signals has been extensively elaborated elsewhere (Plattner 2016a). A typical long-range signal is the docking of trichocysts (Aufderheide 1978) along microtubules which emanate from ciliary basal bodies and, thus, serve as transport rails (Plattner et al. 1982). This has to be complemented by short-range signals. For instance SNAREs and G-proteins are important for vesicle docking and finally membrane fusion. Local Ca2+ increase is another signal which has to arise from a nearby source since Ca2+ signals decay rapidly (Neher 1998). This also guarantees selective activation of distinct sites and also avoids cytotoxicity (Plattner and Verkhratsky 2015). Local restriction of Ca2+ signaling is most obvious, for instance, by the assignment of different CRCs types to different trafficking organelles, from the cell surface to deep inside, in Paramecium (Plattner 2015a). Moreover, ciliates fascinate particularly by their highly regular design that predetermines their vesicle trafficking routes and signaling sites based on epigenetic phenomena (Frankel 2000; Beisson 2008). Accordingly, cilia and secretory organelles are arranged in a strikingly regular surface pattern.
2.2 Overview of Trafficking Regulation Along Different Signaling Pathways
Basic trafficking pathways in ciliates are outlined in Fig. 2.1a. Box 1 outlines different kinds of cytoplasmic signaling operating in ciliates. Despite the old evolutionary age of ciliates, signaling mechanisms are quite similar to those in animals and—with exceptions—in plants. Similarities encompass the role of monomeric GTP-binding proteins (G-proteins acting as GTPases) (Bright et al. 2010), H+-ATPase, SNARE proteins and their chaperone, NSF, as well as the regulation of membrane fusion by a local Ca2+ signal (Plattner 2010). The importance of luminal acidification of trafficking vesicles is derived from the observation that a transmembrane signal generated by the conformational change of H+-ATPase intramembranous V0 part causes binding of GTPase modulators (Hurtado-Lorenzo et al. 2006), thus facilitating docking and membrane fusion. Specificity of vesicle interaction is finally mediated by SNAREs (Plattner 2010) and GTPases (Bright et al. 2010). Sequences encoding GTPases and GTPase modulators, such as GAP (guanine nucleotide activation protein) and GEF (guanine nucleotide exchange factor), also occur in the P. tetraurelia database (Plattner and Kissmehl 2003b).
Signaling pathways in the Paramecium cell. a Vesicle trafficking pathways encompass different main streams, such as the exocytotic, the endocytotic, the phagocytotic pathway and less overt trafficking in the contractile vacuole complex. Dotted arrows are less well established, particularly membrane input into this organelle via acidosomes, as derived from various recent papers about other protists. Also for proteins passing or bypassing the Golgi apparatus has not yet been sufficiently specified in detail. b Cortial organelles, such as cilia and exocytosis sites are regulated separately. Depolatization induces ciliary beat reversal by Ca2+ influx via ciliary voltage-dependent Ca2+ channels, abolished via negative feedback (Θ) by intraciliary [Ca2+] increase. CRCs in alveolar sacs, type RyR-LPs, are facing the plasmamembrane, opposite to the SERCA pump. Alveolar sacs contain a calsequestrin-like high capacity/low affinity CaBP. Trichocyst exocytosis is governed by a SOCE mechanism (store-operated Ca2+-entry), i.e. Ca2+ release from alveolar sacs in a first step, followed by Ca2+ influx via somatic (non-ciliary) channels in a tightly coupled second step. c Summary of events during trichocyst exocytosis. Top Freeze-fracture images of fusion/resealing stages and their estimated duration, derived from synchronous stimulation/quenched-flow/rapid freezing analysis. Note decay of rosette particle aggregates and rapid formation of a fusion pore which expands and, thus, allows Ca2+ access to the secretory contents which triggers their explosive discharge by densondensation (stretching). Below Parallel situations seen on ultrathin sections. a Data pertinent to trichocyst processing are based on previous reviews (Plattner et al. 1993; Plattner 2014), those for endo-/phagocytotic trafficking are mainly derived from Allen and Fok (2000) and c trafficking in context of the contractile vacuole complex is based on recent reviews (Plattner 2015b, 2016a) b is modified from Plattner (2014), c is modified from Plattner et al. (1993, 1997)
The Ca2+ signal is generated by intracellular CRCs of which different types are assigned to different organelles (Ladenburger and Plattner 2011; Plattner and Verkhratsky 2013). The Ca2+-sensor causing fusion, as known from higher eukaryotes, is a low capacity/high affinity Ca2+-binding protein (CaBP) which usually contains two high affinity Ca2+-binding C2 domains (β-barrels with a Ca2+-binding loop), such as synaptotagmin (Rizo et al. 2006; Südhof 2014). Although such CaBPs have not yet been specified in ciliates, equivalents of synaptotagmin occur in the P. tetraurelia database (Farrell et al. 2012). Extended synaptotagmins (e-syntag) with more than two C2 domains are known from some mammalian cells (Min et al. 2007), but they also occur in the Paramecium database (H. Plattner and R. Kissmehl, unpublished observations). Calmodulin (CaM) is another low capacity/high affinity CaBP, with four EF-hand type loops, each with high affinity Ca2+-binding capacity. CaM operates at many sites also in ciliates. In the CaM molecule, the extensive conformational change upon hierarchical Ca2+ binding in the EF-hand loops I to IV represents the transduction of a chemical to a molecular-mechanical signal (Park et al. 2008). Thus, CaM can regulate a variety of surface influx channels (Saimi and Kung 2002), phagocytosis (Gonda et al. 2000) and probably endocytosis, also in ciliates.
Box 1 also shows that Ca2+ for activation may eventually also come from the outside medium for some specific effects, e.g. for activating some nucleotide cyclases, kinases and phosphatases, in the context of ciliary activity. This includes the signaling function of cyclic nucleotides, such as cyclic adenosine monophosphate (cAMP) and cyclic guanosine monophosphate (cGMP) and activation of the respective protein kinases, protein kinase A (PKA) and protein kinase G (PKG) (Bonini and Nelson 1990); for review see Plattner (2016a). Also some metabolic Ca2+ channel activators, such as cyclic adenosinediphosphoribose (cADPR) and nicotinic acid adenine dinucleotidephosphate (NAADP) are derived from nucleotides, i.e. nicotinamide adeninedinucleotide (NAD) and nicotinic acid adenine dinucleotidephosphate (NAADP), respectively, as known from vertebrates (Lee 2012). For cADPR and NAADP effects there is only circumstantial evidence in Paramecium (Plattner et al. 2012).
A total of 2600 kinases has been found in the P. tetraurelia genome (Bemm et al. 2009), thus contributing by 7 % to the macronuclear genome. In T. thermophila the proportion is 3.8 % (Tian et al. 2014). Both values stress their importance for signal transduction. The difference between the two genera may originate from whole genome duplication in Paramecium. A considerable difference between protein kinases in animal cells and in ciliates is the absence in the latter of a “CaM kinase”, i.e. a kinase activated by a complex of calmodulin (CaM) and Ca2+. Whereas such CaM-kinases in metazoans contribute to the regulation of neuronal activity, they are replaced in ciliates by “Ca2+-dependent protein kinases” (CDPKs). These contain CaM-like sequences integrated in the kinase molecule (Kim et al. 1998).
Box 1 also indicates the occurrence in ciliates of protein phosphatases (PPs), e.g. PP1, PP2A and PP2B. PP2B, which is identical with calcineurin, encompasses two subunits, catalytical subunit A and regulatory subunit B, from ciliates (Fraga et al. 2010) to man where it regulates immune-response and long term potentiation, i.e. learning. In ciliates, multiple roles can be expected for calcineurin, including exo-/endocytosis regulation (Momayezi et al. 1987; Fraga et al. 2010).
2.3 Subcompartmentalization of Signaling Including Signaling in Cilia
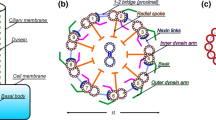
Signals can be rather precisely restricted to subcompartments, e.g. cilia (Box 2), for which Box 3 shows details. Mechanical stimulation of a ciliated protozoan cell causes depolarization or hyperpolarization, depending on whether stimulation occurs at the anterior or posterior part of the cell (Eckert and Brehm 1979; Machemer 1988a, b). This is enabled by a graded differential distribution of specific ion channels over the somatic (non-ciliary) cell membrane. The respective receptor potential formed by different ion conductances activates different mechanisms in cilia. For instance, depolarization activates voltage-dependent Ca2+-channels selectively occurring in cilia (Machemer and Ogura 1979) and, thus, a Ca2+-carried action potential. (This signaling occurs no more in metazoans beyond Ctenophores.) Increased intraciliary Ca2+ shuts off this Ca2+ influx (Brehm and Eckert 1978). Hyperpolarization accelerates forward swimming (Preston et al. 1992).
During de- and hyperpolarization, different cyclic nucleotides are formed, activating PKG and PKA, respectively (Box 3). Ciliary activation mechanisms are independent of Ca2+-activated processes during exocytosis, except when massive exocytosis stimulation entails an exuberant Ca2+ signal (Husser et al. 2004). In summary, a mechanical signal is transformed into a long-range electrical signal generated at the somatic cell membrane that is transduced into cilia where it causes short-range Ca2+ signaling and a mechanical response in ciliary activity.
Mechanisms described for basic ciliary activity (Fig. 2.1b) also apply to chemotaxis and to gravitaxis/gravikinesis (Box 3). Chemotaxis requires the activation of distinct ion conductances to achieve specific behavioral responses (Saimi and Kung 2002; Bell et al. 2007; Yano et al. 2015). Positive gravitaxis is rare in ciliates where negative gravitaxis, causing upward swimming in the gravity field, by far prevails. For this, Paramecium is the best analyzed example (Machemer et al. 1998; Hemmersbach and Braun 2006; Machemer 2014). Accordingly cAMP and PKA are assumed to be involved in negative gravitaxis (Hemmersbach et al. 2002). Investigators assume that, for sensing gravity, channels have to be linked to filamentous actin (F-actin) (Machemer 2014). In fact, actin has been localized to the cell cortex (Sehring et al. 2007) and, even more precisely, to the narrow space between cell membrane and alveolar sacs (Kissmehl et al. 2004).

2.4 Organelle Trafficking Signals
2.4.1 Molecular Background
Long-range signals, such as firmly installed microtubules, can guide vesicles to their target sites (Box 4). This is true of trichocysts (Aufderheide 1978; Plattner et al. 1982) and organelles of the phagocytotic cycle (Allen and Fok 2000). Short-range signals involved are GTPases, SNAREs, H+-ATPase, as outlined in Sect. 2.1, together with actin. For GTPases (Bright et al. 2010) and the other key players, organelle specific isoforms are available (Plattner 2010). The multimeric H+-ATPase molecule is composed of an intramembranous V0 basepiece and a catalytic head, V1, which may dis- and re-assemble by interaction with an elongate, variable a-SU (Sun-Wada and Wada 2015). Considering the key role of H+-ATPase (Sect. 2.2), the unsurpassed number of 17 a-subunits in Paramecium may mediate adjustment to local requirements (Wassmer et al. 2005, 2006, 2009). Among SNAREs, longin-type sequences in Paramecium’s “synaptobrevins” may contribute to organelle specificity, in addition to the usual domain sequences (Schilde et al. 2006, 2010). In P. tetraurelia, plasmalemmal Syntaxin 1 (PtSyx1) is engaged in trichocyst exocytosis (Kissmehl et al. 2007). For more details, see Plattner (2010, 2016a).
Vesicles undergoing trafficking are endowed with CRCs identical with, or related to InsP3Rs and RyRs (Ladenburger and Plattner 2011; Plattner and Verkhratsky 2013); see Box 5. An exception are trichocysts which seem to be devoid of luminal Ca2+, in contrast to what is known from some other dense core-secretory vesicles, endosomes and phagocytotic organelles of higher eukaryotes (Hay 2007). The presence of the key players mentioned above, including CRCs, in the endo-/phagocytotic cycle of Paramecium may reflect the intensity and multitude of vesicle trafficking known from ultrastructural studies (Allen and Fok 2000) [In Paramecium, not all of these vesicles are acidic (Wassmer et al. 2009), and not all lysosomal enzymes have an acidic pH-optimum (Fok and Paeste 1982; Fok 1983)]. Appropriate CRCs may drive membrane interactions in concert with, or independently from other key players. The importance of local availability and regulation of Ca2+ during membrane docking and fusion is discussed in the accompanying paper (Plattner 2016b). The numerous members of the six CRC subfamilies found in Paramecium may fine tune Ca2+ signals and membrane interactions depending on local requirements.
2.4.2 Dense Core-Secretory Vesicle Exocytosis
Ca2+ regulation of trichocyst exocytosis involves three steps (Box 5, Fig. 2.1b, c): (i) Ca2+ release from alveolar sacs via RyR-like proteins and (ii) immediately superimposed Ca2+ influx from the outside medium (Klauke and Plattner 1997; Ladenburger and Plattner 2011; Plattner 2014). Both mechanisms acting in concert are called store-operated Ca2+ entry, SOCE—a mechanism maintained up to mammals. A large excess of Ca2+, much more than seen by fluorochromes, has to flood trichocyst exocytosis sites to become activated, just as in some neuroendocrine cells (for details, see Plattner 2016a). (iii) Discharge of contents follows formation of an exocytotic opening and requires the entry of Ca2+ from the outside and binding to some secretory components, thus causing decondensation by conformational change (Plattner et al. 1997; Klauke et al. 1998; Plattner 2014). This in turn depends on proper processing of secretory protein precursors (Pouphile et al. 1986; Bowman et al. 2005).
2.4.3 The Phagocytotic Cycle
This aspect is reviewed here in more detail, as it demonstrates the complex sequence of interacting signaling molecules although these are only partially known.
The phagocytotic cycle in Paramecium requires multiple signaling (Allen and Fok 2000), including firmly established microtubules as long-range signals and variable stage-dependent short-range signals. In detail the sequence is as follows. (i) At the cytopharynx, at the bottom of the cytostome, vesicles recycling from advanced stages of food vacuoles, together with vesicles from the cytoproct, deliver membrane material for a bulging nascent food vacuole. Thus, a phagosome is formed at converging microtubular rails, the “postoral fibers”. (ii) After detachment, acidosomes (late endosomes) fuse with the phagosome, thus endowing it with H+-ATPase molecules for luminal acidification. (iii) This is followed by fusion with lysosomes, thus forming phagolysosomes. (iv) Lysosomal enzymes are retrieved later on during cyclosis, (v) as are parts of the membrane for delivery to the cytopharynx. (vi) The contents of spent food vacuoles are released by exocytosis at the cytoproct and membranes are recycled as indicated for step (i) (Allen and Fok 2000).
In Paramecium tetraurelia, key players for signaling in the different stages (Box 6) encompass exchanging sets of SNAREs (Schilde et al. 2006, 2010; Kissmehl et al. 2007), subunits (SU) of H+-ATPase (Wassmer et al. 2005, 2006), and actin, as outlined in a separate chapter (Plattner 2016b). In Tetrahymena, different types of GTPases are exchanged during cyclosis (Bright et al. 2010). In Paramecium, the exchange of numerous actin isoforms, types 1, 3, 6, 8, 11–14 and 16 as well as their patchy or unilateral arrangement in some stages is a most striking phenomenon (Sehring et al. 2007). This may serve propulsion of the organelle and/or regulation of accessibility to fusion and/or budding of vesicles. All this documents a series of interacting long- and short-range signaling during cyclosis.
2.4.4 The Contractile Vacuole Complex
Surprisingly, the contractile vacuole complex contains all components relevant for vesicle trafficking, except actin, in even higher variability and with strict localization to specific substructures, such as the vacuole, the pore and the meshwork of the smooth spongiome (Box 7). The organelle has a very complex design (Allen and Naitoh 2002). It not only can expel fluid with an excess of Ca2+ and other ions (Stock et al. 2002), but it also shows some reflux of Ca2+ into the cytosol via constitutively active InsP3Rs (Ladenburger et al. 2006). This may serve not only for fine tuning of cytosolic Ca2+ but also to drive the extensive membrane fusion and fission processes within the organelle during systole/diastole cycles (Plattner 2015b).
2.4.5 Additional Signals
Little is known about other types of Ca2+ release channels in ciliates, such as two pore-channels (TPC) and transient receptor potential-channels (TRPC) and their activators (Box 5). Particularly metabolic CRC activators (Lee 2012), such as cADPR, NAADP, remain to be assigned to different channels and organelles in ciliates. Such channels have to be expected also in ciliates, based on microinjection studies (Plattner et al. 2012).
Vesicle budding at the Golgi apparatus and other organelles as well as at the plasmamembrane requires a set of additional proteins, such as coatamer proteins (COPs) and clathrin, together with their adaptor proteins known from higher eukaryotes up to mammals (Rothman 2014). In ciliate cells, coatamer coats are suggested to occur by electron microscopy in the cis- and trans-side of the Golgi apparatus (Allen and Fok 1993; Garreau De Loubresse 1993) and clathrin coats in addition by molecular biology according to Elde et al. (2005) who also reported the expression of adaptor proteins, AP-1, AP-2, AP-3 and AP-4 in T. thermophila. While none of them appear important for lysosome biogenesis (Briguglio et al. 2013), AP-2 is important for endocytosis via coated pits (Elde et al. 2005). Sequences encoding all these adaptor proteins have also been found in the P. tetraurelia database, in addition to the ARF/SAR-type G-protein known as a target of the drug, brefeldin A (Plattner and Kissmehl 2003b). The same is true of clathrin heavy chains and of COPs.
In summary, for vesicle trafficking ciliates have at their disposal most of the signaling components known from multicellular organisms. Note, however, that InsP3R/RyR-like molecules are absent from higher plants (Plattner and Verkhratsky 2015), whereas they occur in some green algae (Wheeler and Brownlee 2008). Globally a ciliate’s signaling machinery closely resembles that of metazoans.
2.5 Protein Phosphorylation for Activation and Deactivation of Signaling Processes
2.5.1 Phosphorylation Processes
As mentioned in Sect. 2.3, signaling in cilia includes PKA and PKG activity for enhanced forward and backward swimming, respectively (Kim et al. 1998; Kutomi et al. 2012). Activating cyclic nucleotides are generated within one ciliary stroke (Yang et al. 1997). Together with CDPKs they belong to the superfamily of Seryl/Threonyl kinases (Box 8). Phosphoproteins are substrates of the different phosphatases. Among them, PP1 dephosphorylates a ciliary phosphoprotein formed during ciliary reversal in Paramecium (Klumpp et al. 1990). PP2B/calcineurin probably has a broad spectrum of activity, depending on its A-subunit, whereas the two genes for the B-SU in Paramecium result in an identical translation product, with a well conserved binding domain in the A-SU (Fraga et al. 2010).
As indicated in Box 8 and discussed in more detail somewhere else (Plattner 2016a), the occurrence of Tyrosyl phosphorylation may be largely restricted in ciliates to cell cycle and mitosis regulation. Work with mammalian cells exposed to Euplotes gamones indicates signaling via a mitogen-activated protein kinase (MAPK) cascade with Tyrosine phosphorylation (Vallesi et al. 2010; Cervia et al. 2013). See also chapter by Luporini.
2.5.2 Signal Downregulation
Also ciliates possess different ways to downregulate signals (Box 9). Cyclic nucleotides are deactivated by diesterases and phosphoproteins are dephosphorylated by protein phosphatases. For instance, the association of calcineurin with parasomal sacs (Momayezi et al. 2000), the clathrin-coated pits in ciliates, is compatible with dynamin dephosphorylation known from mammalian coated pits.
Ca2+ signals are downregulated by different mechanisms with different kinetics (Box 9). The most rapid is binding to centrin (Sehring et al. 2009) a CaBP with high capacity/low affinity (in addition to low capacity/high affinity) binding sites localized in the cell cortex of Paramecium (see Plattner 2016a). This is orders of magnitude more rapid than downregulation by Ca2+-ATPases/pumps (Plattner 2016a) of which type Sarcoplasmic/Endoplasmic Reticulum Ca2+-ATPase (SERCA) (Hauser et al. 1998) or plasmamembrane Ca2+-ATPase (PMCA) (Elwess and Van Houten 1997) have been analyzed in Paramecium. PMCA also occurs in cilia of Tetrahymena (Dentler 1988) and Paramecium (Yano et al. 2013). These two are P-type ATPases because they autocatalytically form a phospho-intermediate which then dephosphorylates itself. Ca2+ exchangers, though not yet identified, show up in ciliate databases; they are driven by a H+-gradient formed by a H+-ATPase (V-type, in vesicles) operating without a phospho-intermediate formation. Although such exchangers urgently call for scrutiny in ciliates it appears that they are much more efficient in signal downregulation than the pumps (Ladenburger et al. 2006; Plattner 2016a).
2.6 Signaling by Surface Receptors
These aspects are summarized in Box 10. The occurrence of trimeric GTP-binding proteins (G-proteins) is likely (De Ondarza et al. 2003; Lampert et al. 2011), but not firmly established in protozoa in general (Krishnan et al. 2015) and in ciliates in particular since important details have not been examined yet, as discussed in more detail elsewhere (Plattner 2016a). The same is true of G-protein-coupled receptors (GPCRs). All this also applies to the secretagogue, aminoethyldextran, which, in Paramecium, is most efficient in activating highly synchronous exocytosis (Plattner et al. 1985; Plattner and Kissmehl 2003; Knoll et al. 1991) by a SOCE mechanism for trichocyst exocytosis (Hardt and Plattner 2000; Plattner 2014). For hints to MAPK activity and Tyrosyl phosphorylation, see Sect. 2.5.
Purinergic receptors can be assumed to occur in Paramecium as these cells, upon exposure to ≥10 µM GTP, perform periodic back- and forward swimming accompanied by depolarization (Clark et al. 1993) and Ca2+ waves oscillating with the same periodicity (Sehring and Plattner 2004). This is unusual insofar as purinergic receptors normally respond to ATP or, less common, to UTP. We assume a function in keeping cells from dispersal to low density which is known to inhibit cell division and maintenance of the population.
2.7 Conclusions
Intracellular signaling by pheromones (gamones) in ciliates (Luporini et al. 2014) is summarized separately in this volume. Epigenetic signaling is also covered separately in this volume by Nowacki; for surveys, also see Chalker et al. (2013) and Simon and Plattner (2014). Most of the other signaling mechanisms described here seem to be evolutionarily old and maintained from protozoa on, particularly ciliates, up to top-ranking metazoans. The impressive complexity of ciliate cells and their elaborate trafficking system may have required a complex signaling system—an old heritage from early eukaryotic ancestors (Dacks and Field 2007; Plattner and Verkhratsky 2015).
References
Allen RD, Fok AK (1993) Nonclathrin vesicle coats and filament networks in the transition zone and trans-Golgi region of the Golgi complex of Paramecium. J Struct Biol 110:215–226
Allen RD, Fok AK (2000) Membrane trafficking and processing in Paramecium. Int Rev Cytol 198:277–318
Allen RD, Naitoh Y (2002) Osmoregulation and contractile vacuoles of protozoa. Int Rev Cytol 215:351–394
Allen PD, Tominaga T, Naitoh Y (2009) The contractile vacuole complex and cell volume control in protozoa. In: Evans DH (ed) Osmotic and ionic regulation: cells and animals. CRC Press, Taylor and Francis Group, Boca Raton, FL, pp 69–106
Aufderheide KJ (1978) Motility events of trichocyst insertion in Paramecium tetraurelia. J Protozool 25:362–365
Beisson J (2008) Preformed cell structure and cell heredity. Prion 2:1–8
Bell WE, Preston RR, Yano J, Van Houten JL (2007) Genetic dissection of attractant-induced conductances in Paramecium. J Exp Biol 210:357–365
Bemm F, Schwarz R, Forster F, Schultz J (2009) A kinome of 2600 in the ciliate Paramecium tetraurelia. FEBS Lett 583:3589–3592
Bonini NM, Nelson DL (1990) Phosphoproteins associated with cyclic nucleotide stimulation of ciliary motility in Paramecium. J Cell Sci 95:219–230
Bowman GR, Elde NC, Morgan G, Winey M, Turkewitz AP (2005) Core formation and the acquisition of fusion competence are linked during secretory granule maturation in Tetrahymena. Traffic 6:303–323
Brehm P, Eckert R (1978) Calcium entry leads to inactivation of calcium channel in Paramecium. Science 202:1203–1206
Bright LJ, Kambesis N, Nelson SB, Jeong B, Turkewitz AP (2010) Comprehensive analysis reveals dynamic and evolutionary plasticity of Rab GTPases and membrane traffic in Tetrahymena thermophila. PLoS Genet 6:e1001155
Briguglio JS, Kumar S, Turkewitz AP (2013) Lysosomal sorting receptors are essential for secretory granule biogenesis in Tetrahymena. J Cell Biol 203:537–550
Cervia D, Catalani E, Belardinelli MC, Perrotta C, Picchietti S, Alimenti C, Casini G, Fausto AM, Vallesi A (2013) The protein pheromone Er-1 of the ciliate Euplotes raikovi stimulates human T-cell activity: involvement of interleukin-2 system. Exp Cell Res 319:56–67
Chalker DL, Meyer E, Mochizuki K (2013) Epigenetics of ciliates. Cold Spring Harb Perspect Biol 5:a017764
Clark KD, Hennessey TM, Nelson DL (1993) External GTP alters the motility and elicits an oscillating membrane depolarization in Paramecium tetraurelia. Proc Natl Acad Sci USA 90:3782–3786
Dacks JB, Field MC (2007) Evolution of the eukaryotic membrane-trafficking system: origin, tempo and mode. J Cell Sci 120:2977–2985
Dentler WL (1988) Fractionation of Tetrahymena ciliary membranes with triton X-114 and the identification of a ciliary membrane ATPase. J Cell Biol 107:2679–2688
De Ondarza J, Symington SB, Van Houten JL, ClarK JM (2003) G-protein modulators alter the swimming behavior and calcium influx of Paramecium tetraurelia. J Eukaryot Microbiol 50:349–355
Eckert R, Brehm P (1979) Ionic mechanisms of excitation in Paramecium. Annu Rev Biophys Bioeng 8:353–383
Elde NC, Morgan G, Winey M, Sperling L, Turkewitz AP (2005) Elucidation of clathrin-mediated endocytosis in Tetrahymena reveals an evolutionarily convergent recruitment of dynamin. PLoS Genet 1:e52
Elwess NL, Van Houten JL (1997) Cloning and molecular analysis of the plasma membrane Ca2+-ATPase gene in Paramecium tetraurelia. J Eukaryot Microbiol 44:250–257
Farrell A, Thirugnanam S, Lorestani A, Dvorin JD, Eidell KP, Ferguson DJP, Anderson-White BR, Duraisingh MT, Marth GT, Gubbels MJ (2012) A DOC2 protein identified by mutational profiling is essential for apicomplexan parasite exocytosis. Science 335:218–221
Flötenmeyer M, Momayezi M, Plattner H (1999) Immunolabeling analysis of biosynthetic and degradative pathways of cell surface components (glycocalyx) in Paramecium cells. Eur J Cell Biol 78:67–77
Fok AK (1983) An inhibition and kinetic study of acid phosphatase in Paramecium caudatum and Paramecium tetraurelia. J Protozool 30:14–20
Fok AK, Paeste RM (1982) Lysosomal enzymes of Paramecium caudatum and Paramecium tetraurelia. Exp Cell Res 139:159–169
Fraga D, Sehring IM, Kissmehl R, Reiss M, Gaines R, Hinrichsen R, Plattner H (2010) Protein phosphatase 2B (PP2B, calcineurin) in Paramecium: partial characterization reveals that two members of the unusually large catalytic subunit family have distinct roles in calcium-dependent processes. Eukaryot Cell 9:1049–1063
Frankel J (2000) Cell biology of Tetrahymena thermophila. Meth Cell Biol 62:27–125
Garreau de Loubresse N (1993) Early steps of the secretory pathway in Paramecium: Ultrastructural, immunocytochemical, and genetic analysis of trichocyst biogenesis. In: Plattner H (ed) Membrane traffic in protozoa. JAI Press, Greenwich, CT, USA, pp 27–59
Gonda K, Komatsu M, Numata O (2000) Calmodulin and Ca2+/calmodulin-binding proteins are involved in Tetrahymena thermophila phagocytosis. Cell Struct Funct 25:243–251
Hardt M, Plattner H (2000) Sub-second quenched-flow/X-ray microanalysis shows rapid Ca2+ mobilization from cortical stores paralleled by Ca2+ influx during synchronous exocytosis in Paramecium cells. Eur J Cell Biol 79:642–652
Hauser K, Pavlovic N, Kissmehl R, Plattner H (1998) Molecular characterization of a sarco(endo)plasmic reticulum Ca2+-ATPase gene from Paramecium tetraurelia and localization of its gene product to sub-plasmalemmal calcium stores. Biochem J 334:31–38
Hay JC (2007) Calcium: a fundamental regulator of intracellular membrane fusion? EMBO Rep 8:236–240
Hemmersbach R, Braun M (2006) Gravity-sensing and gravity-related signaling pathways in unicellular model systems of protists and plants. Sign Transduct 6:432–442
Hemmersbach R, Wilczek M, Stieber C, Bräucker R, Ivanova K (2002) Variable acceleration influences cyclic AMP levels in Paramecium biaurelia. J Gravit Physiol 9:P267–P268
Hurtado-Lorenzo A, Skinner M, El Annan J, Futai M, Sun-Wada GH, Bourgoin S, Casanova J, Wildeman A, Bechoua S, Ausiello DA, Brown D, Marshansky V (2006) V-ATPase interacts with ARNO and Arf6 in early endosomes and regulates the protein degradative pathway. Nat Cell Biol 8:124–136
Husser MR, Hardt M, Blanchard MP, Hentschel J, Klauke N, Plattner H (2004) One-way calcium spill-over during signal transduction in Paramecium cells: from the cell cortex into cilia, but not in the reverse direction. Cell Calcium 36:349–358
Kim K, Messinger LA, Nelson DL (1998) Ca2+-dependent protein kinases of Paramecium—cloning provides evidence of a multigene family. Eur J Biochem 251:605–612
Kissmehl R, Sehring IM, Wagner E, Plattner H (2004) Immunolocalization of actin in Paramecium cells. J Histochem Cytochem 52:1543–1559
Kissmehl R, Schilde C, Wassmer T, Danzer C, Nuehse K, Lutter K, Plattner H (2007) Molecular identification of 26 syntaxin genes and their assignment to the different trafficking pathways in Paramecium. Traffic 8:523–542
Klauke N, Plattner H (1997) Imaging of Ca2+ transients induced in Paramecium cells by a polyamine secretagogue. J Cell Sci 110:975–983
Klauke N, Kissmehl R, Plattner H, Haga N, Watanabe T (1998) An exocytotic mutant of Paramecium caudatum: membrane fusion without secretory contents release. Cell Calcium 23:349–360
Klumpp S, Cohen P, Schultz JE (1990) Okadaic acid, an inhibitor of protein phosphatase 1 in Paramecium, causes sustained Ca2+-dependent backward swimming in response to depolarizing stimuli. EMBO J 9:685–689
Knoll G, Braun C, Plattner H (1991) Quenched flow analysis of exocytosis in Paramecium cells: time course, changes in membrane structure, and calcium requirements revealed after rapid mixing and rapid freezing of intact cells. J Cell Biol 113:1295–1304
Krishnan A, Mustafa A, Sällman Almén M, Fredriksson R, Williams MJ, Schiöth HB (2015) Evolutionary hierarchy of vertebrate-like heterotrimeric G protein families. Mol Phylogenet Evol 91:27–40
Kutomi O, Hori M, Ishida M, Tominaga T, Kamachi H, Koll F, Cohen J, Yamada N, Noguchi M (2012) Outer dynein arm light chain 1 is essential for controlling the ciliary response to cyclic AMP in Paramecium tetraurelia. Eukaryot Cell 11:645–653
Ladenburger EM, Plattner H (2011) Calcium-release channels in Paramecium. Genomic expansion, differential positioning and partial transcriptional elimination. PLoS ONE 6:e27111
Ladenburger EM, Korn I, Kasielke N, Wassmer T, Plattner H (2006) An Ins(1,4,5)P3 receptor in Paramecium is associated with the osmoregulatory system. J Cell Sci 119:3705–3717
Ladenburger EM, Sehring IM, Korn I, Plattner H (2009) Novel types of Ca2+ release channels participate in the secretory cycle of Paramecium cells. Mol Cell Biol 29:3605–3622
Lampert TJ, Coleman KD, Hennessey TM (2011) A knockout mutation of a constitutive GPCR in Tetrahymena decreases both G-protein activity and chemoattraction. PLoS ONE 6:e28022
Lee HC (2012) Cyclic ADP-ribose and nicotinic acid adenine dinucleotide phosphate (NAADP) as messengers for calcium mobilization. J Biol Chem 287:31633–31640
Luporini P, Alimenti C, Vallesi A (2014) Ciliate mating types and pheromones. In: Hausmann K, Radek R (eds) Cilia and flagella, ciliates and flagellates. Schweizerbart Science Publishers, Stuttgart, pp 95–118
Machemer H (1988a) Electrophysiology. In: Görtz HD (ed) Paramecium. Springer, Berlin, Heidelberg, pp 185–215
Machemer H (1988b) Motor control of cilia. In: Görtz HD (ed) Paramecium. Springer, Berlin, Heidelberg, pp 216–235
Machemer H (2014) How do protists keep up? In: Hausmann K, Radek R (eds) Cilia and flagella, ciliates and flagellates. Schweizerbart Science Publishers, Stuttgart, pp 133–146
Machemer H, Ogura A (1979) Ionic conductances of membranes in ciliated and deciliated Paramecium. J Physiol 296:49–60
Machemer H, Bräucker R, Machemer-Röhnisch S, Nagel U, Neugebauer DC, Weskamp M (1998) The linking of extrinsic stimuli to behaviour: roles of cilia in ciliates. Eur J Protistol 34:254–261
Min SW, Chang WP, Südhof TC (2007) E-Syts, a family of membranous Ca2+-sensor proteins with multiple C2 domains. Proc Natl Acad Sci USA 104:3823–3828
Momayezi M, Lumpert CJ, Kersken H, Gras U, Plattner H, Krinks MH, Klee CB (1987) Exocytosis induction in Paramecium tetraurelia cells by exogenous phosphoprotein phosphatase in vivo and in vitro: possible involvement of calcineurin in exocytotic membrane fusion. J Cell Biol 105:181–189
Momayezi M, Kissmehl R, Plattner H (2000) Quantitative immunogold localization of protein phosphatase 2B (calcineurin) in Paramecium cells. J Histochem Cytochem 48:1269–1281
Neher E (1998) Vesicle pools and Ca2+ microdomains: new tools for understanding their roles in neurotransmitter release. Neuron 20:389–399
Park HY, Kim SA, Korlach J, Rhoades E, Kwok LW, Zipfle WR, Waxham MN, Webb WW, Pollack L (2008) Conformational changes of calmodulin upon Ca2+ binding studied with a microfluidic mixer. Proc Natl Acad Sci USA 105:542–547
Plattner H (2010) Membrane trafficking in protozoa SNARE proteins, H+-ATPase, actin, and other key players in ciliates. Int Rev Cell Mol Biol 280:79–184
Plattner H (2014) Calcium regulation in the protozoan model, Paramecium tetraurelia. J Eukaryot Microbiol 61:95–114
Plattner H (2015a) Molecular aspects of calcium signalling at the crossroads of unikont and bikont eukaryote evolution—the ciliated protozoan Paramecium in focus. Cell Calcium 57:174–185
Plattner H (2015b) The contractile vacuole complex of protists—new cues to function and biogenesis. Crit Rev Microbiol 41:218–227
Plattner H (2016a) Signalling in ciliates: Long- and short-range signals and molecular determinants for cellular dynamics. Biol Rev (in press). doi: 10.1111/brv.12218
Plattner H (2016b) Signals regulating vesicle trafficking in Paramecium cells. This volume
Plattner H, Klauke N (2001) Calcium in ciliated protozoa: sources, regulation, and calcium-regulated cell functions. Int Rev Cytol 201:115–208
Plattner H, Kissmehl R (2003a) Dense-core secretory vesicle docking and exocytotic membrane fusion in Paramecium cells. Biochim Biophys Acta (Mol Cell Res) 1641:183–193
Plattner H, Kissmehl R (2003b) Molecular aspects of membrane trafficking in Paramecium. Int Rev Cytol 232:185–216
Plattner H, Verkhratsky A (2013) Ca2+ signalling early in evolution—all but primitive. J Cell Sci 126:2141–2150
Plattner H, Verkhratsky A (2015) The ancient roots of calcium signalling evolutionary tree. Cell Calcium 57:123–132
Plattner H, Westphal C, Tiggemann R (1982) Cytoskeleton-secretory vesicle interactions during the docking of secretory vesicles at the cell membrane in Paramecium tetraurelia cells. J Cell Biol 92:368–377
Plattner H, Stürzl R, Matt H (1985) Synchronous exocytosis in Paramecium cells. IV. Polyamino-compounds as potent trigger agents for repeatable trigger-redocking cycles. Eur J Cell Biol 36:32–37
Plattner H, Knoll G, Pape R (1993) Synchronization of different steps of the secretory cycle in Paramecium tetraurelia: trichocyst exocytosis, exocytosis-coupled endocytosis, and intracellular transport. In: Plattner H (ed) Membrane traffic in protozoa. JAI Press, Greenwich (CT) and London, pp 123–148
Plattner H, Braun C, Hentschel J (1997) Facilitation of membrane fusion during exocytosis and exocytosis-coupled endocytosis and acceleration of “ghost” detachment in Paramecium by extracellular calcium. A quenched-flow/freeze-fracture analysis. J Membr Biol 158:197–208
Plattner H, Sehring IM, Mohamed IK, Miranda K, De Souza W, Billington R, Genazzani A, Ladenburger EM (2012) Calcium signaling in closely related protozoan groups (Alveolata): non-parasitic ciliates (Paramecium, Tetrahymena) vs. parasitic Apicomplexa (Plasmodium, Toxoplasma). Cell Calcium 51:351–382
Pouphile M, Lefort-Tran M, Plattner H, Rossignol M, Beisson J (1986) Genetic dissection of the morphogenesis of exocytosis sites in Paramecium. Biol Cell 56:151–162
Preston RR, Saimi Y, Kung C (1992) Calcium current activated upon hyperpolarization of Paramecium tetraurelia. J Gen Physiol 100:233–251
Reuter AT, Stuermer CAO, Plattner H (2013) Identification, localization, and functional impliclations of the microdomain-forming stomatin family in the ciliated protozoan Paramecium tetraurelia. Eukaryot Cell 12:529–544
Rizo J, Chen X, Arac D (2006) Unraveling the mechanisms of synaptotagmin and SNARE function in neurotransmitter release. Trends Cell Biol 16:339–350
Rothman JE (2014) The principle of membrane fusion in the cell (Nobel lecture). Angew Chemie Int Ed 53:12676–12694
Saimi Y, Kung C (2002) Calmodulin as an ion channel subunit. Annu Rev Physiol 64:289–311
Schilde C, Wassmer T, Mansfeld J, Plattner H, Kissmehl R (2006) A multigene family encoding R-SNAREs in the ciliate Paramecium tetraurelia. Traffic 7:440–455
Schilde C, Schönemann B, Sehring IM, Plattner H (2010) Distinct subcellular localization of a group of synaptobrevin-like SNAREs in Paramecium tetraurelia and effects of silencing SNARE-specific chaperone NSF. Eukaryot Cell 9:288–305
Sehring IM, Plattner H (2004) Ca2+ oscillations mediated by exogenous GTP in Paramecium cells: assessment of possible Ca2+ sources. Cell Calcium 36:409–420
Sehring IM, Reiner C, Mansfeld J, Plattner H, Kissmehl R (2007) A broad spectrum of actin paralogs in Paramecium tetraurelia cells display differential localization and function. J Cell Sci 120:177–190
Sehring IM, Klotz C, Beisson J, Plattner H (2009) Rapid downregulation of the Ca2+-signal after exocytosis stimulation in Paramecium cells: essential role of a centrin-rich filamentous cortical network, the infraciliary lattice. Cell Calcium 45:89–97
Simon M, Plattner H (2014) Unicellular eukaryotes as models in cell and molecular biology: critical appraisal of their past and future value. Int Rev Cell Mol Biol 309:141–198
Stock C, Grønlien HK, Allen RD (2002) The ionic composition of the contractile vacuole fluid of Paramecium mirrors ion transport across the plasma membrane. Eur J Cell Biol 81:505–515
Südhof TC (2014) The molecular machinery of neurotransmitter release (Nobel lecture). Angew Chemie Int Ed 53:12696–12717
Sun-Wada GH, Wada Y (2015) Role of vacuolar-type proton ATPase in signal transduction. Biochim Biophys Acta 1847:1166–1172
Tian M, Chen X, Xiong Q, Xiong J, Xiao C, Ge F, Yang F, Miao W (2014) Phosphoproteomic analysis of protein phosphorylation networks in Tetrahymena thermophila, a model single-celled organism. Mol Cell Proteom 13:503–519
Vallesi A, Di Pretoro B, Ballarini P, Apone F, Luporini P (2010) A novel protein kinase from the ciliate Euplotes raikovi with close structural identity to the mammalian intestinal and male-germ cell kinases: characterization and functional implications in the autocrine pheromone signaling loop. Protist 161:250–263
Wassmer T, Froissard M, Plattner H, Kissmehl R, Cohen J (2005) The vacuolar proton-ATPase plays a major role in several membrane-bounded organelles in Paramecium. J Cell Sci 118:2813–2825
Wassmer T, Kissmehl R, Cohen J, Plattner H (2006) Seventeen a-subunit isoforms of Paramecium V-ATPase provide high specialization in localization and function. Mol Biol Cell 17:917–930
Wassmer T, Sehring IM, Kissmehl R, Plattner H (2009) The V-ATPase in Paramecium: functional specialization by multiple gene isoforms. Eur J Physiol 457:599–607
Wheeler GL, Brownlee C (2008) Ca2+ signalling in plants and green algae—changing channels. Trends Plant Sci 13:506–514
Yang WQ, Braun C, Plattner H, Purvee J, Van Houten JL (1997) Cyclic nucleotides in glutamate chemosensory signal transduction of Paramecium. J Cell Sci 110:2567–2572
Yano J, Rajendran A, Valentine MS, Saha M, Ballif BA, Van Houten JL (2013) Proteomic analysis of the cilia membrane of Paramecium tetraurelia. J Proteom 78:113–122
Yano JY, Valentine MS, Van Houten JL (2015) Novel insights into the development and function of cilia using the advantages of the Paramecium cell and its many cilia. Cells 4:297–314
Zhen Y, Stenmark H (2015) Cellular functions of Rab GTPases at a glance. J Cell Sci 128:3171–3176
Acknowledgements
Experimental work by the author cited herein has been supported by the German Research Council.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2016 Springer International Publishing Switzerland
About this chapter
Cite this chapter
Plattner, H. (2016). Principles of Intracellular Signaling in Ciliated Protozoa—A Brief Outline. In: Witzany, G., Nowacki, M. (eds) Biocommunication of Ciliates. Springer, Cham. https://doi.org/10.1007/978-3-319-32211-7_2
Download citation
DOI: https://doi.org/10.1007/978-3-319-32211-7_2
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-32209-4
Online ISBN: 978-3-319-32211-7
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)