Abstract
All evidence points to Dupuytren disease being a complex genetic trait in which a large number of genetic factors contribute to disease susceptibility in combination with nongenetic (environmental) factors. A small number of risk alleles have been identified through the first genome-wide association study explaining a small portion of the estimated heritability of the disease. Similar but much larger efforts have been initiated to leverage this genome-wide systematic search for risk alleles, which will certainly reveal and provide new molecular insights into the etiology of Dupuytren disease. The contribution of rare coding variants may also play a role in familial forms of Dupuytren disease in a subset of pedigrees that show a Mendelian inheritance of the disease.
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
Keywords
1 A Simple Principle of Human Genetic Studies
Human genetics is the scientific study of heredity as it occurs in human beings. The value of human genetics lies in the fact that it addresses the issue of causality. Whenever we study biology and observe a phenomenon, for example, a behavioral trait or a disorder that may be life threatening such as Huntington’s disease, we may be able to identify a biological hallmark linked to the trait of interest. But simply the fact that there is a biological observation in a group of patients does not mean that this is causing the disease. First, we need to establish that the biological marker is truly linked with the disease, and, secondly, we need to find out whether the biomarker is causing the disease or a secondary effect of having the disease. The beauty of human genetics is that it provides the tools to answer both of these questions and aims to identify the genetic origin of molecular pathways and mechanisms resulting in a specific and individual observable trait, also called a phenotype.
The observation that individual traits are passed down through from generation to generation is not new. As long as humans have habited the earth, they have observed similarities and dissimilarities between parents and children, between peoples from different regions or continents. We have records of Plato’s writing almost 2,500 years ago (in Politikos) where he explains in detail the task of carefully selecting spouses to reproduce children who will develop into bodily and ethically eminent personalities. We can easily dismiss this as a bad example of the earliest eugenics movement and not taking into account the effect of nurture on human beings, but he must have observed a connection between parents and offspring, the inherited features that impact our physical appearance and our behavioral features. In today’s world, the observations are the same, but now we have the knowledge and the tools to specifically study the inheritance of phenotypes and identify the causal relationship between genotype (genetic variation) and phenotype. And this is a simple outline, a principle of human genetic studies (Fig. 12.1). It is significant, because identification of genes involved in disease gives us direction for studying the underlying molecular mechanisms, which in turn will hopefully result in discovery of new therapeutic targets. In short, genetic research is important for discovery of new and improvement of existing treatment of disease.
2 Dupuytren Disease as Genetic Trait
Studying the genetic basis of Dupuytren Disease is no different from studying the genetics of other human disorders. First of all, it requires a proper understanding of the clinical manifestations. A proper diagnosis is the foundation of a good human genetic study of a disorder; without this, a study is doomed to fail. A trained physician can readily make the Dupuytren diagnosis. The disease is a benign, chronic, slowly progressive disease affecting the hands. There is accumulation of fibrous tissue beneath the skin of the palm. This tissue shrinks along its length, pulling the fingers into a permanently bent position. Over time, Dupuytren contracture progresses toward a crippling deformity.
In addition to a proper diagnosis, for genetic studies we need to know more about the incidence of the disease, the age of onset, gender differences, and if there are known environmental risk factors for developing Dupuytren Disease. It would be helpful if tools are available to quantify disease severity or understand if there are other disorders that are comorbid with Dupuytren Disease. The big question for genetic studies, however, is the issue of heritability. Is there evidence that genetic factors do contribute to the disease? The measure to which extent genetic variation can explain phenotypic variation is captured by the heritability estimate. Heritability is a statistical measure to explain how much of the variation observed in a phenotype in a population is due to genetic variation in that population. A simple approach is to examine the familial occurrence and inheritance of Dupuytren Disease in order to get taste of the genetic contribution.
Dupuytren Disease has repeatedly been observed and described to occur in large families across different generation. The first substantial evidence that familial Dupuytren Disease may be a Mendelian trait, i.e., caused by a single genetic factor, transmitted from generation to generation in a pedigree, comes from a study a decade ago (Hu et al. 2005). A genetic linkage study was performed in a large Swedish family with Dupuytren Disease, resulting in the identification of a genetic disease locus on the long arm of chromosome 16. A genetic locus is a specific region on a chromosome and may contain multiple genes. Even though there was (and still is) no known gene with a mutation causing Dupuytren Disease, the genetic evidence showed that in this pedigree, Dupuytren Disease is inherited as an autosomal dominant trait.
Another important contribution to our understanding of the role of genetic factors and Dupuytren Disease came from a recent study by Becker et al. (2015). They examined a possible link between family history and disease severity of Dupuytren Disease. Individuals undergoing the first surgery for Dupuytren contracture were recruited (n = 801) without prior selection for family history. The mean age at first surgery was 59.0 ± 12.2 years of age with a range 22–87 years. Almost 40 % of probands reported a family history of the disease with a first-degree relative (i.e., parents or siblings) being affected. However, family history of the disease had the strongest effect on the age of first surgery. Affected individuals with a positive family history were on average 5.2 years younger than patients without known family history, a highly significant difference (p = 6.7E–08)2. They also observed that the percentage of familial cases decreased with age of onset from 55 % in the 40–49 age category to 17 % for age 80 or older. Even though this study is not a population-based analysis and may be biased toward the most severe cases of DD, the results strongly suggest that a positive family history of the disease is the most prominent risk factor for surgical intervention at an earlier age.
Even though these studies point specifically toward a genetic contribution to disease, the question of heritability remained largely unanswered – until earlier this year. Larsen and colleagues performed the largest twin study of Dupuytren Disease thus far in a population-based twin registry in Denmark (Larsen et al. 2015). The size and scope of the study with >30,000 twins in the general population make this study very valuable for estimating the heritability and population prevalence of Dupuytren Disease. The difference in concordance rates of Dupuytren Disease in monozygotic and dizygotic twins yielded a heritability estimate of approximately 80 % with a prevalence of 0.6 % in the general population (Larsen et al. 2015). The high heritability means that much (but not all) of Dupuytren Disease in the population is due to genetic variation among individuals in that population. It does not necessarily mean that the genetic basis of the disease is simple, however.
These three studies (among some others) show that Dupuytren Disease is a highly heritable trait in which genetic factors are likely to play a role in disease severity. It also leaves room for other, nongenetic factors, such as environmental exposures to contribute as well. Sometimes Dupuytren Disease can manifest itself as a familial disorder inherited in a Mendelian fashion, as seen in the large Swedish family described above. This, however, is rarely the case. The emerging picture of the genetic architecture of Dupuytren Disease is very similar to other human complex traits in which there is a large contribution of many different genetic factors to the genetic risk of the disease (i.e., polygenicity), mixed with a smaller group of monogenic familial forms of the trait.
3 Genome-Wide Association Study of Dupuytren Disease
Even though the genetic evidence was limited a few years ago, we embarked on a genome-wide association study (GWAS) of Dupuytren Disease through a large collaborative effort (Dolmans et al. 2011). The purpose of a GWAS is to identify common risk alleles throughout the human genome contributing to a phenotype. Hundreds of thousands of locations in the genome are each examined for a possible link with disease. Success of a GWAS is largely dependent on sample size of the study since most common risk alleles have only a very modest effect on disease risk. To our surprise, we observed strong evidence of genetic risk factors for Dupuytren Disease, even though the discovery sample included <1,000 patients (Table 12.1). Probably the most exciting observation was that many of the nine loci that we identified contained genes known to be involved in Wnt signaling (Dolmans et al. 2011), pointing to a biological mechanism that is causally involved in the etiology of Dupuytren Disease. Wnt signaling is known to regulate the proliferation and differentiation of fibroblasts in both cancer and fibromatosis; the involvement of the Wnt signaling pathway in the pathogenesis of Dupuytren Disease is consistent with features of the disease and with established aspects of Wnt signaling. The first large-scale genetic study of Dupuytren Disease turned out to be very successful.
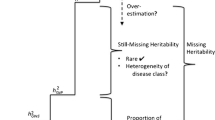
But does this mean that we can mop the floors and close the books and go home because all is known about Dupuytren Disease? No, this is clearly not the case. Genome-wide genotype data of Dupuytren Disease can be used to estimate the proportion of phenotypic variance explained by common alleles (i.e., single nucleotide polymorphisms, SNPs) and to better understand the genetic architecture of Dupuytren Disease as complex trait. Approaches to examine polygenic risk scores from GWAS for complex traits were first proposed for schizophrenia and bipolar disorder (International Schizophrenia Consortium et al. 2009). There is a substantial polygenic contribution to complex traits, and risk alleles are enriched among SNPs selected even for marginal evidence for association from GWAS studies. We used the polygenic score analysis to examine the extent to which the same model can be applied to Dupuytren Disease. We used half of the genome-wide genotyped sample of 856 cases and 2,836 controls as discovery and the other half as target to examine polygenicity. The analysis showed that common alleles may explain up to 14 % of the disease variance, while the nine associated alleles (from the GWAS) account for <2 % of the phenotypic variance. Another common tool used for examining the heritability explained by SNPs with common alleles is GCTA (Yang et al. 2011, 2014). When applied to the overall discovery panel of 856 cases and 2836 control subjects, GCTA estimates the genetic variance (Vg) at 0.163 with standard error (SE) of 0.0136, which is fully in line with the results of the polygenic risk score analysis. While common alleles explain a significant fraction of the overall estimated heritability of Dupuytren Disease (~20 % of total heritability), our results also imply that other types of genetic variation including rare coding variants are likely to play an important role in the disease. The identification of these rare coding variants may require family-based studies, which is a different approach than GWAS.
So instead of mopping the floors to clear out the room, if we are serious about understanding the genetic basis of Dupuytren Disease, we should first aim to expand the GWAS efforts to include much larger sample size for comprehensive discoveries. Other traits report GWAS studies of well over 10,000 patients or even 200,000 subjects (Wood et al. 2014; Schizophrenia Working Group 2014) and are leading the way of genetic discoveries. The road to success for Dupuytren Disease is no different. The second observation is that other types of genetic variation must also contribute to the genetic architecture of disease susceptibility. Only a few years ago, it was first observed how abundant rare coding variations are in human genome, many of which are predicted to be deleterious with likely relevance to understanding disease risk (Nelson et al. 2012). It will take time and effort to fully decipher the genetic architecture of Dupuytren Disease susceptibility, perhaps ranging from polygenic trait in many to Mendelian disorder in a subset of pedigrees. The recent advances in genomic technologies, especially in high-throughput sequencing, provide us with a unique opportunity to identify the genetic origins of Dupuytren Disease.
Conclusions
The evidence is solid that Dupuytren Disease is a highly heritable trait and we already have discovered several genetic factors that play a key role in disease susceptibility. If the initial, relatively small GWAS study was any indicator of success, we have high expectations for future discoveries knowing that larger GWAS efforts are being prepared and are underway. It gives us hope that new therapeutic targets will be discovered and effective ways of prevention and treatment will be developed. This is the best of times for genetic studies of Dupuytren Disease.
References
Becker K, Tinschert S, Lienert A et al (2015) The importance of genetic susceptibility in Dupuytren’s disease. Clin Genet 87(5):483–487
Dolmans GH, Werker PM, Hennies HC et al (2011) Wnt signaling and Dupuytren’s disease. N Engl J Med 365(4):307–317
Hu FZ, Nystrom A, Ahmed A et al (2005) Mapping of an autosomal dominant gene for Dupuytren’s contracture to chromosome 16q in a Swedish family. Clin Genet 68(5):424–429
International Schizophrenia Consortium, Purcell SM, Wray NR, Stone JL et al (2009) Common polygenic variation contributes to risk of schizophrenia and bipolar disorder. Nature 460(7256):748–752
Larsen S, Krogsgaard DG, Aagaard Larsen L et al (2015) Genetic and environmental influences in Dupuytren’s disease: a study of 30,330 Danish twin pairs. J Hand Surg Eur 40(2):171–176
Nelson MR, Wegmann D, Ehm MG et al (2012) An abundance of rare functional variants in 202 drug target genes sequenced in 14,002 people. Science 337(6090):100–104
Schizophrenia Working Group of the Psychiatric Genomics C (2014) Biological insights from 108 schizophrenia-associated genetic loci. Nature 511(7510):421–427
Wood AR, Esko T, Yang J, Vedantam S et al (2014) Defining the role of common variation in the genomic and biological architecture of adult human height. Nat Genet 46(11):1173–1186
Yang J, Lee SH, Goddard ME, Visscher PM (2011) GCTA: a tool for genome-wide complex trait analysis. Am J Hum Genet 88(1):76–82
Yang J, Zaitlen NA, Goddard ME et al (2014) Advantages and pitfalls in the application of mixed-model association methods. Nat Genet 46(2):100–106
Conflict of Interest Declaration
The author declares that there is no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2017 Springer International Publishing Switzerland
About this chapter
Cite this chapter
Ophoff, R.A. (2017). Human Genetic Studies of Dupuytren Disease: A Primer. In: Werker, P., Dias, J., Eaton, C., Reichert, B., Wach, W. (eds) Dupuytren Disease and Related Diseases - The Cutting Edge. Springer, Cham. https://doi.org/10.1007/978-3-319-32199-8_12
Download citation
DOI: https://doi.org/10.1007/978-3-319-32199-8_12
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-32197-4
Online ISBN: 978-3-319-32199-8
eBook Packages: MedicineMedicine (R0)