Abstract
Testis morphogenesis requires the integration and reorganization of multiple cell types from several sources, one of the more notable being the mesonephric-derived cell population. One of the earliest sex-specific morphogenetic events in the gonad is a wave of endothelial cell migration from the mesonephros that is crucial for (1) partitioning the gonad into domains for testis cords, (2) providing the vasculature of the testis, and (3) signaling to cells both within the gonad and beyond it to coordinately regulate testis development. In addition to endothelial cell migration, there is evidence that precursors of peritubular myoid cells migrate from the mesonephros, an event which is also important for testis cord architecture. Investigation of the mesonephric cell migration event has utilized histology, lineage tracing with mouse genetic markers, and many studies of the signaling molecules/pathways involved. Some of the more well-studied signaling molecules involved include vascular endothelial growth factor (VEGF), platelet-derived growth factor (PDGF), and neurotrophins. In this chapter, the morphogenetic events, relevant signaling pathways, mechanisms underlying the migration, and the role of the migratory cells within the testis will be discussed. Overall, the migration of mesonephric cells into the early testis is indispensable for its development and future functionality.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
4.1 Introduction
Mammalian testis development requires the integration of cells from different sources into a complex structure. Cells from the coelomic epithelium and the mesonephros contribute to the gonad via migratory events at multiple points during development. Some of these migrations occur in both male and female gonads, while some are sex specific. In the male, for example, a wave of cell migration from the mesonephros into the gonad initiates key testis architecture changes. This testis-specific mesonephric migration is dependent on prior sex differentiation and occurs in response to chemotactic signaling molecules. The cells that migrate into the early testis are primarily endothelial, and these cells are required for seminiferous cord morphogenesis and testis vascularization. In this chapter, we present the advancements in understanding that have been made regarding the contribution of mesonephric migration to testis development. The studies discussed chiefly focus on mouse and rat models, as much of the work has necessitated knockout and conditional transgenic mouse lines.
4.2 The Timing of Mesonephric Cell Migration and Initial Vascularization of the Developing Gonad
The developmental context of testis-specific mesonephric cell migration is a key part of understanding the importance of this event. The gonad arises as a thickening and restructuring of the coelomic epithelium to form the genital ridge at the 5–6 tail somite developmental stage or around 10.25–10.4 days post coitum (dpc) in the mouse (Hu et al. 2013; Kusaka et al. 2010) (Fig. 4.1). The exact timing of this event in dpc can vary due to subtle differences in embryonic growth rate in various mouse lines, which can be caused by variable accelerated preimplantation development related to the Y chromosome (Burgoyne et al. 1995). Genital ridge formation has been reported from 9 dpc up to 10.5 dpc (Chen et al. 2012; Hacker et al. 1995; Harikae et al. 2013; Karl and Capel 1995; Nef and Parada 2000; Tanaka and Nishinakamura 2014). This event involves proliferation and ingression of cells from the coelomic epithelium to a mesenchymal compartment producing the genital ridge (Harikae et al. 2013; Karl and Capel 1998; Kusaka et al. 2010; Schmahl et al. 2000; Schmahl and Capel 2003). Coelomic epithelial migration prior to 11.5 dpc occurs in both XX and XY gonads, and the migratory cells will eventually become Sertoli cells and interstitial cells in the testis and follicular granulosa cells in the ovary (Karl and Capel 1998; McLaren 2000; Mork et al. 2012). The resultant gonad primordia are initially long, thin structures beside the mesonephros. Primordial germ cells must also migrate into and colonize the gonad starting at 10.0 dpc and continuing up to 11.5 dpc (Ewen and Koopman 2010; Gomperts et al. 1994; Molyneaux et al. 2001) (Fig. 4.1). Importantly, up until 11.5 dpc, the early gonad is morphogenetically identical in males and females as the genital ridge is bipotential and thus can give rise to either a testis or an ovary.
This timeline of testis development shows a progression from the undifferentiated urogenital ridge at 9.5 days postcoitum (dpc) in the mouse (11 dpc in the rat) to the vascularized, compartmentalized testis complete with testis cords at 13.5 dpc in the mouse. Mesonephric cell migration into the male gonad begins in the mouse at 11.5 dpc with the breakdown of the mesonephric vascular plexus (MVP, arrow), and this MVP dissolution and subsequent events do not occur in female embryos. The migrating endothelial cells partition the gonad into avascular domains and form the coelomic vessel (CV, arrowheads) by 12.5 dpc in the mouse. Testis cords then begin to form between 12.5 and 13.5 dpc
Male sexual differentiation is dependent on the expression of the Sry gene and is supported by continued expression of a set of male differentiation maintenance genes (Koopman et al. 1991; Lovell-Badge and Robertson 1990; Sinclair et al. 1990; Ungewitte and Yao 2013). A network of transcription factors including Wilms’ tumor 1 (WT1) initiates Sry mRNA expression at ~10.5 dpc beginning in the center of the gonad and spreading outward in a wave (Bullejos and Koopman 2001; Bradford et al. 2009). The main events in testis development are the specification of the Sertoli cell lineage, testis cord morphogenesis, and then pre-Sertoli cell expansion concurrent with testis cord elongation (Ungewitte and Yao 2013). All of these phases of development are vital to the adult testis structure and function. This chapter focuses on the second event in that series, the formation of testis cords, and the critical role that mesonephric cell migration plays in that process.
While male-specific cellular identities are established earlier, the first two morphogenetic manifestations of testis differentiation are (1) an Sry-dependent thickening of the gonad and the coelomic epithelium due to increased cellular proliferation in preparation for a second coelomic epithelial contribution of cells to the testis and (2) a wave of mesonephric cell migration into the XY gonad that is also dependent on Sry expression (Capel et al. 1999; Schmahl et al. 2000) (Fig. 4.1). The second, Sry-dependent coelomic epithelium migration to the developing testis after 11.5 dpc, does not contribute to the Sertoli cell lineage but rather to the interstitium and serves to assist in the rapid doubling in size of the testis from 11.5 to 13.5 dpc (DeFalco et al. 2011; Karl and Capel 1998; Nel-Themaat et al. 2009; Schmahl et al. 2000). The mesonephric cell migration, on the other hand, has less to do with increasing the overall size of the testis and is instead required for tissue patterning and testis cord morphogenesis (Bott et al. 2006; Combes et al. 2009; Cool et al. 2011).
Mesonephric cell migration into the developing testis shares some basic characteristics in common with other developmental migration events. This migration does not require direct contact with the XY gonad and can actually occur through an XX gonad placed in between a mesonephros and an XY gonad in culture at 11.5 dpc, suggesting that the trigger to migrate must come from a diffusible signaling molecule (Tilmann and Capel 1999). This functions similarly to morphogen gradients that promote and direct migration during embryonic development.
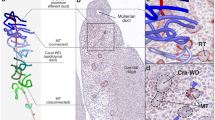
The events that occur during mesonephric cell migration also mirror cell behaviors seen in other migratory events and have been closely observed with time-lapse confocal microscopy from 11.5 to 13.5 dpc in a mouse line with GFP markers conditionally expressed in endothelial cells (Coveney et al. 2008). The majority of the migrating mesonephric cells are endothelial in nature, and thus descriptions of the behavior of the migrating population focus on that cell type (Combes et al. 2009) (Fig. 4.2). First, the endothelial cells that will migrate are released from an existing vessel network within the mesonephros in a sex-specific dissolution of the mesonephric vascular plexus. In XX embryos, the mesonephric vascular network remains intact. In XY embryos, vascular cells that are released from the plexus then undergo an endothelial-to-mesenchymal transition (EMT) by losing their extended squamous shape, detaching from the surrounding endothelial cells, and then by extending long filopodia and beginning to migrate to the coelomic domain of the developing gonad. The migrating population does not move as a unit, but rather individual cells move at different paces along the same general paths. These cells then form the coelomic vessel, the male-specific main testicular artery that persists into adulthood in rodents, and vessels branch off into the interstitium between the testis cords (Figs. 4.1 and 4.2). Ultimately, this process partitions the gonad into approximately ten avascular domains which form the testis cords and subsequently the seminiferous tubules (Coveney et al. 2008) (Fig. 4.2).
Endothelial cells are stained blue in mesonephros (M) and gonads (T testis, O ovary) from transgenic mice expressing KDR-LacZ (a VEGF receptor-driven beta-galactosidase marker). At 11.5 dpc in the male, mesonephric endothelial cells are in the process of migrating into the developing testis (arrowheads). At 12.5 and 13.5 dpc, the coelomic vessel (CV) is visible in the developing testis, avascular domains have segmented, and testis cords are beginning to form. By 16.5 dpc, the male gonad contains seminiferous tubules as seen in the histological section and a defined vasculature with a prominent coelomic vessel (arrows) as seen in the whole mount stained testis with the epididymis (E) attached. Ovaries at 14.5 dpc as shown in histological section and at 16.5 dpc with whole mount staining both lack all male-specific vascular structures and instead have blood vessels spread uniformly throughout the ovary. Microscopy images reprinted with publisher (Springer) permission from Bott et al. (2010)
This wave of patterned endothelial migration does not occur in the XX gonad (Fig. 4.2). During the 11.5–13.5 dpc timeframe, the female mesonephric vascular plexus remains intact (Coveney et al. 2008). In fact, very few morphological changes are seen in the developing ovary until shortly before birth, 18.5 dpc in the mouse (Brennan and Capel 2004). However, there is research on ovarian development in domestic livestock such as cattle and sheep that supports a model involving early migration from the mesonephros. In this model, mesonephric surface epithelial cells at 10–11.5 dpc in mice (days of gestation 30–50 for cattle and 22–38 for sheep) differentiate into the Gonad Ridge Epithelial-Like (GREL) cells (Smith et al. 2014). Based on data primarily from sheep and bovine models, mesonephric stromal cells, endothelial cells, and the GREL cells all migrate into the developing ovary after sex specification, but this process has not been definitively shown in rodent models (Hummitzsch et al. 2013; Smith et al. 2014). The key sex-specific differences are the relatively disorganized mesonephric migration of the developing XX gonad and that the processes are regulated by different signaling pathways (Ungewitte and Yao 2013).
4.3 How the Knowledge of Mesonephric Cell Migration Emerged
Based on histological examination, researchers suspected for years that cells from the mesonephros migrated into and contributed to the structure of both the undifferentiated gonad and sex-differentiated testes (Upadhyay et al. 1979, 1981; Wartenberg et al. 1991). Once the technological tools became available in the 1990s, studies using lineage-tracing markers directly demonstrated mesonephric migration. The first lineage study used mouse testis explants cultured with mesonephros containing a transgenic marker that could be detected by in situ hybridization to show that (1) the mesonephros contributes non-germ and non-Sertoli cells to the testes by migration, (2) this cell migration is required for normal testis architecture, and (3) both XX and XY mesonephroi can contribute cells to a developing testis (Buehr et al. 1993). While the identity of the migrating cells was inconclusive at this point, the demonstration that mesonephric cell migration is required for testis morphogenesis was a significant advancement.
A few years later, researchers used gonad explants cultured with mesonephroi of ROSA26-LacZ mice to show that only XY gonads promote mesonephric cell migration from 11.5 to 16.5 dpc (Martineau et al. 1997). The ROSA26-LacZ transgenic mouse line ubiquitously expresses the enzyme β-galactosidase. When ROSA26-LacZ mesonephric cells migrated into an XY gonad explant lacking the transgene, the substrate for β-galactosidase was provided to selectively stain those cells blue while the surrounding gonad remained unstained. This proved that those blue cells in the gonad originated in the ROSA26-LacZ mesonephros explant. This study also provided more insight into the cell types that undergo migration based on the final morphology and destinations of the migratory cells. The authors suggested that endothelial cells, cells that migrated to positions near areas of Sertoli cell condensation, and other cells that migrated to locations close to endothelial cells composed the migratory population (Martineau et al. 1997).
The exact cell types that migrate from the mesonephros have been a topic of debate over the years. Early on Martineau et al. (1997) and Merchant-Larios et al. (1993) proposed that peritubular myoid cells and other interstitial cells migrate into the gonad along with endothelial cells. Peritubular myoid cells form a thin, flattened layer around the testis cords and future seminiferous tubules, defining the structure of the cords and potentially contributing to the adult functionality of the tubules by muscular contraction to aid movement of sperm through the lumen (Wilhelm et al. 2007). Interestingly, these peritubular myoid cells are the only testis cell type with no known ovarian equivalent. They also have no known cell type-specific genetic markers and can only be identified by genes common to muscle cells such as alpha-smooth muscle actin and desmin (Jeanes et al. 2005).
Later studies argue that the migrating population is primarily or even exclusively endothelial cells based on the expression of endothelial markers before/during the migratory period. In one such article, Combes et al. (2009) performed coculture from 11.5 to 12.5 dpc of mesonephros constitutively expressing green fluorescent protein (GFP) with XY gonads not expressing GFP and showed by immunofluorescence that >99 % of the GFP-labeled cells co-localized with the endothelial marker cadherin-5 (also designated VE-cadherin, CD144 antigen, and Cadherin 5, type 2). These data support the claim that the majority of the initial wave of migratory cells is endothelial in nature, but does not rule out subsequent migration or differentiation of other cell types. In a separate study, Cool et al. (2008) utilized lineage tracing with an EYFP alpha-smooth muscle actin (α-Sma) marker of interstitial cells expressed in transgenic mesonephroi cultured with wild-type XY gonads. This experiment showed that no cells already expressing alpha-smooth muscle actin were part of the migrating population, which the authors argue demonstrates that no peritubular myoid cells in the testis originate in the mesonephros (Combes et al. 2009; Cool et al. 2008). However, this finding does not rule out the possibility of precursors that do not yet express the interstitial actin marker migrating into the gonad and subsequently differentiating. Overall, the literature supports the idea that the wave of mesonephric cell migration into the gonad concurrent with male sex specification is primarily endothelial in nature but does not rule out subsequent migration of other testis cell types or their precursors. In addition to the evidence for a large migratory endothelial component, there is also support for the idea that the precursors of peritubular myoid cells migrate from the mesonephros in response to neurotrophins, though the timing of the migration is difficult to determine. As will be discussed in more detail in the section specific to neurotrophin signaling, mesonephric-derived peritubular myoid cells express the low-affinity neurotrophin receptor NGFR (formerly p75NTR), and these cells do not appear in the fetal testis unless cocultured with a mesonephros explant (Russo et al. 1999; Campagnolo et al. 2001). Neurotrophin-3 (NT3) signaling inhibition via antisense nucleotides against NT3, a pharmacological inhibitor of the receptor NGFR, and receptor-IgG fusion dominant negative expression also disrupt seminiferous tubule formation and testis architecture (Cupp et al. 2000, 2003; Levine et al. 2000). Additionally, a small proportion of GFP-expressing mesonephric cells that migrated into the gonad and subsequently were isolated by fluorescence-assisted cell sorting (FACS) were shown to express the muscle-specific intermediate filament desmin, which is a general marker for myoid cells, including the peritubular myoid cells (Nishino et al. 2001). Furthermore, there is evidence that some cells that will contribute to the Leydig cell (an interstitial cell adjacent to the mature seminiferous tubules) population migrate into the gonad from the mesonephric border alongside vascular cells (DeFalco et al. 2011). Thus, the mesonephros contributes migratory cells of both endothelial and non-endothelial cell types, though the bulk of the migratory population express endothelial cell markers during migration.
Migration from the mesonephros into the gonad after the period of sex specification also occurs in the developing ovary. After 12.5 dpc the transcription factor GLI1 is expressed in the mesonephros but not the ovary. GLI1+ mesonephric cells then migrate into the XX gonad starting at 18.5 dpc, just before birth, to contribute a small but crucial subpopulation to the theca cells of the ovarian follicles (Liu et al. 2015). Mesonephric migration into the gonad thus has the possibility to occur at multiple points during development and contribute to its architecture and function.
4.4 Signals That Promote Mesonephric Cell Migration
The signaling pathways that coordinately regulate testis development are numerous. This section will discuss those pathways that are involved in mesonephric cell migration into the XY gonad and the phenotypic results if those signals are inhibited (Table 4.1). Some signaling pathways are directly required for mesonephric cell migration, while others act in a permissive capacity or modulate the effects of other signals.
4.4.1 Sex Determination and Pleiotropic Regulators of Migration
SRY and SOX9
Male sex determination is a prerequisite for migration of mesonephric cells (Fig. 4.3). The initiator of the male-specific genetic program is Sry expression, which occurs from 10.5 to 12.5 dpc in the mouse and is necessary for cell migration into the testes from the mesonephros (Capel et al. 1999; Koopman et al. 1991). Shortly after the initiation of Sry expression, the SRY-box containing gene 9 (Sox9) is expressed in the pre-Sertoli cells via synergistic action of SRY and the transcription factor steroidogenic factor 1 (SF1, also known as NR5A1) on a Sox9 enhancer (Luo et al. 1994; Sekido and Lovell-Badge 2008). Unlike Sry, Sox9 expression is maintained throughout embryogenesis and into adulthood (Kent et al. 1996). Mutations in Sox9 or its regulatory regions result in male-to-female sex reversal (Wagner et al. 1994). Conversely, transgenic expression of Sry or Sox9 in XX gonads causes female-to-male sex reversal (Koopman et al. 1991; Vidal et al. 2001). The transcription factors SRY and SOX9 do not directly initiate or control the mesonephric cell migration, but they put into effect a male developmental program involving several signaling molecules and pathways that result in the migratory event and seminiferous tubule morphogenesis (Fig. 4.3). These signaling factors will be discussed in more detail in this section.
Mesonephric cell migration and subsequent testis cord formation require the integration of multiple signaling molecules. Many of these signals promote the male gene expression profile necessary for mesonephric migration to occur; others directly participate in promoting cell migration from the mesonephros or control other aspects of testis cord morphogenesis
FGF9
Within the developing testis, the male-specific genes expressed regulate sex specification maintenance and/or the morphogenetic changes crucial for testis architecture. Many of these genes exist in feedback loops and interacting signaling pathways. A prime example of a sex maintenance gene involved in feedback loops and pathway interaction is fibroblast growth factor 9 (FGF9), which upregulates and maintains Sox9 expression after sex determination in the XY gonad (Fig. 4.3). Fgf9 –/– mice can initiate but not maintain Sox9 expression, resulting in impaired Sertoli cell differentiation and testis cord formation (Kim et al. 2006). This signaling occurs primarily via FGF receptor 2 (FGFR2), as Fgfr2 –/– mice have similar phenotypes to Fgf9 –/– mice and experience sex reversal (Bagheri-Fam et al. 2008; Kim et al. 2007; Schmahl et al. 2004). A crucial reason that FGF9 and FGFR2 are required for testis development is that FGF9 signaling actively represses the WNT4 signaling that would otherwise promote female gonad development (Jameson et al. 2012) (Fig. 4.3). Conditional knockout of either Fgf9 or Fgfr2 in the Sf1-expressing cells of XY gonads causes male-to-female sex reversal, while testis morphology is rescued in Fgf9/Wnt4 and Fgfr2/Wnt4 double mutants (Jameson et al. 2012). Together these data suggest that the primary role of FGF signaling is to suppress the female developmental program.
Factors with Pleiotropic Effects in XX and XY Gonads
Additionally, it is important to note that signals from the female gonad can actively inhibit mesonephric cell migration into the XX gonad. WNT4 must be downregulated in the XY gonad in order for endothelial cell migration into the developing testes and the formation of a coelomic blood vessel (Jeays-Ward et al. 2003). Interestingly, signaling pathways in gonad development are often pleiotropic and can function in both the male and female developmental programs (Fig. 4.4). One such example involves the transcription factor SF1 (which is commonly considered a marker for the Sertoli cell lineage) and its cofactor Cbp/p300-interacting transactivator 2 (CITED2). By looking at a Cited2 –/– mouse and the resulting decrease in SF1 activity, researchers discovered that in XY mice SF1/CITED2 function is important for proper testis architecture, but in XX gonads SF1/CITED2 signaling is also crucial for female patterning and without it there is ectopic mesonephric cell migration (Combes et al. 2010) (Fig. 4.4).
Pleiotropic signaling factors such as RSPO1 and SF1/CITED2 have opposite effects in the XY gonad compared to the XX gonad. In the XY gonad in the absence of WNT4 and DKK1, RSPO1 and SF1/CITED2 both promote testis vascularization. In the XX gonad, RSPO1 enhances WNT4 canonical signaling via β-catenin to inhibit male sex specification and SF1/CITED2 promotes female gonad patterning
Another such factor with pleiotropic effects is R-spondin 1 (RSPO1), which enhances canonical β-catenin/Wnt signaling to drive female gonad development (Carmon et al. 2014; Wilhelm 2007). This is required for ovarian development as masculinization and loss of female morphology is observed in XX gonads in Rspo1 knockout mice (Chassot et al. 2008). In XY gonads of Rspo –/– mice testis morphogenesis appears normal, indicating that RSPO1 is not required in vivo for testis development (Chassot et al. 2008; Lavery et al. 2012). However, a recently published study shows that there may be a complex balance between RSPO1 and Dickkopf homolog 1 (DKK1), an inhibitor of the canonical WNT/RSPO signaling pathway regulating testicular angiogenesis with R-spondin 1 driving vascularization (Caruso et al. 2015) (Fig. 4.4). In cultured explants of XY gonads/mesonephroi, DKK1 treatment inhibits endothelial cell migration into the gonad while RSPO1 treatment rescues this inhibition (Caruso et al. 2015). Thus, molecules that promote mesonephric cell migration in the XY gonad such as SF1/CITED2 or RSPO1 have the opposite effect in the XX gonad and prevent ectopic vascularization.
4.4.2 VEGFA
The VEGF Family
The vascular endothelial growth factor (VEGF) family contains five mammalian proteins: VEGFA, VEGFB, VEGFC, VEGFD, and PGF (placental growth factor, formerly known as PlGF). The factor for which the family is named is VEGFA, originally known simply as VEGF, and it was originally discovered as a gene vital for embryonic blood vessel development (Carmeliet et al. 1996; Ferrara and Henzel 1989; Ferrara et al. 1996). VEGFB and PGF are both involved in blood vessel development, but do not appear to be expressed in the developing gonad at the time of mesonephric cell migration into the testis (Olofsson et al. 1998; Park et al. 1994). VEGFC and VEGFD primarily function in lymphatic angiogenesis (Achen et al. 1998; Jeltsch et al. 1997; Joukov et al. 1996; Karkkainen et al. 2002).
VEGFA
A great deal of investigation has been centered on VEGFA, as it is critical for vascularization in many contexts (Ferrara et al. 2003). The function of VEGFA is also multifaceted because it undergoes alternative splicing of its eight exons that produces many isoforms with varying functions (Dehghanian et al. 2014; Olsson et al. 2006). The known human isoforms include VEGFA-111, -121, -145, -148, -165, -183, -189, and -206 (named for the length of the protein in amino acids), while the mouse isoforms are one amino-acid residue shorter than the corresponding human isoform and named accordingly (Dehghanian et al. 2014). However, there is an additional alternative splicing variation in which the previously known version of exon 8 of the VEGFA transcript, now called exon 8a, is replaced with exon 8b which gives the protein anti-angiogenic properties (Bates et al. 2002). This creates a set of anti-angiogenic isoforms corresponding to the pro-angiogenic isoforms containing exon 8: VEGFA-121b, -145b, -165b, -183b, and -189b (Dehghanian et al. 2014). Most of the research regarding mesonephric endothelial migration and the role that VEGFA plays in this process has looked at broad inhibition of VEGF receptors or other methods that are not able to distinguish the function of individual isoforms. Thus, as the field moves forward, it will be interesting to discover how much the complexity and nuance in signaling is available from so many signaling molecules factors into testis vascularization.
What is currently known is that manipulations of VEGFA can have strong effects on testis vascularization. Immunohistochemistry and explant culture in the presence of signaling inhibitors SU1498 (a selective inhibitor of one VEGF receptor), VEGFR-TKI (a general VEGFA receptor antagonist), Je-11 (competitively binds VEGFA to prevent receptor binding), and LY 294002 (a phosphoinositide 3-kinase (PI3K) pathway inhibitor) demonstrated that angiogenic isoforms of VEGFA including Vegfa120, Vegfa164, and Vegfa188 are expressed in the developing testis in the rat and that VEGFA signaling via KDR is required for vascularization and testis cord formation (Bott et al. 2006) (Fig. 4.3). Inhibition of VEGFA signaling with pharmacological treatments blocks the action of both angiogenic and antiangiogenic isoforms, showing that the vascularization and structural defects are due to impaired VEGFA signaling as a whole without distinguishing the roles of individual variants (Bott et al. 2006; Cool et al. 2011). Specific inhibition of anti-angiogenic VEGFA using antibody treatment, on the other hand, results in excessive vascularization and thus suggests that those isoforms inhibit over-vascularization (Baltes-Breitwisch et al. 2010). VEGFA signaling can thus either promote vascularization or inhibit it based on the splice variants produced.
VEGFA Signaling Transduction
In addition to the variety of VEGFA isoforms possible, the ways in which VEGFA signaling can occur are quite varied and include both canonical ligand–receptor interactions and ligand-independent signaling, involving various receptor dimers and interactions with co-receptors (Domigan et al. 2015). Many of these unconventional signaling pathways are recently discovered, and the extent to which they might function in the developing gonad is not yet known. Within the early gonad, most of the research to date has focused on traditional ligand–receptor signaling. Two major receptors play important roles in transduction VEGFA signaling in the early gonad: the fms-like tyrosine kinase receptor (FLT1, sometimes cited as VEGFR-1) and the kinase insert domain receptor (KDR, alternatively called FLK1 or VEGFR-2) (de Vries et al. 1992; Shibuya et al. 1990; Terman et al. 1992). KDR and FLT1 have different modes of signal transduction and binding affinities for the VEGFA ligand. Binding analyses showed that VEGFA has a ~50-fold greater binding affinity for FLT1 than for KDR (Waltenberger et al. 1994). However, KDR is the primary receptor through which VEGFA signal transduction occurs (Kroll and Waltenberger 1997; Waltenberger et al. 1994). VEGFA binding-induced tyrosine kinase autophosphorylation of KDR results in its downstream signal transduction in endothelial cells (Waltenberger et al. 1994). When VEGFA binds to FLT1 in endothelial cells autophosphorylation and signal transduction do not occur, though in non-endothelial cells FLT1 can transduce VEGFA signaling (Caires et al. 2012).
The expression patterns of FLT1 and KDR impart critical sex-specific differences to VEGFA signaling. In the XY gonad and mesonephros, FLT1 is not expressed until after testis cord formation (18 dpc in the rat) (Bott et al. 2006). In XX embryos, FLT1 is expressed much earlier and may prevent ectopic endothelial cell migration by acting as a “decoy” receptor that sequesters VEGFA away from KDR to attenuate the signal transduction (Fong et al. 1999; Gille et al. 2000; Hiratsuka et al. 1998; McFee et al. 2009). Interestingly, this FLT1 decoy function/lack of kinase activity is defined by a single amino acid (Meyer et al. 2006).
There are many proteins that can modulate VEGFA signaling in addition to its receptors. VEGFA itself can bind to heparan sulfate proteoglycans within the extracellular matrix, which can alter its diffusion through tissues (Park et al. 1993; Vieira et al. 2010). In addition, the heparan sulfate proteoglycan binding domain of some VEGFA isoforms allows them to bind to co-receptors of the neuropilin family which includes neuropilin 1 (NRP1) and neuropilin 2 (NRP2) (Chen et al. 1997; Fidder 1996; Gitay-Goren et al. 1996; Kawasaki et al. 1999). The way that NRP1 functions to modulate VEGFA signaling is to bind VEGFA and form complexes with either KDR or FLT1 (without directly binding to either receptor) (Soker et al. 1998, 2002). The angiogenic isoforms of VEGFA such as Vegfa-165 can bind NRP1 and KDR, and proteins that interact with the C-terminus of NRP1 can then enhance KDR signal transduction (Wang et al. 2006). Additionally, NRP1 binding may promote KDR trafficking and recycling and thereby further enhance VEGFA signaling (Kofler and Simons 2015). The anti-angiogenic isoforms, on the other hand, cannot bind to NRP1 which may help explain why those isoforms do not have strong KDR signaling properties (Kawamura et al. 2008).
VEGFA in Mesonephric Chemotaxis
Within the developing testis, the most studied VEGFA signaling pathway is the traditional angiogenic ligand–KDR receptor interaction. Based on a transgenic marker line (KDR-LacZ), mesonephric endothelial cells that undergo migration express KDR prior to and after migration (Bott et al. 2010) (Fig. 4.2). KDR as a lineage marker, however, may also include hematopoetic stem cells due to KDR expression beginning in a shared precursor, the hemangioblasts (Ziegler et al. 1999). Importantly, pharmacological inhibition of KDR alone with SU1498 or KDR and FLT1 both with VEGFR-TKI (a general VEGFA receptor antagonist) and VEGF-Trap (which contains domains of KDR and FLT1) prevent endothelial migration and testis cord formation (Bott et al. 2006, 2010; Cool et al. 2011). VEGFA does not act in isolation, however, as both platelet-derived growth factor (PDGF) and NRP1 act in concert with VEGFA. As will be discussed further in the next section, PDGF is crucial for coelomic vessel formation and likely participates in VEGFA-mediated cross talk between the migrating endothelial cells and the mesenchymal cells of the gonad (Brennan et al. 2003; Cool et al. 2011; Uzumcu et al. 2002). NRP1, on the other hand, can function as both a modulator of canonical VEGFA signaling and can regulate endothelial cell function independently of KDR (Kofler and Simons 2015). NRP1 can regulate adhesion and motility of endothelial cells by interacting with integrins and the non-receptor tyrosine kinase ABL1 (Fukasawa et al. 2007; Murga et al. 2005; Raimondi et al. 2014; Valdembri et al. 2009). Thus, VEGFA signaling plays a major role in testis vascularization and testis cord morphogenesis, but it is supported by other signaling pathways and potentially made redundant based on the observation that a pDmrt1-Cre-driven Vegfa knockout (excision of exon 3 specifically in the Sertoli and germ cells) does not prevent vascularization or testis cord formation (Lu et al. 2013).
4.4.3 PDGF
Platelet-derived growth factor (PDGF) is a family of signaling molecules that are made of dimers of four subunits (A, B, C, and D), resulting in PDGF-AA, -BB, -CC, -DD, and -AB (Heldin 2013). The receptors for these factors are also dimers with alpha and beta subunits corresponding to separate PDGF subunits (Heldin 2013). Within the developing testis, PDGF-AA, -BB, and -CC have been shown to be expressed and to play a role in testis cord morphogenesis (Hoch and Soriano 2003; Puglianiello et al. 2004). PDGF-BB has various functions in vasculature throughout embryonic development. In the developing testis, selective PDGF signaling inhibition with AG1296 (tyrphostin) prevents testis cord formation while treatment with PDGF factors in culture partially rescues testis cord morphogenesis, but the resulting cords are of a smaller diameter (Ricci et al. 2004; Smith et al. 2005) (Fig. 4.3). Additionally, genetic knockouts of the alpha PDGF receptor subunit show disrupted vascularization and compartmentalization prior to testis cord formation in XY gonads at 13.5 dpc (Brennan et al. 2003). The interactions between PDGF signaling and VEGF signaling, both of which are known regulators of testis cord morphogenesis, are a great example of cooperative signaling driving two interdependent morphological processes. Using inhibition of VEGF signaling with VEGF-Trap (which inhibits both KDR and FLT1) and mouse transgenic lineage markers for various cell types, Cool et al. (2011) showed that somatic cells of the early testis express VEGFA to promote endothelial migration and respond, by proliferation, to that endothelial migration (Fig. 4.3). Interestingly, there is some cell culture-based evidence that VEGFA can directly signal via PDGF receptors in cells lacking VEGF receptors (Ball et al. 2007). Overall, the interactions and complexities of these interconnected signaling pathways likely provide precision of control and potential redundancy in the regulation of mesonephric endothelial migration, vascularization of the testis, and testis cord morphogenesis.
4.4.4 Neurotrophins
Another chemotactic factor that has been shown to be involved in mesonephric cell migration into the early testis, but not endothelial cells, are the neurotrophins (Fig. 4.3). Beginning at 13 dpc in the developing rat testis and 11.5 dpc in the mouse, immunohistochemistry can be used to localize neurotrophin 3 in the Sertoli cells and the neurotrophin receptors in the mesonephros and subsequently in the peritubular myoid cells (Campagnolo et al. 2001; Cupp et al. 2000; Levine et al. 2000; Russo et al. 1999). In fact, Campagnolo et al. (2001) showed that the low-affinity neurotrophin receptor NGFR can be used as a lineage marker for mesonephric-derived peritubular myoid cells and that these cells do not appear in the fetal testis unless cocultured with a mesonephros explant. Neurotrophin signaling inhibition with antisense nucleotides against NT3, a pharmacological inhibitor of the receptor NGFR, and receptor-IgG fusion dominant negative expression in rat testis explants also inhibit seminiferous tubule formation, and mouse genetic knockout of the neurotrophin ligand or its receptor results in decreased testis interstitial volume (Cupp et al. 2000, 2003; Levine et al. 2000). In addition, neurotrophin receptor knockout mice (Ntrk1, Ntrk3) show impaired seminiferous tubule formation and reduced interstitial area in the testis at 13–14 dpc (Cupp et al. 2002). Thus, the mesonephros contributes both endothelial cells and at least part of the peritubular myoid cell population to testis architecture.
4.4.5 Other Signaling Factors Contributing to Mesonephric Migration
In addition to the more well-studied contributors to mesonephric cell migration into the testis, there are other signaling pathways that can influence this migration. Anti-Müllerian hormone (AMH) deficient mice do not have testis abnormalities, but exogenous AMH in an organ culture can induce both mesonephric cell migration into XX gonads and testis cord formation (Ross et al. 2003; Vigier et al. 1987). Amh mRNA is expressed at 12.5 dpc in the mouse testis and thus could play a contributing role in inducing mesonephric migration into the XY gonad, though it is likely not the main initiator of the migration in vivo due to the timing of the expression (Hacker et al. 1995; Münsterberg and Lovell-Badge 1991) (Fig. 4.3).
Another pathway with an incompletely defined role in testis cord formation is Hedgehog signaling involving two ligands: desert hedgehog (DHH) and sonic Hedgehog (SHH). DHH is required for the formation of the basal lamina that surrounds the testis cords and is also involved in testicular somatic cell fate specification (Clark et al. 2000; Pierucci-Alves et al. 2001; Yao et al. 2002). In fact, DHH expression in the XY gonad shortly after Sry expression is one of the earliest signs of sex-specific differentiation of Sertoli cells (Bitgood et al. 1996). SHH, on the other hand, is expressed in the Wolffian duct epithelium of the mesonephros (Bitgood and McMahon 1995). The Hedgehog receptor Patched, which binds and transduces signals for both DHH and SHH, is expressed in the mesonephric mesenchyme as well as the testis interstitium (Franco and Yao 2012; Yao et al. 2002). Importantly, the migration of mesonephric endothelial cells into the early testis and its vascularization do not depend on Hedgehog signaling based on data from DHH genetic null mice and pharmacological inhibition of the Hedgehog co-receptor Smoothened (Yao et al. 2002; Yao and Capel 2002). Instead, a primary role of DHH appears to be to promote differentiation of fetal Leydig cells (Barsoum et al. 2009; Yao et al. 2002). However, DHH also plays a role in peritubular myoid cell function or differentiation, as Dhh –/– mice have peritubular myoid defects (Clark et al. 2000; Pierucci-Alves et al. 2001). In addition to the likely DHH signaling to peritubular myoid cells from Sertoli cells, it is possible that SHH from the mesonephric epithelium may play a role in peritubular myoid cell function either through direct interaction with myoid cells in the early testis or by its function within the mesonephros. NOTCH signaling (like DHH) is also a key regulator of the Leydig cell lineage, though NOTCH acts to promote maintenance of the progenitor line by inhibiting Leydig cell differentiation (Tang et al. 2008). Interestingly, Notch1 receptor and the ligands Jagged1 and Jagged2 have been reported to be expressed in the testis vasculature during the period of mesonephric endothelial migration and testis cord formation (Brennan et al. 2002). There is also evidence that this results in active NOTCH signaling due to coincident expression of a downstream target, Hes-5 (Brennan et al. 2002). Other cells such as Sertoli cells and the interstitium can also express Notch2 and Notch3 with corresponding expression of the downstream target genes Hes-1 and Hes-5 (Tang et al. 2008). Inhibition of NOTCH signaling with mouse genetic knockout of Hes-1 and pharmacological treatment with DAPT (a gamma-secretase inhibitor) results in increases in differentiated Leydig cell numbers. Constitutive overexpression of Notch driven by the Sf1 promoter, on the other hand, results in a loss of Leydig cells. And, ultimately, both Hes-1 –/– and ectopic Notch result in smaller and irregularly shaped testis cords (Tang et al. 2008). However, the possible connection between the mesonephric-derived endothelial cell migration and regulation of Leydig cell differentiation in the testis via NOTCH signaling has not been thoroughly investigated.
4.5 Mechanisms of Mesonephric Cell Migration
Actively migrating cells must undergo several coordinated changes to detach from the original tissue, receive and interpret polarizing cues to orient their movement, translocate, and integrate into the target tissue (Ridley et al. 2003). The first step, disengaging from the original tissue, often requires the change from an epithelial state to a mesenchymal cell morphology. In the case of the mesonephric endothelial cells that migrate into the XY gonad, this is actually an endothelial-to-mesenchymal transition (EMT), though it still follows the same pattern of events based on immunofluorescence and cell shape changes (Combes et al. 2009; Thiery et al. 2009). The vascular plexus of the mesonephros and the endothelial cells that migrate into the early testis express classic endothelial cell adhesion molecules such as VE-cadherin (Combes et al. 2009). Though there is currently little direct evidence, it is conceivable that the endothelial-to-mesenchymal transition that allows migration would cause a change in cell–cell binding by internalizing adhesion molecules from the membrane and sequestering or degrading them temporarily. Simultaneously, the endothelial cells lose their extended squamous shape and become elongated with filopodia-like extensions (Combes et al. 2009; Coveney et al. 2008). The loss of existing cell adhesion and changes in cytoskeletal structure required for these cell shape changes permit the cell to undergo directed movements with polarized adhesion formation and cytoskeletal filament elongation (Fig. 4.5).
During the process of migrating into the XY gonad, endothelial cells within the vascular plexus of the mesonephros must lose their existing adhesions and structure and acquire a mesenchymal phenotype with greater cytoskeletal dynamics and multiple filopodia-like extensions. In response to a gradient of a signaling molecule such as VEGFA, the closer filopodia extend toward the source of the morphogen and the further edges retract. This results in directional migration
4.5.1 Cues to Orient Mesonephric Migration
An obvious candidate for an endothelial chemotactic signal is VEGF. Neovascularization in many contexts is controlled by VEGFA gradients, and it serves both to orient migration and to promote the necessary cellular changes to cause cell movement. For example, VEGFA signaling via human KDR expressed in porcine endothelial cells resulted in changes in cell morphology, actin reorganization, membrane ruffling, and chemotaxis while FLT1 signaling did not have the same responses (Waltenberger et al. 1994). Other data from human umbilical endothelial cells show that signaling via KDR may regulate focal adhesion complexes while FLT1 affects cytoskeletal dynamics in this context (Kanno et al. 2000). Additionally, VEGFA can promote chemotactic migration of non-endothelial human cells including osteoblasts, lung cancer cells, and mesenchymal progenitor cells (Chen et al. 2009; Fiedler et al. 2005; Mayr-Wohlfart et al. 2002). This role for VEGFA chemotaxis has also been shown for endothelial cells in other developmental systems such as the kidney (Li et al. 2005). All of this, combined with the previously demonstrated role of VEGFA in mesonephric endothelial migration into the testis, makes it highly likely to be a major directional cue to those migrating cells.
The way in which a gradient of signaling ligands is interpreted varies based on the context. Unlike cells with established adhesions in a tissue, migrating cells must respond to directional cues autonomously. Migrating endothelial tip cells in the retina and other contexts do this by extruding filopodia in multiple directions (Gerhardt et al. 2003). Further cytoskeletal polymerization is encouraged in the filopodia that receive the most signal transduction, thus elongating the cell toward the source of the signaling ligand (Fig. 4.5). VEGFA signaling gradients in the developing brain cause endothelial tip cells to migrate toward them because they express KDR and NRP1, both of which are required for migration (Fantin et al. 2013; Gerhardt et al. 2003, 2004). The mosaic studies that show that endothelial migration has a cell autonomous dependence on KDR and NRP1 signaling have not been performed in the developing testis/mesonephros, but it is likely that migration in that system occurs with a similar mechanism. It is also possible that other signaling pathways that promote mesonephric cell migration into the XY gonad do so through an analogous system of random filopodial extension, greater signaling response nearer the chemotactic source, and reinforcement of cytoskeletal dynamics in that direction.
The way in which a filopodium seeking and finding a greater source of a chemotactic factor like VEGFA responds and causes migration varies based on the cell type, but there are some common themes. Such a localized area of signaling activation results in phosphorylation/activation of downstream signal transducers and in proteins that regulate cytoskeletal dynamics. For example, endothelial cell culture assays show that a VEGF/KDR-initiated MAP-Kinase signal transduction cascade phosphorylates LIM-Kinase which inhibits cofilin, preventing cofilin from depolymerizing actin filaments and thus forming actin stress fibers that further stabilize the local filopodium (Kobayashi et al. 2006). This is just one of many potential avenues by which VEGFA or other signaling molecules regulate cytoskeletal dynamics in a complex network of interacting signal transductions and cytoskeleton-interacting proteins.
4.5.2 Narrowing the Migratory Path
One possibility that has been proposed to control the breadth of the migration path is that the individual migrating endothelial cells follow prelaid extracellular matrix scaffolding paths to partition the gonad into avascular domains prior to testis cord formation (Coveney et al. 2008). These extracellular matrix paths would be, in a sense, the path of least resistance with more matrix molecules that the leading edge of the migrating cell could bind to more easily than the surrounding matrix in order to narrow the directional movement toward a more general chemotactic signal. Another possibility is that the very first migratory endothelial cells would only have a broad directional cue to follow but would modify the surrounding tissue as they passed to guide subsequent migratory cells along the same path (Combes et al. 2009; Murphy and Gavrilovic 1999).
4.5.3 Reintegration into Target Tissue
Endothelial cells migrate individually rather than as a unit and must integrate into the target tissue (the XY gonad) and aggregate to form the coelomic vessel. Smaller vessels then branch off of the coelomic vessel between the avascular domains that will form the testis cords. In order to establish the new vasculature of the testis, VE-cadherin binding is crucial as treatment with an antibody blocking that binding prevents the formation of a nascent vascular plexus in the testis that would give rise to the coelomic vessel (Combes et al. 2009). The localization of endothelial cells between partitioned sections occurs by 12.25 dpc, and by 12.5 dpc, the endothelial cells have become interconnected again organized into vessels, so the reestablishment of cell–cell adhesion is coordinated and relatively fast (Combes et al. 2009). Both the act of partitioning the domains of the future testis cords and the presence of the endothelial cells themselves are important for the rest of the testis architecture formation.
4.6 The Role of Mesonephric-Derived Cells in the Testis
Once cells have migrated into the early XY gonad from the mesonephros, they take their place in the developing testis. Endothelial cells that were dissociated reform vasculature. The main purpose of the vasculature, of course, is to allow blood flow. Before 12 dpc, blood flows into the developing testis from the mesonephros vascular plexus through the gonad/mesonephros boundary (Brennan et al. 2002). After mesonephric endothelial cell migration and remodeling of the testis, blood flow is redirected into the testis via the arterial coelomic vessel (Brennan et al. 2002). Funneling blood flow in this manner is necessary for an external organ that will both need a sufficient blood supply and need to transport secreted hormones into circulation.
The importance of the testis vasculature to the development and functionality of the gonad is clear when vasculature formation is inhibited. When mesonephros–gonad organ cultures are treated with an antibody against the extracellular domain of VE-cadherin (thereby preventing endothelial cells from forming adherens junctions), mesonephric endothelial cell migration into the testis is reduced by 63 % (Combes et al. 2009). The coelomic vessel, which like other large vessels utilizes tight junctions in addition to adherens junctions, still forms despite inhibited VE-cadherin binding. However, the branches from the coelomic vessel that separate the avascular domains do not form, and this lack of segmentation prevents testis cord development entirely (Combes et al. 2009). And, as discussed in Sect. 4.4.2, inhibition of the VEGFA signaling that is important for endothelial migration into the gonad and its vascularization also results in inhibition of testis cord formation. Thus, the testis vasculature plays a key developmental role in establishing testis architecture in addition to its role in the circulatory system. Importantly, once endothelial cells reach the gonad, they are still active in controlling testis morphogenesis. This occurs because endothelial cells secrete signaling molecules that allow cross talk with other cells such as the mesenchymal cells of the interstitium. Using fluorescent cell type markers and inhibition of signaling pathways, Cool et al. (2011) showed that VEGFA secreted from the testis interstitium recruits endothelial cells to migrate into the testis, but then PDGF from those endothelial cells also acts on the mesenchymal cells to promote their proliferation. This feed-forward signaling drives testis cord morphogenesis, and disruption of any part of the loop inhibits cord formation. This signaling loop does not represent a self-contained system by which morphogenesis occurs, as other cells from outside the gonad continue to migrate in and contribute to testis morphogenesis. Notably, fetal macrophages have migrated to the vascular plexus developing into the coelomic vessel as early as 10.5 dpc and appear to participate in vascularization of the testis and cord formation by promoting tissue clearance and remodeling (DeFalco et al. 2014). The recruitment of those macrophages is dependent on endothelial cell presence (DeFalco et al. 2014). Thus, the mesonephric-derived endothelial cell population plays many crucial roles in (1) partitioning the testis into domains for cords, (2) providing the vasculature of the testis, and (3) signaling to cells already present and recruiting cells from outside the gonad to coordinately regulate testis morphogenesis.
4.7 Conclusion
Testis architecture and vascularization is a complex morphogenetic event that cannot occur without a key migratory influx of cells from the mesonephros that begins at 11.5 dpc in the mouse. The majority of these cells are endothelial and come from the breakdown of the mesonephric vascular plexus. These cells then migrate into the XY gonad along paths that partition the early testis into avascular domains. This migration and compartmentalization are required for testis cord formation, which is necessary for the subsequent development of the seminiferous tubules. Male-specific gene expression in the gonad is a prerequisite for mesonephric migration, and many secreted signaling molecules are part of the signaling network that controls the migration including VEGFA, PDGF, neurotrophins, FGF9, AMH, DHH, and SHH. Based on the current body of evidence, the main directional cue that guides endothelial cell migration is a VEGFA morphogen gradient originating from the XY gonad. The cellular behaviors that occur during this specific migration event seem to parallel other well-studied cellular migrations during development in which the adhesions and structure of the endothelial cell are lost, a mesenchymal phenotype is adopted, and migration occurs in the direction of the target tissue via polarized cytoskeletal dynamics. Once the endothelial cell arrives, it interacts with the existing cells in the gonad to promote interstitial cell proliferation and restructure the early testis. The vasculature established during this time also serves as the foundation for the future vasculature of the testis. Thus, mesonephric cell migration into the early gonad is central to testis development and function.
References
Achen M, Jeltsch M, Kukk E, Mäkinen T, Vitali A, Wilks A, Alitalo K, Stacker S (1998) Vascular endothelial growth factor D (VEGF-D) is a ligand for the tyrosine kinases VEGF receptor 2 (Flk1) and VEGF receptor 3 (Flt4). Proc Natl Acad Sci U S A 95:548–553. doi:10.1073/pnas.95.2.548
Bagheri-Fam S, Sim H, Bernard P, Jayakody I, Taketo MM, Scherer G, Harley VR (2008) Loss of Fgfr2 leads to partial XY sex reversal. Dev Biol 314:71–83. doi:10.1016/j.ydbio.2007.11.010
Ball SG, Shuttleworth CA, Kielty CM (2007) Vascular endothelial growth factor can signal through platelet-derived growth factor receptors. J Cell Biol 177:489–500. doi:10.1083/jcb.200608093
Baltes-Breitwisch M, Artac RA, Bott RC, McFee RM, Kerl JG, Clopton DT, Cupp AS (2010) Neutralization of vascular endothelial growth factor antiangiogenic isoforms or administration of proangiogenic isoforms stimulates vascular development in the rat testis. Reproduction 140:319–329. doi:10.1530/REP-09-0456.Neutralization
Barsoum IB, Bingham NC, Parker KL, Jorgensen JS, Yao H (2009) Activation of the Hedgehog pathway in the mouse fetal ovary leads to ectopic appearance of fetal Leydig cells and female pseudohermaphroditism. Dev Biol 329:96–103. doi:10.1016/j.ydbio.2009.02.025
Bates DO, Cui T-G, Doughty JM, Winkler M, Sugiono M, Shields JD, Peat D, Gillatt D, Harper SJ (2002) VEGF165b, an inhibitory splice variant of vascular endothelial growth factor, is down-regulated in renal cell carcinoma. Cancer Res 62:4123–4131
Bitgood M, McMahon A (1995) Hedgehog and Bmp genes are coexpressed at many diverse sites of cell-cell interaction in the mouse embryo. Dev Biol 172:126–138. doi:10.1006/dbio.1995.0010
Bitgood M, Shen L, McMahon A (1996) Sertoli cell signaling by Desert hedgehog regulates the male germline. Curr Biol 6:298–304. doi:10.1016/S0960-9822(02)00480-3
Bott RC, McFee RM, Clopton DT, Toombs C, Cupp AS (2006) Vascular endothelial growth factor and kinase domain region receptor are involved in both seminiferous cord formation and vascular development during testis morphogenesis in the rat. Biol Reprod 75:56–67. doi:10.1095/biolreprod.105.047225
Bott R, Clopton D, Fuller A, McFee R, Lu N, Cupp A (2010) KDR-LacZ-expressing cells are involved in ovarian and testis-specific vascular development, suggesting a role for VEGFA in the regulation of this vasculature. Cell Tissue Res 342:117–130. doi:10.1007/s00441-010-1038-9
Bradford ST, Wilhelm D, Bandiera R, Vidal V, Schedl A, Koopman P (2009) A cell-autonomous role for WT1 in regulating Sry in vivo. Hum Mol Genet 18:3429–3438. doi:10.1093/hmg/ddp283
Brennan J, Capel B (2004) One tissue, two fates: molecular genetic events that underlie testis versus ovary development. Nat Rev Genet 5:509–521. doi:10.1038/nrg1381
Brennan J, Karl J, Capel B (2002) Divergent vascular mechanisms downstream of Sry establish the arterial system in the XY gonad. Dev Biol 244:418–428. doi:10.1006/dbio.2002.0578
Brennan J, Tilmann C, Capel B (2003) Pdgfr-α mediates testis cord organization and fetal Leydig cell development in the XY gonad. Genes Dev 17:800–810. doi:10.1101/gad.1052503
Buehr M, Gu S, McLaren A (1993) Mesonephric contribution to testis differentiation in the fetal mouse. Development 117:273–281
Bullejos M, Koopman P (2001) Spatially dynamic expression of Sry in mouse genital ridges. Dev Dyn 221:201–205. doi:10.1002/dvdy.1134
Burgoyne PS, Thornhill AR, Boudrean SK, Darling SM, Bishop CE, Evans EP, Capel B, Mittwoch U (1995) The genetic basis of XX-XY differences present before gonadal sex differentiation in the mouse [and discussion]. Philos Trans R Soc B Biol Sci 350:253–261. doi:10.1098/rstb.1995.0159
Caires KC, De Avila JM, Cupp AS, McLean DJ (2012) VEGFA family isoforms regulate spermatogonial stem cell homeostasis in vivo. Endocrinology 153:887–890. doi:10.1210/en.2011-1323
Campagnolo L, Russo MA, Puglianiello A, Favale A, Siracusa G (2001) Mesenchymal cell precursors of peritubular smooth muscle cells of the mouse testis can be identified by the presence of the p75 neurotrophin receptor. Biol Reprod 64:464–472
Capel B, Albrecht KH, Washburn LL, Eicher EM (1999) Migration of mesonephric cells into the mammalian gonad depends on Sry. Mech Dev 84:127–131. doi:10.1016/S0925-4773(99)00047-7
Carmeliet P, Ferreira V, Breier G, Pollefeyt S, Kieckens L, Gertsenstein M, Fahrig M, Vandenhoeck A, Harpal K, Eberhardt C, Declercq C, Pawling J, Moons L, Collen D, Risau W, Nagy A (1996) Abnormal blood vessel development and lethality in embryos lacking a single VEGF allele. Nature 380:435–439
Carmon KS, Gong X, Yi J, Thomas A, Liu Q (2014) RSPO-LGR4 functions via IQGAP1 to potentiate Wnt signaling. Proc Natl Acad Sci U S A 111:E1221–E1229. doi:10.1073/pnas.1323106111
Caruso M, Ferranti F, Corano Scheri K, Dobrowolny G, Ciccarone F, Grammatico P, Catizone A, Ricci G (2015) R-spondin 1/dickkopf-1/beta-catenin machinery is involved in testicular embryonic angiogenesis. PLoS One 10:e0124213. doi:10.1371/journal.pone.0124213
Chassot AA, Ranc F, Gregoire EP, Roepers-Gajadien HL, Taketo MM, Camerino G, de Rooij DG, Schedl A, Chaboissier MC (2008) Activation of β-catenin signaling by Rspo1 controls differentiation of the mammalian ovary. Hum Mol Genet 17:1264–1277. doi:10.1093/hmg/ddn016
Chen H, Chédotal A, He Z, Goodman CS, Tessier-Lavigne M (1997) Neuropilin-2, a novel member of the neuropilin family, is a high affinity receptor for the semaphorins Sema E and Sema IV but not Sema III. Neuron 19:547–559. doi:10.1016/S0896-6273(00)80371-2
Chen CH, Lai JM, Chou TY, Chen CY, Su LJ, Lee YC, Cheng TS, Hong YR, Chou CK, Whang-Peng J, Wu YC, Huang CYF (2009) VEGFA upregulates FLJ10540 and modulates migration and invasion of lung cancer via PI3K/AKT pathway. PLoS One. doi:10.1371/journal.pone.0005052
Chen H, Palmer JS, Thiagarajan RD, Dinger ME, Lesieur E, Chiu H, Schulz A, Spiller C, Grimmond SM, Little MH, Koopman P, Wilhelm D (2012) Identification of novel markers of mouse fetal ovary development. PLoS One. doi:10.1371/journal.pone.0041683
Clark AM, Garland KK, Russell LD (2000) Desert hedgehog (Dhh) gene is required in the mouse testis for formation of adult-type Leydig cells and normal development of peritubular cells and seminiferous tubules. Biol Reprod 63:1825–1838. doi:10.1095/biolreprod63.6.1825
Combes AN, Wilhelm D, Davidson T, Dejana E, Harley V, Sinclair A, Koopman P (2009) Endothelial cell migration directs testis cord formation. Dev Biol 326:112–120. doi:10.1016/j.ydbio.2008.10.040
Combes AN, Spiller CM, Harley VR, Sinclair AH, Dunwoodie SL, Wilhelm D, Koopman P (2010) Gonadal defects in Cited2-mutant mice indicate a role for SF1 in both testis and ovary differentiation. Int J Dev Biol 54:683–689. doi:10.1387/ijdb.092920ac
Cool J, Carmona FD, Szucsik JC, Capel B (2008) Peritubular myoid cells are not the migrating population required for testis cord formation in the XY gonad. Sex Dev 2:128–133. doi:10.1159/000143430
Cool J, DeFalco TJ, Capel B (2011) Vascular-mesenchymal cross-talk through Vegf and Pdgf drives organ patterning. Proc Natl Acad Sci U S A 108:167–172. doi:10.1073/pnas.1010299108
Coveney D, Cool J, Oliver T, Capel B (2008) Four-dimensional analysis of vascularization during primary development of an organ, the gonad. Proc Natl Acad Sci U S A 105:7212–7217. doi:10.1073/pnas.0707674105
Cupp AS, Kim GH, Skinner MK (2000) Expression and action of neurotropin-3 and nerve growth factor in embryonic and early postnatal rat testis development. Biol Reprod 63:1617–1628. doi:10.1095/biolreprod63.6.1617
Cupp AS, Tessarollo L, Skinner MK (2002) Testis developmental phenotypes in neurotropin receptor trkA and trkC null mutations: role in formation of seminiferous cords and germ cell survival. Biol Reprod 66:1838–1845. doi:10.1095/biolreprod66.6.1838
Cupp AS, Uzumcu M, Skinner MK (2003) Chemotactic role of neurotropin 3 in the embryonic testis that facilitates male sex determination. Biol Reprod 68:2033–2037. doi:10.1095/biolreprod.102.012617
DeFalco T, Takahashi S, Capel B (2011) Two distinct origins for Leydig cell progenitors in the fetal testis. Dev Biol 352:14–26. doi:10.1016/j.ydbio.2011.01.011
DeFalco T, Bhattacharya I, Williams AV, Sams DM, Capel B (2014) Yolk-sac-derived macrophages regulate fetal testis vascularization and morphogenesis. Proc Natl Acad Sci U S A. doi:10.1073/pnas.1400057111
Dehghanian F, Hojati Z, Kay M (2014) New insights into VEGF-A alternative splicing: key regulatory switching in the pathological process. Avicenna J Med Biotechnol 6:192–199
de Vries C, Escobedo J, Ueno H, Houck K, Ferrara N, Williams L (1992) The fms-like tyrosine kinase, a receptor for vascular endothelial growth factor. Science 255:989–991. doi:10.1126/science.1312256
Domigan CK, Ziyad S, Iruela-Arispe ML (2015) Canonical and noncanonical vascular endothelial growth factor pathways: new developments in biology and signal transduction. Arterioscler Thromb Vasc Biol 35:30–39. doi:10.1161/ATVBAHA.114.303215
Ewen KA, Koopman P (2010) Mouse germ cell development: from specification to sex determination. Mol Cell Endocrinol 323:76–93. doi:10.1016/j.mce.2009.12.013
Fantin A, Vieira JM, Plein A, Denti L, Fruttiger M, Pollard JW, Ruhrberg C (2013) NRP1 acts cell autonomously in endothelium to promote tip cell function during sprouting angiogenesis. Blood 121:2352–2362. doi:10.1182/blood-2012-05-424713
Ferrara N, Henzel WJ (1989) Pituitary follicular cells secrete a novel heparin-binding growth factor specific for vascular endothelial cells. Biochem Biophys Res Commun 161:851–858. doi:10.1016/j.bbrc.2012.08.021
Ferrara N, Carver-Moore K, Chen H, Dowd M, Lu L, O’Shea KS, Powell-Braxton L, Hillan KJ, Moore MW (1996) Heterozygous embryonic lethality induced by targeted inactivation of the VEGF gene. Nature 380:439–442
Ferrara N, Gerber H-P, LeCouter J (2003) The biology of VEGF and its receptors. Nat Med 9:669–676. doi:10.1038/nm0603-669
Fidder H (1996) Characterization of novel vascular endothelial growth factor (VEGF) receptors on tumor cells that bind VEGF[IMAGE] via its exon 7-encoded domain. J Biol Chem 271:5761–5767. doi:10.1074/jbc.271.10.5761
Fiedler J, Leucht F, Waltenberger J, Dehio C, Brenner RE (2005) VEGF-A and PlGF-1 stimulate chemotactic migration of human mesenchymal progenitor cells. Biochem Biophys Res Commun 334:561–568. doi:10.1016/j.bbrc.2005.06.116
Fong GH, Zhang L, Bryce DM, Peng J (1999) Increased hemangioblast commitment, not vascular disorganization, is the primary defect in flt-1 knock-out mice. Development 126:3015–3025
Franco HL, Yao HHC (2012) Sex and hedgehog: roles of genes in the hedgehog signaling pathway in mammalian sexual differentiation. Chromosome Res 20:247–258. doi:10.1007/s10577-011-9254-z
Fukasawa M, Matsushita A, Korc M (2007) Neuropilin-1 interacts with integrin beta1 and modulates pancreatic cancer cell growth, survival and invasion. Cancer Biol Ther 6:1173–1180. doi:10.4161/cbt.6.8.4363
Gerhardt H, Golding M, Fruttiger M, Ruhrberg C, Lundkvist A, Abramsson A, Jeltsch M, Mitchell C, Alitalo K, Shima D, Betsholtz C (2003) VEGF guides angiogenic sprouting utilizing endothelial tip cell filopodia. J Cell Biol 161:1163–1177. doi:10.1083/jcb.200302047
Gerhardt H, Ruhrberg C, Abramsson A, Fujisawa H, Shima D, Betsholtz C (2004) Neuropilin-1 is required for endothelial tip cell guidance in the developing central nervous system. Dev Dyn 231:503–509. doi:10.1002/dvdy.20148
Gille H, Kowalski J, Yu L, Chen H, Pisabarro MT, Davis-Smyth T, Ferrara N (2000) A repressor sequence in the juxtamembrane domain of Flt-1 (VEGFR-1) constitutively inhibits vascular endothelial growth factor-dependent phosphatidylinositol 3′-kinase activation and endothelial cell migration. EMBO J 19:4064–4073. doi:10.1093/emboj/19.15.4064
Gitay-Goren H, Cohen T, Tessler S, Soker S, Gengrinovitch S, Rockwell P, Klagsbrun M, Levi BZ, Neufeld G (1996) Selective binding of VEGF121 to one of the three vascular endothelial growth factor receptors of vascular endothelial cells. J Biol Chem 271:5519–5523
Gomperts M, Garcia-Castro M, Wylie C, Heasman J (1994) Interactions between primordial germ cells play a role in their migration in mouse embryos. Development 120:135–141
Hacker A, Capel B, Goodfellow P, Lovell-Badge R (1995) Expression of Sry, the mouse sex determining gene. Development 121:1603–1614
Harikae K, Miura K, Kanai Y (2013) Early gonadogenesis in mammals: significance of long and narrow gonadal structure. Dev Dyn 242:330–338. doi:10.1002/dvdy.23872
Heldin C-H (2013) Targeting the PDGF signaling pathway in tumor treatment. Cell Commun Signal 11:97. doi:10.1186/1478-811X-11-97
Hiratsuka S, Minowa O, Kuno J, Noda T, Shibuya M (1998) Flt-1 lacking the tyrosine kinase domain is sufficient for normal development and angiogenesis in mice. Proc Natl Acad Sci U S A 95:9349–9354. doi:10.1073/pnas.95.16.9349
Hoch RV, Soriano P (2003) Roles of PDGF in animal development. Development 130:4769–4784. doi:10.1242/dev.00721
Hu YC, Okumura LM, Page DC (2013) Gata4 is required for formation of the genital ridge in mice. PLoS Genet 9:1–12. doi:10.1371/journal.pgen.1003629
Hummitzsch K, Irving-Rodgers HF, Hatzirodos N, Bonner W, Sabatier L, Reinhardt DP, Sado Y, Ninomiya Y, Wilhelm D, Rodgers RJ (2013) A new model of development of the mammalian ovary and follicles. PLoS One. doi:10.1371/journal.pone.0055578
Jameson SA, Lin YT, Capel B (2012) Testis development requires the repression of Wnt4 by Fgf signaling. Dev Biol 370:24–32. doi:10.1016/j.ydbio.2012.06.009
Jeanes A, Wilhelm D, Wilson MJ, Bowles J, McClive PJ, Sinclair AH, Koopman P (2005) Evaluation of candidate markers for the peritubular myoid cell lineage in the developing mouse testis. Reproduction 130:509–516. doi:10.1530/rep.1.00718
Jeays-Ward K, Hoyle C, Brennan J, Dandonneau M, Alldus G, Capel B, Swain A (2003) Endothelial and steroidogenic cell migration are regulated by WNT4 in the developing mammalian gonad. Development 130:3663–3670. doi:10.1242/dev.00591
Jeltsch M, Kaipainen A, Joukov V, Meng X, Lakso M, Rauvala H, Swartz M, Fukumura D, Jain RK, Alitalo K (1997) Hyperplasia of lymphatic vessels in VEGF-C transgenic mice. Science 276:1423–1425. doi:10.1126/science.276.5317.1423
Joukov V, Pajusola K, Kaipainen A, Chilov D, Lahtinen I, Kukk E, Saksela O, Kalkkinen N, Alitalo K (1996) A novel vascular endothelial growth factor, VEGF-C, is a ligand for the Flt4 (VEGFR-3) and KDR (VEGFR-2) receptor tyrosine kinases. EMBO J 15:290–298
Kanno S, Oda N, Abe M, Terai Y, Ito M, Shitara K, Tabayashi K, Shibuya M, Sato Y (2000) Roles of two VEGF receptors, Flt-1 and KDR, in the signal transduction of VEGF effects in human vascular endothelial cells. Oncogene 19:2138–2146. doi:10.1038/sj.onc.1203533
Karkkainen MJ, Mäkinen T, Alitalo K (2002) Lymphatic endothelium: a new frontier of metastasis research. Nat Cell Biol 4:E2–E5. doi:10.1038/ncb0102-e2
Karl J, Capel B (1995) Three-dimensional structure of the developing mouse genital ridge. Philos Trans R Soc Lond B Biol Sci 350:235–242. doi:10.1098/rstb.1995.0157
Karl J, Capel B (1998) Sertoli cells of the mouse testis originate from the coelomic epithelium. Dev Biol 203:323–333. doi:10.1006/dbio.1998.9068
Kawamura H, Li X, Harper SJ, Bates DO, Claesson-Welsh L (2008) Vascular endothelial growth factor (VEGF)-A165b is a weak in vitro agonist for VEGF receptor-2 due to lack of coreceptor binding and deficient regulation of kinase activity. Cancer Res 68:4683–4692. doi:10.1158/0008-5472.CAN-07-6577
Kawasaki T, Kitsukawa T, Bekku Y, Matsuda Y, Sanbo M, Yagi T, Fujisawa H (1999) A requirement for neuropilin-1 in embryonic vessel formation. Development 126:4895–4902
Kent J, Wheatley SC, Andrews JE, Sinclair AH, Koopman P (1996) A male-specific role for SOX9 in vertebrate sex determination. Development 122:2813–2822
Kim Y, Kobayashi A, Sekido R, DiNapoli L, Brennan J, Chaboissier MC, Poulat F, Behringer RR, Lovell-Badge R, Capel B (2006) Fgf9 and Wnt4 act as antagonistic signals to regulate mammalian sex determination. PLoS Biol 4:1000–1009. doi:10.1007/11558958_121
Kim Y, Bingham N, Sekido R, Parker KL, Lovell-Badge R, Capel B (2007) Fibroblast growth factor receptor 2 regulates proliferation and Sertoli differentiation during male sex determination. Proc Natl Acad Sci U S A 104:16558–16563. doi:10.1073/pnas.0702581104
Kobayashi M, Nishita M, Mishima T, Ohashi K, Mizuno K (2006) MAPKAPK-2-mediated LIM-kinase activation is critical for VEGF-induced actin remodeling and cell migration. EMBO J 25:713–726. doi:10.1038/sj.emboj.7600973
Kofler NM, Simons M (2015) Angiogenesis versus arteriogenesis: neuropilin 1 modulation of VEGF signaling. F1000Prime Rep 7:26. doi:10.12703/P7-26
Koopman P, Gubbay J, Vivian N, Goodfellow P, Lovell-Badge R (1991) Male development of chromosomally female mice transgenic for Sry. Nature 351:117–121. doi:10.1038/351117a0
Kroll J, Waltenberger J (1997) The vascular endothelial growth factor receptor KDR activates multiple signal transduction pathways in porcine aortic endothelial cells. J Biol Chem 272:32521–32527
Kusaka M, Katoh-Fukui Y, Ogawa H, Miyabayashi K, Baba T, Shima Y, Sugiyama N, Sugimoto Y, Okuno Y, Kodama R, Iizuka-Kogo A, Senda T, Sasaoka T, Kitamura K, Aizawa S, Morohashi KI (2010) Abnormal epithelial cell polarity and ectopic Epidermal Growth Factor Receptor (EGFR) expression induced in Emx2 KO Embryonic Gonads. Endocrinology 151:5893–5904. doi:10.1210/en.2010-0915
Lavery R, Chassot A-A, Pauper E, Gregoire EP, Klopfenstein M, de Rooij DG, Mark M, Schedl A, Ghyselinck NB, Chaboissier M-C (2012) Testicular differentiation occurs in absence of R-spondin1 and Sox9 in mouse sex reversals. PLoS Genet 8:e1003170. doi:10.1371/journal.pgen.1003170
Levine E, Cupp AS, Skinner MK (2000) Role of neurotropins in rat embryonic testis morphogenesis (cord formation). Biol Reprod 62:132–142. doi:10.1095/biolreprod62.1.132
Li ZD, Bork JP, Krueger B, Patsenker E, Schulze-Krebs A, Hahn EG, Schuppan D (2005) VEGF induces proliferation, migration, and TGF-beta1 expression in mouse glomerular endothelial cells via mitogen-activated protein kinase and phosphatidylinositol 3-kinase. Biochem Biophys Res Commun 334:1049–1060. doi:10.1016/j.bbrc.2005.07.005
Liu C, Peng J, Matzuk MM, Yao HH-C (2015) Lineage specification of ovarian theca cells requires multicellular interactions via oocyte and granulosa cells. Nat Commun 6:6934. doi:10.1038/ncomms7934
Lovell-Badge R, Robertson E (1990) XY female mice resulting from a heritable mutation in the primary testis-determining gene, Tdy. Development 109:635–646
Lu N, Sargent KM, Clopton DT, Pohlmeier WE, Brauer VM, McFee RM, Weber JS, Ferrara N, Silversides DW, Cupp AS (2013) Loss of vascular endothelial growth factor A (VEGFA) isoforms in the testes of male mice causes subfertility, reduces sperm numbers, and alters expression of genes that regulate undifferentiated spermatogonia. Endocrinology 154:4790–4802. doi:10.1210/en.2013-1363
Luo X, Ikeda Y, Parker KL (1994) A cell-specific nuclear receptor is essential for adrenal and gonadal development and sexual differentiation. Cell 77:481–490. doi:10.1016/0092-8674(94)90211-9
Martineau J, Nordqvist K, Tilmann C, Lovell-Badge R, Capel B (1997) Male-specific cell migration into the developing gonad. Curr Biol 7:958–968. doi:10.1016/S0960-9822(06)00415-5
Mayr-Wohlfart U, Waltenberger J, Hausser H, Kessler S, Günther KP, Dehio C, Puhl W, Brenner RE (2002) Vascular endothelial growth factor stimulates chemotactic migration of primary human osteoblasts. Bone 30:472–477. doi:10.1016/S8756-3282(01)00690-1
McFee RM, Artac RA, McFee RM, Clopton DT, Smith RAL, Rozell TG, Cupp AS (2009) Inhibition of vascular endothelial growth factor receptor signal transduction blocks follicle progression but does not necessarily disrupt vascular development in perinatal rat ovaries. Biol Reprod 81:966–977. doi:10.1095/biolreprod.109.078071
McLaren A (2000) Germ and somatic cell lineages in the developing gonad. Mol Cell Endocrinol 163:3–9. doi:10.1016/S0303-7207(99)00234-8
Merchant-Larios H, Moreno-Mendoza N, Buehr M (1993) The role of the mesonephros in cell differentiation and morphogenesis of the mouse fetal testis. Int J Dev Biol 37:407–415
Meyer RD, Mohammadi M, Rahimi N (2006) A single amino acid substitution in the activation loop defines the decoy characteristic of VEGFR-1/FLT-1. J Biol Chem 281:867–875. doi:10.1074/jbc.M506454200
Molyneaux KA, Stallock J, Schaible K, Wylie C (2001) Time-lapse analysis of living mouse germ cell migration. Dev Biol 240:488–498. doi:10.1006/dbio.2001.0436
Mork L, Maatouk DM, McMahon JA, Guo JJ, Zhang P, McMahon AP, Capel B (2012) Temporal differences in granulosa cell specification in the ovary reflect distinct follicle fates in mice. Biol Reprod 86:37. doi:10.1095/biolreprod.111.095208
Münsterberg A, Lovell-Badge R (1991) Expression of the mouse anti-Müllerian hormone gene suggests a role in both male and female sexual differentiation. Development 113:613–624
Murga M, Fernandez-capetillo O, Tosato G (2005) Neuropilin-1 regulates attachment in human endothelial cells independently of vascular endothelial growth factor receptor-2. Cell 105:1992–1999. doi:10.1182/blood-2004-07-2598.Reprints
Murphy G, Gavrilovic J (1999) Proteolysis and cell migration: creating a path? Curr Opin Cell Biol 11:614–621. doi:10.1016/S0955-0674(99)00022-8
Nef S, Parada L (2000) Hormones in male sexual development. Genes Dev 14:3075–3086. doi:10.1101/gad.843800.genital
Nel-Themaat L, Vadakkan TJ, Wang Y, Dickinson ME, Akiyama H, Behringer RR (2009) Morphometric analysis of testis cord formation in sox9-egfp Mice. Dev Dyn 238:1100–1110. doi:10.1002/dvdy.21954
Nishino K, Yamanouchi K, Naito K, Tojo H (2001) Characterization of mesonephric cells that migrate into the XY gonad during testis differentiation. Exp Cell Res 267:225–232. doi:10.1006/excr.2001.5238
Olofsson B, Korpelainen E, Pepper MS, Mandriota SJ, Aase K, Kumar V, Gunji Y, Jeltsch MM, Shibuya M, Alitalo K, Eriksson U (1998) Vascular endothelial growth factor B (VEGF-B) binds to VEGF receptor-1 and regulates plasminogen activator activity in endothelial cells. Proc Natl Acad Sci U S A 95:11709–11714. doi:10.1073/pnas.95.20.11709
Olsson A-K, Dimberg A, Kreuger J, Claesson-Welsh L (2006) VEGF receptor signalling—in control of vascular function. Nat Rev Mol Cell Biol 7:359–371. doi:10.1038/nrm1911
Park JE, Keller GA, Ferrara N (1993) The vascular endothelial growth factor (VEGF) isoforms: differential deposition into the subepithelial extracellular matrix and bioactivity of extracellular matrix-bound VEGF. Mol Biol Cell 4:1317–1326. doi:10.1091/mbc.4.12.1317
Park JE, Chen HH, Winer J, Houck KA, Ferrara N (1994) Placenta growth factor: potentiation of vascular endothelial growth factor bioactivity, in vitro and in vivo, and high affinity binding to Flt-1 but not to Flk-1/KDR. J Biol Chem 269:25646–25654. doi:10.1371/journal.pone.0018076
Pierucci-Alves F, Clark AM, Russell LD (2001) A developmental study of the Desert hedgehog-null mouse testis. Biol Reprod 65:1392–1402. doi:10.1095/biolreprod65.5.1392
Puglianiello A, Campagnolo L, Farini D, Cipollone D, Russo MA, Siracusa G (2004) Expression and role of PDGF-BB and PDGFR-beta during testis morphogenesis in the mouse embryo. J Cell Sci 117:1151–1160. doi:10.1242/jcs.00981
Raimondi C, Fantin A, Lampropoulou A, Denti L, Chikh A, Ruhrberg C (2014) Imatinib inhibits VEGF-independent angiogenesis by targeting neuropilin 1-dependent ABL1 activation in endothelial cells. J Exp Med 211:1167–1183. doi:10.1084/jem.20132330
Ricci G, Catizone A, Galdieri M (2004) Embryonic mouse testis development: role of platelet derived growth factor (PDGF-BB). J Cell Physiol 200:458–467. doi:10.1002/jcp.20035
Ridley AJ, Schwartz MA, Burridge K, Firtel RA, Ginsberg MH, Borisy G, Parsons JT, Horwitz AR (2003) Cell migration: integrating signals from front to back. Science 302:1704–1709. doi:10.1126/science.1092053
Ross AJ, Tilman C, Yao H, MacLaughlin D, Capel B (2003) AMH induces mesonephric cell migration in XX gonads. Mol Cell Endocrinol 211:1–7. doi:10.1016/j.mce.2003.09.021
Russo MA, Giustizieri ML, Favale A, Fantini MC, Campagnolo L, Konda D, Germano F, Farini D, Manna C, Siracusa G (1999) Spatiotemporal patterns of expression of neurotrophins and neurotrophin receptors in mice suggest functional roles in testicular and epididymal morphogenesis. Biol Reprod 61:1123–1132. doi:10.1095/biolreprod61.4.1123
Schmahl J, Capel B (2003) Cell proliferation is necessary for the determination of male fate in the gonad. Dev Biol 258:264–276. doi:10.1016/S0012-1606(03)00122-2
Schmahl J, Eicher EM, Washburn LL, Capel B (2000) Sry induces cell proliferation in the mouse gonad. Development 127:65–73
Schmahl J, Kim Y, Colvin JS, Ornitz DM, Capel B (2004) Fgf9 induces proliferation and nuclear localization of FGFR2 in Sertoli precursors during male sex determination. Development 131:3627–3636. doi:10.1242/dev.01239
Sekido R, Lovell-Badge R (2008) Sex determination involves synergistic action of SRY and SF1 on a specific Sox9 enhancer. Nature 453:930–934. doi:10.1038/nature07622
Shibuya M, Yamaguchi S, Yamane A, Ikeda T, Tojo A, Matsushime H, Sato M (1990) Nucleotide sequence and expression of a novel human receptor-type tyrosine kinase gene (flt) closely related to the fms family. Oncogene 5:519–524
Sinclair AH, Berta P, Palmer MS, Hawkins JR, Griffiths BL, Smith MJ, Foster JW, Frischauf AM, Lovell-Badge R, Goodfellow PN (1990) A gene from the human sex-determining region encodes a protein with homology to a conserved DNA-binding motif. Nature 346:240–244. doi:10.1038/346240a0
Smith CA, McClive PJ, Hudson Q, Sinclair AH (2005) Male-specific cell migration into the developing gonad is a conserved process involving PDGF signalling. Dev Biol 284:337–350. doi:10.1016/j.ydbio.2005.05.030
Smith P, Wilhelm D, Rodgers RJ (2014) Development of mammalian ovary. J Endocrinol. doi:10.1530/JOE-14-0062
Soker S, Takashima S, Miao HQ, Neufeld G, Klagsbrun M (1998) Neuropilin-1 is expressed by endothelial and tumor cells as an isoform-specific receptor for vascular endothelial growth factor. Cell 92:735–745. doi:10.1016/S0092-8674(00)81402-6
Soker S, Miao HQ, Nomi M, Takashima S, Klagsbrun M (2002) VEGF165 mediates formation of complexes containing VEGFR-2 and neuropilin-1 that enhance VEGF165-receptor binding. J Cell Biochem 85:357–368. doi:10.1002/jcb.10140
Tanaka SS, Nishinakamura R (2014) Regulation of male sex determination: genital ridge formation and Sry activation in mice. Cell Mol Life Sci 71:4781–4802. doi:10.1007/s00018-014-1703-3
Tang H, Brennan J, Karl J, Hamada Y, Raetzman L, Capel B (2008) Notch signaling maintains Leydig progenitor cells in the mouse testis. Development 135:3745–3753. doi:10.1242/dev.024786
Terman BI, Dougher-Vermazen M, Carrion ME, Dimitrov D, Armellino DC, Gospodarowicz D, Böhlen P (1992) Identification of the KDR tyrosine kinase as a receptor for vascular endothelial cell growth factor. Biochem Biophys Res Commun 187:1579–1586. doi:10.1016/0006-291X(92)90483-2
Thiery JP, Acloque H, Huang RYJ, Nieto MA (2009) Epithelial-Mesenchymal Transitions in Development and Disease. Cell 139:871–890. doi:10.1016/j.cell.2009.11.007
Tilmann C, Capel B (1999) Mesonephric cell migration induces testis cord formation and Sertoli cell differentiation in the mammalian gonad. Development 126:2883–2890
Ungewitte EK, Yao H (2013) How to make a gonad: cellular mechanisms governing formation of the testes and ovaries. Sex Dev 7:7–20. doi:10.1159/000338612
Upadhyay S, Luciani JM, Zamboni L (1979) The role of the mesonephros in the development of indifferent gonads and ovaries of the mouse. Ann Biol Anim Biochim Biophys 19:1179–1196. doi:10.1051/rnd:19790802
Upadhyay S, Luciani JM, Zamboni L (1981) The role of the mesonephros in the development of the mouse testis and its excurrent pathways. In: Byskov AG, Peters H (eds) Development and function of reproductive organs. Excerpta Medica, Amsterdam, pp 18–27
Uzumcu M, Dirks KA, Skinner MK (2002) Inhibition of platelet-derived growth factor actions in the embryonic testis influences normal cord development and morphology. Biol Reprod 66:745–753. doi:10.1095/biolreprod66.3.745
Vainio S, Heikkilä M, Kispert A, Chin N, McMahon AP (1999) Female development in mammals is regulated by Wnt-4 signalling. Nature 397:405–409. doi:10.1038/17068
Valdembri D, Caswell PT, Anderson KI, Schwarz JP, König I, Astanina E, Caccavari F, Norman JC, Humphries MJ, Bussolino F, Serini G (2009) Neuropilin-1/GIPC1 signaling regulates α5β1 integrin traffic and function in endothelial cells. PLoS Biol. doi:10.1371/journal.pbio.1000025
Vidal VPI, Chaboissier M-C, de Rooij DG, Schedl A (2001) Sox9 induces testis development in XX transgenic mice. Nat Genet 28:216–217. doi:10.1038/90046
Vieira JM, Ruhrberg C, Schwarz Q (2010) VEGF receptor signaling in vertebrate development. Organogenesis 6:97–106. doi:10.4161/org.6.2.11686
Vigier B, Watrin F, Magre S, Tran D, Josso N (1987) Purified bovine AMH induces a characteristic freemartin effect in fetal rat prospective ovaries exposed to it in vitro. Development 100:43–55
Wagner T, Wirth J, Meyer J, Zabel B, Held M, Zimmer J, Pasantes J, Bricarelli FD, Keutel J, Hustert E, Wolf U, Tommerup N, Schempp W, Scherer G (1994) Autosomal sex reversal and campomelic dysplasia are caused by mutations in and around the SRY-related gene SOX9. Cell 79:1111–1120. doi:10.1016/0092-8674(94)90041-8
Waltenberger J, Claesson-Welsh L, Siegbahn A, Shibuya M, Heldin CH (1994) Different signal transduction properties of KDR and Flt1, two receptors for vascular endothelial growth factor. J Biol Chem 269:26988–26995
Wang L, Mukhopadhyay D, Xu X (2006) C terminus of RGS-GAIP-interacting protein conveys neuropilin-1-mediated signaling during angiogenesis. FASEB J 20:1513–1515. doi:10.1096/fj.05-5504fje
Wartenberg H, Kinsky I, Viebahn C, Schmolke C (1991) Fine structural characteristics of testicular cord formation in the developing rabbit gonad. J Electron Microsc Tech 19:133–157. doi:10.1002/jemt.1060190203
Wilhelm D (2007) R-spondin1—discovery of the long-missing, mammalian female-determining gene? Bioessays 29:314–318. doi:10.1002/bies.20553
Wilhelm D, Palmer S, Koopman P (2007) Sex determination and gonadal development in mammals. Physiol Rev 87:1–28. doi:10.1152/physrev.00009.2006
Yao HH-C, Capel B (2002) Disruption of testis cords by cyclopamine or forskolin reveals independent cellular pathways in testis organogenesis. Dev Biol 246:356–365. doi:10.1006/dbio.2002.0663
Yao HHC, Whoriskey W, Capel B (2002) Desert Hedgehog/Patched 1 signaling specifies fetal Leydig cell fate in testis organogenesis. Genes Dev 16:1433–1440. doi:10.1101/gad.981202
Ziegler BL, Valtieri M, Porada GA, De Maria R, Müller R, Masella B, Gabbianelli M, Casella I, Pelosi E, Bock T, Zanjani ED, Peschle C (1999) KDR receptor: a key marker defining hematopoietic stem cells. Science 285:1553–1558. doi:10.1126/science.285.5433.1553
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2016 Springer International Publishing Switzerland
About this chapter
Cite this chapter
Romereim, S.M., Cupp, A.S. (2016). Mesonephric Cell Migration into the Gonads and Vascularization Are Processes Crucial for Testis Development. In: Piprek, R. (eds) Molecular Mechanisms of Cell Differentiation in Gonad Development. Results and Problems in Cell Differentiation, vol 58. Springer, Cham. https://doi.org/10.1007/978-3-319-31973-5_4
Download citation
DOI: https://doi.org/10.1007/978-3-319-31973-5_4
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-31971-1
Online ISBN: 978-3-319-31973-5
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)