Abstract
The present study has been undertaken to investigate the physicochemical properties of soil samples of agricultural region collected from the Amaravathi river basin. The soil characterization was carried out for the parameters like pH, turbidity, total dissolved solids (TDS), total hardness (TH), calcium (Ca), magnesium (Mg), sodium (Na), potassium (K), bicarbonate \( ({{{\text{HCO}}_{3}}^{ - }} ) \), nitrate \( {{ ( {\text{NO}}_{3}}^{ - }} ) \), sulfate \( \left( {{{{\text{SO}}_{4}}^{2 - } }} \right) \), chloride (Cl−), fluoride (F−), phosphate \( \left( {{{{\text{PO}}_{4}}^{3 - } }} \right) \), iron (Fe), manganese (Mn), biochemical oxygen demand (BOD), and chemical oxygen demand (COD). A variety of values were observed in the different physicochemical parameters due to the soil quality in different sampling locations. Values of pH, chloride, fluoride, sulfate, BOD and COD concentration in the soil samples also showed wide variations. The groundwater samples of Amaravathi river were also analyzed for different water quality parameters. Comparing the analytical data with WHO and BIS standards, elevated concentration was observed in parameters like TDS, TH, Ca2+, Na+, Cl−, \( {{{\text{SO}}_{4}}^{2 - }} \), and COD which exemplify the degradation of water quality. Correlation analysis was carried out for soil samples and groundwater samples to investigate the relationship between the variables. Chlorinity and salinity index were performed for both soil and groundwater samples, and it was observed that most of the groundwater samples have medium to high chlorinity and salinity hazards. The microbiological characteristics of soil samples were carried out, and Bacillus and Pseudomonas species were the predominant bacteria genera isolated.
Access provided by Autonomous University of Puebla. Download conference paper PDF
Similar content being viewed by others
Keywords
12.1 Introduction
Soil is a dynamic, living natural body and a key factor in the sustainability of terrestrial ecosystems, fulfilling many functions including those that are essential for sustaining plant growth. The main components of soil include organic matter, inorganic mineral matter, water, gases, and living organisms such as earthworms, insects, bacteria, fungi, algae, etc. [1]. Worldwide, there is a significant decline in soil productive capacity due to the changes in its physicochemical and biological properties and contamination by organic and inorganic chemicals [2]. According to Rodale Institute [3], the three basic components of a soil productive capacity as well as of quality are (i) the capacity of the soil to enhance crop production; (ii) the capacity of soil to function in the reduction of environmental contaminants, pathogens, and off-site damage; and (iii) the linkage between soil properties/quality and plant, animal, and human health namely, productivity component, environment component, and health component, respectively.
The soil fertility is primarily influenced by soil properties like soil depth, organic matter, bulk density, soil reaction, texture, nutrient availability, infiltration, and retention capacity. The variation in soil profile can be identified by observing community-level physiological profiles of soil bacterial communities [4]. Scrutinization of the physiological activity of soil bacteria may reveal important information about soil quality which may go undetected by physicochemical evaluation, because soil bacterial action reacts differently to impacts than physicochemical parameters do [5].
Water chemistry is influenced by environmental factors such as precipitation, stratum, soil, vegetation, bedrock lithology and shows a sensitive response to regional and global environment change [6]. The study of rivers and its basin groundwater provides valuable information on dissolved load sources, the extent of physical and chemical weathering, and leaching of landfills [7]. Due to the rapid increase in nonpoint anthropogenic pollutant sources arising from urbanization and development of riverine areas, the data must be interpreted carefully to know the reasons. Poorly or untreated human sewage, animal wastes, agricultural runoff, partially treated effluents from the dyeing and bleaching industries and direct mixing of sewage from municipality contribute to the pollution load on the environment. Apart from pollutants, groundwater chemistry may be altered by continuous water supply, dam construction for flood control and hydroelectric generations. However, the poor quality of both surface and groundwater is not only a limiting factor in crop production but its indiscriminate and constant use cause salinity and alkalinity [8]. During the last few decades, numerous studies have been carried out on the geochemistry of rivers in India [9–12]. On the other hand, little attempt has been made on small watersheds. The objective of this study is to determine the quality of soil, frequency of microbes, and suitability of groundwater and also to know the relationship between soil and groundwater in the Amaravathi river basin of Karur District.
12.2 Description of the Study Area
12.2.1 Location
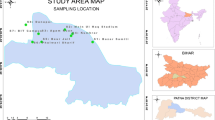
Amaravathi river rises from Naimakad at an elevation of 2300 m above mean sea level in the Western Ghats in Idukki district of Kerala state. It flows toward northeast and finally merges with the river Cauvery on its right bank. Amaravathi river lies between the latitudes 11.20°N and 12.00°N and longitudes 77.28°E and 78.50°E in Karur district. It receives water from a number of small streams through the course of 282 km and covers a total area of 8280 km2 mainly constituting four districts namely Coimbatore, Erode, Dindigul, and Karur in Tamil Nadu. Amaravathi is a tributary of Shanmuganadhi, Nankanchi, and Kodaganar rivers, which join at 60, 40, and 20 km upstream of Karur town, respectively. Amaravathi river enters into Karur district near Aravakurichi and merges with river Cauvery near Kattali village, and the flow in the river is seasonal from late October to early February.
12.2.2 Drainage
The major part of Karur district is drained by Cauvery river. Amaravathi, Kodavanar, and Nanganji are the important rivers draining the western part of the district and the river Pungar drains in the eastern part of the district. The drainage pattern, in general, is dendritic. All the rivers are seasonal and carry substantial flows during the monsoon period.
12.2.3 Geomorphology and Hydrogeology
The entire area of the district is a pediplain. The Rangamalai hills and Kadavur hills occurring in the southern side of the district constitute the remnants of the much denuded Eastern Ghats and rise to heights of over 1031 m above mean sea level. There are numerous small residual hills represented by Ayyarmalai, Thanthonimalai, and Velayuthampalayam. The general elevation of the area ranges between 100 and 200 m above mean sea level. The prominent geomorphic units identified in the district are structural hills, pediments, shallow pediments, buried pediments, and alluvial plain. Karur district is underlain entirely by Archaean crystalline formations with recent alluvial deposits occurring along the river and stream courses. The hard consolidated crystalline rocks of Archaean age represent weathered, fissured, and fractured formations of gneisses, granites, charnockites, and other associated rocks. Groundwater occurs under phreatic conditions, and the maximum saturated thickness of these aquifers is up to 10 m depending upon the topographic conditions. The thickness of the weathered mantle of the hard rocks varies from less than a meter to as much as 20.10 m.
12.2.4 Soil and Vegetation
Red soil is the predominant one covering a major part of the district followed by thin red soil and red loam. The red soil is predominantly seen in Kadavur, Kulithalai, Krishnarayapuram, Thanthoni, and Thogamalai blocks. The thin red soils are seen in Aravakurichi and K. Paramathy blocks. A major portion of the Karur block is covered by red loam. The major crops under cultivation in this area are paddy (16.30 %), groundnut (6.90 %), sugar cane (6.40 %), jowar (22.60 %), and banana (5.30 %). Total geographical area is 289 557 ha, of which the area utilized for cultivation is 114 554 ha, 37 264 ha of land is put into nonagricultural uses, and the remaining is used for other activities (Table 12.1).
12.2.5 Irrigation Practices
The data available indicate that an area of about 54,709 ha, which is about 18.89 % of the total geographical area of the district is under irrigated agriculture. Dug wells are the major source of water for irrigation in the district, accounting for about 59.97 % of the total area irrigated in the district and tube wells about 9.48 %. On the net area irrigation, the canal irrigates only 29.45 %. The area irrigated under the tank is 1.10 %.
12.2.6 Rainfall and Climate
The Amaravathi river basin is subjected to four distinct seasons, namely southwest monsoon from June to early September, northeast monsoon from October to December, winter season from January to February, and summer season from March to May. The district receives the rain under the influence of both southwest and northeast monsoons. The northeast monsoon chiefly contributes to the rainfall in the district. Most of the precipitation occurs in the form of cyclonic storms due to the depressions in the Bay of Bengal. The southwest monsoon rainfall is highly erratic and summer rains are negligible. The normal annual rainfall over the district from 1901 to 2011 varies from about 620 to 745 mm, and in 2012, it was 527.6 mm, much less than the normal average of 652.20 mm [13], and it is the minimum around Aravakurichi (622.7 mm) in the western part of the district. It gradually increases toward the east and attains a maximum around Kulithalai (744.6 mm). The district enjoys a tropical climate, and the relative humidities are generally between 40 and 80 %. The mean maximum temperature ranges from 26.7 to 38.6 °C, and the mean minimum temperature ranges from 18.7 to 29.3 °C. The daytime heat is tyrannical and the temperature is as high as 43.9 °C. The lowest temperature recorded is of the order of 13.9 °C [14].
12.3 Materials and Methods
12.3.1 Soil Sampling
Soil samples were collected from 36 different places (including river bed soil) of the Amaravathi river basin area of Karur district. Soil samples were collected from a depth of 5–10 cm during February 2014 in labeled sterile polyethylene bags and taken in ice-packed coolers to the laboratory for physicochemical and microbiological analysis. For chemical measurements, the soil samples were air-dried and then sieved through a 2 mm sieve.
12.3.2 Analysis of Soil Samples
An aqueous extract of the samples was prepared by mixing 80 g of the air-dried sample with 400 ml double-distilled water in a 500 ml beaker. Using a hot plate magnetic stirrer, the mixture was stirred for 30 min at 40 °C, and then the mixture was allowed to settle for 1 hour. Filtrates of soil–water slurry (1:5 w/v) were used for chemical analysis. The same procedure was adopted for each sample. Soil pH, electrical conductivity (EC), and total dissolved solids (TDS) were determined using a combined water quality multiparameter probe Elico PE 138 make. The sum of the calcium (Ca2+) and magnesium (Mg2+) contents in the extract were determined by the Eriochrome black T titration method, and the Ca2+ content was then subtracted from the sum to determine the Mg2+ content. Total alkalinity (TA) was determined by titrating with 0.1 M HCl using methyl orange as indicator. Chloride content of the soil samples was determined by Mohr’s method. Turbidity and sulfate were estimated using a nephelometer. Fluoride was estimated colorimetrically by SPADNS [2-(p-sulphophenylazo) 1,8-dihydroxynaphthalene-3,6-disulphonic acid trisodium salt, C16H9N2O11S3Na3]. Nitrate was analyzed by spectrophotometric determination at 520 nm. A value for biochemical oxygen demand (BOD) was obtained using the Winkler’s titration method. Chemical oxygen demand (COD) was determined by the dichromate open reflux method. Iron and manganese were analyzed using the atomic absorption spectrometer Perkin Elmer A Analyst 3600.
12.3.3 Microbiological Analysis of the Soil Samples
The soil microbiological analyses of the samples were carried out according to the methods of Rabah et al. [15] and Oyeleke and Manga [16]. The bacterial isolates were identified and characterized using standard biochemical tests [17]. The tests employed include gram stain, motility, catalase, oxidase, methyl red, nitrate, Voges–Proskauer, indole production, urease activity, and citrate utilization tests.
12.3.4 Groundwater Sample Collection and Analysis
Sixteen samples were collected for assessment of groundwater quality during the post-monsoon season (February 2014) from the different deep bore and hand pumps which are shown in Fig. 12.1. Hand pumps for sampling were selected on the basis of industrial unit as well as different land use patterns. Groundwater samples were collected in clean polyethylene bottles. At the time of sampling, bottles were thoroughly rinsed two or three times with groundwater to be sampled. The water samples were collected after flushing water for 10–15 min to remove the stagnant water as per standard procedure [18]. The samples were filtered using 0.45 μm Millipore filter paper and were stored at 4 °C before analysis. pH, EC, and TDS were measured within few hours using an Elico Multi-water analyzer PC tester. Total hardness (TH), Ca2+, and Mg2+ were determined titrimetrically using the standard EDTA method, and chloride was determined by AgNO3 titration method [19]. Bicarbonate was estimated by hydrochloric acid, and sulfate was determined by precipitating BaSO4 using BaCl2. Na+ and K+ were determined using an Elico flame photometer [18]. Nitrate determination was performed using the Brucine method. Fluoride was determined by SPADN’S method using a spectrophotometer. Phosphate was estimated by the spectrophotometric method using stannous chloride. BOD analysis was carried out after 5 days incubation at 20 °C. Similarly, analysis of COD was carried out by acidifying sample at pH <2 with sulphuric acid and titrated with an excess of K2Cr2O7 within 7 days. Iron and manganese were measured by atomic absorption spectroscopy (Perkin Elmer A Analyst 3600). The analytical precision for ions was determined by the ionic balances calculated as 100 × Σ(cations − anions)/Σ(cations + anions), which is generally within ±5 %. The mathematical and statistical results have been derived from the statistical package for social sciences (SPSS version 16) software.
12.3.5 Reagents
The reagents including indicators and buffers were of analytical grade (Merck). The aqueous solutions were prepared using double-distilled deionized water. The glassware employed in this study was of Borosil (India) grade. The standardization of reagents and solutions was carried out in accordance with standard methods of water chemical analysis [18].
12.4 Results and Discussion
The statistical gauges such as maximum, minimum, average, standard deviation, skewness, and kurtosis of the analyzed physicochemical parameters are represented in Tables 12.2 and 12.3 for soil and groundwater samples, respectively.
12.4.1 Soil Chemistry
Soil chemistry is an important factor in determining the yield of agricultural crops. On this basis, the soil samples were collected from irrigation lands of the Amaravathi river basin and the results are discussed. The turbidity of the soil samples ranges from 1.3 to 3.6 NTU with a mean value of 2.6 NTU. Soil pH is a measure of soil acidity and most crops grow prominently if the soil pH is between 6.0 and 7.5. Liming can increase the soil pH, and sandy soil has low pH values because sandy soils have low amount of reserve acidity due to low cation exchange capacity [20]. The pH ranges from 7.05 to 8.79 with an average value of 7.77. About 61 % of samples have a pH value above 7.50, indicating that they are moderately alkaline. EC of water is a measure of total dissolved contents and is an indicator of salinity. EC of soil samples ranges between 1210 and 1719 μS/cm (Mean: 1420 μS/cm). TDS levels have an average value of 984 ppm with a minimum and maximum value of 838 and 1191 ppm, respectively.
Hardness is defined as the sum of the concentration of Ca2+ and Mg2+ ions expressed in ppm [21]. TH ranges from 157 to 291 ppm with a mean value of 220 ppm. The high value of TH is due to the presence of the above metallic ions. Concentration of calcium and magnesium ranges from 29 to 116 ppm and 25 to 78 ppm, respectively. Calcium level in the soil samples falls under low category (<1000 ppm), whereas for magnesium, only 20 % of samples are found in the medium category (60–180 ppm) whilst the remaining 80 % of samples are found in the low category (<60 ppm). Sodium content in the soil samples ranges from 63 to 257 ppm, with about 17 % of samples exceeding the Na standard value of 200 ppm. Excess soil potassium levels can result in elevated potassium levels in grass forage crops which may be detrimental to animal and human health. Potassium content in all the soil samples falls under low category (<150 ppm) having a mean value of 37 ppm. Bicarbonate concentration in soil samples ranges from 117 to 279 ppm, showing a slightly elevated level, which may affect plant growth leading to white patches on the leaves. According to Mills [22] and Singh et al. [23], the chloride index can be categorized into five types: <350 ppm (Class I), 350–750 ppm (Class II), 750–900 ppm (Class III), 900–1300 ppm (Class IV), and >1300 ppm (Class V). All the soil samples fall in the class I (<350 ppm) which is suitable for all types of crops.
Fluoride level in 86 % of soil samples was well within 1.5 ppm, which is the maximum tolerable limit, whereas 14 % of samples exceed the limit. Plants absorb sulfur in the form of sulfate (\( {{{\text{SO}}_{4}}^{2 - }} \)–S) and the soils should have adequate sulfate content (>10 ppm). In the study area, sulfate level ranges from 116 to 228 ppm with an average value of 167 ppm. Plant available forms of nitrogen are nitrate (\( {{{\text{NO}}_{3}}^{ - }} {-} {\text{N}} \)) and 10–20 ppm are considered sufficient for plant growth. An average value of nitrate of 50 ppm is observed, which is considered as excessive for plant growth. High soil phosphate content combined with agricultural runoff can cause excessive growth of plants and algae in surface waters which damage aquatic ecosystems. Phosphate level ranges from 0.01 to 0.16 ppm, which is low for growth of plants. Iron and manganese levels in soil samples ranged from 0.05 to 0.69 ppm and 0.02 to 0.37 ppm, respectively. BOD is the amount of dissolved oxygen (DO) needed by aerobic biological organisms to break down organic materials [24]. BOD in the soil samples ranges from 236 to 420 ppm. The high level of BOD values observed is indicative of the presence of high pollution load and high population of microbes. High COD values may cause oxygen depletion due to decomposition by microbes. The COD value ranges from 157 to 337 ppm indicating a high level of organic pollutants from textile and dyeing industries. The cationic composition was dominated by the presence of Na+ and Ca2+ whilst the anionic composition varies from dominant Cl− to \( {{{\text{HCO}}_{3}}^{ - }} \) and \( {{{\text{SO}}_{4}}^{2 - }} \) (Figs. 12.2 and 12.3).
12.4.2 Groundwater Chemistry
The temperature of water collected from the Amaravathi river basin ranges from 28.3 to 28.9 °C; a temperature above 30 °C is unfit for public use [25]. The pH value of the groundwater is an index of the acidity or alkalinity. The pH value of the groundwater in the study area ranges from 6.15 to 8.16 indicating faintly alkaline nature. EC (Fig. 12.4) along the study area ranges from 1492 to 8091 μS/cm (mean: 3093 μS/cm), indicating a strong salinity stratification (Class III–IV). The TDS value represents the concentration of a solution in terms of the total weight of dissolved solids, which in turn reflects the degree of salinity [26]. The determined TDS value of groundwater samples ranges from 1034 to 5607 ppm with an average value of 2143 ppm. The TH value of water is a measure of dissolved Ca2+ and Mg2+ ions, and is also a soap neutralizing power [27]. Hardness of the groundwater ranges from 285 to 3200 ppm, showing hard to very hard types of water. About 96 % of samples exceeded the WHO [28] guideline value for calcium (75 ppm). The average magnesium value is 88 ppm with a minimum value of 13 ppm and maximum value of 396 ppm. The Ca2+ and Mg2+ ions may be derived from calcite and gypsum. The concentrations of sodium range from 105 to 1011 ppm with an average value of 383 ppm. About 75 % of the groundwater samples exceed the maximum permissible limit of 200 ppm [29]. Sodium coupled with a high concentration of chloride results in high salinity. The potassium value ranges from 6 to 100 ppm with a mean value of 28 ppm. The high level of potassium may be due to saline intrusion and anthropogenic sources.
The analyzed results show that chloride and bicarbonate are the dominant anions in groundwater, which ranges from 134 to 3067 ppm and 273 to 636 ppm, respectively. Bicarbonate is mainly derived from the dissolution of atmospheric CO2 and the weathering of carbonate and silicate minerals. The chlorinity index (Fig. 12.5) of the groundwater samples was computed using the measured chloride ion concentrations. Based on the data, 62.5 % of the samples are safe for irrigation (Class I and II) and the remaining are suitable for high salt tolerant crops only. Fluoride level in groundwater samples ranges from 0.1 to 4.1 ppm with about 50 % of samples exceeding the desirable limit of 1.5 ppm. The amount of sulfate ranges from 75 to 362 ppm with 41.6 % of the samples not being within the maximum desirable limit of 200 ppm.
Phosphate and nitrate levels are well within the permissible limits of 1 and 45 ppm as per BIS and WHO values. The ionic assemblage of cations and anions is shown in Fig. 12.2. The observed concentration of DO content of the groundwater varies from 2.56 to 7.49 ppm and is likely due to the low rate of dissolution of ambient oxygen into water and a high rate of microbial degradation. The analyzed BOD values for groundwater samples vary from 0.5 to 4.1 mg/L indicating that they are not higher than 5 ppm which is the limit set by WHO. The BOD concentration is apparently due to the percolation of organic wastes, human and animal excreta into the water resulting in the uptake of oxygen in the oxidative breakdown of these wastes into the groundwater. The values of COD oscillate between 32 and 117 ppm for groundwater samples. The high COD values may be due to the discharge of untreated or incompletely treated industrial effluents into the sewage from various manufacturing plants, thus affecting the status of groundwater.
12.4.3 Microbial Isolates from Soil Samples
Soil microorganisms respond very quickly to various natural and anthropogenic pressures or stresses acting on the soil ecosystem as they have a high surface-to-volume ratio, and thus they are capable of much more intense exchange of matter and energy with their environment [30, 31]. The bacterial cultures were isolated and identified by pure culture technique, gram staining method, and biochemical test methods. Bacterial cultures were isolated from 36 soil samples, identified and maintained by subculturing them in nutrient broth. More than 20 bacterial species were isolated and the most dominant species are taken into account. The results of the percentage frequency of occurrence of the microbial isolates are presented in Table 12.4. From the analyzed results, Escherichia coli had the highest frequency of occurrence (28 %), followed by Bacillus subtilis (22 %), Staphylococcus aureus (20 %), and Bacillus cereus (12 %). The lowest frequency of occurrence of 2 % was observed for Bacillus anthracis and Streptococcus faecalis followed by Pseudomonas fluorescens and Staphylococcus epidermis with 3 % each.
12.4.4 Correlation Analysis
The correlation coefficient is generally used to measure and establish the relationship between two variables. It is used to exhibit how well one variable predicts the other [32]. The correlation matrix of soil and groundwater variables was performed (Tables 12.5 and 12.6) and it reveals that major soil parameters such as EC with TDS (r = 0.998), Na+ with K+ (r = 0.840), EC and TDS with K+ (r = 0.719), EC and TDS with Na+ (r = 0.781, 0.782), EC and TDS with Cl− (r = 0.633, 0.632), and Fe2+ with \( {{{\text{SO}}_{4}}^{2 - }} \) (r = 0.570) exhibit high positive correlation with each other. Moderate correlations were observed for TH with \( {{\text{PO}}_{4}}^{3 - } \) (r = 0.488), EC and TDS with \( {{\text{HCO}}_{3}}^{ - } \) (r = 0.458, 0.459), and F− with \( {{\text{NO}}_{3}}^{ - } \) (r = 0.428). Most of the groundwater parameters were found to bear a statistically significant correlation with each other indicating close association of these parameters with each other. EC and TDS (r = 0.999) had a strong positive correlation with a number of parameters like TH (r = 0.921), Na+ (r = 0.935), Ca2+ (r = 0.878), Mg2+ (r = 0.876), and Cl− (r = 0.993). Same was observed for TH, Ca2+, and Mg2+ with Cl− (r = 0.941, 0.888, 0.902) indicating the high mobility of these ions. Some moderate and negative correlations were observed for TH with Na+ (r = 0.750), K+ with HCO3 − (r = 0.725), Ca2+ and Mg2+ with Na+ (r = 0.703, 0.710), K+ with \( {{\text{HCO}}_{3}}^{ - } \) (r = 0.725), and DO with BOD (r = −0.897).
12.4.5 Industrial Usage
Groundwater is used as one of the main sources for many industries due to lack of sufficient surface water. It is considered to be safe for industrial usage, if it is neither scale forming nor scale removing [23]. Langelier saturation index (LSI) and Ryznar saturation index (RSI) were determined to quantify the water suitability for industrial purposes. LSI [33] values were calculated using Eqs. 12.1 and 12.2.
where pHW is the measured pH of water and pHS is the saturation pH for CaCO3, calculated using Eq. 12.2:
where constant A = (log10[TDS]) − 1)/10, B = −13.12 × log10(°C + 273) + 34.55, C = log10[Ca2+ as CaCO3] − 0.4, and D = log10[Alkalinity as CaCO3]. RSI [34] data were calculated using Eq. 12.3:
LSI may be negative, neutral, or positive indicating, respectively, that the solution is undersaturated with CaCO3, at equilibrium with CaCO3 or supersaturated with CaCO3. All the groundwater samples (100 %) show negative LSI value (Fig. 12.6), indicating that they are of CaCO3 dissolving type. RSI point out the corrosion-level potential and is categorized into (i) 4–5 “Heavy scale;” (ii) 5–6 “Light scale;” (iii) 6–7 “Very light scale;” and (iv) >8 “No scaling” corrosion. From Fig. 12.6, RSI values are >11 indicating there is no scale formation and corrosion, and thus the samples are fit for industrial purposes.
12.5 Conclusions
The physicochemical and bacterial studies were carried out for soil samples along with groundwater samples from the Amaravathi river basin of Karur District. The soil pH is moderately alkaline and the parameters like TH, Na+, \( {{\text{HCO}}_{3}}^{ - } \), \( {{\text{NO}}_{3}}^{ - } \), \( {{\text{SO}}_{4}}^{2-} \), BOD and COD are at high levels due to the agricultural impact in the upper Amaravathi river basin and the effects of dyeing effluents and sewages from various sources causing the pollution in the lower part of the Amaravathi river basin. Most of the groundwater parameters in the river basin are at elevated levels, indicating the groundwater to be in a semi-critical category. However, all the water samples are considered to be safe for industrial usage. Soil management practices alter the physicochemical properties of soil, and the soil microbial community may respond to these changes in ways that affect the ability of the soil to resist soilborne diseases. Soil microbial diversity drops when the soil is subjected to intensive exploitation during agricultural production. Proficient preservation and caring of the significant soil properties are major concerns in rigorous crop cultivation for improving soil productive ability, food security, and environmental superiority.
References
Fageria NK (2002) Soil quality vs. environmentally-based agricultural management practices. Commun Soil Sci Plant Anal 33:2301–2329
Haque AAM, Jayasuriya HPW, Salokhe VM, Tripathi NK, Parkpian P (2007) Assessment of influential soil properties by GIS and factor analysis for irrigated rice production of Bangladesh: a case study. Agr Eng Int CIGR E-J 9:1–17
Rodale Institute (1991) In: Proceedings of international conference on the assessment and monitoring of soil quality, Rodale Institute, Emmaus, PA, USA
Garland JL, Mills AL (1991) Classification and characterization of heterotrophic microbial communities on the basis of patterns of community-level sole-carbon-source utilization. Appl Environ Microbiol 57:2351–2359
Doi R, Ranamukhaarachchi SL (2009) Correlations between soil microbial and physic-chemical variations in a rice paddy: implications for assessing soil health. J Biosci 34:969–976
Pu T, He Y, Zhu G, Xin H, Cao W, Niu H (2013) Hydrochemical characteristics of typical rivers in a temperate glacier basin, China. Environ Earth Sci 68:615–621
Shin WJ, Ryu JS, Lee KS, Chung GS (2013) Seasonal and spatial variations in water chemistry and nitrate sources in six major Korean rivers. Environ Earth Sci 68:2271–2279
Ogunfowokan AO, Obisanya JF, Ogunkoya OO (2013) Salinity and sodium hazards of three streams of different agricultural land use systems in Ile-Ife, Nigeria. Appl Water Sci 3:19–28
Rajmohan N, Elango L (2005) Nutrient chemistry of groundwater in an intensively irrigated region of southern India. Environ Geol 47:820–930
Umar R, Ahmed I, Alam F, Khan MM (2009) Hydrochemical characteristics and seasonal variations in groundwater of an alluvial aquifer in parts of Central Ganga plain, Western Uttar Pradesh, India. Environ Geol 58:1295–1300
Subramani T, Rajmohan N, Elango L (2010) Groundwater geochemistry and identification of hydrogeochemical processes in a hard rock region, Southern India. Environ Monit Assess 162:123–137
Chetia M, Chatterjee S, Banerjee S, Nath MJ, Singh L, Srivastava RB, Sarma HP (2011) Groundwater arsenic contamination in Brahmaputra river basin: a water quality assessment in Golaghat (Assam), India. Environ Monit Assess 173:371–385
Renganathan L (2014) Water table plummets to new low in Karur. The Hindu. http://www.thehindu.com/13 (January)
CGWB (2008) District groundwater brochure of Karur district, Tamil Nadu. Central Ground Water Board, Chennai
Rabah AB, Ijah UJJ, Manga SB, Ibrahim ML (2008) Assessment of physico-chemical and microbiological qualities of abattoir wastewater in Sokoto, Nigeria. Niger J Basic Appl Sci 16:145–150
Oyeleke SB, Manga SB (2008) Essentials of laboratory practicals in microbiology, 1st edn. Tobest Publishers, Minna
Cheesebrough M (2006) District laboratory practice in tropical countries, part 2, Low price edition. Cambridge University Press, London
APHA (2005) Standard methods for the examination of water and wastewater, 21st edn. American Public Health Association, Washington DC
Vogel AI (1968) A text book of quantitative inorganic analysis including elementary instrumental analysis, 3rd edn. ELBS/Longman, p 121
Marx ES, Hart J, Srevens RG (1999) Soil test interpretation guide, EC 1478. Oregon State University Extension Service, USA
Thilagavathi R, Chidambaram S, Prasanna MV, Thovya C, Singarraja C (2012) A study on groundwater geochemistry and water quality in layered aquifers system of Pondicherry region, Southeast India. Appl Water Sci 2:253–269
Mills B (2003) Interpreting water analysis for crop and pasture. File No. FS0334, DPI’s Agency for Food and Fiber Sciences, Toowoomba
Singh VK, Bikundia DS, Sarswat A, Mohan D (2012) Groundwater quality assessment in the village of Lutfullapur Nawada, Loni, District Ghaziabad, Uttar Pradesh, India. Environ Monit Assess 184:4473–4488
Jafar Ahamed A, Loganathan K, Vijayakumar P (2013) Bio-chemical investigation of selected water quality parameters in Amaravathi river basin, Karur district, Tamil Nadu, India. Int J Curr Res 5:3100–3103
Khan I, Javed A, Khurshid S (2013) Physico-chemical analysis of surface and groundwater around Singrauli coal field, District Singrauli, Madhya Pradesh, India. Environ Earth Sci 68:1849–1861
Mitra BK, Sasaki C, Enari K, Matsuyama N, Fujita M (2007) Suitability assessment of shallow groundwater for agriculture in sand dune area of Northwest Honshu Island, Japan. Appl Ecol Environ Res 5:177–188
Li P, Wu J, Qian H (2013) Assessment of groundwater quality for irrigation purposes and identification of hydrogeochemical evolution mechanisms in Pengyang County, China. Environ Earth Sci 69:2211–2225
WHO (2005) International standards for drinking water. World Health Organization, Geneva
BIS (2003) Indian standards specification for drinking water 15:10500. Bureau of Indian Standards, New Delhi
Gomoryova E, Strelcova K, Fleischer P, Gomory D (2011) Soil microbial characteristics at the monitoring plots on windthrow areas of the Tatra National Park (Slovakia): their assessment as environmental indicators. Environ Monit Assess 174:31–45
Nielsen MN, Winding A (2002) Microorganisms as indicators of soil health. NERI Technical Reports 388
Jafar Ahamed A, Ananthakrishnan S, Loganathan K, Manikandan K (2013) Assessment of groundwater quality for irrigation use in Alathur Block, Perambalur District, Tamil Nadu, South India. Appl Water Sci 3:763–771. doi:10.107/s132-013-0124-z
Langelier WF (1936) The analytical control of anti-corrosion water treatment. J Am Water Works Assoc 28:1500–1521
Ryznar JW (1944) A new index for determining the amount of calcium carbonate scale formed by water. J Am Water Works Assoc 36:472–494
Acknowledgements
The authors are thankful to the University Grants Commission (UGC), New Delhi for providing major research fund (F. No.41-337/2012) and the members of the Management Committee and the Principal of Jamal Mohamed College for providing the necessary facilities. We are grateful to the anonymous reviewers who have offered their valuable suggestions to improve the manuscript.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2016 Springer International Publishing Switzerland
About this paper
Cite this paper
Jafar Ahamed, A., Loganathan, K., Ananthakrishnan, S., Manikandan, K. (2016). Physicochemical and Microbiological Studies of Soils in Amaravathi River Bed Area, Karur District, Tamil Nadu, India. In: Ramasami, P., Gupta Bhowon, M., Jhaumeer Laulloo, S., Li Kam Wah, H. (eds) Crystallizing Ideas – The Role of Chemistry. Springer, Cham. https://doi.org/10.1007/978-3-319-31759-5_12
Download citation
DOI: https://doi.org/10.1007/978-3-319-31759-5_12
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-31758-8
Online ISBN: 978-3-319-31759-5
eBook Packages: Chemistry and Materials ScienceChemistry and Material Science (R0)