Abstract
The knowledge of the host diversity in the Tuber genus, an economically important group of ectomycorrhizal fungi, is of crucial importance for efficient cultivation system establishment. Centuries of field observations as well as scientific researches provided the information on ectomycorrhizal associations formed by these interesting fungi. Besides this, some recent works indicate that Tuber mycelia may be associated not only with roots of woody plants traditionally considered as ectomycorrhizal hosts but also with root tissues of herbaceous plants and with some kinds of soil organic matter. At the same time, some species of the Tuber genus tend to form scarce ectomycorrhizas. These facts suggest that Tuber mycelia may exploit some auxiliary sources of nutrition in a commensalistic, parasitic, or necrotrophic manner. This would make the concept of the host in the Tuber life cycle more complex than just a source of the nutrition provided via ectomycorrhizas. A possible change of the host concept in Tuber symbiosis may result in an increase in the host diversity, compared to the diversity based on the concept that is recently accepted.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
1 Introduction
Mycorrhizal symbiosis represents a type of coexistence of the host plant root with a specialized fungus. In general, the mycorrhizal fungus colonizes rhizodermis and primary root cortex of the finest roots, forming specific communication structures: mycorrhizas. In typical ectomycorrhizal (ECM) symbiosis, unlike the endomycorrhizal one, the fungus does not penetrate the host cell wall and stays in the intercellular spaces of the root cortex, forming a hyphal network called Hartig net. The root surface is covered by compact hyphal mantle. The mycorrhizas formed in ECM symbiosis are called ectomycorrhizas (ECMs) and are morphologically distinct from roots not colonized by the fungus (Smith and Read 2008).
In common view, the host plant in ECM symbiosis represents a partner responsible for energy supply, the saprotrophic nutrition being of marginal importance (Baldrian 2009). The solar energy is captured in the process of photosynthesis and is delivered to the mycorrhizal fungus in the form of energy-rich organic compounds, mostly carbohydrates, via ECMs. Ectomycorrhizal symbiosis involves complex physiological and ecological adaptations in both partners, which results in physiological and ecological specificity of the interaction (Smith and Read 2008). This means that, under field conditions, not all combinations of potential hosts with ECM fungi are possible. Instead, each fungal species can live in symbiosis with a specific range of hosts only. This is true also for true truffles as representatives of ECM fungi. Because the truffles are economically important fungal group, knowledge of the diversity of their host plant species is of a crucial importance.
2 Concept of the Host in ECM Symbiosis with True Truffles
The concept of the ECM nutrition of true truffles (members of the genus Tuber, Pezizales, Ascomycota) and their close relation to the host has not been always fully accepted as valid (Barry et al. 1994; Callot 1999). Tuber fruiting bodies are formed on soil mycelium undoubtedly connected to a tree root via ECMs. In the course of their development, they become large carbon sink that may be localized far from active ECMs, apparently without sufficient physiological connection with them. It is not completely known whether they are always fed via direct transfer of carbohydrates from the host tree through the ECM and mycelial network or whether they become saprotrophic, independent on the symbiotic nutrition at some time after their formation. The existence of hypothetical saprotrophic nutrition of true truffles is suggested by the formation of extensive external mycelium clumps emerging from the ascoma peridium surface as well as by in vitro utilization of starch, cellobiose, and, probably in some extent, also cellulose as carbon sources (Mamoun and Olivier 1991). Saprotrophic capacity of Tuber melanosporum Vittad. pure cultures was also indicated by production of hydrolytic enzymes (unpublished results).
In the light of the newly gained information, however, the hypothesis of saprotrophic nature of the true truffle mycelia producing ascomata seems to be surpassed. It seems now that at least the mycelium of T. melanosporum at fructification stage gains its nutrition mainly from the host. The concept of colonized tree as exclusive energy supplier for ECM fungus was first experimentally supported by Zeller et al. (2008) who suggested, on the basis of the analysis of stable isotopes 15N and 13C , that nutrition of growing ascomata of four Tuber spp. is not saprotrophic. Similarly, the data obtained by Le Tacon et al. (2013, 2015) from a labeling experiment using the same stable isotopes indicates that almost all carbon allocated to the truffles (T. melanosporum ascomata) came from the tree-bearing ECMs.
Further, the sequencing of the T. melanosporum genome shows rather limited range of genes coding for hydrolytic enzymes involved in saprotrophic exploitation of complex forms of organic matter (Martin et al. 2010). This may be taken as other information supporting the idea of predominantly biotrophic nature of T. melanosporum. Evolution of ECM fungi has led to a loss of some enzymes involved in degradation of the plant cell wall. Most ECM fungi, including T. melanosporum, lack genes coding for cellobiohydrolases GH6 and GH7 but can maintain others, such as polysaccharide monooxygenases (Kohler et al. 2015). On the other hand, Martin et al. (2010) mentioned very high upregulation of endoglucanase and laccase genes in symbiotically living mycelium, in comparison to free-living mycelium of T. melanosporum. The same was not observed for another ECM fungus, Laccaria bicolor (Maire) P.D. Orton (Martin et al. 2008, 2010). Upregulation of the laccase gene transcription in ECM of T. melanosporum relative to free-living mycelium and non-colonized roots was independently confirmed by Zarivi et al. (2013) using quantitative PCR. As the laccase is involved in degradation of lignin components, its upregulation in fungus suggests the active utilization of the material of lignified host cell walls.
These findings indicate a potential of T. melanosporum ECM to hydrolyze particular macromolecules which can be met in proximity of the root and exploited for nutrition. At the same time, they reveal a difference between the physiology of T. melanosporum symbiosis and physiology of symbiosis of L. bicolor as representative of a “model” ECM fungus.
The above text might invoke the feeling that the mode of the fungal life is either (most likely) symbiotic/biotrophic or (less probably) saprotrophic. However, the reality might be much more complex. It seems that true truffles might use different life strategies involving, besides the mycorrhizal symbiosis, also commensalism, parasitism, or necrotrophy. If so, the Tuber mycelium utilizes soil organic matter as a source of nutrition auxiliary to the symbiotic one. The hypothesis of auxiliary saprotrophic nutrition of Tuber mycelium is supported, for example, by the existence of eccentric mycelial colonies (manifested as brûlé areas in some Tuber species) which may be very distant from host trees (Callot 1999, see Fig. 3–18B on p. 110; Sourzat 2002, see Fig. on p. 39). In these distant areas, the host root density may be very low which may result in symbiotic nutrition shortage and consecutive need for exploitation of other organic nutrients than those provided by ECMs.
The hyphae of T. melanosporum were observed to heavily colonize roots of Thymus sp. (Callot 1999, see Fig. on p. 109), whose tissues obviously undergo a kind of degradation, perhaps releasing mineral and organic nutrients that might be utilized by the mycelium. Formation of necroses probably induced by the same fungus has been observed in roots of plants traditionally not considered as hosts (Plattner and Hall 1995). These effects of Tuber mycelium on herbaceous plants may be due to the production of allelopathic compounds by the Tuber hyphae (Streiblová et al. 2012). A question appears whether these infected plants can be considered as symbiotic hosts of the invading fungus. It seems that, at least in the case of herbaceous plants that do not form ECM, the fungus may behave as a necrotroph exploiting mineral and/or organic nutrients released from moribund root tissues. This hypothesis has not been exhaustively tested till recent days but deserves attention because the fungus has the potential to induce the host cell death. This is documented by the formation of necroses induced by the interaction between the host and Tuber mycelia during the morphogenesis of ECM (Ragnelli et al. 2014).
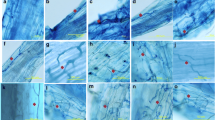
The mycelium of Tuber aestivum Vittad. was detected in the microbial film attached to the surface of decaying layers of suberinized cells of host roots other than ECM (Gryndler et al. 2013) as well as in the roots of many herbaceous plant species present at the natural locality of T. aestivum (Gryndler et al. 2014) without signs of penetration of fungal hyphae into the tissue volume (Fig. 16.1).
Mycelium on the root surface of nonhost plants: Silene vulgaris (Moench) Garcke (a), Carex muricata L. (b), and Achillea millefolium L. (c) collected within the soil mycelial colony of T. aestivum. The root surface is covered by microbial film containing hyphae of various fungi. Highly dense mycelia of T. aestivum were detected there using quantitative real-time PCR (Gryndler et al. 2014). Bar: 1 mm
This superficial contact may be either ineffective to induce plant defense reaction or the plant defense is induced but is not manifested in any visible change in root morphology. Again, it is unclear whether the plant, whose roots are superficially colonized, may be taken as a symbiotic host but it would be reasonable if a kind of signalization exists between such a “nonhost” root and the fungus. For example, bioactive compounds produced by the root tissues during secondary growth might serve as hypothetical signal mediators.
Similar, non-ECM association has been observed in T. melanosporum , which forms stromatic structures attached to the root bark surface (Pargney and Jalade 1995). These structures may represent an important, long-living contact surface between the mycelium and decaying root bark, distinct from ECMs. It is very interesting in this regard that the development of the Tuber soil mycelial colony seems to be stimulated by the presence of dead wood pieces, such as wooden fence pillar (Sourzat 2004), which may simulate the decaying root tissues. If this observation will be confirmed experimentally, it may indicate an association of the truffle mycelium with degradation of wooden root tissues.
It is also possible that the saprotrophic abilities vary among different Tuber species. In genomic data, different sets of genes coding for cazymes (carbohydrate active enzymes) involved in cellulose degradation were identified in different Tuber species (Claude Murat, pers comm; see also Chap. 9). This may be reflected in varying abundance of mycelia and ECMs in the soil. As the ECMs formed by T. aestivum (Pruett et al. 2009; Gryndler et al. 2013) or T. melanosporum (Rubini et al. 2011) are very abundant and can be easily extracted from the soil, the ECMs of other species, such as Tuber magnatum Pico, seem to be scarce or are even almost absent from soils in productive areas (Murat et al. 2005; Bertini et al. 2006; Leonardi et al. 2013; see Chap. 6). There may be no direct linkage among soil space occupied by T. magnatum mycelium and spatial distribution of mycorrhizas (Murat et al. 2005; Zampieri et al. 2010; Iotti et al. 2014).
The above text is dealing with only one aspect of mycorrhizal symbiosis: the nutrition. However, another important aspect should be taken into account when the host concept in mycorrhizal symbiosis is mentioned: the recognition of both partners. The partners must interchange the information on their identity, which results in specific modification of physiology at the level of cell, hyphae, or root tissues. The root may be colonized by either vegetative mycelium already established in soil or by the mycelium originating from germinating spores (ascospores, basidiospores). It was reported that the germination of spores of ECM fungi often requires the presence of a specific signal produced by a potential host root (Fries et al. 1987). Moreover, the germination may be enhanced in the presence of soil saprotrophic microflora (Birraux and Fries 1981). A cascade of events follows, which leads to the formation of ECMs and beginning of the nutrient exchange. This represents an important attribute of mycorrhizal symbiosis: the partners are tuned to each other, can accept a signal from each other, and produce an adequate response to the signal (Plett et al. 2011, 2014).
What we know about initial pre-colonization events occurring between Tuber spp. and their plant partners? The germination of Tuber ascospores under defined conditions is an extremely difficult task, which demands the prolonged storage at 5 °C (Grente et al. 1972), but is possible in the absence of any plant tissues, as it was proved also in our laboratory (Fig. 16.2). This fact indicates that Tuber ascospore germination is not induced by the root (does not depend on the presence of a potential host), though the germination may be enhanced by the root products (Guiochon 1959).
Germination of ascospores, explanted from ripe ascoma of T. melanosporum, on water agar in the absence of the root. Ascospores were stored at 4 °C for 2 months and incubated at room temperature for 4 weeks. The uppermost photograph shows formation of multiple germ tubes per one ascospore. The germ tubes appear aseptate, in contradiction to figures presented in Granetti et al. (2005), p. 34, and in Delmas (1976), p. 15. The growth of these mycelia was limited and attempts to subculture them failed. Photographed by E. Streiblová and M. Gryndler, using Nomarski interference contrast optics. Bar: 50 μm
Similarly to the ascospore germination, the growth of vegetative mycelium is not dependent on the presence of host roots under axenic conditions. Tuber mycelia can be transferred to pure culture as it was demonstrated mainly for T. aestivum (e.g., by the author of this text and by Iotti et al. 2002), T. melanosporum (Fontana 1971; Iotti et al. 2002), Tuber brumale Vittad. (Chevalier 1972, 1973; Iotti et al. 2002), and Tuber maculatum Vittad., Tuber macrosporum Vittad., and Tuber rufum Pico (Iotti et al. 2002). Some strains can be maintained in pure culture infinitely, which is the proof of their saprotrophic capacity.
However, the above facts do not indicate the ability of free-living Tuber mycelia to compete with other soil microorganisms and a signal produced by the host may be necessary in this respect. The woody plant species traditionally accepted as hosts of true truffles may provide such a signal activating the growth of mycelial biomass which is nourished both saprotrophically and symbiotically. The role of other plants present at the locality, which are conventionally classified as “nonhost” species, is not known and is mostly neglected by scientific community.
3 Hosts of True Truffles in Natural and Man-Made Environments
True truffles are inhabitants of various environments occupied by different known host plants (Table 16.1) as well as other plant species forming plant communities. These communities constitute a complex biological environment which, as a whole, support the existence and development of the Tuber mycelia. The communities associated with particular Tuber species were frequently studied, but the obtained data have been mostly published at national or even regional level and are thus hardly accessible to the majority of international scientific community.
One of the best accessible analysis of plant communities associated with T. aestivum , Tuber mesentericum Vittad., and T. melanosporum in Burgundy is presented by Chevalier and Frochot (1997, pp. 117–128) with conclusion that T. aestivum (syn. Tuber uncinatum Chatin) is typically – but not exclusively – found in the alliance Carpinion betuli, in particular in the association Scillo-bifoliae-Carpinetum, where Carpinus betulus, a European hornbeam, dominates as a host.
Voluminous list of plant species found in six T. aestivum localities in Poland is given by Hilszczanska et al. (2014). The authors registered the occurrence of five Tuber species on their localities but did not provide the details on possible host-fungus relationships.
Tuber spp. are also reported as associates or symbionts of orchids and some ericoid plants and are supposed to form typical orchideoid mycorrhizas (Selosse et al. 2004) and arbutoid mycorrhizas (Lancellotti et al. 2014; Taschen et al. 2015). This indicates that true truffles are able to produce at least three types of morphologically and functionally very different associations.
Among the orchid hosts, the presence of T. aestivum and Tuber excavatum Vittad. has been documented in roots of Epipactis microphylla (Ehrh.) Sw. (Selosse et al. 2004; Ouanphanivanh et al. 2008). Fungi similar to T. rufum and Tuber maculatum Vittad. have been detected in Epipactis helleborine (L.) Crantz using molecular identification (Ogura-Tsujita and Yukawa 2008). Tuber maculatum was confirmed in the root system of E. helleborine and Cephalanthera damasonium (Mill.) Druce by Ouanphanivanh et al. (2008).
It is obvious from the above reports and from the literature data sample summarized in Table 16.1 that true truffles are not strictly specialized on a particular host tree species and that the real range of hosts in the nature is relatively wide. However, the presence of Tuber species in the root system is not always accompanied by the production of fruiting bodies (Parádi and Baar 2006; Bonito et al. 2011).
Such a wide range of hosts of many Tuber species represents a factor which probably enhanced domestication of T. melanosporum and T. aestivum. Efficient hosts have been recruited during the decades of the field experimentation and this effort led to utilization of several woody species that could be easily managed in semiculture. Quercus pubescens Willd., Quercus ilex L., and Corylus avellana L. can be taken as examples of such suitable hosts. Even at the intraspecific level, the selection of efficient hosts was successful as documented for first time on C. avellana clone H219 with increased receptiveness toward T. melanosporum and enhanced resistance to colonization by competing ECM fungi (Mamoun and Olivier 1996).
The process of the host recruitment continues till present days as the new host species are tested and experimentally introduced to truffle grounds (Benucci et al. 2012; Turgeman et al. 2012). This effort is reasonable when the cultivation of truffles radiates to regions where traditionally used hosts do not perform well.
4 Colonization of Host Roots in vitro
Though the identity of the fungi present in ECM can be evaluated using efficient and reliable molecular methods (see Chap. 15), the experimental synthesis of ECM symbiosis should be performed under axenic conditions to prove the ECM status of the fungus. This will exclude the possibility that Tuber mycelium is present in the ECMs as an opportunist accompanying other ECM fungi (Murat et al. 2005). Generally, the mycorrhizal synthesis under fully controlled conditions is difficult and the failure to obtain ECMs with a particular combination of putative host with the tested fungus cannot be taken as a proof of impossibility of the association under natural conditions. Relatively few combinations of host plant/ECM fungus were hitherto tested for their ability to successfully form mycorrhizal symbiosis in vitro and the genus Tuber is not an exception of this rule.
First successful synthesis of Tuber axenic ECM symbiosis has been performed by Fontana (1967) and Fassi and Fontana (1967) with Pinus strobus L. and T. maculatum . Shortly after this initial success, Fontana and Palenzona (1969) continued these works by obtaining axenic mycorrhization of P. strobus and Populus nigra L. with Tuber albidum Pico (syn. Tuber borchii Vittad.). Chevalier (1973) used T. brumale as a fungal symbiont and C. avellana, Pinus sylvestris L., Pinus halepensis Mill., and Pinus nigra J. F. Arnold as hosts to fulfill the Koch’s postulates necessary for ultimate proof of ECM status of T. brumale as ECM fungus. After that, axenic cultures have been reached for T. melanosporum, T. mesentericum, and T. aestivum with C. avellana, P. sylvestris, P. halepensis, P. nigra, P. strobus, Q. pubescens, Quercus robur L. (syn. Quercus pedunculata Ehrh.), Quercus sessiliflora Salisb, Q. ilex, Quercus coccifera L. Fagus sylvatica L., and Tilia cordata Mill. (Chevalier et al. 1973). These cultures were based on sterilized mineral substrate (vermiculite) or sterilized soil. Later, the synthesis of ECMs of T. melanosporum was obtained on agar medium (overlayered with sterilized soil) with C. avellana by Chevalier and Desmas (1977). In this case, very small fraction of ascospores present in the agar medium germinated. However, this approach confirmed that inoculation by ascospores in vitro may be successful. In vitro synthesis of ECMs of T. borchii with white poplar (Zambonelli et al. 1989) or with Pinus radiata D. Don (Duñabeitia et al. 1996) has been reached in axenic conditions in test tubes. This work confirmed that mycorrhization under axenic conditions can be met even with fungal isolates poorly growing in pure culture.
Structures morphologically resembling typical ECMs of T. melanosporum have been demonstrated by (Wenkart et al. 2001) on hairy (root-inducing T-DNA transformed) roots of Cistus incanus L. in liquid and solid cultures in vitro. The attempts to further cocultivate other clones of transformed roots of C. incanus with mycelia of T. melanosporum and T. borchii under various nutrition regimes resulted in obtaining structures corresponding to endomycorrhizas with hyphae penetrating root cells, or “pseudo-ectomycorrhizas” with hyphae forming loose hyphal mantle but without typical Hartig net (Pacioni et al. 2014). In this case, interestingly, the character of T. melanosporum colonization was determined by the root genotype: the C. incanus clone M2 tended to form preferentially the structures corresponding to endomycorrhizas, whereas the clone W51 was mostly willing to form “pseudo-ectomycorrhizas,” the structures resembling the morphology of ECMs but lacking the typical fungal colonization. This indicates that behavior of the fungus may be dependent on its host genotype also under natural conditions. If this is the case, one fungal individuum (mycelial colony) may form a wide range of morphological structures with different plant genotypes.
5 Conclusions
In traditional view, true truffles represent a genus of ECM fungi which live in mycorrhizal symbiosis with a wide range of host woody plants, as it has been confirmed by axenic as well as greenhouse experiments. Besides this, however, the mycelium of true truffles may be associated with root tissues of “nonhost” woody and herbaceous plants. At the same time, some species of Tuber tend to form scarce ECMs. These facts suggest that Tuber mycelia may exploit some auxiliary sources of nutrition, other than these provided by the ECMs. Then, the dependence of free-living mycelia of some true truffles on their hosts may be relatively low, whereas the development of ascomata is probably indispensable from the symbiotic nutrition. The genus Tuber seems to possess relatively high biological multiformity, which may have enhanced the domestication of the economically most important species. This multiformity may induce the change of the host concept in Tuber symbiosis and enlarge the range of hosts by acceptation of other, hitherto neglected plants as symbiotic partners which do not form typical ECMs with Tuber mycelia but live in a functional interaction with them.
References
Baldrian P (2009) Ectomycorrhizal fungi and their enzymes in soils: is there enough evidence for their role as facultative soil saprotrophs? Oecologia 161(4):657–660. doi:10.1007/s00442-009-1433-7
Barry D, Staunton S, Callot G (1994) Mode of the absorption of water and nutrients by ascocarps of Tuber melanosporum and Tuber aestivum. A radioactive tracer technique. Can J Bot 72(3):317–322. doi:10.1139/b94-041
Benucci GMN, Bonito G, Falini LB, Bencivenga M (2012) Mycorrhization of Pecan trees (Carya illinoinensis) with commercial truffle species: Tuber aestivum Vittad. and Tuber borchii Vittad. Mycorrhiza 22(5):383–392. doi:10.1007/s00572-011-0413-z
Bertini L, Rossi I, Zambonelli A, Amicucci A, Sacchi A, Cecchini M, Gregori G, Stocchi V (2006) Molecular identification of Tuber magnatum ectomycorrhizae in the field. Microbiol Res 161(1):59–64. doi:10.1016/j.micres.2005.06.003
Birraux D, Fries N (1981) Germination of Thelephora terrestris basidiospores. Can J Bot 59(11):2062–2064. doi:10.1139/b81-267
Bonito G, Brenneman T, Vilgalys R (2011) Ectomycorrhizal fungal diversity in orchards of cultivated pecan (Carya illinoinensis; Juglandaceae). Mycorrhiza 21(7):601–612. doi:10.1007/s00572-011-0368-0
Callot G (1999) La truffe, la terre, la vie. INRA Editions, Paris
Chevalier G (1972) Obtention des cultures de mycélium de truffe à partir du carpophore et des mycorhizes. Académie d´Agriculture de France, extrait procès-verbal séance, 28 Juin 1972, pp 981–989
Chevalier G (1973) Synthèse axenique des mycorhizes de Tuber brumale Vitt. a partir de cultures pures du champignon. Ann Phytopathol 5:163–182
Chevalier G, Desmas C (1977) Synthèse des mycorhizes de Tuber melanosporum avec Corylus avellana sur gélose à partir de spores. Ann Phytopathol 9:531–540
Chevalier G, Frochot H (1997) La truffe de Bourgogne (Tuber uncinatum Chatin). Pétrarque, Levallois-Perret
Chevalier G, Grente J, Polacsek A (1973) Obtention de mycorhizes de différents Tuber par synthèse à partir de spores en conditions gnotoxéniques et à partir de cutures pures de mycélium en conditions axéniques et gnotoxéniques. Ann Phytopatol 5:107–108
Delmas J (1976) La truffe et sa culture. Editions SEI, INRA, Versailles, Etude No 60
Duñabeitia MK, Hormilla S, Salcedo I, Peña JI (1996) Ectomycorrhizae synthesised between Pinus radiata and eight fungi associated with Pinus spp. Mycologia 88(6):897–908. doi:10.2307/3761052
Fassi B, Fontana A (1967) Sintesi micorrizica tra Pinus strobus e Tuber maculatum. I. Micorrize e sviluppo dei semenzali nel secondo anno. Allionia 13:177–186
Fontana A (1967) Sintesi micorrizica tra Pinus strobus e Tuber maculatum. Giornale Botanico Italiano 101:298–299
Fontana A (1971) Il micelio di “Tuber melanosporum” Vitt. in coltura pura. Allionia 17:19–23
Fontana A, Palenzona M (1969) Sintesi di Tuber albidum in coltura pura con Pinus strobus e pioppo americano. Allionia 15:99–104
Fries N, Serck-Hanssen K, Häll-Dimberg L, Theander O (1987) Abietic acid an activator of basidiospore germination in ectomycorrhizal species of the genus Suillus (Boletaceae). Exp Mycol 11(4):360–363. doi:10.1016/0147-5975(87)90024-7
Granetti B, DeAngelis A, Materozzi G (2005) Umbria, terra di tartufi. Regione Umbria, Assessorato Regionale Agricoltura, Foreste, Caccia e Pesca
Grente J, Chevalier G, Pollacsek A (1972) La germination de l´ascospore de Tuber melanosporum et al. synthése sporale des mycorhizes. CR Acad Sci Paris Ser D 275:743–746
Gryndler M, Trilčová J, Hršelová H, Streiblová E, Gryndlerová H, Jansa J (2013) Tuber aestivum Vittad. mycelium quantified: advantages and limitations of a qPCR approach. Mycorrhiza 23(5):341–348. doi:10.1007/s00572-012-0475-6
Gryndler M, Černá L, Bukovská P, Hršelová H, Jansa J (2014) Tuber aestivum association with non-host roots. Mycorrhiza 24(8):603–610. doi:10.1007/s00572-014-0580-9
Guiochon P (1959) Germination monospermes de la truffe, Tuber melanosporum Vitt. Mushroom Sci 4:294–297
Hall IR, Brown GT, Zambonelli A (2007) Taming the truffle. The history, lore, and science of the ultimate mushroom. Timber Press Inc, Portland
Hilszczanska D, Rosa-Gruszecka A, Szmidla H (2014) Characteristic of Tuber spp. localities in natural stands with emphasis on plant species composition. Acta Mycol 49(2):267–277. doi:10.5586/am.2014.024
Iotti M, Amicucci A, Stocchi V, Zambonelli A (2002) Morphological and molecular characterisation of mycelia of some Tuber species in pure culture. New Phytol 155(3):499–505. doi:10.1046/j.1469-8137.2002.00486.x
Iotti M, Leonardi M, Lancellotti E, Salerni E, Oddis M, Leonardi P, Perini C, Pacioni G, Zambonelli A (2014) Spatio-temporal dynamic of Tuber magnatum mycelium in natural truffle grounds. PLoS One 9(12), e115921. doi:10.1371/journal.pone.0115921
Kohler A, Kuo A, Lg N, Morin E, Kw B, Buscot F, Canbäck B, Choi C, Cichocki N, Clum A, Colpaert J, Copeland A, Costa MD, Doré J, Floudas D, Gay G, Girlanda M, Henrissat B, Herrmann S, Hess J, Högberg N, Johansson T, Khouja HR, LaButti K, Lahrmann U, Levasseur A, Lindquist EA, Lipzen A, Marmeisse R, Martino E, Murat C, Ngan CY, Nehls U, Plett JM, Pringle A, Ohm RA, Perotto S, Peter M, Riley R, Rineau F, Ruytinx J, Salamov A, Shah F, Sun H, Tarkka M, Tritt A, Veneault-Fourrey C, Zuccaro A, Mycorrhizal Genomics Initiative Consortium, Tunlid A, Grigoriev IV, Hibbett DS, Martin F (2015) Convergent losses of decay mechanisms and rapid turnover of symbiosis genes in mycorrhizal mutualists. Nat Genet 47(4):410–415. doi:10.1038/ng.3223
Lancellotti E, Iotti M, Zambonelli A, Franceschini A (2014) Characterization of Tuber borchii and Arbutus unedo mycorrhizas. Mycorrhiza 24(6):481–486. doi:10.1007/s00572-014-0564-9
Le Tacon F, Zeller B, Plain C, Hossann C, Bréchet C, Robin C (2013) Carbon transfer from the host to Tuber melanosporum mycorrhizas and ascocarps followed using a 13C pulse-labeling technique. PLoS One 8(5), e64626. doi:10.1371/journal.pone.0064626
Le Tacon F, Zeller B, Plain C, Hossann C, Bréchet C, Martin F, Kohler A, Villerd J, Robin C (2015) Study of nitrogen and carbon transfer from soil organic matter to Tuber melanosporum mycorrhizas and ascocarps using 15N and 13C soil labelling and whole-genome oligoarrays. Plant Soil 395(1):351–373. doi:10.1007/s11104-015-2557-7
Leonardi M, Iotti M, Oddis M, Lalli G, Pacioni G, Leonardi P, Maccherini S, Perini C, Salerni E, Zambonelli A (2013) Assessment of ectomycorrhizal fungal communities in the natural habitats of Tuber magnatum (Ascomycota, Pezizales). Mycorrhiza 23(5):349–358. doi:10.1007/s00572-012-0474-7
Mamoun M, Olivier JM (1991) Influence du substrat carbone et de la forme d’azote mineral sur la croissance de T. melanosporum Vittad. en culture pure. Application à la production de biomasse mycelienne. Agronomie 11(6):521–527
Mamoun M, Olivier JM (1996) Receptivity of cloned hazels to artificial ectomycorrhizal infection by Tuber melanosporum and symbiotic competitors. Mycorrhiza 6(1):15–19. doi:10.1007/s005720050100
Martin F, Aerts A, Ahrén D, Brun A, Danchin EG, Duchaussoy F, Gibon J, Kohler A, Lindquist E, Pereda V, Salamov A, Shapiro HJ, Wuyts J, Blaudez D, Buée M, Brokstein P, Canbäck B, Cohen D, Courty PE, Coutinho PM, Delaruelle C, Detter JC, Deveau A, DiFazio S, Duplessis S, Fraissinet-Tachet L, Lucic E, Frey-Klett P, Fourrey C, Feussner I, Gay G, Grimwood J, Hoegger PJ, Jain P, Kilaru S, Labbé J, Lin YC, Legué V, Le Tacon F, Marmeisse R, Melayah D, Montanini B, Muratet M, Nehls U, Niculita-Hirzel H, Oudot-Le Secq MP, Peter M, Quesneville H, Rajashekar B, Reich M, Rouhier N, Schmutz J, Yin T, Chalot M, Henrissat B, Kües U, Lucas S, Van de Peer Y, Podila GK, Polle A, Pukkila PJ, Richardson PM, Rouzé P, Sanders IR, Stajich JE, Tunlid A, Tuskan G, Grigoriev IV (2008) The genome of Laccaria bicolor provides insights into mycorrhizal symbiosis. Nature 452(7183):88–92. doi:10.1038/nature06556
Martin F, Kohler A, Murat C, Balestrini R, Coutinho PM, Jaillon O, Montanini B, Morin E, Noel B, Percudani R, Porcel B, Rubini A, Amicucci A, Amselem J, Anthouard V, Arcioni S, Artiguenave F, Aury JM, Ballario P, Bolchi A, Brenna A, Brun A, Buee M, Cantarel B, Chevalier G, Couloux A, Da Silva C, Denoeud F, Duplessis S, Ghignone S, Hilselberger B, Iotti M, Marcais B, Mello A, Miranda M, Pacioni G, Quesneville H, Riccioni C, Ruotolo R, Splivallo R, Stocchi V, Tisserant E, Viscomi AR, Zambonelli A, Zampieri E, Henrissat B, Lebrun MH, Paolocci F, Bonfante P, Ottonello S, Wincker P (2010) Perigord black truffle genome uncovers evolutionary origins and mechanisms of symbiosis. Nature 464(7291):1033–1038. doi:10.1038/nature08867
Murat C, Vizzini A, Bonfante P, Mello A (2005) Morphological and molecular typing of the below-ground fungal community in a natural Tuber magnatum truffle-ground. FEMS Microbiol Lett 245(2):307–313. doi:10.1016/j.femsle.2005.03.019
Ogura-Tsujita Y, Yukawa T (2008) Epipactis helleborine shows strong mycorrhizal preference towards ectomycorrhizal fungi with contrasting geographic distributions in Japan. Mycorrhiza 18(6):331–338. doi:10.1007/s00572-008-0187-0
Ouanphanivanh N, Merényi Z, Orczán AK, Bratek Z, Szigeti Z, Illyés Z (2008) Could orchids indicate truffle habitats? Mycorrhizal association between orchids and truffles. Acta Biologica Szegediensis 52(1):229–232
Pacioni G, Comandini O (1999) Tuber. In: Cairney JWG, Chambers SM (eds) Ectomycorrhizal fungi: key genera in profile. Springer, Berlin, pp 163–186. doi:10.1007/978-3-662-06827-4_6
Pacioni G, Ragnelli AM, Aimola P, Leonardi M, Marinucci D, Roth-Bejerano N, Kagan-Zur V (2014) Endomycorrhiza and pseudo-ectomycorrhiza produced in vitro by two species of Tuber on transformed Cistus incanus roots. Int J Plant Biol Res 2(4):1021
Palenzona M (1969) Sintesi micorrizica tra Tuber aestivum Vitt, Tuber brumale Vitt, Tuber melanosporum Vitt. e semenzali di Corylus avellana L. Allionia 15:121–131
Parádi I, Baar J (2006) Mycorrhizal fungal diversity in willow forests of different age along the river Waal, The Netherlands. For Ecol Manage 237:366–372. doi:10.1016/j.foreco.2006.09.059
Pargney JC, Jalade M (1995) Cytological study of fungal structures forming stromas on roots of truffle mycorrhizal plantlets. Bulletin des Academie Societe Lorraines des Sciences 34(1):27–44
Plattner I, Hall IR (1995) Parasitism of non-host plants by the mycorrhizal fungus Tuber melanosporum. Mycol Res 99(11):1367–1370. doi:10.1016/S0953-7562(09)81223-9
Plett JM, Kemppainen M, Kale SD, Kohler A, Legue V, Brun A, Tyler BM, Pardo AG, Martin F (2011) A secreted effector protein of Laccaria bicolor is required for symbiosis development. Curr Biol 21(14):1197–1203. doi:10.1016/j.cub.2011.05.033
Plett JM, Khachane A, Ouassou M, Sundberg B, Kohler A, Martin F (2014) Ethylene and jasmonic acid act as negative modulators during mutualistic symbiosis between Laccaria bicolor and Populus roots. New Phytol 202(1):270–286. doi:10.1111/nph.12655
Pruett GE, Bruhn JN, Mihail JD (2009) Greenhouse production of Burgundy truffle mycorrhizae on oak roots. New For 37(1):43–52. doi:10.1007/s11056-008-9108-5
Ragnelli AM, Aimola P, Maione M, Zarivi O, Leonardi M, Pacioni G (2014) The cell death phenomenon during Tuber ectomycorrhiza morphogenesis. Plant Biosyst 148(3):473–482. doi:10.1080/11263504.2013.788575
Riousset LG, Chevalier G, Bardet MC (2001) Truffles d´Europe et de Chine. INRA, Paris
Rubini A, Belfiori B, Riccioni C, Arcioni S, Martin F, Paolocci F (2011) Tuber melanosporum: mating type distribution in a natural plantation and dynamics of strains of different mating types on the roots of nursery-inoculated host plants. New Phytol 189(3):723–735. doi:10.1111/j.1469-8137.2010.03493.x
Selosse MA, Faccio A, Scappaticci G, Bonfante P (2004) Chlorophyllous and achlorophyllous specimens of Epipactis microphylla (Neottieae, Orchidaceae) are associated with ectomycorrhizal septomycetes, including truffles. Microb Ecol 47(4):416–426. doi:10.1007/s00248-003-2034-3
Smith SE, Read DJ (2008) Mycorrhizal symbiosis, 3rd edn. Academic, New York
Sourzat P (2002) Guide pratique de trufficulture. Quatrième edition. Lacoste, Le Montat
Sourzat P (2004) Questions d´écologie appliquées à la trufficulture. Lycée professionnel agricole de Cahors-Le Montat, Impression 43029, Le Montat
Stobbe U, Büntgen U, Sproll L, Tegel W, Egli S, Fink S (2012) Spatial distribution and ecological variation of re-discovered German truffle habitats. Fungal Ecol 5(5):591–599. doi:10.1016/j.funeco.2012.02.001
Stobbe U, Egli S, Tegel W, Peter M, Sproll L, Büntgen U (2013) Potential and limitations of Burgundy truffle cultivation. Appl Microbiol Biotechnol 97(12):5215–5224. doi:10.1007/s00253-013-4956-0
Streiblová E, Gryndlerová H, Gryndler M (2012) Truffle brûlé: an efficient fungal life strategy. FEMS Microbiol Ecol 80(1):1–8. doi:10.1111/j.1574-6941.2011.01283.x
Taschen E, Sauve M, Taudiere A, Parlade J, Selosse MA, Richard F (2015) Whose truffle is this? Distribution patterns of ectomycorrhizal fungal diversity in Tuber melanosporum brûlés developed in multi-host Mediterranean plant communities. Environ Microbiol 17(8):2747–2761. doi:10.1111/1462-2920.12741
Turgeman T, Sitrit Y, Danai O, Luzzati Y, Bustan A, Roth-Bejerano N, Kagan-Zur V, Masaphy S (2012) Introduced Tuber aestivum replacing introduced Tuber melanosporum: a case study. Agrofor Syst 84(3):337–343. doi:10.1007/s10457-011-9478-0
Wenkart S, Roth-Bejerano N, Mills D, Kagan-Zur V (2001) Mycorrhizal association between Tuber melanosporum mycelia and transformed roots of Cistus incanus. Plant Cell Rep 20(4):369–373. doi:10.1007/s002990100325
Zambonelli A (2010) Lecture de l´approche de la trufficulture dans l´hémisphère austral à la lumière de l´experience Italienne. In: Olivier JM, Vilatte C (eds) Les nouvelles techniques de culture de la truffe. MGD Imprimeurs à Sarlat, Sarlat, pp 68–77
Zambonelli A, Govi G, Previati A (1989) Micorrizazione in vitro di piantine micro-propagate di Populus alba con micelio di Tuber albidum in coltura pura. Micol Ital 18(3):105–111
Zampieri E, Murat C, Cagnasso M, Bonfante P, Mello A (2010) Soil reveals the presence of an extended mycelial network in a Tuber magnatum truffle-ground. FEMS Microbiol Ecol 71(1):43–49. doi:10.1111/j.1574-6941.2009.00783.x
Zarivi O, Bonfigli A, Colafarina S, Aimola P, Ragnelli AM, Miranda M, Pacioni G (2013) Transcriptional, biochemical and histochemical investigation on laccase expression during Tuber melanosporum Vittad. development. Phytochemistry 87:23–29. doi:10.1016/j.phytochem.2012.11.019
Zeller B, Bréchet C, Maurice JP, Le Tacon F (2008) Saprotrophic versus symbiotic strategy during truffle ascocarp development under holm oak. A response based on 13C and 15N natural abundance. Ann For Sci 65(6):607. doi:10.1051/forest:2008037
Acknowledgment
The work was supported by the Czech Science Foundation within the frame of the project P504/10/0382.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2016 Springer International Publishing Switzerland
About this chapter
Cite this chapter
Gryndler, M. (2016). True Truffle Host Diversity. In: Zambonelli, A., Iotti, M., Murat, C. (eds) True Truffle (Tuber spp.) in the World. Soil Biology, vol 47. Springer, Cham. https://doi.org/10.1007/978-3-319-31436-5_16
Download citation
DOI: https://doi.org/10.1007/978-3-319-31436-5_16
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-31434-1
Online ISBN: 978-3-319-31436-5
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)