Abstract
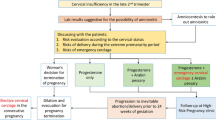
There are 15 million babies delivered prematurely every year, and the incidence of preterm birth is rising. Each year, 1.1 million babies die of complications from preterm birth, making preterm birth one of the important issues in the obstetrics field worldwide [1]. Preterm birth complicates between 5 and 12 % of all pregnancies and is associated with high perinatal morbidity and mortality. The inability of the uterine cervix to retain a pregnancy in the second trimester is referred to as cervical incompetence (Fig. 12.1). At less than 23 weeks of gestation, the fetus is not able to survive, and even if it does, there is very high morbidity. Among the topics related to preterm birth, cervical incompetence is a very important keyword. However, controversy in the medical literature exists pertaining to issues of pathophysiology, screening, diagnosis, and management (especially with cerclage) of cervical incompetence. Many reviews concerning cervical cerclage have been published, most of them based on randomized controlled studies and the Society for Maternal-Fetal Medicine and American College of Obstetricians and Gynecologists guidelines. Most of the guidelines suggest that the decision to perform cerclage should not be based solely on a poor obstetric history, because of the availability of TVU surveillance [2, 3] of cervical length (CL) and progesterone prophylaxis. However some clinicians have been performing cerclage based on the history of classic cervical insufficiency only, not following this suggestion [4]. We would like here to review the literature on cervical insufficiency and other clinical points of view and then discuss in detail the major surgical techniques for cerclage. Although the term “cervical incompetence” has been used for many years, this condition is now referred to as “cervical insufficiency” to avoid the negative connotations that the term “incompetence” may have for patients [5].
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
12.1 Introduction
There are 15 million babies delivered prematurely every year, and the incidence of preterm birth is rising. Each year, 1.1 million babies die of complications from preterm birth, making preterm birth one of the important issues in the obstetrics field worldwide [1]. Preterm birth complicates between 5 and 12 % of all pregnancies and is associated with high perinatal morbidity and mortality. The inability of the uterine cervix to retain a pregnancy in the second trimester is referred to as cervical incompetence (Fig. 12.1). At less than 23 weeks of gestation, the fetus is not able to survive, and even if it does, there is very high morbidity. Among the topics related to preterm birth, cervical incompetence is a very important keyword. However, controversy in the medical literature exists pertaining to issues of pathophysiology, screening, diagnosis, and management (especially with cerclage) of cervical incompetence. Many reviews concerning cervical cerclage have been published, most of them based on randomized controlled studies and the Society for Maternal-Fetal Medicine and American College of Obstetricians and Gynecologists guidelines. Most of the guidelines suggest that the decision to perform cerclage should not be based solely on a poor obstetric history, because of the availability of TVU surveillance [2, 3] of cervical length (CL) and progesterone prophylaxis. However some clinicians have been performing cerclage based on the history of classic cervical insufficiency only, not following this suggestion [4]. We would like here to review the literature on cervical insufficiency and other clinical points of view and then discuss in detail the major surgical techniques for cerclage. Although the term “cervical incompetence” has been used for many years, this condition is now referred to as “cervical insufficiency” to avoid the negative connotations that the term “incompetence” may have for patients [5].
12.2 Definition
Cervical insufficiency has no consistent definition, but some authorities have suggested it to be characterized usually by dilatation and shortening of the cervix before 37 weeks of gestation in the absence of preterm labor and to be most classically associated with painless, progressive dilatation of the uterine cervix in the second or early third trimester, resulting in membrane prolapse (Fig. 12.2), premature rupture of membranes, midtrimester loss, or preterm birth [6, 7]. Others have suggested that the definition should include a functional component of repeat pregnancy loss [8]. To complicate matters further, the advent of ultrasonic cervical length measurement has reframed the concept of the definition of cervical insufficiency.
12.3 Cervical Remodeling
It is essential to understand the physiology of the normal cervix (Fig. 12.3), because any untimely disarray in cervical remodeling could end in cervical insufficiency and preterm delivery. Although complex biochemical and hormonal changes are involved with the cervical ripening, these effects on cervical change are still not fully understood.
The cervix is a dynamic organ responsible for the physiology of gestation and parturition (Fig. 12.4); it has to be firm enough to retain the fetus from the beginning (Fig. 12.5) until the term and to soften during the labor for the delivery of the infant. The cervix consists of fibrous connective tissue and an extracellular matrix (70 % type I and 30 % type III), along with elastin, proteoglycans, and cellular compartments [9]. The cervical remodeling can be subclassified into four sequential phases: softening, ripening, dilation, and postpartum repair [10]. Cervical remodeling was once considered to be a passive process, which was induced by uterine contraction. But by now many investigators have confirmed that it is a complex process that can occur independent of uterine contractions [11, 12]. The uterine body and the cervix undergo separate functional changes in preparation for the labor (Fig. 12.6). Cervical remodeling might begin due to hormonal changes (e.g., a loss of progesterone), genetic predisposition, or infection and inflammation. During the cervical softening phase, poorly cross-linked collagen and extensive changes in the extracellular matrix lead progressively to a weakening of the tensile strength of the cervix and result in a cascade of cervical ripening and dilation [11, 12]. Other cellular compartments of the cervix also seem to be involved with cervical remodeling, but their roles are generally unknown.
12.4 Risk Factors for Cervical Insufficiency
Risk factors for cervical insufficiency include uterine anomaly, previous cervical surgery (conization or trachelectomy), prior induced (Fig. 12.7) or spontaneous abortions (Fig. 12.8), genetic defects in collagen and in elastin synthesis (e.g., Ehlers-Danlos and Marfan syndromes), a history of cervical insufficiency or midtrimester short cervix, and in utero diethylstilbestrol (DES) exposure [13–15]. Cervical competence is also influenced by infection (Fig. 12.9) and inflammation (Fig. 12.10) [16]. It however also occurs in a substantial number of patients without there being any identifiable risk factors.
12.5 Diagnosis
Although numerous investigators have tried to find accurate means of cervical insufficiency diagnosis, there is no reliable and objective standard as of yet. Because cervical insufficiency is usually diagnosed retrospectively, it is difficult to lay out any well-defined diagnostic criteria. Furthermore, there is difficulty in the diagnosis or prediction of cervical insufficiency in the nonpregnant state. Patients often have vaginal pressures without vaginal bleeding or labor pains. Obstetricians coincidentally find a short cervix or membrane protruding into the vagina in such patients. In the past, digital palpation of the cervix or pull-through techniques using a Hegar dilator were performed as tools for CI diagnosis [17], and some investigators sought to develop a cervical compliance score [18]. Currently, however, these techniques are not recommended for use in the diagnosis of cervical insufficiency, as they are subjective and not well reproducible [19].
Transvaginal ultrasonography (TVU) is the most powerful diagnostic tool for the assessment of cervical competence [20]. TVU is superior to transabdominal or translabial ultrasound for the assessment of the cervix. It is an accurate, reliable, and reproducible method for the measurement of cervical length (CL) and funneling. It has been well documented that a short CL (<25 mm) preferentially increases the risk of midtrimester birth [21–24]. The risk of preterm birth is inversely associated with CL, from <1 % at 30 mm to 80 % at 5 mm [25]. Serial measurements of CL can help identify high-risk patients for whom cerclage placement is beneficial, given that 12–40 % of “at-risk” patients will not present CI in subsequent pregnancies [19]. In 2012, the Society for Maternal-Fetal Medicine stated that universal CL screening in singleton pregnancies without prior PTB is controversial, while CL screening in singleton pregnancies with prior preterm birth is beneficial for the prevention of preterm birth [26].
However, they also emphasized that CL screening in singleton pregnancies without prior preterm birth should be considered when necessary [26]. CL measurement usually begins at 15 weeks, as CL screening prior to 15 weeks does not predict the risk of PTB [27].
It is recommended that TVU CL in singleton pregnancies with prior preterm birth can be started at 16 weeks and be repeated every 2 weeks until 23 weeks; TVU CL <25 mm is detected; the placement of cerclage should be considered, as cerclage significantly reduces the risk of PTB at less than 35 weeks [28]. Unfortunately, TVU CL screening in twin pregnancies cannot be recommended due to a lack of evidence [23]. For as a CL screening, the proper TVU technique is pivotal. The bladder should be emptied before TVU, as a full bladder compresses and elongates the cervix. After obtaining a sagittal long-axis view of the cervix, excessive pressure against the cervix should be avoided as it exaggerates CL [29].
Funneling, which is defined as the opening of the internal cervix, is characterized by funnel length and funnel width. Funneling occurs along with cervical effacement, which is easily remembered by the use of the mnemonic “trust your vaginal ultrasound” [30] (Fig. 12.11).
The T shape represents a normal closed cervix. The Y shape represents a small breaking funnel, and further funneling is shown by the V shape. A more advanced funnel takes the shape of a U, which is the worrisome finding of PTB [30, 31]. Unlike CL, there is high interobserver variability in measuring funneling [22]. Despite the high interobserver variability, funneling is useful for predicting preterm birth when combined with CL. The combination of a short CL (<25 mm) and the presence of funneling increases the sensitivity of predicting preterm birth compared to when there is a short CL alone [32].
In some cases, microbial invasion of the amniotic cavity (MIAC) is found in women with painless cervical dilation in the midtrimester [33]. It may be beneficial to have an amniotic fluid culture in the case of CI. However, there is the limitation that amniotic fluid bedside testing is not available for prompt decisions on management. Cervicovaginal fetal fibronectin (fFN) may be positive in some women with cervical insufficiency [34]. Positive fFN could predict an increased risk of preterm birth in women with prior preterm birth history [34].
Genetic predisposition is also helpful for the diagnosis of cervical insufficiency (Fig. 12.12). Polymorphisms in the promoter region of the interleukin-10 (IL-10) gene are found to be more common in women with cervical insufficiency compared to the controls [35]. Collagen 1alpha1 and transforming growth factor-beta polymorphisms are also related to cervical insufficiency [36]. These results suggest that cervical insufficiency is partly mediated by alterations in inflammatory processes or familial genetic factors [35, 36]. Women with Ehlers-Danlos syndrome and Marfan syndrome have genetic predispositions for cervical insufficiency [37, 38].
12.6 Treatment of Cervical Insufficiency
Several nonsurgical and surgical modalities have been proposed for treating cervical insufficiency. Certain nonsurgical approaches, including activity restriction, bed rest, and pelvic rest, have not been proven effective for the treatment of cervical insufficiency, and their uses are discouraged. Another nonsurgical treatment to be considered in patients at risk of cervical insufficiency is a vaginal pessary [39]. Vaginal pessaries are intended to alter the axis of the cervical canal and displace the weight of the uterine contents away from the cervix. Evidence is limited as to the potential benefits of pessary placement in select high-risk patients.
12.7 Cerclage
Cervical cerclage has become the mainstay for management of cervical insufficiency, but it remains one of the more controversial surgical interventions. Cervical cerclage is a surgical procedure that is carried out during pregnancy to position a suture around the neck of the cervix (Fig. 12.13). The purpose of this procedure is to provide a mechanical support to the cervix and so reduce the risk of preterm birth. During a normal pregnancy, the neck of the cervix stays tightly closed (Fig. 12.14), allowing the pregnancy to reach full term. Toward the end of pregnancy, the cervix then starts to shorten and becomes progressively softer in preparation for normal labor and delivery. Sometimes, the cervix begins to shorten and dilates too early, causing either late miscarriage or preterm birth. Cervical cerclage has been the treatment of choice for patients in this situation, although the effectiveness and safety of this procedure remain controversial.
Cerclage placement may be indicated based on a history of cervical insufficiency (history-indicated cerclage), on a history of preterm birth and certain ultrasonographic findings (ultrasound-indicated cerclage), and on a physical examination findings (physical examination-indicated cerclage). Transabdominal cerclage may be indicated in women having had prior transvaginal cerclage resulting in preterm birth at less than 33 weeks. Cerclage should be limited to pregnancies in the second trimester, before fetal viability has been achieved.
12.8 History-Indicated Cerclage
Patient selection for history-indicated cerclage (also known as prophylactic cerclage) is based on classic historical features of cervical insufficiency, and history-indicated cerclages are typically placed at approximately 13–14 weeks of gestation. Cerclage indicated based solely on poor obstetric history is less commonly performed, however, given the availability of transvaginal ultrasonographic surveillance of cervical length and progesterone prophylaxis [2, 3]. Based on trials of the efficacy of cerclage indicated by obstetric history, history-indicated cerclage can be considered in a patient with either of the following [40]: history of one or more second-trimester pregnancy losses, when risk factors for cervical insufficiency are present and other differential diagnoses have been ruled out, and prior cerclage due to painless cervical dilation in the second trimester.
Lotgering’s review of the clinical aspects of cervical insufficiency claimed that cerclage is regarded as ineffective because the data of randomized controlled trials is pooled and as a result shows no reduction in fetal loss [41]. This raises the question of whether the absence of proof from randomized controlled trials should be taken as proof of an absence of reduction in fetal loss, thanks to cervical cerclage in cases at high risk for cervical insufficiency. One may wonder why no large-scale randomized controlled trials have been performed to definitively prove the effectiveness of cervical cerclage, while there is such an obvious need for these studies. One reason could be that patients at high risk of yet another fetal loss are unwilling to give their consents to randomization after being informed that observational studies have shown approximately 90 % infant viability after cerclage and a low rate of procedure-related complications. This may explain why studies of the effectiveness of cerclage have been relatively small scale and/or have not been performed on truly high-risk patients. Lotgering also points out that small-scale studies of relatively low-risk patients are minimally informative of the true value of cerclage, as their power is low and both groups in the studies will have relatively good outcomes. He also notes the obvious reluctance of women and their doctors “to wait till the diagnosis of classic cervical insufficiency has been established by recurrence of fetal loss.” He points out the finding of uncontrolled studies that infant viability is around 25 % if cerclage is not used, but 75–90 % when it is. He stresses the critical fact that “without prophylactic cerclage, one accepts the risk that the cervix may open quite suddenly within days after the documented absence of funneling and normal cervical length.” He notes in conclusion that fetal loss is a painful experience. In cases of classic cervical insufficiency, recurrence is high, and a policy of prophylactic cerclage may be safer than one of serial cervical length measurement followed by cerclage, tocolysis, and bed rest in cases of cervical shortening or dilation. In low-risk cases, however, he states that prophylactic cerclage is not useful.
Fox et al. did a study entitled “History-Indicated Cerclage: Practice Patterns of Maternal-Fetal Medicine Specialists in the USA” [4]. They performed a mail-based survey of 827 specialists in the USA, asking them whether they would recommend history-indicated cerclage at 12–14 weeks, in a patient whose prior pregnancy was her first and had ended in spontaneous, painless loss at 19 weeks with no identifiable causes. Of the specialists surveyed, 75 % said that they would recommend a history-indicated cerclage for this patient. Twenty-one percent meanwhile indicated that they would not recommend it, but would place one if desired by the patient. Only 4 % said they would not place a history-indicated cerclage in this scenario. In reality, then, many clinicians seem to perform cerclage, not following the ACOG or textbook guidelines.
A large-scale randomized controlled trial is needed of high-risk classic cervical insufficiency that responded to history-indicated cerclage, to obtain exact data on the side effects of cerclage and on the progesterone effect.
Transvaginal methods currently use either the McDonald (Fig. 12.15) or the Shirodkar techniques. In the McDonald procedure, a simple purse-string suture of non-resorbable material is placed in four to six bites circumferentially at the cervicovaginal junction (Fig. 12.16). The Shirodkar procedure involves the dissection of the vaginal mucosa off the bladder and the rectum cephalad, so as to place the suture as close to the cervical internal os as is safely possible. The superiority of either technique over the other has not been established [42, 43]. The McDonald technique is preferred over the Shirodkar because of its ease of placement and removal. Mersilene 5-mm tape is most commonly used for cerclage, as it provides better tensile strength and is less likely to pull through the cervix in later gestation. The suture is placed below the level of the internal os and must be placed deep into the substance of the cervix to prevent lacerations. Suture removal is recommended at 36–37 weeks of gestation.
Although no trials have evaluated the efficacy of 17-alpha-hydroxyprogesterone caproate weekly supplementation after the cerclage procedure, progesterone can be administered after history-indicated cerclage [44].
12.9 Ultrasound-Indicated Cerclage
Ultrasound-indicated cerclage is often recommended for women with short cervical length on second-trimester transvaginal ultrasonography. Meta-analyses of multiple randomized trials comparing cerclage versus no cerclage in patients with short cervical length during the second trimester have reached the following conclusions [2, 45, 46]: Ultrasound-indicated cerclage may be effective in women with current singleton pregnancies, prior spontaneous preterm birth at less than 34 weeks of gestation, and short cervical length (less than 25 mm) before 24 weeks of gestation. Between 30 and 40 % of women with singleton gestations who have had prior spontaneous preterm birth will develop a short cervix (<25 mm) before 24 weeks. In these cases ultrasound-indicated cerclage is associated with significant decreases in preterm birth outcomes, as well as improvements in composite neonatal morbidity and mortality. On the other hand, ultrasound-indicated cerclage in women without history of prior spontaneous preterm birth and with cervical length less than 25 mm detected between 16 and 24 weeks of gestation has not been associated with any significant reduction in preterm birth [46]. Therefore, incidentally detected short cervical length in the second trimester in the absence of a prior singleton preterm birth is not diagnostic of cervical insufficiency, and cerclage is not indicated. Vaginal progesterone is recommended as a management option for reducing the risk of preterm birth in this setting [47].
There has until now been no mention in the literature of how to perform ultrasound-indicated cerclage. When we do perform it, the cervix can have various different shapes—such as a short cervix (e.g., 2.1 cm) with funneling or a very short cervix (0.5 cm) with funneling. Iatrogenic membrane can sometimes occur during this procedure, when the membrane is located very near the cervix. We have used four different small-size uniconcave balloons in ultrasound-indicated cerclage, for protection of the amniotic membrane.
Prior to or concurrent with any cerclage, the mother should be screened and as necessary treated for genitourinary tract infection, bacteriuria, vaginitis, bacterial vaginosis, cervicitis, and sexually transmitted infections. After receiving the results, we have used perioperative antibiotics, tocolytics, and progesterone.
12.10 Physical Examination-Indicated Cerclage
Occasionally, women presenting with advanced cervical dilation on speculum or digital examination with minimal or no symptoms before 24 weeks have been candidates for physical examination-indicated cerclage (known as emergency or rescue cerclage). Limited data from one small randomized trial and retrospective cohort studies have suggested that placement of cerclage in women with dilated cervix and visible membranes appears to prolong pregnancy by about 1 month and improve pregnancy outcomes compared with the use of expectant management [48]. Emergency cerclage is recognized as an essential procedure for prolonging gestation in women with advanced cervical changes and/or prolapsed membranes in the second trimester. After intra-amniotic infection, ruptured membranes, advanced labor, and significant hemorrhage are ruled out, physical examination-indicated cerclage placement may be beneficial. The rate of emergency cerclage success is relatively low, however, certainly compared with elective cerclage. Membranes are easily ruptured intraoperatively, especially when the cervix is widely dilated and the fetal membranes are prolapsed beyond the cervix [49, 50]. Pushing bulging fetal membranes back into the uterine cavity during cerclage with a sponge swab or Foley catheter is difficult. Overfilling the urinary bladder to reduce prolapsed fetal membranes without direct mechanical contact is often insufficient as a single method [51]. Other less utilized techniques include inflatable devices such as a metreuryter or a rubber balloon, although few studies of their use have as yet appeared [50, 52, 53]. Recently Son et al. have developed a new uniconcave balloon device for repositioning fetal membranes into the uterus during emergency cerclage and reported its use in 103 patients who underwent emergency cerclage [54]. This device has a shape similar to that of a red blood cell or a donut, providing maximum surface area to allow the force exerted on the membranes to push them back into the uterus safely and effectively (Fig. 12.17).
A uniconcave balloon. (a) This device is composed of a balloon, a shaft, and a valve for air injection. The inflated balloon is not deformed or moved backward when pushing the bulging fetal membranes because of the supportive part on the rear side of the balloon. This device has centimeter gradations on the shaft, so that the depth of insertion can be noted. (b) Deflated balloon. (c) Inflated balloon, shaped like a red blood cell or a donut (Copyright permission obtained from: Am J Obstet Gynecol 212:114, 2015)
Cerclage was technically successful in all cases, and there were no ruptures of membranes in any patients and no operative or anesthetic complications. Son et al. concluded that obstetricians could perform emergency cerclage with this uniconcave balloon easily and safely with few complications (Fig. 12.18).
McDonald operation using uniconcave balloon. Illustration of a uniconcave balloon used in cerclage procedure. (a) Bulging fetal membranes are visualized. (b) The cervix is grasped and retracted with two atraumatic forceps, and adequately inflated balloon then gently pushes fetal membranes back into the uterus. (c, d) After fetal membranes are replaced in the uterus, sutures are placed as high as possible in accordance with McDonald technique. (e) Balloon is deflated. Purse-string suture is tied as instrument is withdrawn from the cervix (Copyright permission obtained from: Am J Obstet Gynecol 212:114, 2015)
Amniocentesis before emergency cerclage is not obligatory, but has two important benefits. One is the decompression of amniotic fluid to place a satisfactory cerclage, especially for hourglassing bulging membranes, and the other is the detection of intra-amniotic infection. Data from uncontrolled retrospective studies has suggested the perioperative use of tocolytics and broad spectrum antibiotics [55–58]. There are no studies of emergency cerclage comparing general with regional anesthesia, but in the writers’ experience, general anesthesia is better for performing cerclage with marked membrane bulging [59]. The recommended gestational age for emergency cerclage is less than 24 weeks, the threshold of fetal viability (i.e., >24 weeks of gestation), because the potential for harm likely outweighs the potential benefit [60, 61]. All contraindications to emergency cervical cerclage should be excluded—preterm labor, evidence of intra-amniotic infection, unexplained vaginal bleeding (abruption), preterm premature rupture of the membrane, fetal demise, and major fetal anomalies [59, 62].
Emergency cerclage in twin pregnancies with membrane bulging had not appeared useful and has not been studied in a dictated trials. Recently, however, Rebarber et al. [63] performed emergency cerclage on 12 women with twin gestation and cervical dilation and showed that emergency cerclage can be associated with favorable outcomes including a high likelihood of delivery at >32 weeks and high likelihood of survival. Levin et al. [64] and Zanardini et al. [65] also found favorable outcomes.
Kuon et al. [66] studied neonatal outcomes after emergency cerclage with a special focus on adverse effects in very low birth weight infants. Neonates of less than 1500 g after rescue cerclage showed significantly impaired outcomes, i.e., need for respiratory support and higher rates of chorioamnionitis after rescue cerclage. They concluded that the higher incidence of chorioamnionitis indicates a potential inflammatory factor in the pathogenesis. Several predictors for emergency cerclage success have been reported, such as intra-amniotic markers of infection and systematic markers of infection. Lee et al. [67] reported that elevated amniotic IL-6 predicts a cerclage short-interval latency. Linear regression analysis with latency as the independent variable revealed a significant relationship (r = −6.62, p < 0.001). Study of intra-amniotic markers of infection and their correlations with perinatal outcomes appears important.
Cases with bulging membranes following prior cerclage are also surgically challenging, as there are no relevant guidelines. Song et al. [68] evaluated 22 women with bulging membranes after primary cerclage, comparing 11 women with repeat cerclage and 11 with bed rest [28]. After repeat cerclage the median gestational age at delivery (p = .004), average birth weight (p < .01), and median prolongation of pregnancy (<.01) were higher, and the neonatal survival rate was also significantly higher (p < .009).
12.11 Transabdominal Cervicoisthmic Cerclage
Transabdominal cervicoisthmic cerclage is indicated for patients in whom cerclage is required but cannot be placed because of anatomical limitations of the cervix or in cases of prior failed transvaginal cervical cerclage procedures that resulted in the delivery before 33 weeks [69]. In patients having had prior failed transvaginal cerclage, transabdominal cerclage was associated with fewer recurrent preterm births compared to undergoing another history-indicated transvaginal cerclage [69]. Transabdominal cerclage can be performed through open laparotomy or operative laparoscopy. It is usually performed between 10 and 14 weeks of gestation or in the nonpregnant state. The suture may be removed by posterior colpotomy or laparoscopy to allow vaginal delivery, but is more often left in place, with cesarean section planned before the labor. There are different techniques for cerclage. The classic approach is transabdominal cerclage during pregnancy, while some authors support the procedure prior to pregnancy [70]. More recently, laparoscopic transabdominal cervicoisthmic cerclage (Figs. 12.19, 12.20, 12.21, 12.22, 12.23, 12.24, 12.25, and 12.26) and even robotic techniques have been described. In addition, one may consider performing transabdominal cerclage in the same session with trachelectomy performed for malignancy.
Laparoscopic cervical cerclage. A 5-mm nonabsorbable Mersilene polyester suture, with adjacent straightened blunt needles to allow passage through the trocar, is introduced into the abdominal cavity. The stitch is placed from posterior to anterior, at the level of the internal cervical os bilaterally (Courtesy of Dr. Helena Ban Frangež, Department of Reproduction, University Medical Center Ljubljana, Slovenia)
Laparoscopic cervical cerclage. A distance of 1.5 cm superior and 1 cm lateral to the insertion of the uterosacral ligament on the posterior uterus is a good initial guide for needle placement (Courtesy of Dr. Helena Ban Frangež, Department of Reproduction, University Medical Center Ljubljana, Slovenia)
Laparoscopic cervical cerclage. The vesicouterine peritoneum is opened and dissected off the lower uterine segment, exposing the uterine vessels anteriorly on both sides, before holding the thread ends of Mersilene (Courtesy of Dr. Helena Ban Frangež, Department of Reproduction, University Medical Center Ljubljana, Slovenia)
Although successful outcomes of transabdominal cervical cerclage have been reported, predictors of the success of transabdominal cerclage have not been thoroughly evaluated. Lee et al. [71] investigated pregnancy outcomes following transabdominal cerclage in 161 women with cervical insufficiency and explored parameters for predicting pregnancy outcomes following TAC. The mean gestational age at delivery after transabdominal cerclage was 36.3 weeks, with a neonatal survival rate of 96 %. Univariate analysis demonstrated that a short CL (<25 mm) at 20–24 weeks and adenomyosis were associated with delivery at <34 weeks of gestation following transabdominal cerclage (p = 0.015 and p = 0.005, respectively). They found that maternal adenomyosis was a good predictor of TAC. However, multivariate analysis demonstrated that only a short CL (<25 mm) at 20–24 weeks was a significant predictor (p = 0.005). In their study there were only 15 cases of adenomyosis, and they postulated that the small number of patients with adenomyosis might not have been sufficient for evaluating its effects as a predictor of transabdominal cerclage outcome.
12.12 Cervical Cerclage in Multiple Gestations
The use of cervical cerclage to prevent preterm delivery is still controversial, particularly in multiple pregnancies. According to the available systematic reviews, cervical cerclage in twin pregnancies seems to be associated with a significant increased risk of preterm birth [72]. Cerclage based solely on the presence of a twin gestation has not been shown to be beneficial [72], and in women with twins and a short cervix, it is potentially harmful. There have however been some published studies showing different results. Zanardini et al. [65] reported on 28 cases of ultrasound-indicated cerclage and 14 of physical examination-indicated cerclage, finding 96 % perinatal survival in the former group and 86 % in the latter. Cervicovaginal and rectal swabs were gotten preoperatively, and perioperative antibiotics and tocolysis were administered. They noted Berghella’s conclusion that ultrasound-indicated cerclage in twin pregnancies is associated with a higher risk of preterm delivery (75 % before 35 weeks). However, this data is related to a relatively small population of 49 pregnancies from two randomized controlled trials, all of which had different inclusion criteria and management protocols and neither of which was intended to specifically evaluate the role of cervical cerclage in twin pregnancies [73]. Zanardini et al. [65] concluded that their data stressed the importance of reevaluating the efficacy of cerclage in twin pregnancies through properly designed clinical trials, particularly if cerclage is physical examination indicated.
Data on transabdominal cerclage in twin gestation is scarce. We reported one case of “successful twin pregnancy after vaginal radical trachelectomy using transabdominal cervico isthmic cerclage” [74]. Kyvernitakis also reported a similar case [75]. From now on transabdominal cervicoisthmic cerclage should be considered in twin pregnancies in cases of extreme short cervix after radical trachelectomy and previous transvaginal cerclage failure, as it would also be considered in single pregnancy. We have had some experience of transabdominal cervicoisthmic cerclage in twin pregnancy, although it is as yet not published.
12.13 Clinical Considerations for Cervical Insufficiency
Cervical insufficiency is a very important keyword related to preterm birth and is not uncommonly encountered. Despite this, however, there have been many controversies about diagnosis and treatment. A thorough obstetric history and risk factors for cervical insufficiency should be reviewed and all possible options for treatment discussed. Treatment should be decided based mainly upon the obstetric history and risk factors for cervical insufficiency and monitoring of cervical length and shape (TVU) by ultrasound. After careful review of all obstetric historic risk factors, a therapy plan should be agreed on with the patient involving cerclage and/or other methods. All women having high-risk factors should have CL checked by ultrasound especially during the period from 16 to 24 weeks of gestation. When finding a short cervix of less than 25 mm, we should discuss cerclage and progesterone therapy with uterine monitoring and vaginal examination. A Cochrane review regarding “cervical cerclage for preventing preterm birth” suggests that “The decision on how best to minimize the risk of recurrent preterm birth in women at risk, either because of poor history or a short or dilated cervix, should be ‘personalized’, based on the clinical circumstances, the skill and expertise of the clinical team, and, most importantly, the woman’s informed choice” [76].
Emergency cerclage may be the best hope for rescuing pregnancy in women with advanced cervical changes and prolapsed membranes in the midtrimester. The operative risk is surgically challenging, but recent new devices may be of great help to the patient.
Transabdominal cervicoisthmic cerclage is beneficial to a patient with extremely short cervix or in cases of prior failed transvaginal cervical cerclage procedures.
In the future, intensive study is needed to determine the true pathogenesis of cervical insufficiency. Some new standard treatment protocol is needed as well. The discovery of new biomarkers for cervical insufficiency will of course also be essential for reducing cervical insufficiency.
References
March of Dimes, PMNCH, Save the Children, WHO. Born too soon: the global action report on preterm birth. Howson CP, Kinney MV, Lawn JE (eds) World Health Organization, Geneva, 2012
Berghella V, Mackeen AD (2011) Cervical length screening with ultrasound-indicated cerclage compared with history-indicated cerclage for prevention of preterm birth: a meta-analysis. Obstet Gynecol 118(1):148–155
Berghella V, Rafael TJ, Szychowski JM et al (2011) Cerclage for short cervix on ultrasonography in women with singleton gestation and previous preterm birth: a meta-analysis. Obstet Gynecol 117(3):663–671
Fox NS, Gelber SE, Kalish RB, Chasen ST (2008) History-indicated cerclage: practice patterns of maternal-fetal medicine specialists in the USA. J Perinat Med 36(6):513–517
Romero R, Espinoza J, Erez O, Hassan S (2006) The role of cervical cerclage in obstetric practice: can the patient who could benefit from this procedure be identified? Am J Obstet Gynecol 194:1–9
McDonald IA (1978) Incompetence of the cervix. Aust N Z J Obstet Gynecol 18:34–37
Shennan A, John B (2004) The cervix and prematurity: aetiology, prediction and prevention. Semin Fetal Neonatal Med 9:471–479
Szychowski JM, Owen J, Hankins G, Iams J et al (2009) Timing of mid-trimester cervical length shortening in high risk women. Ultrasound Obstet Gynecol 33(1):70–75
Junqueira LC, Zugaib M, Montes GS, Toledo OM, Krisztan RM, Shigihara KM (1989) Morphologic and biochemical evidence for the occurrence of collagenolysis and for the role of neutrophilic polymorphonuclear leukocytes during cervical dilatation. Am J Obstet Gynecol 138:273–281
Read CP, Word RA, Ruscheinsky MA, Timmons BC, Mahendroo MS (2007) Cervical remodeling during pregnancy and parturition: molecular characterization of the softening phase in mice. Reproduction 134:327–340
Danforth DN (1995) The morphology of the human cervix. Clin Obstet Gynecol 38:267–279
Leppert PC (1998) The biochemistry and physiology of the uterine cervix during gestation and parturition. Prenat Neonat Med 3:103–105
Romero R, Espinoza J, Kusanovic JP et al (2006) The preterm parturition syndrome. BJOG 113:17–42
Kiefer DG, Keeler SM, Rust OA, Waycck CP, Vintzileos AM, Hanna N (2009) Is midtrimester short cervix a sign of intraamniotic inflammation? Am J Obstet Gynecol 200:374.e1–5
Anum EA, Hill LD, Pandya A, Streauss JF III (2009) Connective tissue and related disorders and preterm birth: clues to genes contributing to prematurity. Placenta 30:207–215
Harger JH (2002) Cerclage and cervical insufficiency: an evidence-based analysis. Obstet Gyenecol 100:1313–1327
Toaff R, Toaff ME (1974) Diagnosis of impending late abortion. Obstet Gynecol 43:756–759
Kiwi R, Neuman MR, Merkatz IR et al (1998) Determination of the elastic properties of the cervix. Obstet Gynecol 71:568–574
Debbs RH, Chen J (2009) Contemporary use of cerclage in pregnancy. Clin Obstet Gynecol 52:597–610
Iam JD, Goldenberg RL, Meis PJ et al (1996) The length of the cervix and the risk of spontaneous premature delivery. N Engl J Med 334:567–572
Welsh A, Nicolaides K (2002) Cervical screening for preterm delivery. Curr Opin Obstet Gynecol 14:195–202
Hibbard JU, Tart M, Moawad A (2000) Cervical length at 16–22 weeks’ gestation and risk for preterm delivery. Obstet Gynecol 96:972–978
Owen J, Yost N, Berghella V, Thom E, Swain M et al (2001) Midtrimester endovaginal sonography in women at high risk for spontaneous preterm birth. JAMA 286:1340–1348
Owen J, Yost N, Berghella V et al (2004) Can shortened mid-trimester cervical length predict very early spontaneous preterm birth? Am J Obstet Gynecol 191:298–303, The risk of preterm birth (PTB) is inversely associated with CL, from <1 % at 30 mm to 80 % at 5 mm
Heath VC, Southall TR, Souka AP et al (1998) Cervical length at 23 weeks of gestation: prediction of spontaneous preterm delivery. Ultrasound Obstet Gynecol 12:312–317
Society for Maternal-Fetal Medicine Publications Committee, with the assistance of Vincenzo Berghella (2012) Progesterone and preterm birth prevention: translating clinical trials data into clinical practice. Am J Obstet Gynecol 206:376–386
Berghella V, Talucci M, Desai A (2003) Does transvaginal sonographic measurement of cervical length before 14 weeks predict preterm delivery in high-risk pregnancies? Ultrasound Obstet Gynecol 21:140–144
Berghella V (2012) Universal cervical length screening for prediction and prevention of preterm birth. Obstet Gynecol Surv 67:653–657
Yost NP, Bloom SL, Twickler DM, Leveno KJ (1999) Pitfalls in ultrasonic cervical length measurement for predicting preterm birth. Obstet Gynecol 93:510–516
Zilanti MD, Azuaga A, Calderon F, Pages G, Mendoza G (1995) Monitoring the effacement of the uterine cervix by transperineal sonography: a new perspective. J Ultrasound Med 14:719–724
Berghella V, Owen J, Mac Pherson C et al (2007) Natural history of cervical funneling in women at high risk for spontaneous preterm birth. Obstet Gynecol 109:863–869
Berghella V, Daly SF, Tolosa JE et al (1999) Prediction of preterm delivery with transvaginal ultrasonography of the cervix in patients with high risk pregnancies: does cerclage prevent prematurity? Am J Obstet Gynecol 181:809–815
Romero R, Gonzalez R, Sepulveda W et al (1992) Infection and labor. VIII. Microbial invasion of the amniotic cavity in patients with suspected cervical incompetence: prevalence and clinical significance. Am J Obstet Gynecol 167:1086–1091
Iams JD, Goldenberg RL, Mercer BM et al (2001) The preterm prediction study: can low-risk women destined for spontaneous preterm birth be identified? Am J Obstet Gynecol 184:652
Warren JE, Nelson LM, Stoddard GJ, Esplin MS, Varner MW, Silver RM (2009) Polymorphisms in the promoter region of the interleukin-10 (IL-10) gene in women with cervical insufficiency. Am J Obstet Gynecol 201:372.e1–5
Warren JE, Silver RM, Dalton J, Nelson LT, Branch DW, Porter TF (2007) Collagen 1Alpha1 and transforming growth factor-beta polymorphisms in women with cervical insufficiency. Obstet Gynecol 110:619–624
Leduc L, Wassestrum N (1992) Successful treatment with the Smith-Hodge pessary of cervical incompetence due to defective connective tissue in Ehlers-Danlos syndrome. Am J Perinatol 9:25–27
Meijboom LJ, Drenthen W, Pieper PG et al (2006) Obstetric complications in Marfan syndromes. Int J Cardiol 110:53–59
Abdel-Aleem H, Shaaban OM, Abdel-Aleem MA (2010) Cervical pessary for preventing preterm birth. Cochrane Database Syst Rev CD007873
American College of Obstetricians and Gynecologists: cerclage for the management of cervical insufficiency: practice bulletin no. 142 (2014) Obstet Gynecol 123:372–379
Lotgering FK (2007) Clinical aspects of cervical insufficiency. BMC Pregnancy Childbirth 7:S17
Harger JH (1980) Comparison of success and morbidity in cervical cerclage procedures. Obstet Gynecol 56:543–548
Rozenberg P, Senat MV, Gillet A et al (2003) Comparison of two methods of cervical cerclage by ultrasound cervical measurement. J Matern Fetal Neonatal Med 13:314–317
Da Fonseca EB, Bittar RE, Carvalho MH, Zugaib M (2003) Prophylactic administration of progesterone by vaginal suppository to reduce the incidence of spontaneous preterm birth in women at increased risk: a randomized placebo-controlled double-blind study. Am J Obstet Gynecol 188:419
Owen J, Hankins G, Iams JD et al (2009) Multicenter randomized trial of cerclage for preterm birth prevention in high-risk women with shortened midtrimester cervical length. Am J Obstet Gynecol 201:375.e1–375.e8
Berghella V, Odibo AO, To MS (2005) Cerclage for short cervix on ultrasonography: meta-analysis of trials using individual patient-level data. Obstet Gynecol 106:181
Simcox R, Seed PT, Bennett P et al (2009) A randomized controlled trial of cervical scanning vs history to determine cerclage in women at high risk of preterm birth (CIRCLE trial). Am J Obstet Gynecol 200:623.e1–623.e6
Althuisius SM, Dekker GA, Hummel P et al (2001) Final results of the cervical incompetence prevention randomized cerclage trial (CIPRACT): therapeutic cerclage with bed rest versus bed rest alone. Am J Obstet Gynecol 185:1106–1112
Harger JH (2002) Cerclage and cervical insufficiency: an evidence-based analysis. Obstet Gynecol 100:1313–1327
Kurup M, Goldkrand JW (1999) Cervical incompetence: elective, emergent, or urgent cerclage. Am J Obstet Gynecol 181:240–246
Scheerer LJ, Lam F, Bartolucci L, Katz M (1989) A new technique for reduction of prolapsed fetal membranes for emergency cervical cerclage. Obstet Gynecol 74:408–410
Pereira L, Cotter A, Gomez R et al (2007) Expectant management compared with physical examination-indicated cerclage (EM-PEC) in selected women with a dilated cervix at 14(0/7)-25(6/7) weeks: results from the EM-PEC international cohort study. Am J Obstet Gynecol 197:483.e1–8
Stupin JH, David M, Siedentopf JP, Dudenhausen JW (2008) Emergency cerclage versus bed rest for amniotic sac prolapse before 27 gestational weeks. A retrospective, comparative study of 161 women. Eur J Obstet Gynecol Reprod Biol 139:32–37
Son GH, Chang KH, Song JE, Lee KY (2015) Use of a uniconcave balloon in emergency cerclage. Am J Obstet Gynecol 56(1):8–14
Nelson L, Dola T, Tran T, Carter M, Luu H, Dola C (2009) Pregnancy outcomes following placement of elective, urgent and emergent cerclage. J Matern Fetal Neonatal Med 22(3):269–273
Deb P, Aftab N, Muzaffar S (2012) Prediction of outcomes for emergency cervical cerclage in the presence of protruding membranes. ISRN Obstet Gynecol 2012:842841
Abo-Yaqoub S, Mohammed AB, Saleh H (2012) The effect of second trimester emergency cervical cerclage on perinatal outcome. J Matern Fetal Neonatal Med 25(9):1746–1749
Fuchs F, Senat MV, Fernandez H, Gervaise A, Frydman R, Bouyer J (2012) Predictive score for early preterm birth in decisions about emergency cervical cerclage in singleton pregnancies. Acta Obstet Gynecol Scand 91(6):744–749
Royal College of Obstetricians and Gynecologists. Cervical cerclage: green-top guideline 60. Published May 2011
Norwitz ER, Greene M, Repke JT (1999) Cervical cerclage- elective and emergent. ACOG Update 24:1–11
Norwitz ER (2002) Emergency cerclage: what do the data really show? Contemporary B/Gyn 104:8–66
Liddiard A, Bhattacharya S, Crichton L (2011) Elective and emergency cervical cerclage and immediate pregnancy outcomes: a retrospective observational study. JRSM Short Rep 2(11):91
Rebarber A, Bender S, Silverstein M, Saltzman DH, Klauser CK, Fos NS (2014) Outcomes of emergency or physical examination-indicated cerclage in twin pregnancies compared to singleton pregnancies. Eur J Obstet Gynecol Reprod Biol 173:43–47
Levin I, Salzer L, Maslovitz S, Avni A, Lessing JB, Groutz A, Almog B (2012) Outcomes of mid-trimester emergency cerclage in twin pregnancies. Fetal Diagn Ther 32(4):246–250
Zanardini C, Pagani G, Fichera A, Prefumo F, Frusca T (2013) Cervical cerclage in twin pregnancies. Arch Gynecol Obstet 288(2):267–271
Kuon R, Hualla H, Selz C, Hertler S et al (2015) Impaired neonatal outcome after emergency cerclage adds controversy to prolongation of pregnancy. Plos One 10(6):e0129104. doi:10.1371/journal.pone.0129104
Lee KY, Jun HA, Kim HB, Kang SW (2004) Interleukin-6, but not relaxin, predicts outcome of recue cerclage in women with cervical incompetence. Am J Obstet Gynecol 191(3):784–789
Song JE, Lee KY, Jun HA (2011) Repeat cerclage prolongs in women with prolapsed membranes. Acta Obstet Gynecol Scand 90(1):111–113
Davis G, Berghella V, Talucci M et al (2000) Patients with a prior failed transvaginal cerclage: a comparison of obstetric outcomes with either transabdominal or transvaginal cerclage. Am J Obstet Gynecol 183:836–839
Tulandi T, Alghanaim N, Hakeem G, Tan X (2014) J Minim Invasive Gynecol 21(6):987–993
Song JE, Lee KY, Son GH (2015) Prediction of outcome for transabdominal cerclage in women with cervical insufficiency. Biomed Res Int 2015:985764
Rafael TJ, Berghella V, Alfirevic Z (2014) Cervical stitch (cerclage) for preventing preterm birth in multiple pregnancy. Cochrane Database Syst Rev 9:CD009166
Saccone G, Rust O, Althuisius S, Roman A, Berghella V (2015) Cerclage for short cervix in twin pregnancies: systematic review and meta-analysis of randomized trials using individual patient-level data. Acta Obstet Gynecol Scand 94(4):352–358
Lee KY, Jun HA, Roh JW, Song JE (2007) Successful twin pregnancy after vaginal radical trachelectomy using transabdominal cervicoisthmic cerclage. Am J Obstet Gynecol 197(3):e5–e6
Kyvernitakis I, Lotgering F, Arabin B (2014) Abdominal cerclage in twin pregnancy after radical surgical conization. Case Rep Obstet Gynecol 2014:519826
Alfirevic Z, Stampalija T, Roberts D, Jorgensen AL (2012) Cervical stitch (cerclage) for preventing preterm birth in singleton pregnancy. Cochrane Database Syst Rev 18(4):CD008991
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2016 Springer International Publishing Switzerland
About this chapter
Cite this chapter
Lee, KY., Song, JE., Son, GH., Di Renzo, G.C. (2016). Cervicoisthmic Incompetence. In: Malvasi, A., Tinelli, A., Di Renzo, G. (eds) Management and Therapy of Early Pregnancy Complications. Springer, Cham. https://doi.org/10.1007/978-3-319-31377-1_12
Download citation
DOI: https://doi.org/10.1007/978-3-319-31377-1_12
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-31375-7
Online ISBN: 978-3-319-31377-1
eBook Packages: MedicineMedicine (R0)