Abstract
Heart failure patients retain sodium and fluid and may develop congestive symptoms of dyspnea, fatigue, and peripheral edema. Congestion is associated with increased morbidity and mortality in heart failure patients. Thus, the clinician should routinely assess for evidence of clinical congestion based on history and physical examination. In addition, laboratory and imaging modalities as well as more recently developed implantable device technologies may assist with the diagnostic evaluation of congestion. The management of congestion has historically been based on loop diuretics, however, additional pharmacologic therapies such as thiazide diuretics, vasodilators, vasopressin antagonists, and mineralocorticoid receptor antagonists may provide additional decongestion benefits. If diuretic-based therapies are unsuccessful, ultrafiltration may be considered but should be used with caution in the setting of cardiorenal syndrome. In this chapter, we review the assessment of clinical congestion and highlight recent device-based diagnostic technologies. The approach to volume management is outlined including both pharmacologic and mechanical fluid removal.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
Introduction
The clinical syndrome of heart failure is a constellation of signs and symptoms resulting from a reduced ability of the heart to pump an adequate volume of blood, either due to impaired ventricular filling or impaired ventricular pumping [1]. Heart failure patients retain sodium and fluid and may develop congestive symptoms of dyspnea, fatigue, and peripheral edema. Congestion is associated with increased morbidity and mortality in heart failure patients. Thus, the clinician should routinely assess clinical congestion based on history and physical examination. In addition, laboratory and imaging modalities as well as more recently developed implantable device technologies may assist with the diagnostic evaluation of congestion. The management of congestion has historically been based on loop diuretics, however, additional pharmacologic therapies such as thiazide diuretics, vasodilators, vasopressin antagonists, and mineralocorticoid receptor antagonists may provide additional decongestion benefits. If diuretic-based therapies are unsuccessful, ultrafiltration may be considered. In this chapter, we review the assessment of clinical congestion and highlight recent device-based diagnostic technologies. The approach to volume management is outlined including both pharmacologic and mechanical fluid removal.
Epidemiology of Congestion
Heart failure is a considerable and costly public health problem in the United States and worldwide, affecting more the 5 million American adults, responsible for over 1 million hospitalizations and costing over $30 million in 2012 [2]. Most heart failure hospitalizations are due to volume overload, with adequate decongestion therefore a major goal during hospitalization [3]. Despite inpatient treatment, many patients are discharged with persistent congestion, and congestion at the time of discharge is associated with worse outcomes [3–5]. Therefore, adequate assessment and treatment of volume overload are important factors in the management of patients with heart failure.
Terminology and Pathophysiology of Congestion
First described by Starling in 1914, as the normal heart fills with blood during diastole, the filling pressure in the ventricle increases, and the resultant stroke volume increases proportionally [6]. In heart failure, the ventricle is unable to increase stroke volume, either due to impaired contraction, impaired relaxation, or both (Fig. 4.1). Typically, during diastole the ventricle can accommodate large increases in volume with small increases in pressure. However, as the ventricle fills to capacity and becomes less distensible, the result is a significant rise in end-diastolic pressure. Therefore, the ventricular end-diastolic pressure is a marker of volume status. Congestion, or volume overload, in the setting of left ventricular dysfunction is defined in part based on high left ventricular end diastolic pressure (LVEDp). LVEDp can be measured directly with a catheter passed retrograde through the aortic valve into the left ventricle or estimated via indirect measurements with a pulmonary artery catheter. In the absence of mitral valve disease, the left atrial pressure (LAp) is equal to the LVEDp, and the pulmonary capillary wedge pressure (PCWP) is a surrogate for the LAp and therefore for the LVEDp.
The mechanisms of congestion in heart failure are thought to be a result of neurohormonal activation of the renin-angiotensin-aldosterone system as well as increased circulating levels of vasopressin (Fig. 4.2). Volume overload may occur in isolation, or in conjunction with decreased cardiac output. Causes of congestion and worsening cardiac function can vary and may be multifactorial. Possible precipitating factors including ischemia, infection, hypertension, arrhythmia, and dietary or medication noncompliance [7]. Another proposed mechanism is that a reservoir of blood from the splanchnic circulation gets abnormally distributed to the effective circulating blood volume in the presence of an abnormal hormonal milleau, as occurs in heart failure [8]. Congestion leads to further neurohormonal activation, and results in ventricular remodeling, pulmonary hypertension, and renovascular pathology, all of which contribute to worsening heart failure [9, 10].
Mechanisms of congestive heart failure. Abbreviations: HTN hypertension, JVD jugular venous distension, LA left atrial, LV left ventricular, LVEDP left ventricular end diastolic pressure, PA pulmonary artery, PCWP pulmonary capillary wedge pressure, PND paroxysmal nocturnal dyspnea, RA right atrial, RV right ventricular
Volume Assessment
History and Physical Examination
Symptoms
The clinical assessment can provide important information regarding volume status (Table 4.1). Patient reported symptoms of congestion include dyspnea, dyspnea on exertion, orthopnea, paroxysmal nocturnal dyspnea, bendopnea, and edema [11].
The symptom of dyspnea is frequently reported by patients, and is one of the most common reasons they seek treatment for heart failure. Cardiogenic dyspnea is caused by fluid accumulation in the lungs that reduces lung compliance. Pulmonary edema is a result of high pressure in the pulmonary capillaries causing transudation of fluid into the alveolar walls and the alveolar spaces [12]. In the early stages of volume overload, dyspnea may only occur with exertion, but as congestion worsens, dyspnea can occur with progressively less exertion and even occur at rest. Shortness of breath may also present suddenly, as in “flash pulmonary edema,” caused by acute increases in LVEDp caused by acute ischemia, acute aortic or mitral regurgitation, or severe hypertension. While the symptom of dyspnea is neither sensitive nor specific for volume overload, it can be used to subjectively assess response to therapy and characterize a patient’s clinical course.
Orthopnea—dyspnea when supine—is due to the changes in blood distribution to the pulmonary circulation and increased ventricular pre-load when lying flat. Patients may describe this symptom in terms of the number of pillows required to sleep without experiencing shortness of breath. More severe orthopnea has been shown to correlate with higher pulmonary capillary wedge pressures [13]. A related symptom that occurs in the supine position is paroxysmal nocturnal dyspnea (PND). PND is acute shortness of breath that awakens a patient from sleep and results in an urge to sit upright and breathe cool air. PND is also thought to occur due to fluid shifting from the peripheral circulation.
Bendopnea—dyspnea when bending over—occurs when there are elevated right- and left-sided cardiac filling pressures. Compared to patients without bendopnea, patients with bendopnea have higher supine right atrial and pulmonary capillary wedge pressures, and both right and left sided filling pressures increase when bending over [14].
Physical Exam
Physical exam signs of congestion include peripheral edema, hepatomegaly, a third heart sound, rales, and jugular venous distention. Jugular venous distention and pulmonary rales are the most specific findings, and a third heart sound is the most sensitive finding [11].
Peripheral edema is the result of high right heart filling pressures which increases hydrostatic pressure in the venous circulation, causing fluid to shift into interstitial tissues. Like many signs and symptoms, the exam finding of dependent edema is not sensitive, but can be used to monitor response to treatment [11]. In addition to edema, marked elevation in right-sided filling pressures can also result in congestion of the liver, causing the liver to be enlarged and pulsatile. Prolonged congestive hepatopathy can result in irreversible liver damage, termed cardiac cirrhosis.
A third heart sound, termed an S3 gallop, is caused by rapid ventricular filling during the passive ventricular filling in diastole. The presence of an S3 is associated with elevated left atrial and left ventricular end diastolic pressures and is associated with a poor prognosis. As filling pressures decrease with diuresis, the S3 may diminish.
Pulmonary rales are due to fluid accumulation in the alveoli due to transudation of fluid due to increased pressures in the pulmonary veins. Volume overload causes elevated pressure in the left ventricle which leads to elevated pressures in the left atrium and pulmonary veins. While rales on the examination of the lungs may be heard, this finding can be found with other conditions. Additionally due to a compensatory increase in lymphatic drainage from the lungs in chronic heart failure, rales are often notably absent in many chronic heart failure patients despite significant patient-reported dyspnea [15].
Jugular venous pressure reflects right atrial pressure which typically correlates with pulmonary capillary wedge pressure. However, in approximately 20 % of patients, right atrial pressure and PCWP are discordant, with low RA pressure despite elevated PCWP, or, less commonly, high RA pressure despite low or normal PCWP [16, 17]. Therefore, JVP assessment is an important component of the evaluation of volume status in heart failure patients, but this should not be used in isolation.
Despite low sensitivity and specificity of individual patient-reported symptoms and physical exam findings, taken together, health care providers are commonly able to use these findings to diagnose decompensated heart failure, distinguish it from other disease processes and characterize the severity of congestion. Furthermore, changes in symptoms and exam findings can aid both patients and health care providers in monitoring volume status and response to therapy. Physician assessment of hemodynamics has been shown to correlate with invasive hemodynamic measurements, with clinical findings of congestion correlating with higher PCWP by invasive hemodynamic measurements [18].
Pulmonary Artery Catheters
In addition to noninvasive evaluations, pulmonary artery (PA) catheters can aid in the evaluation and monitoring of volume status. The Evaluation Study of Congestive Heart Failure and Pulmonary Artery Catheterization Effectiveness (ESCAPE) trial, studied heart failure patients hospitalized with congestion, and compared therapy tailored by clinical assessment versus invasive hemodynamic monitoring [13]. In this study, 433 patients were randomized to one of the two strategies with an endpoint of resolution of clinical congestion. While the trial did not show a difference in survival or hospitalization between patients who were treated with the aid of a PA catheter and those who were treated based on clinical assessment alone, the patients whose diuresis was adjusted based on the invasive hemodynamics had greater diuresis and less renal dysfunction with therapy [13, 19]. Furthermore, a review of patients excluded from the trial confirmed they were often more severely decompensated than those included in the trial [20]. Because physical exam findings can be confounded by factors such as discordant hemodynamics or valvular disease, PA catheters are recommended for patients with uncertain clinical pictures such as those with symptoms out of proportion to clinical exam findings and those not responding to therapy as expected based on clinical assessment alone [21].
Biomarkers: Natriuretic Peptides
Serum biomarkers, most notably the natriuretic peptides, can also be used to assess volume status and differentiate between signs and symptoms caused by heart failure versus other etiologies.
Brain Natriuretic Peptide (BNP)
Natriuretic peptides are neurohormones involved in natriuresis and diuresis. Brain natriuretic peptide (BNP) was originally identified in the brain, but is primarily released from the cardiac ventricles in response to volume overload and cardiac wall stress. Pre-proBNP is synthesized in the myocardium, cleaved first to pro-BNP, then cleaved to the biologically active BNP and the inactive NT-proBNP fragment. BNP causes myocardial relaxation and counteracts the effects of the renin-angiotensin-aldosterone system resulting in vasodilation, natriuresis, and diuresis [22].
In two prospective studies of patients presenting to the emergency department with complaints of dyspnea, the Breathing Not Properly (BNP) study and the N-terminal Pro-BNP investigation of dyspnea in the emergency department (PRIDE) study, natriuretic peptides were shown to accurately differentiate between dyspnea due to congestive heart failure versus dyspnea due to other causes [23, 24]. Elevated levels of BNP (>100 pg/mL) and NT-proBNP (>450 pg/mL for patients <50 years of age, >900 pg/mL for patients >50 years of age) were shown to have a high positive predictive value for shortness of breath due to congestive heart failure, while low levels of BNP (<50 pg/mL) and NT-proBNP (<300 pg/mL) had a high negative predictive value indicating dyspnea due to non-cardiac causes [23]. Furthermore, BNP and NT-proBNP levels were superior to other history, physical exam or laboratory findings for diagnosing acute heart failure [23–25].
BNP has been shown to correlate with high LVEDP, with decreases in BNP correlating with decreases in LVEDP [26, 27]. Furthermore, elevated BNP levels have been shown to correlate with heart failure severity and prognosis [24, 28, 29]. It is important to note, however, that BNP is influenced by a number of factors, with the elderly, females, and patients with renal dysfunction have been shown to have higher BNP levels, while obese patients typically have lower BNP levels [30–33]. It has been suggested that the change in BNP level for a particular patient compared to the baseline BNP or the admission BNP may be more accurate than the use of a fixed value for all patients [34]. Furthermore, BNP may not correlate with hemodynamics in patients with advanced heart failure [35], possibly due to changes in BNP clearance in patients with advanced disease [36]. Multiple studies with modest sample sizes have assessed the utility of using natriuretic peptides to guide therapeutic decisions in heart failure patients (Table 4.2). These studies have had variable results and a large-scale clinical trial, Guiding Evidence Based Therapy Using Biomarker Intensified Treatment (GUIDE-IT), is ongoing (clinicaltrials.gov, NCT01685840).
Atrial Natriuretic Peptide (ANP)
ANP is released from cardiomyocytes primarily in the atria. ANP has similar actions as BNP, acting as a vasodilator and increasing natriuresis and diuresis by reducing renal sodium reabsorption and decreasing the activity of the renin-angiotensin-aldosterone system [37, 38]. Despite the similarities, ANP has been shown to be inferior to BNP at predicting volume status and prognosis, and is therefore not used in the clinical setting [34].
Imaging
Chest Radiography
Chest radiographs can provide important clinical information and confirmation of physical exam findings for patients with heart failure and volume overload. The heart size can be evaluated on chest imaging. Cardiomegaly, identified as the cardiac silhouette >50 % of the chest width, is an important clue in the diagnosis of new onset heart failure. Pulmonary findings including evidence of pulmonary hypertension, pulmonary edema, and pleural effusions can also aid in the assessment of patients with volume overload and are helpful in monitoring the efficacy of treatment for volume overload. Furthermore, chest radiographs can often identify other possible sources of the patient’s symptoms.
Echocardiography
Echocardiography is considered the gold standard in identifying depressed ventricular function. Echocardiography is also a tool to assess volume status noninvasively. Size and respirophasic movements of the inferior vena cava (IVC) reflect right atrial pressure. Imaging of the inferior vena cava in the subcostal echocardiogram view can estimate right atrial pressure. A normal sized IVC of 1.5–2.5 cm diameter which collapses completely with inspiration corresponds to a right atrial pressure of 5–10 mmHg. Elevation of right atrial pressure leads to dilation of the vessel and loss of the normal inspiratory collapse. A nondilated IVC (1.5–2.5 cm) with <50 % collapse during inspiration corresponds to a right atrial pressure of 10–15 mmHg, and a dilated IVC (>2.5 cm) with <50 % collapse corresponds to a right atrial pressure of 15–20 mmHg. A dilated IVC of >2.5 cm with no respiratory collapse corresponds to a right atrial pressure of >20 mmHg [39]. As previously stated, right atrial pressure typically corresponds with pulmonary capillary wedge pressure, but can be discordant in some patients [16, 17].
Implantable Devices
Table 4.3 provides a summary of implantable fluid monitoring devices as well as several relevant clinical trials.
Impedance Monitors
Another way to assess fluid status is through devices that measure intrathoracic impedance, which correlates with volume status. Electricity traveling between two points conducts better (i.e. decreased impedance) through water than through air (Fig. 4.3). As fluid accumulates in the lungs, the impedance across the lungs decreases [40, 41]. Devices that monitor impedance and record changes in impedance over time are included on some implantable cardioverter defibrillators.
OptiVol [42] (Medtronic, Inc., Minneapolis, MN) measures intrathoracic impedance between the tip of the right ventricular lead and the implanted device. The utility of the OptiVol device was studied in the Medtronic Impedance Diagnostics in Heart Failure Trial (MIDHeFT) [42], showing a decrease in intrathoracic impedance approximately 2 weeks prior to hospitalization for decompensated heart failure, and more than 1 week prior to the onset of symptoms. Furthermore, with diuresis, there was a correlation with an increase in intrathoracic impedance. This concept was further tested in the Fluid Accumulation Status Trial (FAST) which showed that impedance monitoring was more sensitive than changes in weight for detecting fluid overload and worsening heart failure [43]. Additional studies have demonstrated that OptiVol monitoring can predict heart failure hospitalizations [44, 45], rehospitalizations [46, 47], and mortality [48]. However, when patients were given access to impedance information, via an automated alert for possible fluid accumulation, the result was more outpatient visits and hospitalizations, and no improvement in mortality compared with usual care [49]. OptiVol monitoring is currently available as a diagnostic feature on certain implantable defibrillators, and is being used as an additional diagnostic component in the overall volume assessment of patients.
While impedance monitors measure use algorithms to characterize volume status, there are also direct pressure sensors that can be implanted in the right ventricle, left atrium, or pulmonary artery.
Right Ventricular Pressure Monitor
Similar to an RV pacemaker lead, a right ventricular pressure monitor can be implanted in the right ventricle and continuously measure ventricular filling pressures [50]. The RV pressure monitor, Chronicle (Medtronic, Inc., Minneapolis, MN) was tested in the Chronicle Offers Management to Patients with Advanced Signs and Symptoms of Heart Failure (COMPASS-HF) study. While the hemodynamic data obtained from the device correlates with right heart catheterization data [51], compared to standard care, treatment with the Chronicle device did not reduce hospitalizations and it did not reduce emergency or urgent care visits requiring intravenous therapy [52]. In both the treatment group and the standard of care groups, there was a lower than expected event rate, which may have been due to regular and frequent contact with medical professionals which has previously been shown to improve heart failure outcomes [53, 54]. Thus, the role for RV pressure monitoring devices for the routine assessment of volume status in heart failure patients requires further study.
Left Atrial Pressure Monitor
A left atrial pressure monitor, the HeartPOD (St. Jude Medical Inc., Minneapolis, MN), measures left atrial pressure and is implanted surgically or transvenously via a transseptal puncture [55, 56]. In the Hemodynamically Guided Home Self-Therapy in Severe HF patients (HOMEOSTASIS) trial [57], the use of this device improved patient’s functional status and ejection fraction, and allowed for up titration of heart failure medications and decreases in diuretic doses. The left atrial pressure monitor is currently being further studied in the Left Atrial Pressure Monitoring to Optimize Heart Failure Therapy (LAPTOP-HF) for safety and efficacy in reducing worsening heart failure and hospitalization (clinicaltrials.gov, NCT01121107). It will be necessary to demonstrate the efficacy of these devices to improve outcomes compared with current usual care prior to broad clinical application.
Pulmonary Artery Pressure Sensor
A pulmonary artery pressure monitor can be deployed in a pulmonary artery branch during right heart catheterization and provides accurate pulmonary pressure measurements [58]. A pulmonary artery pressure sensor, CardioMEMS (CardioMEMS, Atlanta, Georgia), was studied in the CardioMEMS Heart Sensor Allows Monitoring of Pressure to Improve Outcomes in NYHA Class III HF Patients (CHAMPION) trial [59, 60]. Use of this device resulted in a reduction in heart failure hospitalizations, pulmonary artery pressures, and an improvement in quality of life and medication utilization. The CardioMEMS device was approved by the FDA in October 2013.
The official practice guidelines for the management of heart failure recognize the advances in technology in the diagnostic evaluations of heart failure [21]. While initial studies suggest that implantable devices can provide accurate measurements that correlate with filling pressures, some of these devices are still being evaluated in larger clinical trials to determine the degree to which they impact outcomes. As these devices are adopted into routine clinical practice, they may be able to provide additional information in the evaluation of patients and the overall assessment of volume status.
Volume Management
Medical Therapy/Diuretics
Diuretics work by limiting sodium reabsorption in the kidney, resulting in increased urinary sodium and water excretion. The mechanism of action and the location of action in the kidney differ between classes of diuretics. Due to a positive charge, sodium can only cross the lipid luminal membrane into the cell by a transmembrane carrier or sodium channel. Sodium is transported out of the cell by Na-K-ATPase pumps in the basolateral cell membrane which return reabsorbed sodium to the systemic circulation. In the kidney, approximately 65–70 % of sodium is reabsorbed in the proximal tubule, 25 % is reabsorbed in the loop of Henle, and the remainder reabsorbed in the distal and collecting tubules [61]. Figure 4.4 presents the sites of diuretic action in the nephron.
Sites of diuretic action in the nephron. Proximal tubular diuretics such as mannitol and acetazolamide, have a modest net negative effect on sodium balance because downstream nephron sites reabsorb much of the sodium that is not reabsorbed in the proximal tubule. Loop diuretics dose-dependently decrease sodium reabsorption in the thick ascending limb of the loop of Henle. Thiazides and metolazone inhibit sodium reabsorption in the early portion of the distal convoluted tubule. Triamterene, amiloride, and spironolactone are potassium-sparing diuretics that work at the late portion of the distal convoluted tubule and the cortical collecting duct. (Reproduced with permission)
Loop Diuretics
Loop diuretics include furosemide, torsemide, and bumetanide, and their mechanism of action is in the loop of Henle. The transmembrane carrier in the thick ascending limb of the loop of Henle is a Na+ K+ 2CL− cotransporter, which is dependent on chloride delivery. Loop diuretics compete for the chloride site on the transporter, thereby limiting the transporter and blocking sodium reabsorption [62]. The pharmacology differs between the loop diuretics. Bumetanide and torsemide have a higher and more predictable bioavailability than furosemide. Torsemide has the longest half-life, but the half-lives of all of the loop diuretics increase with renal or hepatic dysfunction. The onset of action for loop diuretics is similar, 30–60 min if given orally and within minutes if given intravenously [63].
Loop diuretics are often the first line for treatment of volume overload in heart failure and are typically given intravenously in the setting of decompensation due to the need for a rapid onset of action. A pharmacologic review of loop diuretics highlights favorable outcomes in patients with heart failure treated with torsemide over furosemide, with respect to mortality, hospitalization, and functional class [63]. Additionally, in outpatients with heart failure, bumetanide has been shown to be more effective than furosemide at reducing dyspnea [64]. While continuous dosing of loop diuretics has theoretical advantages over intermittent bolus dosing, with a steady delivery of the drug to maintain a constant effect, the Diuretic Optimization Strategies Evaluation (DOSE) trial did not show a significant difference between the two approaches for the co-primary endpoints assessing patients’ symptoms and creatinine change [65, 66]. The DOSE trial was a prospective, randomized trial to evaluate diuretic dosing strategies in patients hospitalized with decompensated heart failure. Three hundred eight patients were randomized in a 2 × 2 factorial design to IV furosemide given as twice daily boluses or continuous infusion, and to either low dose (equivalent dose to home dose) or high dose (2.5 times home dose). There was no significant difference between the bolus versus continuous infusion groups. However, compared to the low dose group, the high dose group had more favorable outcomes in terms of dyspnea relief, weight loss, and net fluid loss. The high dose group, however, had worsening renal function, though this was found to be transient and resolved by the 60-day follow up [65]. Thus, an evidence-based initial approach to congestion management involves high-dose intravenous diuretics, administered as bolus or continuous infusion dosing.
Thiazide Diuretics
Sequential nephron blockade with thiazide-type diuretics may be used in combination with loop diuretics to augment diuresis [67]. Thiazide diuretics including hydrochorothiazide, chlorothiazide, chlorthalidone, and metolazone, act in the distal tubule by inhibiting the Na+ Cl− cotransporter in this location. Because the distal tubule reabsorbs less sodium than the loop of Henle, thiazide diuretics are less potent than loop diuretics. However, if sodium is not absorbed proximally, as during administration of loop diuretics, there is a compensatory response for the excess sodium and water to be absorbed distally [62]. Under normal physiologic conditions, the distal tubule absorbs approximately 5 % of the filtered sodium; the capacity for reabsorption can more than double in response to increased flow to the distal tubule due to the effects of a loop diuretic [62]. Giving a thiazide diuretic in conjunction with a loop diuretic may increase effectiveness of the loop diuretic by preventing distal reabsorption of sodium [68]. Because thiazide diuretics have a longer half-life than loop diuretics, the effect on the distal tubule will continue even after the loop diuretic has worn off [67]. Thus, patients who take loop diuretics chronically may be instructed to take thiazide diuretics on an “as needed” basis for worsening volume overload, though this strategy has not been rigorously evaluated in a clinical trial. Furthermore, the use of thiazide diuretics has been associated with increased arrhythmia risk due to hypokalemia [69, 70].
Potassium Sparing Diuretics
Potassium sparing diuretics include sodium channel blockers and aldosterone antagonists. These groups of medications act at the collecting tubule via different mechanisms. In the collecting tubule, the luminal membrane contains sodium and potassium channels, not transporters. Sodium channel blockers, amiloride and triamterene, directly block the sodium channels in the luminal membrane.
Aldosterone acts as a diuretic by increasing the number of open sodium channels in the collecting tubule. In the setting of loop diuretic use, when sodium is not absorbed proximally in the loop of Henle, it can be absorbed distally via an upregulation of aldosterone-sensitive sodium channels in the collecting tubule. The aldosterone antagonists (also referred to as mineralocorticoid receptor antagonists [MRAs]), spironolactone and eplerenone, block the action of aldosterone resulting in decreased sodium reabsorption in the collecting tubule; therefore, the addition of an MRA to a loop diuretic may result in increased natriuresis and diuresis. Aldosterone antagonists are recommended for patients with heart failure and reduced left ventricular ejection fraction ≤35 % and New York Heart Association class II–IV symptoms, based on several studies which demonstrated a reduction in mortality in patients taking aldactone or eplerenone [71–73]. While the MRAs have both diuretic and potassium-sparing effects, they also offer additional cardiovascular benefits beyond these properties [74]. Heart failure patients taking only non-potassium sparing diuretics without concomitant use of a potassium-sparing diuretic have been shown to have an increased risk of progressive heart failure and death, likely due to deleterious effects of neurohormonal activation that occurs with diuretic use in heart failure [75, 76].
Diuretic Resistance and RAAS Activation
The efficacy of a diuretic depends on many factors: the dose of the drug, the rate of delivery of the drug to the renal tubule, and patient factors including sodium and fluid intake and co-morbidities including heart failure and renal dysfunction [62]. There is a dose response curve that differs between drugs and between oral and intravenous administration. A certain concentration of the drug is required before diuresis occurs. Once that threshold level is reached, the response increases with increasing dose of the drug. There is a ceiling on the dose responsiveness. Once the transporter or channel is saturated, the maximum rate of diuresis is reached, and further dose increases will not result in increased diuresis [62]. The goal with diuresis is to find an effective dose that results in an effect on the ascending portion of the dose-response curve (Fig. 4.5). In patients with heart failure, the dose response curve is shifted downward and to the right and patients become less responsive to diuretics, thus higher dose are often required to achieve effective diuresis [77]. While some observational studies have shown an association between high dose loop diuretics and poor outcomes, these results are cofounded given that patients receiving higher doses of diuretics were likely more sick with more volume overload and possibly more diuretic resistance, requiring higher doses of diuretics to achieve a diuretic response [78].
Dose response curve of loop diuretics. Schematic of dose‐response curve of loop diuretics in heart failure patients compared with controls. In heart failure patients, higher doses are required to achieve a given diuretic effect and the maximal effect is blunted (Reproduced with permission from Felker [77])
Impaired renal function affects the bioavailability of diuretics. If the reduced glomerular filtration rate (GFR) is due to chronic kidney disease, there is impaired delivery of the drug to the kidney. A higher dose of the drug promotes an increased rate of delivery to the tubule and thus may be necessary in order to achieve efficacy in the setting of chronic kidney disease. If the reduced GFR is due to low cardiac output, improving hemodynamics can improve renal perfusion and diuretic efficacy [67]. Additionally, with volume overload resulting in intestinal edema, intestinal absorption of oral drugs may be impaired, therefore intravenous administration is preferred to overcome this issue [62].
In addition to adequately dosing and optimizing delivery of the drug, diuretic resistance may occur in patients being treated for volume overload. Several mechanisms contribute to diuretic resistance with loop diuretics: reduced diuretic efficacy with repeated dosing, rebound sodium retention due to increased sodium reabsorption in the distal nephron, and with chronic use, renal adaptation in the distal tubule resulting in hypertrophy and increased sodium reabsorption [66, 67]. One way to overcome diuretic resistance, in addition to increasing the dose of the drug, is to block sodium reabsorption in the distal tubule by giving a thiazide diuretic in conjunction with a loop diuretic (i.e., dual nephron blockade). However, treatment with combination diuretics can result in electrolyte disturbances, particularly hypokalemia, so electrolytes must be closely monitored and repleted during diuresis. Similarly, blocking downstream sodium reabsorption in the collecting tubule by administering an aldosterone antagonist can help overcome diuretic resistance. Reduced diuretic efficacy can be caused by neurohormonal activation, as diuretics may activate the renin-angiotensin-aldosterone system, which increases sodium reabsorption. This issue can be overcome with concomitant use of other medications that block the cascade, including ACE inhibitors, ARBs, and MRAs [67].
Vasopressin Receptor Antagonists
Vasopressin, or antidiuretic hormone, which is increased in the setting of heart failure has many systemic effects including vasoconstriction, cardiac hypertrophy, platelet aggregation, adrenocorticotropic hormone release, and uterine contraction [79]. Activation of the V2 receptor in the renal collecting tubule effects the aquaporin channels resulting in increased permeability to water which leads to water retention and hyponatremia [80]. Unlike diuretics that promote natriuresis and diuresis, vasopressin receptor antagonists, like tolvaptan, inhibit vasopressin, resulting in selective free water diuresis without natriuresis.
Treatment with tolvaptan has been shown to reduce weight, decrease dyspnea and edema, and normalize serum sodium levels in patients with hyponatremia [81–83]. Weight loss and symptom relief appears to be more significant in patients with hyponatremia. However, in heart failure patients, it has not yet been shown that treatment with tolvaptan improves long term mortality or cardiovascular morbidity [83]. Tolvaptan is approved for the treatment of severe or symptomatic hyponatremia in patients with heart failure.
Ultrafiltration
Ultrafiltration is an alternate strategy for volume removal. During the process of ultrafiltration, plasma water is removed from whole blood across a semipermeable membrane due to a pressure gradient across the membrane. Until recently, ultrafiltration has required central venous, but current devices allow for ultrafiltration through peripheral venous access [84]. In this technique, two peripheral intravenous catheters are placed, one for blood withdrawal and one for blood return, with ultrafiltration through a single-use extracorporeal blood circuit achieving fluid removal of up to 500 mL/h [66, 84]. Anticoagulation is typically required to prevent malfunction of the filter. Contraindications to ultrafiltration include hemodynamic instability, acute renal insufficiency, hypercoagulability, and poor venous access [66].
An advantage of ultrafiltration over diuretics is that ultrafiltrate is isotonic compared with urinary output with diuretics which is hypotonic. Thus, ultrafiltration removes more sodium and less potassium for the same volume compared with diuretics and may offer benefits related to maintain electrolyte balance [85]. Additionally, the rate of fluid removal can be titrated so that it does not does not exceed the interstitial fluid mobilization rate, preserving intravascular volume and avoiding the acute renal insufficiency the may occur with diuretic therapy [66, 86].
The first prospective, randomized, multicenter study comparing ultrafiltration with intravenous diuretic therapy in patients with heart failure and volume overload, the Ultrafiltration versus Intravenous Diuretics for Patients Hospitalized for Acute Decompensated Congestive Heart Failure (UNLOAD) trial, randomized 200 patients within 24 hours of hospital admission to either ultrafiltration or standard care with intravenous diuretics administered via continuous infusion or bolus injections [87]. At 48 hours, both groups had similar relief of dyspnea, but the ultrafiltration group had greater net fluid loss and greater weight loss. Both groups had similar length of hospital stay. At 90 days, the ultrafiltration group had fewer rehospitalizations and unscheduled clinic or emergency department visits. There were no differences in serum creatinine changes between the groups, and both groups had a similar number of deaths [87]. Further analysis comparing ultrafiltration to continuous intravenous diuretic therapy and to bolus intravenous diuretic therapy revealed similar degree of weight and fluid loss between the ultrafiltration and continuous infusion groups and between the continuous infusion and bolus dosing groups, but a greater degree of weight and fluid loss in the ultrafiltration group compared to the bolus dosing group [85]. However, despite similar weight and volume loss in the ultrafiltration and continuous infusion groups, there were fewer rehospitalizations and unscheduled visits to the clinic or emergency room in the ultrafiltration group [85]. Notably, the number of events was low and these findings warrant further validation in larger adequately powered studies.
Despite the favorable outcomes for ultrafiltration in patients with heart failure and volume overload, the outcomes may be different in patients with worsening renal function in the setting of decompensated heart failure and volume overload, as assessed in the Cardiorenal Rescue Study in Acute Decompensated Heart Failure (CARRESS-HF) study [88]. In this prospective randomized study, 188 patients with acute decompensated heart failure, worsening renal function with a rise in serum creatinine ≥0.3 mg/dL from baseline, and persistent congestion were randomized to ultrafiltration or a stepped pharmacologic therapy to maintain a urine output of 3–5 l/day. While the weight loss was similar between the groups at 96 h, the ultrafiltration group experienced a greater increase in serum creatinine. Furthermore, the ultrafiltration group had a higher rate of serious adverse events over the follow-up period of 60 days. At 60 days, there were no significant differences in weight loss, mortality, or rehospitalizations between the groups, and both groups had lower creatinine levels compared to baseline levels [88]. The difference in outcomes in these two trials highlights the complexity of implementing this novel technique to treat patients with volume overload. Current guidelines recommend consideration of ultrafiltration for relief of volume overload or for refractory congestion not responding to medical therapy [21].
Summary
Volume overload occurs in heart failure because of pathologic changes in hemodynamics and neurohormonal activation. Congestion is a major cause of morbidity and mortality in patients with heart failure, and thus it must be accurately recognized and adequately treated. The diagnosis of volume overload is often made based on patient and clinician assessments, though radiographic and echocardiographic findings and serum biomarker measurements can help confirm the diagnosis and monitor the effectiveness of treatment. Implantable devices to measure filling pressures are being developed and tested to provide additional information to incorporate into the overall clinical picture of congestion. Invasive hemodynamic monitoring can be pursued for cases in which noninvasive assessments are inadequate or confounded.
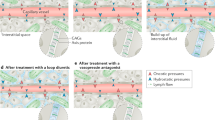
Treatment of volume overload consists of pharmacologic and mechanical strategies (Fig. 4.6) [89]. Diuretics increase urinary sodium and water excretion, with different classes of diuretics acting at different sites in the kidneys—loop diuretics at the loop of Henle, thiazide diuretics at the distal tubule, and potassium sparing diuretics and vasopressor receptor antagonists at the collecting tubule. When escalating doses of diuretics are ineffective, volume removal may be achieved with ultrafiltration, a process in which plasma water is removed from whole blood across a semipermeable membrane. Ultrafiltration, which once require central venous catheter placement, can now be performed through peripheral venous access.
Management of volume overload in heart failure (Modified and reproduced with permission from Mentz et al. [89])
Conclusions
Heart failure is a considerable public health problem worldwide. In this chapter, we reviewed the diagnosis and treatment of volume overload, one of the major sources of morbidity and mortality in heart failure. Despite the current assessment and management tools available to clinicians, the burden of heart failure remains high, highlighting the need for development of novel tools and strategies to improve outcomes in this patient population.
References
Braunwald E. Heart failure. JACC Heart Fail. 2013;1(1):1–20.
Mozaffarian D, Benjamin EJ, Go AS, et al. Executive summary: heart disease and stroke statistics-2016 update: a report from the American Heart Association. Circulation. 2016;133(4):447–54.
Gheorghiade M, Filippatos G, De Luca L, Burnett J. Congestion in acute heart failure syndromes: an essential target of evaluation and treatment. Am J Med. 2006;119(12 Suppl 1):S3–10.
Ambrosy AP, Pang PS, Khan S, et al. Clinical course and predictive value of congestion during hospitalization in patients admitted for worsening signs and symptoms of heart failure with reduced ejection fraction: findings from the EVEREST trial. Eur Heart J. 2013;34(11):835–43.
Lucas C, Johnson W, Hamilton MA, et al. Freedom from congestion predicts good survival despite previous class IV symptoms of heart failure. Am Heart J. 2000;140(6):840–7.
Patterson SW, Piper H, Starling EH. The regulation of the heart beat. J Physiol. 1914;48(6):465–513.
Schiff GD, Fung S, Speroff T, McNutt RA. Decompensated heart failure: symptoms, patterns of onset, and contributing factors. Am J Med. 2003;114(8):625–30.
Fallick C, Sobotka PA, Dunlap ME. Sympathetically mediated changes in capacitance: redistribution of the venous reservoir as a cause of decompensation. Circ Heart Fail. 2011;4(5):669–75.
Schrier RW, Abraham WT. Hormones and hemodynamics in heart failure. N Engl J Med. 1999;341(8):577–85.
Nohria A, Hasselblad V, Stebbins A, et al. Cardiorenal interactions: insights from the ESCAPE trial. J Am Coll Cardiol. 2008;51(13):1268–74.
Ahmed M, Hill J. A rational approach to assess volume status in patients with decompensated heart failure. Curr Heart Fail Rep. 2012;9(2):139–47.
West JB, Mathieu-Costello O. Vulnerability of pulmonary capillaries in heart disease. Circulation. 1995;92(3):622–31.
Binanay C, Califf RM, Hasselblad V, et al. Evaluation study of congestive heart failure and pulmonary artery catheterization effectiveness: the ESCAPE trial. JAMA. 2005;294(13):1625–33.
Thibodeau JT, Turer AT, Gualano SK, et al. Characterization of a novel symptom of advanced heart failure: bendopnea. JACC Heart Fail. 2014;2(1):24–31.
Szidon JP. Pathophysiology of the congested lung. Cardiol Clin. 1989;7(1):39–48.
Drazner MH, Hamilton MA, Fonarow G, Creaser J, Flavell C, Stevenson LW. Relationship between right and left-sided filling pressures in 1000 patients with advanced heart failure. J Heart Lung Transplant. 1999;18(11):1126–32.
Drazner MH, Brown RN, Kaiser PA, et al. Relationship of right- and left-sided filling pressures in patients with advanced heart failure: a 14-year multi-institutional analysis. J Heart Lung Transplant. 2012;31(1):67–72.
Nohria A, Tsang SW, Fang JC, et al. Clinical assessment identifies hemodynamic profiles that predict outcomes in patients admitted with heart failure. J Am Coll Cardiol. 2003;41(10):1797–804.
Stevenson LW. Are hemodynamic goals viable in tailoring heart failure therapy? Hemodynamic goals are relevant. Circulation. 2006;113(7):1020–7; discussion 1033.
Allen LA, Rogers JG, Warnica JW, et al. High mortality without ESCAPE: the registry of heart failure patients receiving pulmonary artery catheters without randomization. J Card Fail. 2008;14(8):661–9.
Yancy CW, Jessup M, Bozkurt B, et al. 2013 ACCF/AHA guideline for the management of heart failure: executive summary: a report of the American College of Cardiology Foundation/American Heart Association Task Force on practice guidelines. Circulation. 2013;128(16):1810–52.
Daniels LB, Maisel AS. Natriuretic peptides. J Am Coll Cardiol. 2007;50(25):2357–68.
Maisel AS, Krishnaswamy P, Nowak RM, et al. Rapid measurement of B-type natriuretic peptide in the emergency diagnosis of heart failure. N Engl J Med. 2002;347(3):161–7.
Januzzi Jr JL, Camargo CA, Anwaruddin S, et al. The N-terminal Pro-BNP investigation of dyspnea in the emergency department (PRIDE) study. Am J Cardiol. 2005;95(8):948–54.
Liquori ME, Christenson RH, Collinson PO, Defilippi CR. Cardiac biomarkers in heart failure. Clin Biochem. 2014;47:327–37.
Maeda K, Tsutamoto T, Wada A, Hisanaga T, Kinoshita M. Plasma brain natriuretic peptide as a biochemical marker of high left ventricular end-diastolic pressure in patients with symptomatic left ventricular dysfunction. Am Heart J. 1998;135(5 Pt 1):825–32.
Kazanegra R, Cheng V, Garcia A, et al. A rapid test for B-type natriuretic peptide correlates with falling wedge pressures in patients treated for decompensated heart failure: a pilot study. J Card Fail. 2001;7(1):21–9.
Di Angelantonio E, Chowdhury R, Sarwar N, et al. B-type natriuretic peptides and cardiovascular risk: systematic review and meta-analysis of 40 prospective studies. Circulation. 2009;120(22):2177–87.
van Veldhuisen DJ, Linssen GC, Jaarsma T, et al. B-type natriuretic peptide and prognosis in heart failure patients with preserved and reduced ejection fraction. J Am Coll Cardiol. 2013;61(14):1498–506.
Redfield MM, Rodeheffer RJ, Jacobsen SJ, Mahoney DW, Bailey KR, Burnett Jr JC. Plasma brain natriuretic peptide concentration: impact of age and gender. J Am Coll Cardiol. 2002;40(5):976–82.
Wang TJ, Larson MG, Levy D, et al. Impact of age and sex on plasma natriuretic peptide levels in healthy adults. Am J Cardiol. 2002;90(3):254–8.
Wang TJ, Larson MG, Levy D, et al. Impact of obesity on plasma natriuretic peptide levels. Circulation. 2004;109(5):594–600.
Drazner MH, de Lemos JA. Unexpected BNP levels in patients with advanced heart failure: a tale of caution and promise. Am Heart J. 2005;149(2):187–9.
de Lemos JA, McGuire DK, Drazner MH. B-type natriuretic peptide in cardiovascular disease. Lancet. 2003;362(9380):316–22.
O’Neill JO, Bott-Silverman CE, McRae 3rd AT, et al. B-type natriuretic peptide levels are not a surrogate marker for invasive hemodynamics during management of patients with severe heart failure. Am Heart J. 2005;149(2):363–9.
Andreassi MG, Del Ry S, Palmieri C, Clerico A, Biagini A, Giannessi D. Up-regulation of ‘clearance’ receptors in patients with chronic heart failure: a possible explanation for the resistance to biological effects of cardiac natriuretic hormones. Eur J Heart Fail. 2001;3(4):407–14.
Goetz KL. Physiology and pathophysiology of atrial peptides. Am J Physiol. 1988;254(1 Pt 1):E1–15.
Cuneo RC, Espiner EA, Nicholls MG, Yandle TG, Livesey JH. Effect of physiological levels of atrial natriuretic peptide on hormone secretion: inhibition of angiotensin-induced aldosterone secretion and renin release in normal man. J Clin Endocrinol Metab. 1987;65(4):765–72.
Solomon SDB, Bernard E, editors. Essential echocardiography: a practical handbook. Totowa: Humana Press; 2007.
Wang L, Lahtinen S, Lentz L, et al. Feasibility of using an implantable system to measure thoracic congestion in an ambulatory chronic heart failure canine model. Pacing Clin Electrophysiol. 2005;28(5):404–11.
Abraham WT. Intrathoracic impedance monitoring for early detection of impending heart failure decompensation. Congest Heart Fail (Greenwich, Conn). 2007;13(2):113–5.
Yu CM, Wang L, Chau E, et al. Intrathoracic impedance monitoring in patients with heart failure: correlation with fluid status and feasibility of early warning preceding hospitalization. Circulation. 2005;112(6):841–8.
Abraham WT, Compton S, Haas G, et al. Intrathoracic impedance vs daily weight monitoring for predicting worsening heart failure events: results of the Fluid Accumulation Status Trial (FAST). Congest Heart Fail (Greenwich, Conn). 2011;17(2):51–5.
Small RS, Wickemeyer W, Germany R, et al. Changes in intrathoracic impedance are associated with subsequent risk of hospitalizations for acute decompensated heart failure: clinical utility of implanted device monitoring without a patient alert. J Card Fail. 2009;15(6):475–81.
Whellan DJ, Ousdigian KT, Al-Khatib SM, et al. Combined heart failure device diagnostics identify patients at higher risk of subsequent heart failure hospitalizations: results from PARTNERS HF (Program to Access and Review Trending Information and Evaluate Correlation to Symptoms in Patients with Heart Failure) study. J Am Coll Cardiol. 2010;55(17):1803–10.
Small RS, Whellan DJ, Boyle A, et al. Implantable device diagnostics on day of discharge identify heart failure patients at increased risk for early readmission for heart failure. Eur J Heart Fail. 2014;16(4):419–25.
Whellan DJ, Sarkar S, Koehler J, et al. Development of a method to risk stratify patients with heart failure for 30-day readmission using implantable device diagnostics. Am J Cardiol. 2013;111(1):79–84.
Tang WH, Warman EN, Johnson JW, Small RS, Heywood JT. Threshold crossing of device-based intrathoracic impedance trends identifies relatively increased mortality risk. Eur Heart J. 2012;33(17):2189–96.
van Veldhuisen DJ, Braunschweig F, Conraads V, et al. Intrathoracic impedance monitoring, audible patient alerts, and outcome in patients with heart failure. Circulation. 2011;124(16):1719–26.
Adamson PB, Magalski A, Braunschweig F, et al. Ongoing right ventricular hemodynamics in heart failure: clinical value of measurements derived from an implantable monitoring system. J Am Coll Cardiol. 2003;41(4):565–71.
Magalski A, Adamson P, Gadler F, et al. Continuous ambulatory right heart pressure measurements with an implantable hemodynamic monitor: a multicenter, 12-month follow-up study of patients with chronic heart failure. J Card Fail. 2002;8(2):63–70.
Bourge RC, Abraham WT, Adamson PB, et al. Randomized controlled trial of an implantable continuous hemodynamic monitor in patients with advanced heart failure: the COMPASS-HF study. J Am Coll Cardiol. 2008;51(11):1073–9.
Ducharme A, Doyon O, White M, Rouleau JL, Brophy JM. Impact of care at a multidisciplinary congestive heart failure clinic: a randomized trial. CMAJ. 2005;173(1):40–5.
McAlister FA, Stewart S, Ferrua S, McMurray JJ. Multidisciplinary strategies for the management of heart failure patients at high risk for admission: a systematic review of randomized trials. J Am Coll Cardiol. 2004;44(4):810–9.
Walton AS, Krum H. The heartpod implantable heart failure therapy system. Heart Lung Circ. 2005;14 Suppl 2:S31–3.
Ritzema J, Melton IC, Richards AM, et al. Direct left atrial pressure monitoring in ambulatory heart failure patients: initial experience with a new permanent implantable device. Circulation. 2007;116(25):2952–9.
Ritzema J, Troughton R, Melton I, et al. Physician-directed patient self-management of left atrial pressure in advanced chronic heart failure. Circulation. 2010;121(9):1086–95.
Verdejo HE, Castro PF, Concepcion R, et al. Comparison of a radiofrequency-based wireless pressure sensor to swan-ganz catheter and echocardiography for ambulatory assessment of pulmonary artery pressure in heart failure. J Am Coll Cardiol. 2007;50(25):2375–82.
Adamson PB, Abraham WT, Aaron M, et al. CHAMPION trial rationale and design: the long-term safety and clinical efficacy of a wireless pulmonary artery pressure monitoring system. J Card Fail. 2011;17(1):3–10.
Abraham WT, Adamson PB, Bourge RC, et al. Wireless pulmonary artery haemodynamic monitoring in chronic heart failure: a randomised controlled trial. Lancet. 2011;377(9766):658–66.
Ernst ME, Moser M. Use of diuretics in patients with hypertension. N Engl J Med. 2009;361(22):2153–64.
Rose BD. Diuretics. Kidney Int. 1991;39(2):336–52.
Wargo KA, Banta WM. A comprehensive review of the loop diuretics: should furosemide be first line? Ann Pharmacother. 2009;43(11):1836–47.
Ramsay F, Crawford RJ, Allman S, Bailey R, Martin A. An open comparative study of two diuretic combinations, frusemide/amiloride (‘Frumil’) and bumetanide/potassium chloride (‘Burinex’ K), in the treatment of congestive cardiac failure in hospital out-patients. Curr Med Res Opin. 1988;10(10):682–9.
Felker GM, Lee KL, Bull DA, et al. Diuretic strategies in patients with acute decompensated heart failure. N Engl J Med. 2011;364(9):797–805.
Felker GM, Mentz RJ. Diuretics and ultrafiltration in acute decompensated heart failure. J Am Coll Cardiol. 2012;59(24):2145–53.
Jentzer JC, DeWald TA, Hernandez AF. Combination of loop diuretics with thiazide-type diuretics in heart failure. J Am Coll Cardiol. 2010;56(19):1527–34.
Cohn JN. The management of chronic heart failure. N Engl J Med. 1996;335(7):490–8.
Duke M. Thiazide-induced hypokalemia. Association with acute myocardial infarction and ventricular fibrillation. JAMA. 1978;239(1):43–5.
Goyal A, Spertus JA, Gosch K, et al. Serum potassium levels and mortality in acute myocardial infarction. JAMA. 2012;307(2):157–64.
Pitt B, Zannad F, Remme WJ, et al. The effect of spironolactone on morbidity and mortality in patients with severe heart failure. Randomized Aldactone Evaluation Study Investigators. N Engl J Med. 1999;341(10):709–17.
Pitt B, Remme W, Zannad F, et al. Eplerenone, a selective aldosterone blocker, in patients with left ventricular dysfunction after myocardial infarction. N Engl J Med. 2003;348(14):1309–21.
Zannad F, McMurray JJ, Krum H, et al. Eplerenone in patients with systolic heart failure and mild symptoms. N Engl J Med. 2011;364(1):11–21.
Rossignol P, Menard J, Fay R, Gustafsson F, Pitt B, Zannad F. Eplerenone survival benefits in heart failure patients post-myocardial infarction are independent from its diuretic and potassium-sparing effects. Insights from an EPHESUS (Eplerenone Post-Acute Myocardial Infarction Heart Failure Efficacy and Survival Study) substudy. J Am Coll Cardiol. 2011;58(19):1958–66.
Domanski M, Norman J, Pitt B, Haigney M, Hanlon S, Peyster E. Diuretic use, progressive heart failure, and death in patients in the Studies Of Left Ventricular Dysfunction (SOLVD). J Am Coll Cardiol. 2003;42(4):705–8.
Domanski M, Tian X, Haigney M, Pitt B. Diuretic use, progressive heart failure, and death in patients in the DIG study. J Card Fail. 2006;12(5):327–32.
Felker GM. Diuretic management in heart failure. Congest Heart Fail (Greenwich, Conn). 2010;16 Suppl 1:S68–72.
Hasselblad V, Gattis Stough W, Shah MR, et al. Relation between dose of loop diuretics and outcomes in a heart failure population: results of the ESCAPE trial. Eur J Heart Fail. 2007;9(10):1064–9.
Greenberg A, Verbalis JG. Vasopressin receptor antagonists. Kidney Int. 2006;69(12):2124–30.
Goldsmith SR, Gheorghiade M. Vasopressin antagonism in heart failure. J Am Coll Cardiol. 2005;46(10):1785–91.
Gheorghiade M, Niazi I, Ouyang J, et al. Vasopressin V2-receptor blockade with tolvaptan in patients with chronic heart failure: results from a double-blind, randomized trial. Circulation. 2003;107(21):2690–6.
Gheorghiade M, Konstam MA, Burnett Jr JC, et al. Short-term clinical effects of tolvaptan, an oral vasopressin antagonist, in patients hospitalized for heart failure: the EVEREST Clinical Status Trials. JAMA. 2007;297(12):1332–43.
Konstam MA, Gheorghiade M, Burnett Jr JC, et al. Effects of oral tolvaptan in patients hospitalized for worsening heart failure: the EVEREST Outcome Trial. JAMA. 2007;297(12):1319–31.
Jaski BE, Ha J, Denys BG, Lamba S, Trupp RJ, Abraham WT. Peripherally inserted veno-venous ultrafiltration for rapid treatment of volume overloaded patients. J Card Fail. 2003;9(3):227–31.
Costanzo MR, Saltzberg MT, Jessup M, Teerlink JR, Sobotka PA. Ultrafiltration is associated with fewer rehospitalizations than continuous diuretic infusion in patients with decompensated heart failure: results from UNLOAD. J Card Fail. 2010;16(4):277–84.
Marenzi G, Lauri G, Grazi M, Assanelli E, Campodonico J, Agostoni P. Circulatory response to fluid overload removal by extracorporeal ultrafiltration in refractory congestive heart failure. J Am Coll Cardiol. 2001;38(4):963–8.
Costanzo MR, Guglin ME, Saltzberg MT, et al. Ultrafiltration versus intravenous diuretics for patients hospitalized for acute decompensated heart failure. J Am Coll Cardiol. 2007;49(6):675–83.
Bart BA, Goldsmith SR, Lee KL, et al. Ultrafiltration in decompensated heart failure with cardiorenal syndrome. N Engl J Med. 2012;367(24):2296–304.
Mentz RJ, Kjeldsen K, Rossi GP, et al. Decongestion in acute heart failure. Eur J Heart Fail. 2014;16(5):471–82.
Sica D. Newer antihypertensive agents. Atlas of Hypertension. Ed. N. Hollenberg. New York: Springer, 2003. p. 301–24.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2016 Springer International Publishing Switzerland
About this chapter
Cite this chapter
Cooper, L.B., Mentz, R.J. (2016). Volume Assessment and Management: Medical and Device Therapies. In: Ventura, H. (eds) Pharmacologic Trends of Heart Failure. Current Cardiovascular Therapy. Springer, Cham. https://doi.org/10.1007/978-3-319-30593-6_4
Download citation
DOI: https://doi.org/10.1007/978-3-319-30593-6_4
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-30591-2
Online ISBN: 978-3-319-30593-6
eBook Packages: MedicineMedicine (R0)