Abstract
Fetal magnetic resonance imaging (MRI) is nowadays an established second-line imaging modality next to prenatal ultrasound (US) [1–3]. Ultrafast fetal MR sequences allow to “picture freeze” the fetus in utero without the need for sedation. The goal of fetal MRI is to offer an enhanced visualization and characterization of the pathologies detected by routine prenatal sonography, and it is therefore used as a problem-solving technique in a selected patient population. Prenatal sonography remains the imaging modality of choice for evaluating disorders related to the fetus and pregnancy; however, occasionally fetal MRI can identify subtle lesions that remained uncovered by US but may be essential for accurate diagnosis. Furthermore, fetal MRI contributes to selection of intrauterine treatment options and obstetric management, determines immediate postnatal care, and enhances parental counseling.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Fetal magnetic resonance imaging
- Fetal development
- Brain malformation
- Spinal malformation
- Brain pathology
- Spinal pathology
Introduction
Fetal magnetic resonance imaging (MRI) is nowadays an established second-line imaging modality next to prenatal ultrasound (US) [1–3]. Ultrafast fetal MR sequences allow to “picture freeze” the fetus in utero without the need for sedation. The goal of fetal MRI is to offer an enhanced visualization and characterization of the pathologies detected by routine prenatal sonography, and it is therefore used as a problem-solving technique in a selected patient population. Prenatal sonography remains the imaging modality of choice for evaluating disorders related to the fetus and pregnancy; however, occasionally fetal MRI can identify subtle lesions that remained uncovered by US but may be essential for accurate diagnosis. Furthermore, fetal MRI contributes to selection of intrauterine treatment options and obstetric management, determines immediate postnatal care, and enhances parental counseling.
MRI of the fetal brain and spine provides a higher sensitivity and specificity compared to prenatal US, because of several inherent US limitations. Depending on the maternal habitus, maternal bowel gasses, amount of amniotic fluid (e.g., oligohydramnios), and positioning of the child in utero (e.g., fetal head descended in the maternal bony pelvis), visualization of the fetal central nervous system (CNS) may be limited by US. Furthermore, the progressing development of the fetal cartilaginous/osseous calvarium and spinal canal during pregnancy may obscure lesions close to the skull or vertebral bodies on US examination [4–6].
The goals of this manuscript are to become familiar with the imaging protocol and indications for fetal MRI of the CNS, enhance your expertise about interpreting fetal MRI of the brain and spine, and become familiar with developmental variants of the fetal brain and spine.
Clinical Indications of Fetal MRI of the Brain and Spine
In general, fetal MRI is indicated in the case of sonographic visualization or suggestion of a CNS pathology of which the etiology or exact constellation of findings remains uncertain [7]. In addition, fetal MRI should be considered if an unexplained spontaneous abortion occurred in a previous pregnancy or if there is a positive family history for CNS abnormalities. Recent studies show that in 30–50% of fetuses undergoing second-line fetal MRI, additional abnormalities of variable clinical significance can be diagnosed [1, 2, 8]. Table 1 summarizes accepted clinical indications for fetal MRI of the CNS. A few specific congenital spinal abnormalities (e.g., non-skin-covered spinal dysraphia) are potentially treatable by means of fetal surgery. In those cases, fetal MRI has become the gold standard for confirming the diagnosis and guides the decision-making for the various surgical options [9, 10].
The appropriate gestational age for fetal MR imaging of the brain and spine is depending on the clinical indication. MR imaging of the fetus prior to 18 weeks of gestational age is of limited diagnostic value because of small fetal size and high fetal mobility. In general, fetal MR imaging of the CNS is performed between 20 and 23 weeks of gestational age to be able to adjust clinical decision-making during pregnancy and prenatal counseling. Fetal MR imaging later than 24 weeks of gestational age can contribute to decisions on treatment options, delivery modus, and prenatal counseling.
Finally, it should never be forgotten that fetal MRI should not only focus on the CNS. Many pathologies may affect multiple functional systems and organs in the fetus simultaneously. Identification of the complete entirety of fetal pathology may not only facilitate final diagnosis but may also have a significant impact on fetal prognosis, obstetric management, and parental counseling. Imaging the fetus should also include evaluation of the umbilical cord, amniotic fluid, placenta, and maternal uterus. Fetal MRI should be performed by experts on fetal pathology combining expertise on fetal CNS and non-CNS pathology.
Imaging Technique of Fetal MRI of the Brain and Spine
Standard MRI Sequences
Typically, fetal MRI is performed at 1.5 T magnetic field strength; 3 T MR units are however progressively used. Multichannel phased array surface coils are used for data collection. Imaging is done without fetal or maternal sedation. Ultrafast single-shot T2-weighted half-Fourier sequences serve as the backbone sequence of fetal MRI. The ultrashort acquisition times per slice (<800 msec) and single-shot approach allow to image the fetal anatomy in exquisite detail. In particular, the fetal brain and spinal cord can be well evaluated due to their high water content which guarantees a strong MR signal. In addition, the T2 hyperintense cerebrospinal fluid which surrounds the fetal brain and spinal cord enhances depiction of the neuroanatomy. Depending on the suspected or encountered pathology (e.g., hemorrhage, fat), additional sequential T1-weighted imaging may be added. Unfortunately, currently no similar ultrafast T1-weighted sequences are available. Consequently, T1-weighted imaging still suffers from some motion artifacts. Triplanar imaging adapted to the fetal brain is collected to facilitate diagnoses.
Advanced MRI Techniques
The application of advanced MRI techniques can serve as problem solver in selected cases and is mainly applied to imaging of the fetal brain. Nevertheless, the use of advanced MRI techniques in assessing the fetal brain and spine needs to be studied more comprehensively before standard application is possible. The relative long acquisition times of advanced MRI techniques result in the principal use of these techniques in the last trimester of pregnancy because of descending of the fetal head and therefore reduced fetal motion.
Diffusion-weighted imaging in general is used to evaluate water motion and tissue characteristics. Including DWI sequences in imaging of the fetal brain provides information about developmental, metabolic, or ischemic brain processes. Diffusion tensor imaging is typically used for fetal CNS malformations.
1H MR spectroscopy is sporadically used to detect the presence of normal or abnormal metabolites in the fetal brain parenchyma. Similar to the DWI/DTI sequences, 1H MRS is due to its longer acquisition time susceptible for fetal motion.
Depending on your local settings and suspected pathology, the imaging protocols will vary combining standard anatomical sequences with a variety of advanced, functional sequences. We refer here to the extensive literature available.
Finally, fetal MRI studies should always be correlated with the prenatal US imaging findings. Fetal MRI should avoid to be considered a “stand-alone” imaging. In many institutions, prenatal US will be either revisited if available by the reading radiologist or will be repeated prior to the fetal MRI study.
Safety of Fetal MRI of the Brain and Spine
The available research studies investigating the safety and potential risks of routine clinical fetal MRI have not been able to determine any adverse effects affecting the mother or developing fetus. The American College of Radiology has published a practice guideline for the safe and optimal performance of fetal MRI in 2010. This guideline was revised collaboratively by the American College of Radiology (ACR) and the Society for Pediatric Radiology (SPR) in 2015. In the guideline is stated that no special consideration is recommended for any trimester in pregnancy to undergo MRI examination involving 3T or weaker magnetic fields if, in the determination of a level 2 MR personnel-designated attending radiologist, the risk-benefit ratio to the patients warrants that the MRI study should be performed [3]. The radiologist should review the indications and document them in the radiology report or the patient’s medical record. The radiologist should be cognizant of the increased power deposition (long exposure times, high specific absorption rate) typically accompanying some higher magnetic field studies and ensure that they do not exceed established guidelines [3]. MRI contrast agents should not be routinely administered to pregnant patients. Gadolinium is a pregnancy class C drug, meaning that the safety in humans has not been proven [3]. In addition due to recirculation phenomena, gadolinium-based contrast agents will remain for an extended period in the fetal circulation if injected to the mother.
In consideration of the possible side effects to the developing fetus as well as the present limitations of fetal MRI of the brain and spine at an early gestational age, fetal MRI examination is preferably not performed prior to 18 weeks of gestation.
Normal Development of the Fetal Brain and Spine
The development of the fetal brain encompasses multiple programmed, interacting, and complex processes including neuronal cell proliferation and migration (histogenesis), cortical sulcation/gyration, and white matter myelination to mention a few key processes. The brain development does not stop postnatally but extends well into the first and partially second decade of life. Familiarity with the normal and abnormal brain embryology and subsequent maturation is a sine qua non to each radiologist for correct identification of pathologies on fetal MRI.
Neuronal Proliferation and Migration
The migration of neurons begins during the sixth week of embryologic development. Neuroectodermal elements proliferate within and migrate from the germinal matrix in the direction of the surface of the brain, developing cortical ribbon. The high cellular germinal matrix is a low T2 signal intensity band surrounding the walls of the lateral ventricles (pronounced at the level of the frontal and temporal horns). During the migration process, the germinal matrix involutes gradually (Fig. 1). The germinal matrix persists in the caudothalamic groove until several months after birth [11–14]. The earliest migrating neurons give rise to the deep primitive cerebral cortex (inner layer) followed by neurons creating the outer superficial layer of the cortex. The next arriving neurons will build op the four intermediate cortical layers in an inside-out manner [7]. The migration process reaches completion around the 24th week of embryologic development. The cortical plate is extremely cellular and is identified as a smooth low T2 signal intensity band. Furthermore, an additional layer of neuroectodermal elements migrates toward the surface of the brain and discontinues their outward migration in the white matter before reaching the peripheral cortex (intermediate layer). This high cell density intermediate layer is seen as a low T2 signal intensity band between developmental weeks 20 and 28. The low cell density adjacent white matter shows high T2 signal intensity [13]. The advancing distribution of glial cells throughout the white matter after the 28th–30th week causes the multilayered pattern to be no longer visible on fetal MRI (Fig. 1). Likewise, by weeks 26–27 of embryologic development, the cerebellar hemispheres show a three-layered pattern matching the high cell density and low T2 signal intensity cerebellar cortex and dentate nuclei alternated with the intervening low cellularity, high T2 signal intensity cerebellar white matter [15].
Sagittal and coronal T2-weighted fetal magnetic resonance images of a normal developing fetal brain at 29 weeks of gestational age. The sagittal image (a) illustrates the normal development of the midline structures and vermis. The T2 hypointense signal in the posterior brain stem is related to the normal myelination process. The parieto-occipital fissure is clearly visible. The coronal image (b) demonstrates the germinal matrix as a thin residual T2 hypointense band lining the lateral ventricles. The low cellularity white matter reveals a T2 hyperintense signal intensity. The T2 hypointense signal of the cortical layer is caused by the high cell density in the cortex. Asymmetry in the closure of the Sylvian fissures is noted as normal variance; the left Sylvian fissure is more advanced in its closure than the right
Cortical Sulcation and Gyration
In the supratentorial brain, the primary lateral fissure and primary parieto-occipital sulcus are the first sulci visible on fetal MRI around the 18th week of embryologic development (Fig. 1). The primary calcarine sulcus, cingulate sulcus, and central sulcus are identified at, respectively, week 24, week 25, and week 27 of development. Cortical sulcation and gyration follow a specific pattern and serve as an indicator for cortical maturation. The number and depth of the primary sulci progress with fetal age, and they gradually mature into secondary and tertiary sulci. The process of sulcation and gyration will reach final contours in the 34th–35th developmental week.
In the infratentorial brain, the primary fissure of the cerebellar vermis can be identified in week 20 of embryologic development, followed by the horizontal fissure at the 21th developmental week. The volume of the fetal cerebellum is relatively small compared to the volume of the supratentorial brain [7, 16].
The appearance of sulci on fetal MRI demonstrates an approximate 2 week time delay compared to anatomic-pathologic identification of sulci in the fetal brain, because of the somewhat limited spatial resolution of fetal MRI of the brain compared to pathological-anatomical studies [17]. Proper knowledge of the temporal/sequential appearance of the sulci and neuronal migration is essential to identify subtle cortical and migrational anomalies.
Myelination
Brain myelination is visualized first in the sensory tracts of the posterior brain stem at week 20 of embryologic development (Fig. 1). The myelination process causes a decrease in water content and increase in cell density and lipid-containing myelin (intensely binding water molecules and thus decreasing the quantity of free water molecules) of the white matter and white matter tracts. Therefore, the myelinated regions of the brain convert to lower T2 signal intensity and higher T1 signal intensity on fetal MRI. Myelination is a strictly programmed process following a specific pattern from the spinal cord to the brain and in any portion of the brain from central to peripheral, caudal to cephalic, and occipital to temporal/frontal [11–13]. The posterior limb of the internal capsule appears myelinated on T1-weighted images in the 33th developmental week. In the subcortical perirolandic white matter, myelination is observed at week 35 of embryologic development on T1-weighted images.
Corpus Callosum
The formation of the corpus callosum starts in the eighth week of embryologic development. As of the 20th week, the completely formed corpus callosum should be detectable at fetal MRI as a low T2 signal intensity midline structure of global uniform thickness [7]. If the intended midline sagittal view is obtained a little off midline (because of fetal motion), the coronal images are helpful in assessing the integrity of the corpus callosum.
Cerebrospinal Fluid (CSF) Spaces
The lateral ventricles, particularly the trigones and occipital horns, as well as the extra-axial CSF spaces are relatively large till about week 24 of embryologic development. Because of increase in brain volume and brain surface, the lateral ventricles and extra-axial CSF spaces appear less prominent with increasing fetal age. The ventricular dimensions are rather stable during fetal brain development, with an upper limit of 10–12 mm measured at the level of the atria in the axial plane [11]. Furthermore, the vacuolization of the primary meninges progresses from ventral to dorsal and posterior to anterior potentially causing accumulation of CSF in temporal and parieto-occipital regions resulting in persistent prominent CSF spaces in some fetuses [16].
Many review articles and textbooks are nowadays available summarizing the normal fetal brain development. In the author’s opinion, it is advisable to consult these documents while reading fetal brain studies. In many cases, “eyeballing” based upon experience may be sufficient; however, subtle deviations from normal development may easily be missed or remain under recognized.
Abnormalities of the Fetal Brain and Spine
Abnormalities of the developing brain and spine involve both so-called “true” congenital brain malformations (programmed maldevelopment) and acquired or secondary brain anomalies (resulting from injuries to already developed fetal brain structures). Between both groups there is however some overlap. A destructive event or injury early during gestation may not only injure already developed structures but may also induce an abnormal subsequent development. For example, a focal stroke early during gestation may result in a focal brain defect, but the concomitant injury to, e.g., the germinal matrix may result in an abnormal or impaired migration of surviving neuronal progenitors. In addition, there is a well-known occasionally confusing overlap between etiologies. Migrational abnormalities may be inherited (e.g., X-linked band heterotopia), part of syndromes (e.g., Aicardi syndrome), and secondary to metabolic disorders (Zellweger syndrome) or result from an infectious process (CMV infection), to mention a few. High-end fetal MRI may identify many abnormalities; however, final recognition of the etiology may be limited.
In the current review, we cannot cover all abnormalities that can be diagnosed by fetal MRI. We will focus on several well-known and more frequently fetal abnormalities that you may see in your daily practice.
Ventriculomegaly
Ventriculomegaly identified on prenatal US is one of the most frequent indications for fetal MRI. The goal is to differentiate between isolated ventriculomegaly (incidental ventriculomegaly due to, e.g., aqueductal stenosis (Fig. 2) or secondary ventriculomegaly due to intraventricular hemorrhage) and syndromal ventriculomegaly in which the observed widening of the ventricles only represents the tip of the iceberg of findings [12, 18]. Differentiation is especially important because of a significant difference in functional prognosis between isolated and syndromal ventriculomegaly. The widespread used definition of fetal ventriculomegaly is a transtrigone diameter of ≥ 10 mm in the axial plane at any stage of embryologic development [18]. The ventriculomegaly is categorized as mild (10–12 mm), moderate (13–15 mm), or severe (≥16 mm) based on the transtrigone diameter. Isolated ventriculomegaly may be secondary to a congenital aqueductal stenosis, postinfectious or posthemorrhagic obstruction of the Sylvian aqueduct, or arachnoid cysts blocking the normal CSF flow dynamics. Syndromal ventriculomegaly may be seen in a wide range of abnormalities including, e.g., Chiari II malformation, Dandy-Walker malformation, rhombencephalosynapsis, commissural anomalies, and migrational abnormalities. Furthermore, ventriculomegaly may be secondary to vascular anomalies like, e.g., a vein of Galen aneurysmal malformation in which the Sylvian aqueduct may be compressed by the dilated vein of Galen and in addition the increased (arterial) pressure in the venous system impairs CSF resorption and the chronic venous hypertension with venous ischemia induces a ventriculomegaly ex vacuo by progressive tissue loss (“melting brain”).
Axial and sagittal T2-weighted fetal magnetic resonance images of a fetus with isolated ventriculomegaly due to aqueductal stenosis. The axial image (a) shows severe supratentorial ventriculomegaly. The T2 hypointense band along the ventricles represents the germinal matrix. The midline sagittal T2-weighted MR image (b) reveals a normal-sized fourth ventricle. Normal anatomy and development of the posterior fossa structures are noted
Commissural Anomalies
Commissural anomalies represent a complex spectrum of malformations in which the various major commissures (corpus callosum, anterior commissure, and hippocampal commissure) may be absent, hypoplastic, malformed, or injured/destructed. Corpus callosum malformations are usually obvious on prenatal US. However, similar to the diagnostic workup of fetal ventriculomegaly, the corpus callosum pathology may just be the most obvious tip of the iceberg. Associated lesions, which may occur in up to 85% of cases, have to be excluded for decision-making and counseling [19]. The number of related abnormalities is extensive and includes Chiari II malformation, Dandy-Walker malformation, septo-optic dysplasia, gray matter heterotopia, gyration abnormalities, holoprosencephaly, schizencephaly, and encephaloceles (Fig. 3). Isolated corpus callosum anomalies are associated with delayed sulcation and encompass a possibility of altered neurodevelopment outcome.
Coronal and sagittal T2-weighted fetal magnetic resonance images of a fetus with corpus callosum agenesis and associated schizencephaly and periventricular heterotopia. The coronal image (a) shows the absence of the corpus callosum with the typical configuration of the moderate enlarged lateral ventricles. Associated periventricular heterotopia is noted. The sagittal T2-weighted MR image (b) reveals an additional unilateral open-lip schizencephaly in the parietal region. Incidental finding of a two-vessel umbilical cord
Indirect evidence of abnormalities of the corpus callosum are the absence of cavum septum pellucidum, dilated ventricular atria and occipital horns (colpocephaly), high-riding third-ventricle radiating medial hemispheric sulci, and the classic staghorn configuration of the lateral ventricles on coronal imaging [11, 19].
Differentiation between “syndromal” corpus callosum agenesis and thinned or destructed corpus callosum due to high-grade ventriculomegaly or adjacent white matter injury (e.g., stroke) is essential for prediction of outcome as well as risk stratification for recurrence in a future pregnancy.
Posterior Fossa Anomalies
A variety of abnormalities of the posterior fossa can be accurately assessed with fetal MRI. Fetal MRI frequently outperforms prenatal US in the correct identification and characterization of posterior fossa pathologies. For the correct identification, a high-resolution evaluation of the various components of the cerebellum, vermis, and brain stem is essential. Fetal MRI typically allows to identify and differentiate Dandy-Walker malformations (Fig. 4), Blake’s pouch cyst, posterior fossa arachnoid cysts, Chiari II/III malformations (Fig. 6), Joubert syndrome, rhombencephalosynapsis, cerebellar hypoplasia/aplasia, and rarer disorders like, e.g., muscle-eye-brain disease with ease. In addition, fetal MRI may identify posterior fossa hemorrhages and stroke. Many posterior fossa anomalies are associated with additional supratentorial brain anomalies (commissural abnormalities, ventriculomegaly, cortical malformations, or migrational disorders), and several entities are related to abnormalities of the spine (open, non-skin-covered spinal dysraphia) [20]. Fetal MRI of the posterior fossa may be limited early in gestation due to the small size of the infratentorial structures which can result in false-positive imaging findings [21]. Therefore, follow-up fetal MRI in case of apparent isolated anomalies of posterior fossa structures should be considered.
Axial and sagittal T2-weighted fetal magnetic resonance images. The axial image (a) shows significant enlargement of the fourth ventricle. The midline sagittal T2-weighted MR image (b) reveals an enlarged posterior fossa with high insertion of the torcular Herophili and tentorium. The vermis is hypoplastic and rotated upward. The fourth ventricle is widened
Cerebral Cortical Malformations or Migrational Anomalies
Fetal MRI is able to demonstrate sulcation/gyration disorders and neuronal migration disorders in better detail than prenatal US. The fetal skull may obscure detection of subtle cortical abnormalities on US. Sulcation/gyration and neuronal migration anomalies may originate from (a combination of) ischemic, infectious, metabolic, or genetic causes. Several more common cortical malformations include focal or diffuse polymicrogyria, focal cortical dysplasia, lissencephaly, and schizencephaly (Fig. 3) [20]. Periventricular or subcortical nodular gray matter heterotopia and band heterotopia are among the relative frequent migrational anomalies (Fig. 3) [22]. Additional associated findings consist of shallow sulci, focal atrophy, and white matter abnormalities [11]. The imaging characteristics of cortical malformations or migrational anomalies are similar to the characteristics described in detail for the postnatal period. The likelihood of detecting cortical malformations and migrational anomalies is determined by fetal age and the severity of the malformation. Identification of the malformation is potentially extremely difficult or impossible before the 20th–21th week of gestation. Subtle shape anomalies of the ventricular system or cerebral sulci may suggest adjacent migrational anomalies.
Ischemia and Hemorrhage
Focal or diffuse ischemia of the fetal brain can be the consequence of maternal hypoxic-ischemic conditions, placental abnormalities, or congenital vascular malformations of the fetal brain [12].
The potential causes of hemorrhage of the fetal brain include fetal stress, focal ischemia, congenital infections, coagulation disorders (congenital or drug induced), or congenital vascular malformations of the fetal brain [12]. Germinal matrix hemorrhage can occur in the intrauterine period with similar features as in premature neonates and likewise result in porencephalic cysts or periventricular leukomalacia. As a result of pronounced volume loss of the germinal matrix with high fibrinolytic activity, there is a significantly increased risk of germinal matrix hemorrhage in developmental weeks 26 to 28 [11]. Intraventricular hemorrhage is a risk factor for developing obstructive hydrocephalus. Cerebellar hemorrhage raises the possibility of an infectious etiology with possible additional extracranial abnormalities [20].
Ischemia as well as hemorrhage of the fetal brain attributes to abnormalities of the developing white matter, for instance, reduced parenchymal thickness, irregular ventricular margins, or destructive white matter lesions. An indicator for white matter damage early in embryologic development is nonappearance of the intermediate layer at fetal age less than 30 weeks [11, 15]. Furthermore, ischemia and hemorrhage in the fetal brain may derange the normal development of the brain, manifested as ventricular dilatation, cortical malformations, and abnormal sulcation [11, 12].
Congenital Infection
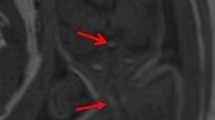
Cytomegalovirus is the most common congenital viral infection followed by varicella zoster virus and rubella (Fig. 5) [23]. A frequent parasitic infectious agent is Toxoplasma gondii. The associated effects of congenital infections on the developing fetal brain may be mild or extensive depending on the time point of infection in relation to the gestational age. The earlier, the more extensive the resultant destructive and malformative features are which may include migrational and cortical abnormalities, parenchymal calcifications, hemorrhages, white matter de-/dysmyelination, ventriculomegaly, microcephaly, cerebellar hypoplasia, and ocular anomalies. Several extracranial manifestations include intrauterine growth retardation, echogenic bowel, and fetal hydrops [19].
Axial and coronal T2-weighted fetal magnetic resonance images of a fetus with congenital cytomegalovirus infection. The axial image (a) shows cystic changes of the right anterior temporal lobe involving the cortical and subcortical region. The coronal image (b) reveals diffuse increase in T2 signal intensity of the cerebral white matter and mild widening of the bilateral Sylvian fissures. Additional subependymal cysts in the bilateral caudothalamic region are identified
Congenital Anomalies of the Spine
Fetal MRI has proven to be especially helpful for the detailed diagnostic workup of a wide range of spinal malformations including open and closed spinal dysraphias (Fig. 6), diastematomyelia, caudal regression syndrome, sacrococcygeal teratomas, segmentation and formation anomalies of the vertebral column, and several rare anomalies including terminal myelocystoceles or neuroenteric fistulas [19]. In addition, associated brain abnormalities (posterior fossa anomalies, Chiari II malformation (Fig. 6)) and assessment and planning of fetal surgery of neural tube defects benefit from high-resolution fetal MRI of the brain and spine [15]. In addition, fetal MRI of the spine serves as a confirmative imaging technique after prenatal US has suggested diagnosis.
Sagittal and axial T2-weighted fetal magnetic resonance images of a fetus with a Chiari II malformation and associated non-skin-covered lumbar myelomeningocele. The sagittal image of the spine (a) shows the spinal defect in the lumbar region with a protruding cystic myelomeningocele. The axial image at the level of the lumbar spinal defect (b) demonstrates the stretched T2 hypointense nerve roots coursing from the widened spinal canal to the neural placode. Incidental finding of a horseshoe kidney. The sagittal image of the craniocervical junction (c) reveals a small posterior fossa and herniation of vermian and cerebellar structures into the upper cervical canal. Additional moderate to severe supratentorial ventriculomegaly is noted [1]
Conclusion
Prenatal sonography and fetal MRI are complimentary imaging techniques for the evaluation of the normal and abnormal development of the fetal CNS. Furthermore, fetal MRI can serve as a confirmative diagnostic tool to validate sonographically detected abnormalities. Definitive and complete knowledge of fetal pathologies is essential for prenatal, perinatal, and postnatal decision-making and for parental counseling.
The technical improvements of fetal MRI of the brain and spine have significantly contributed to the current detailed knowledge of normal fetal development and etiologic factors and pathogenic processes of congenital and acquired abnormalities of the CNS. Additional technical advances and ongoing research allow application of advanced imaging techniques (diffusion tensor imaging, magnetic resonance spectroscopy) in fetal neuroimaging, facilitating a better understanding of fetal pathologies.
References
Breysem L, Bosmans H, Dymarkowski S et al (2003) The value of fast MR imaging as an adjunct to ultrasound in prenatal diagnosis. Eur Radiol 13(7):1538–1548
Frates MC, Kumar AJ, Benson CB, Ward VL, Tempany CM (2004) Fetal anomalies: comparison of MR imaging and US for diagnosis. Radiology 232(2):398–404
Bulas DI, Levine D, Barth RA, Cassady CI, Estroff JA, Victoria D (2015) ACR-SPR practice parameter for the safe and optimal performance of fetal magnetic resonance imaging (MRI). Am College Radiol
Raybaud C, Levrier O, Brunel H et al (2003) MR imaging of fetal brain malformations. Childs Nerv Syst 19:455–470
Coakley FV, Glenn O, Qayyum A et al (2004) Fetal MRI: a developing technique for the developing patient. Am J Roentgenol 182:243–252
Aubry MC, Aubry JP, Dommergues M (2003) Sonographic prenatal diagnosis of central nervous system abnormalities. Childs Nerv Syst 19:391–402
Glenn OA, Barkovich AJ (2006) Magnetic resonance imaging of the fetal brain and spine: an increasing important tool in prenatal diagnosis, part 1. Am J Neuroradiol 27:1604–1611
Levine D, Barnes PD, Madsen JR et al (1997) Fetal central nervous system anomalies: MR imaging augments sonographic diagnosis. Radiology 204:635–642
Saleem SN (2014) Fetal MRI: an approach to practice: a review. JARE 5:507–523
Coakley FV (2001) Role of magnetic resonance imaging in fetal surgery. Top Magn Reson Imaging 12(1):39–51
Girard NJ, Chaumoitre K (2012) The brain in the belly: what and how of fetal neuroimaging? J Magn Reson Imaging 36(4):788–804
Huisman TAGM (2008) Fetal magnetic resonance imaging. Semin Roentgenol 43(4):314–336
Huisman TAGM, Martin E, Kubik-Huch R, Marincek B (2002) Fetal magnetic resonance imaging of the brain: technical considerations and normal brain development. Eur Radiol 12(8):1941–1951
Fogliarini C, Chaumoitre K, Chapon F, Fernandez C, Lévrier O, Figarella-Branger D, Girard N (2005) Assessment of cortical maturation with prenatal MRI. Part I: normal cortical maturation. Eur Radiol 15(8):1671–1685
Huisman TAGM, Martin E, Kubik-Huch R, Marincek B (2002) Fetal magnetic resonance imaging of the central nervous system. Eur Radiol 12(8):1952–1961
Girard NJ, Raybaud CA (2001) Ventriculomegaly and pericerebral CSF collections in the fetus: early stage of benign external hydrocephalus? Childs Nerv Syst 17:239–245
Garel C, Chantrel E, Brisse H et al (2001) Fetal cerebral cortex: normal gestational landmarks identified using prenatal MR imaging. Am J Neuroradiol 22:184–189
Griffiths PD, Reeves MJ, Morris JE, Mason G, Russell SA, Paley MNJ, Whitby EH (2010) A prospective study of fetuses with isolated ventriculomegaly investigated by antenatal sonography and in utero MR imaging. Am J Neuroradiol 31:106–111
Glenn OA, Barkovich AJ (2006) Magnetic resonance imaging of the fetal brain and spine: an increasing important tool in prenatal diagnosis, part 2. Am J Neuroradiol 27:1807–1814
Glen OA (2010) MR imaging of the fetal brain. Pediatr Radiol 40:68–81
Limperopoulos C, Robertson RL, Estroff JA et al (2006) Diagnosis of inferior vermian hypoplasia by fetal magnetic resonance imaging: potential pitfalls and neurodevelopmental outcome. Am J Obstet Gynecol 194:1070–1076
Fogliarini C, Chaumoitre K, Chapon F, Fernandez C, Lévrier O, Figarella-Branger D, Girard N (2005) Assessment of cortical maturation with prenatal MRI. Part II: abnormalities of cortical maturation. Eur Radiol 15(8):1781–1789
Hollier LM, Grissom H (2005) Human herpes viruses in pregnancy: cytomegalovirus, Epstein-Barr virus, and varicella zoster virus. Clin Perinatol 32:671–696
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2016 Springer International Publishing Switzerland
About this chapter
Cite this chapter
Dremmen, M.H.G., Ellen Grant, P., Huisman, T.A.G.M. (2016). Fetal MRI of the Brain and Spine. In: Hodler, J., Kubik-Huch, R., von Schulthess, G. (eds) Diseases of the Brain, Head and Neck, Spine 2016-2019. Springer, Cham. https://doi.org/10.1007/978-3-319-30081-8_23
Download citation
DOI: https://doi.org/10.1007/978-3-319-30081-8_23
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-30080-1
Online ISBN: 978-3-319-30081-8
eBook Packages: MedicineMedicine (R0)