Abstract
Aptamers are defined as new generation of nucleic acids which has recently presented the promising spesifications over to antibodies. They can be produced in vitro by Systematic Evolution of Ligands by EXponential Enrichment (SELEX), and have the ability to recognize selectively and sensitively their targets; protein, toxin, drug or cell targets. Thus, they have a wide range of applications in different areas, such as, drug delivery, imaging and biosensing. Accordingly, an increasing number of studies related to aptamer based sensors “aptasensors” have been introduced in the literature. The recent studies on development of aptasensor technologies, which were applied for toxin detection, have been overviewed herein.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
The rapid detection and monitoring of toxins in clinical fluids, environmental samples and foods require new approaches in order to expedite appropriate detection systems. Many toxins are secreted by bacteria during the course of infection and can be detected in low ng mL−1 quantities in urine or blood samples. Toxins in environmental samples can be introduced by industrial, agricultural, or military activity. Toxic compounds may also be found in environmental samples as a result of terrorist activity. Of particular concern for homeland defense are toxins that can be used as weapons; these include ricin, botulinum toxins, staphylococcal enterotoxin B (SEB), trichothecene mycotoxins, and saxitoxin [1]. Toxins also occur naturally in the food supplies. Mycotoxin contamination is a particular problem due to fungal infection of grains and peanuts and can still be present after food processing [2, 3]. While many cases of foodborne illnesses are caused by bacteria (e.g., salmonellosis, campylobacteriosis), a large number of illnesses are also caused by bacterial toxins, that have been secreted into the foodstuff during growth (e.g., Staphylococcus aureus enterotoxins, botulinum toxins) [4]. They also cause death in longterm. Due to their vital side effects, the advanced and faster detection protocols for toxins with better sensitivity and specifity has become an emerging necessity.
Aptamers are a class of new generation nucleic acids, which can recognize the target molecules specifically. Since their discovery in 1990 by Tuerk and Gold [5] they could be synthesized as single stranded DNA or RNA oligonucleotides using Systematic Evolution of Ligands by EXponential Enrichment (SELEX) method, which mimics the natural selection [5, 6] SELEX comprises tree fundamental steps; (i) the creation of a nucleic acid pool and the incubation with the target molecule, (ii) the generation of the specific bounds and separation of nonspecific bounds and finally (iii) the amplification of the bound molecules. Due to SELEX providing the design of the aptamer molecules, which have strong affinity to their targets, aptamers can be utilized to recognize a variety of (bio)molecules such as toxins [7] proteins [8–33] drugs [34–35] and even whole cells [36, 37]. Therefore, they have a great potential to apply for development of analysis systems toxins in the field of food [7, 34, 35, 38] medicine [8, 39]and environment [40, 41].
Biosensors are analytical devices, that are aimed to detect the analytes sensitively and selectively. Their structure allows to occur a specific response in the presence of the biological recognition element and the target molecule [10, 15, 18, 29–31]. Then, the response is converted into an electrical signal via a transducer. There are different types of transducers designed by using quartz cyrstral microscopy (QCM), surface plasmon resonance (SPR), optical or electrochemical techniques. Aptamers can be succesfully manipulated to develop biosensor systems and their combination is called as “aptasensors”. Aptamers are assessed in a wide range of biosensor designs due to their specifity against to analytes. Moreover, they promote the development more stable and robust platforms in comparison to antibodies which is a result of SELEX method. Consequently, there are many reports emphasizing the development of biosensors in combination with aptamer technology for detection of toxins [7, 42–62].
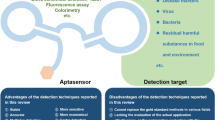
The recent studies on different aptasensor technologies, which were applied for detection of numerous toxins have been overviewed herein, and an aptasensor technology was simply represented in Scheme 1.
1.1 Electrochemical Aptasensors for Detection of Toxins
Toxins are small molecules produced from living organisms such as bacteria and fungus and have extremely serious effects on human health within very short time [42, 63, 64]. Their importance is about medical diagnosis, environmental monitoring, and food safety surveillance [42, 44, 63, 64]. Thus, monitoring of toxins via fast, reliable, sensitive and selective detection platforms has been gained attraction by researchers. In the meantime, aptamers were introduced in the field of development of biosensing platforms. One of them is electrochemical aptasensor technologies. Some approaches in the field of electrochemical aptasensors have been progressed for detection of toxins and given in Table 1 [35, 42–50, 64–70].
Ochratoxins are well-known by-products of numerous fungal species, which can contaminate not only foods, but also beverages including, coffee, beer, and wine. They are mainly produced in the Aspergillus and Penicillium genera [71]. Due to the fact that ochratoxin A (OTA) is known as the most toxic and has hepatotoxic, nephrotoxic, teratogenic and mutagenic effects onto a wide range of mammalian species [71–73], there are many electrochemical aptasensor applications in the literature to detect OTA [35, 65–67]. Zhang et al. [66] developed an electrochemical aptasensor by using gold electrode. They immobilized single stranded thiolated DNA aptamer labelled with biotin group onto the surface of gold electrode. The interaction of OTA and its DNA aptamer was then performed at the electrode surface and the interaction was determined in the presence of the resistance against TaqaI enzyme occurred after interaction process. Then, the enzymatic reaction between streptavidin-HRP and 3,3ʹ,5,5ʹ-tetramethylbenzidine sulfate (TMB) was monitored by using chronoamperometry technique.
In the study reported by Rhouati et al. [35], a fully automated flow electrochemical aptasensor based on the magnetic beads (MBs) was introduced and accordingly, direct and indirect competitive electrochemical assays were developed to monitor OTA. For fabrication of this direct assay, carboxylated aptamer modified MBs were immobilized onto the surface of screen printed carbon electrode (SPCE) placed in a flow cell. After the immobilization of avidin-ALP onto the surface of the electrode, the enzymatic reaction in the presence of 1-naphthyl phosphate was occurred and the oxidation of the electro-active product 1-naphtol phosphate to 1-iminoquinone was detected by using amperometry. For fabrication of indirect assay, OTA modified MBs were immobilized onto the surface of SPCE. The free OTA molecules and the immobilized OTA molecules were competed in the solution for binding of biotinylated DNA aptamer. The avidin-ALP was then conjugated and the enzymatic reaction was utilized. A lower limit of detection (0.05 µg/L) was obtained with the indirect flow-based aptasensor both of the electrochemical assays were tested in the presence of buffer, or beer samples.
Aflatoxins are known to be carcinogen and highly toxic secondary metabolites produced by Aspergillus flavus and Aspergillus parasiticus [46–48]. FDA limited the level of aflatoxins in nuts, seed and legumes. The monitoring and the detection of aflatoxin at low levels has become attractive in the food safety area. Therefore, some studies were reported for development of electrochemical aptasensors for detection of aflatoxins [46–49]. Nguyen et al. [49] fabricated an electrochemical aptasensor platform for monitoring of AFM1. They used Fe3O4 incorporated polyaniline (Fe3O4/PANI) film modified interdigitated electrode (IDE) as electrochemical aptasensor platform. They found the detection limit as 1.98 ng/L. In another study, an impedimetric aptasensor onto SPE surface was developed and used for detection of AFM1 [48]. The detection of AFM1 was achieved based on the changes at the charge transfer resistance (Rct) even in milk samples.
Fumonisin B1 (FB-1) is primarily produced by Fusarium moniliforme and the most abundant and important fumonisin [74]. It has been found in maize, maize products animal feeds [75]. FB1 threats both animal and human health [76, 77]. An impedimetric aptasensor was developed by Chen and coworkers [70] for recognition of FB-1. GCE surface was modified gold nanoparticles (AuNP) and the interaction of DNA aptamer and FB-1 was investigated based on the changes at the Rct value. The selectivity of the aptasensor was then tested against other toxins.
1.2 Optical Aptasensors for Detection of Toxins
Aptamers have been used as bio-probes in optical sensors based primarily on the incorporation of a fluorophore or a nanoparticle. In the case of fluorescence detection, the simplest format is to label the aptamers with both a quencher and a fluorophore. Additionally, many nano-materials, including QDs, AuNPs, CNTs, graphene oxide (GO), polymer nanobelts, and coordination polymers, have been investigated for their fluorescence-quenching effect instead of using a more traditionally quencher [78–84]. Some optical aptasensors developed for detection of toxins were summarized in Table 2.
AuNPs or several polymers that cause color changes, can be applied as novel reagents for the optical detection technique called colorimetry. The highly negatively-charged ssDNA (complementary strand of the aptamer), which is separated from the aptamer by interaction between the aptamer and the target, is stabilized against aggregation, and a color change occurs in conjunction with this phenomenon [85].
The light chain of BoNT/A (LCA) was utilized as target molecules in SELEX process. Overall, Chang et al. [86] identified three RNA aptamer species which have high binding affinity, specificity and strong inhibition activity. They showed that the endopeptidase activity was effectively inhibited by docking of aptamer to BoNT/A (LCA). Their study was the first to confirm that the aptamers for the light chain BoNT/A (LCA) could be used as therapeutic reagents against the deadly botulism [86].
1.3 Other Techniques Developed for Detection of Toxins Using Aptamer Technologies
There are some reports in the literature which can be classified as aptasensors. Nanogold modified piezoresistive microcantilevers (PZR) were used for monitoring of Staphylococcus enterotoxin B (SEB) which is small monomeric protein and a pathogen with high thermal and proteolytic stability [87]. PZR sensor surface was modified with DNA aptamer, then the interaction of SEB and its DNA aptamer was investigated even in milk samples.
Ricin is a plant lectin from the castor bean plant Ricin communis [51]. It consists of two chains, an A chain and B chain linked by a single disulfide bond and the A chain is toxic to cells [52]. Its production is relatively easy and it is a potential threat as a terrorist weapon. Capillary electrophoresis based aptasensor was reported by Haes et al. [52] for monitoring of ricin A chain. The interaction of ricin and DNA aptamer was performed in capillary surface. Detection of ricin could be achieved in nuclease-contaminated sample matrixes. In another study, atomic force microscopy (AFM) based aptasensor was developed for monitoring of ricin [53]. DNA aptamer and ricin interaction was performed at the surface of Au(111) and ricin binding sites to aptamer was predicted.
2 Conclusion
Aptamers have been utilized in biosensor area since their discovery by Tuerk and Gold [5] due to their stability against physical conditions such as ionic strength, temperature and pH and production cost. They have been alternative biorecognition elements for antibodies even their discovery is relatively new [88, 89]. Aptamers synthesized and isolated by SELEX procedure can spesifically recognize their targets even in complex matrix due to characteristic structure generated during SELEX procedure. They have been used for recognition of proteins [21, 29, 30, 32, 33, 63, 90–92], drugs [93–95] and also toxins [7, 42–62, 64, 96, 97] in combination with different detection techniques such as optic, colorimetric, electrochemical, or piezoelectric techniques. Aptasensors developed for toxin analysis have offered the advanced assays for sensitive, selective, fast, reliable and cost-effective monitoring of numerous toxins as well as their application into the real samples such as food matrices, or biological fluids.
In another aspect, aptasensors can be miniaturized and adaptable for chip technologies for development of aptasensors based on point of care systems which are portable, compatible and having an easy-to-use design. Thus, their application to the environmental or food samples such as water, milk, nuts etc. could be performed and toxins could be sensitively and selectively analyzed with on-line measurements via aptamer based chip technologies in a short time.
References
Federation of American scientists special weapons primer (2015) http://www.fas.org/nuke/intro/bw/agent.htm. Accessed 22 Oct 2015
Sweeney MJ, White S, Dobson ADW (2000) Mycotoxins in agriculture and food safety. Irish J Agric Food Res 29:235–244
Richard JL, Fleetwood K (Dec 2001–Jan 2002) Current Trends in Mycotoxin Analysis Food Safety Magazine pp 18–21
Balaban N, Rasooly A (2000) Staphylococcal enterotoxins. Int J Food Microbiol 61:1–10
Tuerk C, Gold L (1990) Systematic evolution of ligands by exponential enrichment: RNA ligands to bacteriophage T4 DNA polymerase. Science 249:505–510
Ellington AD, Szostak JW (1990) In vitro selection of RNA molecules that bind specific ligands. Nature 346:818–822
Shim WB, Mun H, Joung HA et al (2014) Chemiluminescence competitive aptamer assay for the detection of aflatoxin B1 in corn samples. Food Control 36:30–35
Liu J, Yu J, Chen J et al (2014) Signal-amplification and real-time fluorescence anisotropy detection of apyrase by carbon nanoparticle. Mater Sci Eng C 38:206–211
Zhou N, Wang J, Zhang J et al (2013) Selection and identification of streptomycin-specific single-stranded DNA aptamers and the application in the detection of streptomycin in honey. Talanta 108:109–116
Wang J (2007) Nanoparticle-based electrochemical bioassays of proteins. Electroanalysis 19:769–776
Xiang Y, Zhang Y, Qian X et al (2010) Ultrasensitive aptamer-based protein detection via a dual amplified biocatalytic strategy. Biosens Bioelectron 25:2539–2542
Hianik T, Wang J (2009) Electrochemical aptasensors—recent achievements and perspectives. Electroanalysis 21:1223–1235
Numnuam A, Chumbimuni-Torres KY, Xiang Y et al (2008) Aptamer-based potentiometric measurements of proteins using ion-selective microelectrodes. Anal Chem 80:707–712
Xiang Y, Xie M, Bash R et al (2007) Ultrasensitive label-free aptamer-based electronic detection. Angew Chem Int Edit 46:9054–9056
Palchetti I, Mascini M (2012) Electrochemical nanomaterial-based nucleic acid aptasensors. Anal Bianal Chem 402:3103–3114
Mascini M, Ilaria P, Sara T (2011) Aptamers smart molecules for biosensing clinical samples. Chim Oggi 29:16–18
Polonschii C, David S, Tombelli S et al (2010) A novel low-cost and easy to develop functionalization platform. Case study: aptamer-based detection of thrombin by surface plasmon resonance. Talanta 80:2157–2164
Centi S, Sanmartin LB, Tombelli S et al (2009) Detection of C reactive protein (CRP) in serum by an electrochemical aptamer-based sandwich assay. Electroanalysis 21:1309–1315
Tombelli S, Mascini M (2009) Aptamers as molecular tools for bioanalytical methods. Curr Opin Mol Ther 11:179–188
Centi S, Messina G, Tombelli S et al (2008) Different approaches for the detection of thrombin by an electrochemical aptamer-based assay coupled to magnetic beads. Biosens Bioelectron 23:1602–1609
Bini A, Minunni M, Tombelli S et al (2007) Analytical performances of aptamer-based sensing for thrombin detection. Anal Chem 79:3016–3019
Centi S, Tombelli S, Minunni M et al (2007) Aptamer-based detection of plasma proteins by an electrochemical assay coupled to magnetic beads. Anal Chem 79:1466–1473
Tombelli S, Minunni M, Mascini M (2005) Analytical applications of aptamers. Biosens Bioelectron 20:2424–2434
Miodek A, Castillo G, Hianik T et al (2013) Electrochemical aptasensor of human cellular prion based on multiwalled carbon nanotubes modified with dendrimers: a platform for connecting redox markers and aptamers. Anal Chem 85:7704–7712
Evtugyn G, Porfireva A, Sitdikov R et al (2013) Electrochemical aptasensor for the determination of ochratoxin A at the Au electrode modified with Ag nanoparticles decorated with macrocyclic ligand. Electroanalysis 25:1847–1854
Castillo G, Trnkova L, Hrdy R et al (2012) Impedimetric Aptasensor for thrombin recognition based on CD support. Electroanalysis 24:1079–1087
Evtugyn G, Kostyleva V, Sitdikov R et al (2012) Electrochemical aptasensor based on a macrocyclic ligand bearing neutral red. Electroanalysis 24:91–100
Porfireva SV, Evtugyn GA, Ivanov AN et al (2010) Impedimetric aptasensors based on carbon nanotubes—poly(methylene blue) composite. Electroanalysis 22:2187–2195
Erdem A, Karadeniz H, Mayer G et al (2009) Electrochemical sensing of aptamer-protein interactions using a magnetic particle assay and single-use sensor technology. Electroanalysis 21:1278–1284
Rohrbach F, Karadeniz H, Erdem A et al (2012) Label-free impedimetric aptasensor for lysozyme detection based on carbon nanotube-modified screen-printed electrodes. Anal Biochem 421:454–459
Erdem A, Congur G (2014) Voltammetric aptasensor combined with magnetic beads assay developed for detection of human activated protein C. Talanta 128:428–433
Erdem A, Congur G (2014a) Dendrimer enriched single-use aptasensor for impedimetric detection of activated protein C. Coll Surf B 117:338–345
Erdem A, Congur G (2014b) Dendrimer modified 8-channel screen-printed electrochemical array system for impedimetric detection of activated protein C. Sens Actuat B-Chem 196:168–174
Zhou N, Zhang J, Tian Y (2014) Aptamer-based spectrophotometric detection of kanamycin in milk. Anal Method 6:1569–1574
Rhouati A, Hayat A, Hernandez DB et al (2013) Development of an automated flow-based electrochemical aptasensor for on-line detection of Ochratoxin A. Sensor Actuat B-Chem 176:1160–1166
Kim YS, Chung J, Song MY et al (2014) Aptamer cocktails: Enhancement of sensing signals compared to single use ofaptamers for detection of bacteria. Biosens Bioelectron 54:195–198
Ma X, Jiang Y, Ji F et al (2014) An aptamer-based electrochemical biosensor for the detection of salmonella. J Microbiol Meth 98:94–98
Dong Y, Xu Y, Yong W et al (2014) Aptamer and its potential applications for food safety. Crit Rev Food Sci 54:1548–1561
Xing H, Hwang K, Li J et al (2014) DNA aptamer technology for personalized medicine. Curr Opin Chem Eng 4:79–87
Alsager OA, Kumar S, Willmott GR et al (2014) Small molecule detection in solution via the size contraction response of aptamer functionalized nanoparticles. Biosens Bioelectron 57:262–268
Shi H, Zhao G, Liu M et al (2013) Aptamer-based colorimetric sensing of acetamiprid in soil samples: sensitivity, selectivity and mechanism. J Hazard Mater 260:754–761
Elshafey R, Siaj M, Zouro M (2015) DNA aptamers selection and characterization for development of label free impedimetric aptasensor for neurotoxin anatoxin-a. Biosens Bioelectron 68:295–302
Fetter L, Richards J, Daniel J et al (2015) Electrochemical aptamers caffold biosensors for detection of botulism and ricintoxins. Chem Commun 51:15137–15140
Wei F, Bai B, Ho C-M (2011) Rapid lyoptimizing an aptamer based BoNTsensor by feedback system control (FSC) scheme. Biosens Bioelectron 30:174–179
Halliwell J, Savage AC, Buckley N et al (2014) Electrochemical impedance spectroscopy biosensor for detection of active botulinum neurotoxin. Sens Bio-Sens Res 2:12–15
Castillo G, Spinella K, Poturnayova A et al (2015) Detection of aflatoxin B1 by aptamer-based biosensor using PAMAM dendrimers as immobilization platform. Food Control 52:9–18
Evtugyn G, Porfireva A, Stepanova V et al (2014) Electrochemical aptasensor based on polycarboxylic macrocycle modified with neutral red for aflatoxin B1 detection. Electroanalysis 26:2100–2109
Istamboulié G, Paniel N, Zara L, Granados LR, Barthelmebs L, Noguer T (2016) Development of an impedimetric aptasensor for the determination of AflatoxinM1 in milk. Talanta 146:464–469
Nguyen B, DaiTran L, Do QP, Nguyen HL, Tran NH, Nguyen PX (2013) Label-free detection of aflatoxin M1 with electrochemical Fe3O4/polyaniline-based aptasensor. Mater Sci Eng C 33:2229–2234
Jiang H-L, Liu X-Y, Qiu Y, Yao D-S, Xie C-F, Liu D-L (2015) Development of an aptasensor for the fastdetection of versicolorin A. Food Control 56:202–210
Eitzen E (2001) Medical Management of Biological Casualties Handbook, 4th edn. U.S. Army Medical Research Institute of Infectious Diseases, Frederick, MD. ter Sci Eng C 33:2229–2234.
Haes AJ, Giordano BC, Collins GE (2006) Aptamer-based detection and quantitative analysis of ricin using affinity probe capillary electrophoresis. Anal Chem 78:3758–3764
Wang B, Guo C, Zhang M et al (2012) High-resolution single-molecule recognition imaging of the molecular details of ricin—aptamer interaction. J Phys Chem B 116:5316–5322
Zhu Z, Feng M, Zuo L et al (2015) An aptamer based surface plasmon resonance biosensor for the detection of ochratoxin A in wine and peanut oil. Biosens Bioelectron 65:320–326
Kim SE, Su W, Cho M et al (2012) Harnessing aptamers for electrochemical detection of endotoxin. Anal Biochem 424:12–20
Sheng LF, Ren JT, Miao YQ et al (2011) PVP-coated graphene oxide for selective determination of ochratoxin A via quenching fluorescence of free aptamer. Biosens Bioelectron 26:3494–3499
Wu SJ, Duan N, Ma XY et al (2012) Multiplexed fluorescence resonance energy transfer aptasensor between upconversion nanoparticles and graphene oxide for the simultaneous determination of mycotoxins. Anal Chem 84:6263–6270
Tang J, Yu T, Guo L et al (2007) In vitro selection of DNA aptamer against abrin toxin and aptamer-based abrin direct detection. Biosens Bioelectron 22:2456–2463
Bruno JG, Richarte AM, Carrillo MA, Edge A (2012) An aptamer beacon responsive to botulinum toxins. Biosens Bioelectron 31:240–243
He L, Lamont E, Veeregowda B et al (2011) Aptamer-based surface-enhanced Raman scattering detection of ricin in liquid foods. Chem Sci 2:1579–1582
Lamont E, He L, Warriner K et al (2011) A single DNA aptamer functions as a biosensor for ricin. Analyst 136:3884–3895
Zengin A, Tamer U, Caykara T (2015) Fabrication of a SERS based aptasensor for detection of ricin B toxin. J Mater Chem B 3:306–315
Zhang Z, Yu L, Xu L et al (2014) Biotoxin sensing in food and environment via microchip. Electrophoresis 35:1547–1559
Chan C, Guo J, Sun C et al (2015) A reduced graphene oxide-Au based electrochemical biosensor for ultrasensitive detection of enzymatic activity of botulinum neurotoxin A, Sens Act B 220:131–137
Kuang H, Chen W, Xu D et al (2010) Fabricated aptamer-based electrochemical “signal-off” sensor of ochratoxin A. Biosens Bioelectron 26:710–716
Zhang J, Chen J, Zhang X et al (2012) An electrochemical biosensor based on hairpin-DNA aptamer probe and restriction endonuclease for ochratoxin A detection. Electrochem Commun 25:5–7
Yang X, Qian J, Jiang L et al (2014) Ultrasensitive electrochemical aptasensor for ochratoxin A based on two-level cascaded signal amplification strategy. Bioelectrochemistry 96:7–13
Luo P, Liu Y, Xia Y et al (2014) Aptamer biosensor for sensitive detection of toxin A of Clostridium difficile using gold nanoparticles synthesized by Bacillus stearothermophilus. Biosens Bioelectron 54:217–221
Eissa S, Siaj M, Zourob M (2015) Aptamer based competitive electrochemical biosensor for brevetoxin-2, Biosens Bioelectron 69:148–154
Chen X, Huang Y, Ma X et al (2015) Impedimetric aptamer-based determination of the moldtoxin fumonisin B1. Microchim Acta 182:1709–1714
Cruz-Aguado JA, Penner G (2008) Fluorescencepolarization based displacementassay for the determination of smallmolecules with aptamers. Anal Chem 80:8853–8855
Barna-Vetro I, Solti L, Teren J et al (1996) Sensitive ELISA test for determination of ochratoxin A. J Agric Food Chem 44:4071–4074
O’Brien E, Dietrich DR (2005) Ochratoxin A: the continuing enigma. Cr Rev Toxicol 35:33–60
Visconti A, Bruno Doko M, Solfrizzo M et al (1996) European inter comparison study for the determination of fumonisins in maize. Microchim Acta 123:55–61
Nelson PE, Desjardins AE, Plattner RD (1993) Fumonisins, mycotoxins produced by Fusarium species: biology, chemistry and significance. Annu Rev Phytopathol 31:233–252
Ross PF, Nelson PE, Richard JL et al (1990) Production of fumonisins by Fusarium moniliforme and Fusarium proliferatum isolates associated with Impedimetric aptamer-based determination of FB-1 1713 equine leukoencephalomalacia and a pulmonary edema syndrome in swine. Appl Eniron Microbiol 56:3225–3226
Yoshizawa T, Yamashita A, Luo Y (1994) Fumonisin occurrence in corn from high- and low-risk areas for human esophageal cancer in China. Appl Environ Microbiol 60:1626–1629
Chhabra R, Sharma J, Wang H et al (2009) Distance-dependent interactions between gold nanoparticles and fluorescent molecules with DNA as tunable spacers. Nanotechnology 20(48):485201–485211
Habenicht BF, Prezhdo OV (2008) Nonradiative quenching of fluorescence in a semiconducting carbon nanotube: a time-domain ab initio study. Phys Rev Lett (100):197402.
Huang Y, Zhao S, Liang H et al (2011) Multiplex detection of endonucleases by using a multicolor gold nanobeacon. Chemistry 17:7313–7319
Liu M, Zhao H, Chen S et al (2011) A “turn-on” fluorescent copper biosensor based on DNA cleavage-dependent graphene-quenched DNAzyme. Biosens Bioelectron 26:4111–4116
Luo Y, Liao F, Lu W et al (2011) Coordination polymer nanobelts for nucleic acid detection. Nanotechnology 22:195502–195508
Olek M, Büsqen T, Hilgendorff M, Giersiq M (2006) Quantum dot modified multiwall carbon nanotubes. J Phys Chem B 110:12901–12904
Chang H, Tang L, Wang Y et al (2010) Graphene fluorescence resonance energy transfer aptasensor for the thrombin detection. Anal Chem 82:2341–2346
Zhao W, Chiuman W, Brook MA, Li Y (2007) Simple and rapid colorimetric biosensors based on DNA aptamer and non cross linking gold nanoparticle aggregation. Chem Bio Chem 8:727–731
Chang TW, Blank M, Janardhanan P et al (2010) In vitro selection of RNA aptamers that inhibit the activity of type A botulinum neurotoxin. Biochem Biophys Res Commun 396:854–860
Zhao R, Wen Y, Yang J et al (2014) Aptasensor for staphylococcus enterotoxin B detection using high SNR piezoresistive microcantilevers. JMEMS 23:1054–1062
Hall B, Micheletti JM, Satya P, Ogle K, Pollard J, Ellington AD (2009) Design, synthesis, and amplification of DNA pools for in vitro selection. In: Current Protocols in Nucleic Acid Chemistry, chapter 9, unit 9.2. iochem Biophys Res Commun 396:854-860
Patel DJ, Suri AK, Jiang F et al (1997) Structure, recognition and adaptive binding in RNA aptamer complexes. J Mol Biol 272:645–664
Bini A, Mascini M, Mascini M et al (2011) Selection of thrombin-binding aptamers by using computational approach for aptasensor application. Biosens Bioelectron 26:4411–4416
Erdem A, Eksin E, Muti M (2014) Chitosan–graphene oxide based aptasensor for the impedimetric detection of lysozyme. Coll Surf B 115:205–211
Zhang Z, Yang W, Wang J et al (2009) A sensitive impedimetric thrombin aptasensor based on polyamidoamine dendrimer. Talanta 78:1240–1245
Fernandez EG, Santos-lvarez N, Lobo-Casta MJ et al (2011) Aptamer-based inhibition assay for the electrochemical detection of tobramycin using magnetic microparticles. Electroanalysis 23:43–49
Yang F, Wang P, Wang R et al (2016) Label free electrochemical aptasensor for ultrasensitive detection of ractopamine. Biosens Bioelectron 77:347–352
Emrani AS, Danesh NM, Lavaee P et al (2016) Colorimetric and fluorescence quenching apta sensors for detection of streptomycin in blood serum and milk based on double-stranded DNA and gold nanoparticles. Food Chem 190:115–121
Bazin I, Nabais E et al (2010) Rapid visual tests: Fast and reliable detection of ochratoxin A. Toxins 2:2230–2241
Cella LN, Sanchez P, Zhong W et al (2010) Nanoaptasensor for protective antigen toxin of anthrax. Anal Chem 82:2042–2047
Acknowledgments
A.E. would like to express her gratitude to the Turkish Academy of Sciences (TUBA) as an Associate member for its partial support.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2016 Springer International Publishing Switzerland
About this chapter
Cite this chapter
Eksin, E., Congur, G., Erdem, A. (2016). Aptasensor Technologies Developed for Detection of Toxins. In: Nikolelis, D., Nikoleli, GP. (eds) Biosensors for Security and Bioterrorism Applications. Advanced Sciences and Technologies for Security Applications. Springer, Cham. https://doi.org/10.1007/978-3-319-28926-7_12
Download citation
DOI: https://doi.org/10.1007/978-3-319-28926-7_12
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-28924-3
Online ISBN: 978-3-319-28926-7
eBook Packages: Physics and AstronomyPhysics and Astronomy (R0)