Abstract
The gastrointestinal motility, exocrine secretions, and endocrine cells are controlled by an integrative nervous system, under the central command of the central nervous system. The enteric nervous system is considered to be quasi-autonomous and in certain circumstances may be self-sustained. The connections of the enteric nervous system with the central nervous system are through afferent and efferent neurons of the parasympathetic and sympathetic nerves, the two major pathways of the autonomous nervous system. The enteric neurons function to control the tonus of the smooth muscle in the intestinal wall and the vascular muscle motor activity and the secretory function of the gastric and intestinal glands and endocrine products and carry sensory information to the central nervous system and some function as communicators between the neurons of the intestinal wall (interneurons). Disorders of the enteric neurons may comprise dysfunctions of the secretory, motor, or immunologic functions. In this chapter, we briefly discuss some more common motility disorders.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Parasympathetic
- Sympathetic
- Enteric nervous system
- Myenteric plexus
- Submucosal plexus
- Interstitial cell of Cajal
- Neuroendocrine cells
- Motility
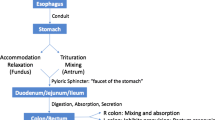
The main role of the gastrointestinal system is the digestion and absorption of the food. The food that we enjoy through our senses is softened, processed by the enzymes secreted by salivary glands, and mixed with gastric, intestinal, biliary, and/or pancreatic juices and enzymes to achieve the end result of transporting the nutrients through the mucosal lining followed by the expelling of the unabsorbable matter by defecation. The passage of food is performed under the nervous system and endocrine control of the gastrointestinal system and its regulation of the contraction and relaxation of the digestive system muscle layers and/or sphincters. Within the peripheral nervous system, the nervous system of the gut is considered the most complex.
There are two major components: the extrinsic system that in turn is divided into two components, the sympathetic and the parasympathetic nerves, and the intrinsic system also known as enteric nervous system, considered a remote portion of the central nervous system that communicates with the central nervous system via sympathetic and parasympathetic extrinsic component neurons. The central nervous system interaction with the enteric neurons is known as “central autonomic neural network” [1]. The major physiologic processes regulated by the intricate nervous system of the gastrointestinal system include (a) the smooth muscle necessary to control motility and sphincters, (b) the mucosal role in secretion of juices and fluid-electrolyte homeostasis, (c) the cells participating in mucosal immunity, and (d) the vascular network [2].
Central Autonomic Neural Network
The enteric nervous system can function autonomously. However, the central nervous system has a major role in the control of the enteric nervous system and its functional role. There are interconnections that bring in concert the motor and sensory pathways in the central nervous system and the enteric nervous system [1, 3].
There are two major pathways of such interactions. One is the parasympathetic or craniosacral pathway. The other is the sympathetic or thoracolumbar pathway. The parasympathetic pathway has a vagal component and a sacral or pelvic component. The vagal component, the predominant participant in the parasympathetic pathway, consists of preganglionic neuron bodies in the nucleus ambiguous and the dorsal vagal nucleus in the medulla. The preganglionic neurons are cholinergic and generally have excitatory effect, increasing the gastrointestinal tract motility. The sympathetic pathway or the thoracolumbar pathway has an inhibitory role, decreasing the motility. The sympathetic system is adrenergic and consists of postganglionic fibers that innervate the gut and have the neuronal bodies in the prevertebral ganglia [1].
The innervation of the esophagus is supplied by the vagus nerve (predominantly parasympathetic) and sympathetic nerves that, through afferent and efferent fibers, control the muscular layer of the esophagus, its glands, and its blood vessels. The vagus nerve receives some filaments from the paravertebral sympathetic system, and, as such, it contains mixed parasympathetic and sympathetic fibers. The right vagus participates, along with the left vagus, in forming the esophageal plexus. Thereafter, the right vagus reforms as the posterior vagal trunk just before passing through the diaphragmatic esophageal hiatus. The left vagus reforms also just above the diaphragm as the anterior vagal trunk. The surgical procedure of vagotomy was in the past more extensively used as therapeutic option for duodenal ulcer, but now is employed only occasionally. However, it is important to be aware that there are anatomic variations in the esophageal plexus and anterior and posterior trunks [4]. The esophageal sympathetic innervation is comprised of fibers with origin in the cervical and paravertebral chains. The upper portion of the esophagus is supplied by filaments from the pharyngeal plexus, and lower portions are supplied by branches from superior, middle, and vertebral ganglia of the sympathetic trunk. Esophageal filaments are also supplied by the stellate ganglia and the splanchnic nerves.
The innervation of the stomach and duodenum consists also of parasympathetic and sympathetic efferents and afferents. Their sympathetic nerve supply follows the gastric artery and the gastroepiploic artery and is derived predominantly from the celiac plexus. This plexus has a right and left portion, each with a celiac ganglion, with the aorticorenal ganglion and a single, unpaired superior mesenteric ganglion. The superior, middle, and inferior thoracic splanchnic nerves along with parasympathetic fibers from the posterior and anterior vagal trunk contribute to the interconnections of the celiac plexus. The celiac plexus forms nerves and networks of nerves including the left gastric plexus, right gastric plexus, hepatic plexus, superior mesenteric plexus, and splenic plexus. The phrenic plexus provides filaments to the cardia [4]. The stomach and the duodenum parasympathetic supply is provided by the anterior vagal trunk (e.g., greater anterior gastric nerve branches and pyloric branches) and by the posterior vagal trunk (e.g., greater posterior gastric nerve).
The innervation of the small and large intestine consists as well of sympathetic and parasympathetic nerve fibers. The sympathetic pathway has cell bodies in the prevertebral ganglia (nodose ganglia). The postganglionic fibers are reaching the small or large intestine wall through the celiac ganglia, superior mesenteric ganglion, inferior mesenteric ganglion, and superior and inferior hypogastric plexi branches and their interconnecting nerves. The parasympathetic innervation of the intestines is through the posterior vagal trunk that relays to the celiac plexus. The descending colon, the sigmoid colon, and the rectum have parasympathetic supply from the second, third, and fourth sacral spinal cord segments through the pelvic splanchnic nerves and the inferior hypogastric plexus. While the rectum is supplied from this plexus, the anal sphincter and the perianal area have direct somatic efferents and afferents from the central nervous system via the pudendal nerves [5].
Enteric Nervous System (Intrinsic System)
At the beginning of the last century, Bayliss and Starling, Magnus and Langley, and Trendelenburg found that extrinsically denervated intestines had coordinated reflex contractions (peristalsis) that were taking place by the nerves present in the intestinal wall and that the intramural intestinal neurons did not communicate with the parasympathetic neurons from the central nervous system. Millions of neurons are identified in the gastrointestinal system. The functional and chemical makeup of the intestine showed that, like the central nervous system, the enteric nervous system contains sensory and motor neurons and interneurons and that the synaptic chemical connections direct the integrative network from sensory to interneurons to motor neurons and to effector system in a similar manner to the central nervous system [6].
In the intestinal wall, there are small ganglia composed of groups of nerve cell bodies. Nerve processes from these small ganglia form three main intramural plexi (Figs. 2.1, 2.2, 2.3 and 2.4). The submucosal plexus is named Meissner plexus. The deep submucosal plexus is known as Henle’s plexus (1871). The intermuscular, or myenteric plexus is also known as Auerbach plexus. It is the most easily seen histologically and is found throughout the gastrointestinal system between the circular smooth muscle layer of the muscularis propria and the longitudinal muscle layer. It also innervates the motor end plates of the striatal muscle portion of the esophagus with the release of the nitric oxide [1]. Its main role is to provide innervation to the two muscle layers in the muscularis propria, and it has an additional role in the innervation of the mucosa. Immediately beneath the muscularis mucosa is the Meissner plexus, the submucosal plexus. It is composed of neurons or ganglion cells and glial cells (Schwann cells). These are interspersed among the loose stromal elements of the submucosa [7]. Occasionally, ganglion cells may be seen in the lamina propria of the mucosa, but it is abnormal to find an increased number of ganglion cells here or clustered ganglion cells in the lamina propria. In such cases, conditions such as ganglioneuroma, inflammatory bowel disease, or neurofibromatosis should be considered. The deep submucosal plexus of Henle is located along the inner portion of the muscularis mucosa. The deep submucosal plexus has fewer small neurons compared to the Meissner plexus. In the latter, approximately 50 % of the ganglia were associated with single fiber tracts, as compared to the deep submucosal plexus (Henle’s) in which approximately 75 % were associated with single fiber tracts [8].
The Enteric Neurons
The enteric neurons have been classified in primary afferent neurons, excitatory circular muscle motor neurons, inhibitory circular muscle motor neurons, longitudinal muscle motor neurons, ascending interneurons, descending interneurons, secretomotor and vasomotor neurons, and intestinofugal neurons [3].
Primary afferent neurons or the intrinsic primary afferent neurons may be seen in ganglia of both the Meissner and the Auerbach plexi. Approximately 30 % of myenteric neurons and 14 % of submucosal ganglia neurons are primary afferent neurons. These neurons respond to chemical or mechanical stimuli of the mucosa to muscle tension and radial stretch of the intestinal wall [3].
The excitatory circular muscle motor neurons are the final effector in the circular layer of the muscularis propria. They are considered to receive fast nicotinic and slow synaptic input from the intrinsic primary afferent neurons. In the deep myenteric plexus, they are denser, and, via acetylcholine and tachykinin transmitters, they act predominantly directly on the circular smooth muscle.
Inhibitory circular muscle motor neurons also receive fast nicotinic input from the intrinsic primary efferent neurons. They also receive noncholinergic input. They act directly and indirectly, by the production of nitric oxide, adenosine triphosphate, vasoactive intestinal peptide (VIP), and other peptides, on the circular smooth muscle, having an inhibitory effect.
The longitudinal muscle motor neurons receive their input from the intrinsic primary afferent neurons and from interneurons.
Ascending interneurons are forming chains of excitatory interneurons with other interneurons and receive fast nicotinic synaptic input and slow synaptic input from the intrinsic primary afferent neurons. They are participating in the action of the excitatory circular muscle neurons. These interneurons contain, along with the enzymes needed for acetylcholine synthesis, opioid peptides and tachykinins.
The descending interneurons are more likely cholinergic. There are some containing choline acetyltransferase and some containing somatostatin as neurotransmitters. Some contain also serotonin. They receive input from non-primary afferent neurons and make synapses with submucosal and myenteric neurons.
Some of the secretomotor neurons are cholinergic, while some contain VIP. They project to the mucosa, to the myenteric plexus ganglia, and to the submucosal plexus ganglia. The secretomotor neurons that contain VIP have inhibitory synapses from the extrinsic sympathetic pathway and possibly from other myenteric neurons. Some submucosal neurons are cholinergic and project to the small blood vessels.
The intestinofugal neurons are cholinergic neurons that project to the prevertebral ganglia from myenteric plexus ganglia [3].
Interstitial Cells of Cajal
In the gastrointestinal motility, besides the extrinsic and intrinsic nerve supply, other components also participate. The interstitial cells of Cajal (ICC) are an important part of the gastrointestinal neuromuscular function. When Cajal described these cells in 1893, he identified them as “primitive neurons.” Electron microscopy showed that these cells are either fibroblast-like cells or primitive muscle cells. These cells express c-kit (CD117), a tyrosine kinase receptor. It was shown that these cells are pacemaker cells for the gastrointestinal tract. They are organized in a network that propagates slow wave electrical activity. The slow waves propagate from ICC to the smooth muscle fibers. Slow waves produce depolarization, followed by the entrance of calcium into the muscle cells, resulting in phasic contraction, or peristalsis. Another role of ICC is in the mediation of the input from the enteric motor neurons to the smooth muscle. ICC also provides sensitivity to stretch. Most ICCs are located at the periphery of myenteric plexus (Auerbach plexus). They form a network from the branched processes of these multipolar ICC. Other ICCs are located within the circular or longitudinal smooth muscle layers, in the connective tissue, within the submucosal plexus (Meissner plexus), or within the deep muscular plexus [9]. The gastrointestinal stromal tumors (GISTs) are mesenchymal tumors of the gastrointestinal system that express c-kit (CD117), CD34, and DOG-1. In 1984, Herrera described such tumors as “plexosarcoma,” indicating that these tumors are originating in the enteric plexus [10]. Today it is widely accepted that GIST is derived from ICC (Figs. 2.5, and 2.6). In addition to the surgical treatment, the current therapy for GIST includes a c-kit tyrosine kinase inhibitor, imatinib, with a good response [11].
Neurotransmitters of Enteric Motor Neurons
As we have seen, the motor neurons may be excitatory or inhibitory. The neurotransmitters released by excitatory neurons are necessary for contraction and mucosal gland secretion. Their main neurotransmitters are acetylcholine and substance P. Enteric gland secretions are stimulated by excitatory neurotransmitters acetylcholine, ATP, and the vasoactive intestinal peptide (VIP).
The inhibitory neurotransmitters for inhibitory neurons that suppress the smooth muscle contraction are ATP, VIP, and nitric oxide.
Sensory Information (Extrinsic Afferent Supply) of the Gastrointestinal Tract
As previously noted, there is a rich afferent innervation that carries the sensory information from the gut to the central nervous system and mediates the sensations from the gastrointestinal organs. The afferents are carried by the vagal and splanchnic and pelvic nerves. The vagus nerves have the cell bodies in the nodose ganglia and then end centrally in the nucleus of the tractus solitarius. The splanchnic afferent neurons have their cell bodies in the dorsal root ganglia. The vagal afferents are predominantly in the upper part of the gastrointestinal system, while the pelvic afferents supply mainly the lower portion of the large intestine. The entire gastrointestinal system appears to send extrinsic afferent information through the splanchnic nerves [12]. The vagal afferent neurons are detecting mechanical distension and are sensitive to the glucose, amino acid, or long-chain fatty acid concentrations in the intestinal lumen. The mucosal neuroendocrine cells release chemical transmitters necessary for the vagal afferent activity. An example is the increased release of serotonin by the enterochromaffin endocrine cells, due to mucosal damage during chemotherapy, with consequently severe emesis. Increased serotonin acts on 5-hydroxytryptamine-3 receptors (5-HT3) located on the vagal neurons. The primary vagal afferent neurons, in turn, are connected with the neurons in the brain stem that control vomiting. The antiemetic drug ondansetron is a 5-HT3 receptor antagonist; therefore, it inhibits the vagal primary afferent neurons. The splanchnic primary afferent neurons are sensing pain (nociceptive receptors). They are activated by chemical, mechanical, or thermal stimuli. Some of their neurotransmitters include calcitonin gene-related peptide and substance P.
In the myenteric plexus, some neurons contain opioid type of peptides (e.g., enkephalins, dynorphin, and endorphin). The opioid receptors are localized principally to the enteric neurons, and opioid receptor agonists produce a decreased neuronal excitability, resulting in motor inhibition and constipation [13].
Neuroendocrine Cells or Endocrine Cells?
The neuroendocrine cells in the gastrointestinal system have important functions. Their embryological derivation has been controversial. These cells have endocrine and paracrine, and some have neurotransmitter functions. Many of these cells have distinctive neural markers. When immunohistochemistry stains are performed, they express either chromogranin or synaptophysin or both. Synaptophysin stains synaptic neural granules and chromogranin granules that are found in neurons or endocrine cells (granins). However, while some neuroendocrine cells (e.g., those in the thyroid) have more neural-like features (neural crest rather than endodermal origin), those located in the gastrointestinal tract display more epithelial-like properties (endodermal origin rather than neural crest) [14]. In the gastrointestinal tract, they have wide distribution in the distal esophagus, stomach, small intestine, large intestine, and anus. Many are in the epithelium or lamina propria of the mucosal lining. They have sensitivity for thermal, chemical, and mechanical stimuli. Some of their products are true peptide hormones with release through the bloodstream to reach their targeted tissue into the circulation (e.g.. gastrin, secretin). Other paracrine cells have only local effect on the nearby cells (e.g., somatostatin). There are known interactions between the endocrine cells and enteric neurons, between neurons and endocrine cells, and among various endocrine cells [2]. The neuroendocrine proliferation in the gastrointestinal tract results in neuroendocrine tumors. In the stomach, there are early precursors noted, enterochromaffin-like cell (ECL) proliferation. These occur predominantly in the context of atrophic gastritis as ECL hyperplasia that may progress to ECL dysplasia and then to well-differentiated neuroendocrine tumors (carcinoid) [15] (Figs. 2.7, 2.8, 2.9, 2.10, and 2.11).
Selected Disorders of the Nervous System of the Gut
Gastrointestinal Neuromuscular Disorders
This is a very heterogeneous group of conditions that have as common denominator alterations in the motility function of the gastrointestinal tract resulting in inadequate propulsion and emptying functions.
Achalasia
In the lower esophagus, nonselective loss of all the enteric neurons from the Auerbach’s plexus (myenteric plexus) results in achalasia, a condition characterized by unrelaxed lower esophagus sphincter, with a persistent tonic contraction. As a consequence, there is a functional esophageal obstruction (cardiospasm), with esophageal dilation and hypertrophy. Achalasia may also occur when there is selectively a dysfunction or loss of the inhibitory myenteric plexus neurons that contain VIP or nitric oxide. Histologically, there is significant decrease or absence and/or degeneration of the myenteric plexus neurons [16].
Pyloric Stenosis
Pyloric stenosis is a rather common condition in infants (1/3000) that has a high familial predilection and is more common in males. Its etiology is disputed, but one theory is the delay in the innervation of the pyloric region and decreased or absent nerves or neurotransmitters such as nitric oxide, absence of ICC, and other supporting cells. The pyloric muscle is hypertrophic. Histologically, there is degeneration of glial cells, increased number of Schwann cells, and hypertrophy of the nerves. ICCs are absent or significantly decreased. ICCs contain the inhibitory neurotransmitters nitric oxide [16].
Intestinal Pseudo-obstruction
Intestinal pseudo-obstruction is a group of acute or chronic disorders that in the absence of mechanical obstructions show an absence of the propulsive function of the intestine. The chronic pseudo-obstruction may be idiopathic, of unknown pathogenesis. There are several rare diseases included under this classification. They include conditions such as idiopathic megacolon or megaduodenum. As a prototype we will use Hirschsprung’s disease. It is by definition a disease of newborn or infant involving the sigmoid and rectum, predominantly in males. Characteristically a segment of distal colon or rectum lacks ganglion cells, especially the ganglion cells of the Meissner (submucosal) plexus. This segment is aperistaltic and narrow. Proximal to it, the colon dilates (congenital megacolon) [16].
Chronic or acute pseudo-obstructions may be also secondary to systemic diseases: endocrine disease such as hypothyroidism; systemic disorders involving the smooth muscle such as amyloidosis or myotonic dystrophies; systemic conditions involving the extrinsic nervous system such as stroke, diabetes, or orthostatic hypotension; or systemic intrinsic enteric nervous system disorders such as paraneoplastic syndrome, viruses, drugs, or Chagas’ disease. This latter group of conditions is generally characterized by multiple segments of the gastrointestinal tract with loss of ganglion cells [1, 16].
Neuronal Dysplasia (Ganglioneuromatosis)
Ganglioneuromatosis may be either nodular or diffuse and consists of a proliferation of ganglion cells, nerve fibers, and Schwann cells, either at myenteric plexus level or throughout the thickness of the intestinal wall (Figs. 2.12 and 2.13). It may be a cause of pseudo-obstruction. In some patients, this condition has been associated with multiple endocrine neoplasia type 2B (MEN-2B) [1].
Other Entities
Other enteric neuron disorders such as neurogenic secretory diarrhea and neurogenic constipation, abdominal pain, inflammatory bowel disease, irritable bowel syndrome, and neuroimmunological modulation are discussed elsewhere in this book.
Conclusion
This chapter has briefly demonstrated that gastrointestinal function is rigorously controlled and integrated with the central nervous system. The enteric neurons that participate in motility, the neurons that help regulate the glandular and endocrine secretions, the vasomotor neurons, the afferent sensory neurons bringing information from the gut, and the interneurons and transmitters with role to facilitate the communication between neurons are working in concert for the complex function of the gastrointestinal system.
References
Goyal RK, Hirano I. The enteric nervous system. N Engl J Med. 1996;334:1106–15.
Noffsinger A, Fenoglio-Preiser C, Maru D, Gilinsky N. Atlas of nontumor pathology: gastrointestinal disease. Washington, DC: American Registry of Pathology and Armed Forces Institute of Pathology; 2007.
Costa M, Brookes SJ, Hennig GW. Anatomy and physiology of the enteric nervous system. Gut. 2000;47(S 4):iv15–9.
Netter FH. Digestive system. Upper digestive system (part 1). Summit, ng: The CIBA Collection of Medical Illustrations; 1959.
Netter FH. Digestive system. Lower digestive tract (part 2). Summit, ng: The CIBA Collection of Medical Illustrations; 1962.
Wood JD. Neuropathophysiology of functional gastrointestinal disorders. World J Gastroenterol. 2007;13:1313–32.
Sternberg SS, editor. Histology for pathologists. Philadelphia/New York: Lippincott Raven; 1997.
Hoyle CH, Burnstock G. Neuronal populations in the submucous plexus of the human colon. J Anat. 1989;166:7–22.
Al-Shboul OA. The importance of interstitial cells of Cajal in the gastrointestinal tract. Saudi J Gastroenterol. 2013;19:3–15.
Herrera GA, de Pinto MH, Grizle WE, et al. Malignant small bowel neoplasm of enteric plexus derivation (plexosarcoma): light and electronic microscopic study confirming the origin of the neoplasm. Dig Dis Sci. 1984;29:275–84.
Streutker CJ, Hulzinga JD, Driman DK, et al. Interstitial cells of Cajal in health and disease: part II, ICC and gastrointestinal stromal tumours. Histopathology. 2007;50:190–202.
Berthoud HR, Blackshaw LA, Brookes SJ, et al. Neuroanatomy of extrinsic afferents supplying the gastrointestinal tract. Neurogastroenterol Motil. 2004;16(S1):28–33.
Galligan JJ, Akbarali HI. Molecular physiology of enteric opioid receptors. Am J Gastroenterol Suppl. 2014;2:17–21.
Rosai J. The origin of neuroendocrine tumors and the neural crest saga. Mod Pathol. 2011;24:S53–7.
Hamilton SR, Aaltonen LA, editors. Pathology and genetics. Tumors of the digestive system. World Health Organization classification of tumors. Lyon: IARC Press; 2000.
Riddell R, El-Zimaity H, Jain D. Motility disorders, in Lewin, Weinstein and Riddell’s gatrointestinal pathology and its clinical implications, vol. I. Philadelphia: Wolters Kluwer Health; 2014. p. 226–89.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Additional information
Disclaimer
This work is performed as an individual Board Certified pathologist and not as an employee of the Overton Brooks VA Medical Center. The views expressed herein are not approved nor adopted by the US Government nor its agents or employees.
Rights and permissions
Copyright information
© 2016 Springer International Publishing Switzerland
About this chapter
Cite this chapter
Constantinescu, M. (2016). The Enteric Nervous System. In: Constantinescu, C., Arsenescu, R., Arsenescu, V. (eds) Neuro-Immuno-Gastroenterology. Springer, Cham. https://doi.org/10.1007/978-3-319-28609-9_2
Download citation
DOI: https://doi.org/10.1007/978-3-319-28609-9_2
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-28607-5
Online ISBN: 978-3-319-28609-9
eBook Packages: MedicineMedicine (R0)