Abstract
Multiple sclerosis (MS) is an autoimmune disease affecting the central nervous system, in which both genetic and environmental factors are involved. Prevalence of MS is increasing remarkably in Asian countries including Japan, indicating a role of environmental factors related to westernization of lifestyle. Recent studies in immunology have demonstrated the dependency of pathogenic or regulatory lymphocytes on the gut microbiota component. Based on the epidemiological data in human and mouse immunology studies, we have been hypothesizing that alterations in the gut microbiota may underlie the pathogenesis of MS at least in Japan. Very recently, analysis of the bacterial 16S ribosomal RNA (rRNA) gene by using a high-throughput culture-independent pyrosequencing method provided evidence of a moderate dysbiosis in the structure of gut microbiota in Japanese patients with MS. Furthermore, we have identified 21 species that showed significant differences in relative abundance in MS as compared with healthy subjects, 2 increased and 19 reduced. The taxa reduced in MS comprised primarily of clostridial species belonging to Clostridia clusters XIVa and IV. Correcting the dysbiosis and altered gut microbiota might deserve consideration as a potential strategy for the prevention and treatment of MS.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Multiple sclerosis
- Gut microbiota
- Dysbiosis
- Autoimmune disease
- Clostridium
- Regulatory T cells
- Faecalibacterium prausnitzii
Introduction
Human gut is inhabited and colonized by trillions of commensal bacteria, fungi, and viruses, which are collectively referred to as the gut microbiota. Recent studies have demonstrated that gut microbiota interacts with the host immune system and plays an essential role in keeping the health and preventing disease conditions [1]. Previous works in the field of gastroenterology showed that compositions of fecal microbiota are significantly biased in inflammatory bowel diseases (IBD), including ulcerative colitis and Crohn’s disease [2, 3]. Parallel research in rodent demonstrated that intestinal bacteria are involved in the pathogenesis of IBD models [4, 5]. More recently, it has been revealed that an alteration in the gut commensal flora is not only associated with IBD, but also with obesity, diabetes, cancer, and autoimmune diseases like multiple sclerosis (MS) [6, 7]. Curiously, patients with these diseases are increasing in developed countries, including Japan, where “westernized” lifestyle, higher intake of high-fat, low-fiber food, or early exposure to antibiotics is prevailing over last decades [8]. Nowadays, dysbiosis of human gut microbiota can be demonstrated by comprehensive genome analysis for bacterial 16S sequences or metagenome analysis. In this short review article, current understanding on the role of gut microbiota in MS is overviewed.
What Is the Relationship Between MS and Inflammatory Bowel Disease?
MS is a chronic autoimmune disease of the central nervous system (CNS), typically characterized by recurrent episodes of neurological disabilities and presence of multiple demyelinating foci in the CNS. MS is continuously increasing in developed countries over last decades [8, 9]. Although MS used to be a very rare disease in Japan, we are witnessing a very remarkable increase of patients with MS in Japan (Fig. 10.1a). This increase could not be explained by better awareness of the disease or advancement in diagnostic skills or facilities [10], and therefore nongenetic, environmental factors should be considered. Known environmental risk factors for MS include lower exposure to sunlight resulting in vitamin D3 deficiency, EB virus infection, and cigarette smoking [11]. There is no evidence that Japanese are exposed more significantly to these risk factors during last decades. High salt intake is also proposed to be a risk factor for MS [12]. However, salt intake is actually decreasing in Japan.
At the turn of centuries, the role of commensal bacteria in shaping lymphocyte repertoire started to attract the attention of immunologists [1], opening the gate to the research into microbiota-immune interactions. We are aware that Crohn’s disease and ulcerative colitis are also increasing in Japan in parallel with MS (Fig. 10.1b). Is it reasonable to hypothesize that MS and IBD may share common pathways leading to occurrence of the inflammatory destruction? Although even nonexperts accepted the possible pathogenic role of altered gut environments in IBD, it took some more years till people started to consider if a brain disease MS could be associated with altered gut flora.
Roles of Gut Commensal Flora in Animal Models of MS
It remained obscure for years whether the gut microbiota could affect systemic immune responses beyond the gut. To answer the question, an animal model of MS, experimental autoimmune encephalomyelitis (EAE), has significantly contributed to our understanding. In 2008, we investigated if antibiotic treatment altering gut flora compositions might affect development of EAE. As reported by Yokote et al. [13], we induced EAE with MOG 35–55 peptide in B6 mice. Before and during induction phase, drinking water, containing nonabsorbing antibiotics kanamycin, colistin, and vancomycin, was given to the mice. The antibiotics treatment significantly altered the composition of intestinal microbiota but also reduced the clinical and pathological signs of EAE [13]. The suppressed signs of EAE were associated with reduced Th1 and Th17 responses to MOG35–55 in the draining lymph nodes. T cells isolated from the gut-associated lymph nodes showed a selectively suppressed IL-17 production. Interestingly, the effect of antibiotics was not observed in mice whose invariant NKT (iNKT) cells are genetically depleted, indicating the role for iNKT cells in this antibiotic-mediated suppression of EAE. In 2011, Lee et al. [14] reported that although germ-free mice are very resistant against induction of EAE, colonizing the mice with segmented filamentous bacteria (SFB), that is essential for induction of Th17 cells in mice [15], would restore the susceptibility to EAE. Berer et al. used T cell receptor (TCR) transgenic mice that spontaneously develop EAE to address the role of gut microbiota in EAE [16]. They showed that the commensal flora greatly affects the development of disease in their spontaneous EAE model created by genetic engineering.
Analysis of Fecal Samples of MS
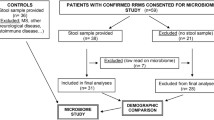
These results obtained from rodent EAE experiments indicate a role for the indigenous gut microbiota in the pathogenesis of EAE, and raised the possibility that an altered gut microbiota might be an environmental risk factor for MS. However, analysis of human fecal commensal microbiota has been a challenge till lately, as the large majority of the gut bacteria is anaerobic and has not been isolated in culture. To overcome the problem, we have used a high-throughput culture-independent pyrosequencing method to compare the gut microbiota of MS patients and healthy subjects [17]. Samples were obtained from 20 patients with relapsing-remitting type of MS during remission and from 40 healthy subjects. In addition, we used 158 control samples from 18 healthy subjects who repeatedly provided the fecal samples.
Bacterial 16S ribosomal RNA (rRNA) gene analysis of fecal DNA revealed that species diversity and richness were not altered in MS (Fig. 10.2). This feature is in striking contrast to the gut microbiota of patients with inflammatory bowel disease (IBD), which is characterized by lower species richness compared with healthy controls. However, UniFrac analysis revealed a significant difference in the overall gut microbiota structure between MS and healthy subjects (Fig. 10.3), indicating that the gut microbiota in MS is significantly altered.
Bacterial species diversity and richness were not altered in MS. Number of operational taxonomic units (OTUs) generated by clustering of 3000 16S reads of gut microbiota samples from 40 healthy control subjects and 20 patients with multiple sclerosis (Revised from Ref. [17])
Moderate dysbiosis found in the gut microbiota of MS. Open and closed circles indicate individual subjects from Healthy controls and MS, respectively. (a) The two components of the unweighted PCoA plot explained 6.96 and 4.30 % of the variance. ANOSIM statistic, R = 0.239, P ≤ 0.0009. (b) Mean unweighted UniFrac distances for HC-HC, HC-MS, and MS-MS subjects (Revised from Ref. [17])
More strikingly, we detected a significant difference in the abundance of 21 bacterial species; two increased and 19 decreased in MS. On comparing MS samples to the 158 longitudinal samples from 18 healthy subjects, the differences were found to be reproducibly significant for most of the species. The taxa reduced in MS comprised primarily of clostridial species belonging to Clostridia clusters XIVa and IV and Bacteroidetes. Among the reduced clostridial strains, the proportions of Faecalibacterium prausnitzii and Eubacterium rectale were reduced in fecal and mucosa-associated microbiota in patients with IBD and were associated with a higher risk of postoperative recurrence of ileal Crohn’s disease [18, 19].
Clostridial species including Faecalibacterium prausnitzii [18] are involved in fermenting digestion of diet fiber, which leads to production of short chain fatty acids (SCFA), including acetate, propionate, and butyrate. Butyrate is known to exert anti-inflammatory functions via inhibition of NF-κB activation and IκB degradation [20]. Interestingly, Atarashi et al. [21] have succeeded in identifying 14 clostridial strains from human feces that are capable of inducing foxp3+ regulatory T cells. Although most of these strains were reduced in IBD samples, they were not phylogenetically close to those that were reduced in MS.
Implications
Results of experimental works as well as epidemiological studies prompted us to evaluate the importance of gut microbiota in MS. As described above, we have found that potentially immunosuppressive clostridial strains are reduced in the gut microbiota from Japanese patients with MS [17]. We could now speculate that the remarkable increase of MS in Japan might result from alterations in the gut microbiota due to the change of lifestyle. If all the bacteria reduced in MS have immune-regulatory potentials like Faecalibacterium prausnitzii [18], not only diet therapy but more drastic therapy such as fecal transplantation may deserve consideration for preventive or therapeutic strategies in MS.
Gut commensal microbiota are not harmful, but accomplish beneficial functions for promoting and maintaining health. For example, they would serve for hosts through synthesizing vitamins and producing short chain fatty acids (SCFA) with anti-inflammatory activity. Of particular interest, nutritional factors previously reported to show protective effects on MS include vegetable protein, dietary fiber, cereal fiber, vitamin C, thiamin, riboflavin, calcium, and potassium [22]. Dietary fibers are a source of butyrate capable of maintaining intestinal homeostasis. It is also of note that green vegetables contain ligands for aryl hydrocarbon receptor expressed by Th17 cells [23]. Along with rapid progress in basic research, anecdotal or fragmental works, supporting diet therapy of MS, could be now re-evaluated based on more solid scientific background [24, 25]. Ongoing works may lead to development of sophisticated approaches for correcting dysbiosis that may lead to cure of MS in the future.
References
Macpherson AJ, Harris NL. Interactions between commensal intestinal bacteria and the immune system. Nat Rev Immunol. 2004;4:478–85.
Manichanh C, Rigottier-Gois L, Bonnaud E, Pelletier E, Frangeul L, Nalin R, Jrrin C, Chardon P, Marteau P, Roca J, Dore J. Reduced diversity of faecal microbiota in Crohn’s disease revealed by a metagenomic approach. Gut. 2006;55:205–11.
Xavier RJ, Podolsky DK. Unravelling the pathogenesis of inflammatory bowel disease. Nature. 2007;448:427–34.
Peloquin JM, Nguyen DD. The microbiota and inflammatory bowel disease: insights from animal models. Anaerobe. 2013;24:102–6.
Chow J, Tang H, Mazmanian SK. Pathobionts of the gastrointestinal microbiota and inflammatory disease. Curr Opin Immunol. 2011;23:473–80.
Moreno-Indias I, Cardona F, Tinahones FJ, Queipo-Ortuno MI. Impact of the gut microbiota on the development of obesity and type 2 diabetes mellitus. Front Microbiol. 2014;5:190.
Joyce SA, Gahan GM. The gut microbiota and the metabolic health of the host. Curr Opin Gastroenterol. 2014;30:120–7.
Yamamura T, Miyake S. Diet, gut flora, and multiple sclerosis: current research and future perspectives. In: Takashi Y, Bruno G, editors. Multiple sclerosis immunology. Heidelberg: Springer; 2013. p. 115–26.
Bach J-F. The effect of infections on susceptibility to autoimmune and allergic diseases. N Engl J Med. 2002;347:911–20.
Houzen H, Niino M, Hirotani M, Fukazawa T, Kikuchi S, Tanaka K, Sasaki H. Increased prevalence, incidence and female predominance in multiple sclerosis in northern Japan. J Neurol Sci. 2012;323:117–22.
Ascherio A, Munger KL. Environmental risk factors for multiple sclerosis. Part II: noninfectious factors. Ann Neurol. 2007;61:504–13.
Kleinewietfeld M, Manzel A, Titze J, Kvakan H, Yosef N, Linker RA, Muller DN, Hafler DA. Sodium chloride drives autoimmune disease by the induction of pathogenic TH17 cells. Nature. 2013;496:518–22.
Yokote H, Miyake S, Croxford JL, Oki S, Mizusawa H, Yamamura T. NKT cell-dependent amelioration of a mouse model of multiple sclerosis by altering gut flora. Am J Pathol. 2008;173:1714–23.
Lee YK, Menezes JS, Umesaki Y, Mazmanian SK. Proinflammatory T-cell responses to gut microbiota promote experimental autoimmune encephalomyelitis. Proc Natl Acad Sci U S A. 2011;108:4615–22.
Ivanov II, Atarashi K, Manel N, Brodie EL, Shima T, Karaoz U, Wei D, Goldfarb KC, Santee CA, Lynch SV, Tanoue T, Imaoka A, Itoh K, Takeda K, Umesaki Y, Honda K, Littman DR. Induction of intestinal Th17 cells by segmented filamentous bacteria. Cell. 2009;139:485–98.
Berer K, Mues M, Koutrolos M, Rasbi ZA, Boziki M, Johner C, Wekerle H, Krishnamoorthy G. Commensal microbiota and myelin autoantigen cooperate to trigger autoimmune demyelination. Nature. 2011;479:538–41.
Miyake S, Kim S, Suda W, Oshima K, Nakamura M, Matsuoka T, Chihara N, Tomita A, Sato W, Kim S-W, Morita H, Hattori M, Yamamura T. Dysbiosis in the gut microbiota of patients with multiple sclerosis, with a striking depletion of species belonging to Clostridia XIVa and IV clusters. PLoS One. doi:10.1371/journal.pone.0137429.
Sokol H, et al. Faecalibacterium prausnitzii is an anti-inflammatory commensal bacterium identified by gut microbiota analysis of Crohn disease patients. Proc Natl Acad Sci U S A. 2008;105:16731–6 (17).
Mondot S, Kang S, Furet JP, Aguirre de Carcer D, McSweeney C, Morrison M, et al. Highlighting new phylogenetic specificities of Crohn’s disease microbiota. Inflamm Bowel Dis. 2011;17:185–92.
Segain J-P, de la Bletiere DR, Bourreille A, Leray V, Gervois N, Rosales C, Ferrier L, Bonnet C, Blottiere HM, Galmiche J-P. Butyrate inhibits inflammatory responses through NFkB inhibition: implications for Crohn’s disease. Gut. 2000;47:397–403.
Atarashi K, Tanoue T, Shima T, Imaoka A, Kuwahara T, Momose Y, Cheng G, Yamasaki S, Saito T, Ohba Y, Taniguchi T, Takeda K, Hori S, Ivanov II, Umesaki Y, Itoh K, Honda K. Induction of colonic regulatory T cells by indigenous Clostridium species. Science. 2011;331:337–41.
Ghadirian P, Jain M, Ducic S, Shatenstein B. Morisset. Nutritional factors in the aetiology of multiple sclerosis: a case-control study in Montreal, Canada. Int J Epidemiol. 1998;27:845–52.
Veldhoen M, Hirota K, Westendorf AM, Buer J, Dumoutier L, Renauld J-C, Stockinger B. The arylhydrocarbon receptor links TH17-cell-mediated autoimmunity to environmental toxins. Nature. 2008;453:106–10.
Turnbaugh PJ, Ridaura VK, Faith JJ, Rey FE, Knight R, Gordon JI. The effect of diet on the human gut microbiome: a metagenomic analysis in humanized gnotobiotic mice. Sci Transl Med. 2009;1:6ra14.
David LA, et al. Diet rapidly and reproducibly alters the human gut microbiome. Nature. 2014;505:559–63.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2016 Springer International Publishing Switzerland
About this chapter
Cite this chapter
Yamamura, T. (2016). Gut Microbiota: A Possible Role in the Pathogenesis of Multiple Sclerosis. In: Constantinescu, C., Arsenescu, R., Arsenescu, V. (eds) Neuro-Immuno-Gastroenterology. Springer, Cham. https://doi.org/10.1007/978-3-319-28609-9_10
Download citation
DOI: https://doi.org/10.1007/978-3-319-28609-9_10
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-28607-5
Online ISBN: 978-3-319-28609-9
eBook Packages: MedicineMedicine (R0)