Abstract
Organ procurement is the first stage of the transplant procedure, and it decisively contributes to transplantation success or failure. In stable donors, we recommend first gaining access to the retroperitoneal space with control of the inferior vena cava and the abdominal aorta, and soon after, we recommend accurate dissection of the hepatic hilum and the recognition of any vascular abnormalities before in situ cooling. The purpose of organ preservation is to slow the unavoidable biological deterioration and damage that occurs between harvesting and reperfusion, thus buying time to organize staff and facilities, to transport organs, and, when necessary, to perform histological examinations. Simple cold storage is the most widely used preservation method, which relies on the effects of hypothermia supplemented by the use of special preservation solutions. Machine perfusion provides the unique opportunity to evaluate the functional performance of the graft prior to transplantation, likely allows a longer and safer cold ischemia time, and, in the near future, will allow the administration in the perfusion system of innovative pharmacological agents for ex vivo organ damage repair.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Delay Graft Function
- Organ Preservation
- Organ Procurement
- Preservation Solution
- Donation After Cardiac Death
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
FormalPara Key Points-
Organ procurement is the first stage of the transplant procedure, and it decisively contributes to transplantation success or failure.
-
In stable donors, we recommend first gaining access to the retroperitoneal space with control of the inferior vena cava and the abdominal aorta, and soon after, we recommend accurate dissection of the hepatic hilum and the recognition of any vascular abnormalities before in situ cooling.
-
The purpose of organ preservation is to slow the unavoidable biological deterioration and damage that occurs between harvesting and reperfusion, thus buying time to organize staff and facilities, to transport organs, and, when necessary, to perform histological examinations.
-
Simple cold storage is the most widely used preservation method, which relies on the effects of hypothermia supplemented by the use of special preservation solutions.
-
Machine perfusion provides the unique opportunity to evaluate the functional performance of the graft prior to transplantation, likely allows a longer and safer cold ischemia time, and, in the near future, will allow the administration in the perfusion system of innovative pharmacological agents for ex vivo organ damage repair.
1 General Principles
1.1 Organizational and Preoperative Aspects
Organ procurement has been defined as “the lifeblood of organ transplantation.” It is the first part of the whole transplant procedure and decisively contributes to its success or failure, because every mistake at this stage can render an organ unsuitable for transplantation or lead to serious complications in the recipient [1].
Ideally, the retrieval team should be self-sufficient and not require any support from the donor hospital other than an operating theater and a local staff member. In practice, most retrieval teams also require a donor hospital anesthetist and a scrub nurse to be present during the procedure. Surgical teams from the transplant center to which the graft will be transplanted usually harvest the organs. Teams from cardiothoracic transplant centers almost always perform cardiothoracic organ retrievals. For the retrieval of all other organs, ideally a single abdominal organ retrieval team should be available. The presence of a single retrieval team, rather than individual organ teams, streamlines the process and ensures a uniform approach to the abdominal retrieval, which is an important factor, particularly when operating in different environments. Local teams sometimes retrieve kidneys for other transplant centers [2]. The donor transplant coordinator plays an important role in organizing organ procurement, arranging the transport of the surgical team to and from the donor hospital, and performing much of the administrative tasks [1, 3]. All retrieval teams should be equipped with a bag containing surgical instruments needed for the procedure. Perfusion solutions are kept refrigerated with ice in proper thermal containers. Some instruments are not always available in the donor hospital, and therefore, the contents of the bag should be checked periodically, in particular before leaving from the transplant center. The bag should also contain drugs that may be difficult to obtain in the donor hospital, for example, prostacyclin, antifungal agents, and some antibiotics for the decontamination of the duodenal lumen during pancreas procurement [2]. Surgical instruments vary according to the necessities and preferences of each retrieval team. Table 8.1 shows a representative list of the retrieval team equipment.
Organ procurement should be performed in a peaceful and dignified atmosphere. In general, a respectful treatment of the organ donor is a condition sine qua non for each person involved in the organ donation, and the remaining wishes of the donor or the relatives must be respected. Communication is essential to ensure that a smooth retrieval process is achieved. Competition or a lack of communication between the members of organ retrieval teams may lead to surgical injury of the organs or inadequate preservation [1, 3]. After arrival in the donor hospital, the procurement surgeon should introduce himself or herself and the procurement team. If different surgical teams are involved in the procurement, they must discuss the details of the technique and sequence they want to adopt before starting the procedure [3]. Every organ is important and must be removed from the donor without jeopardy to any of the individual grafts, especially in the case of anatomical or vascular conflict detected during preliminary dissection. In general, the order of multiple organ procurement gives priority to the heart and lungs; then the liver, pancreas, and small bowel; and finally to the kidneys, vascular grafts, and tissues [2, 4].
The key responsibility and an absolute imperative for the lead surgeon of the retrieval team is the correct identification of the donor prior to the operation and the check of its blood group compatibility. The lead surgeon must also check that the diagnosis of death has been made appropriately and documented correctly and that the consent or authorization for donation has been obtained and documented. Preoperative checks should also ensure that all other necessary information about the donor is available, as summarized in Table 8.2. Clinical examination of the donor should be performed, including inspection of the entire body for any skin lesions, palpation of the breast (in both male and female donors!), and digital rectal examination to identify possible malignancies.
1.2 Preventable Errors in Organ Procurement
Severe adverse events in organ transplantation are quite rare. Nevertheless, the scientific literature and the media have reported a small number of cases where organ recipients died or had serious injuries as a result of errors during the transplantation process [5]. Clinicians and surgeons traditionally equate medical errors with human shortcomings and often fail to understand the importance of redesigning the systems and processes of care to anticipate potential failures that can lead to errors. Organ transplantation is a particularly complex procedure of healthcare and routinely stresses nearly all of the systems and processes of surgical care, offering a unique opportunity to proactively identify vulnerabilities and potential failures. Initial steps have been taken to understand these issues through the United Network for Organ Sharing (UNOS) Operations and Safety Committee, which has collected data about preventable errors in organ transplantation and shown that 55 % of reported adverse events are due to miscommunication and errors in documentation and data entry. Separate data about kidney and liver transplantation have confirmed that failures in communication and the coordination of care are significant contributors to preventable errors. Moreover, errors with labeling and packing have been reported by the UNOS Operations and Safety Committee as a recurrent problem across transplant centers, and between 2006 and 2010, they accounted for 38 % of the reported errors [6]. A national commission instituted in Italy after three cases in 2007 of transplant-related human immunodeficiency virus (HIV) transmission released some recommendations to improve transplant safety: automatic transcription of test results from laboratory instruments to laboratory information systems and donors’ medical records, centralization of donor laboratory tests, and training to develop a proactive quality and safety culture in regional donation and transplantation networks [5]. Checklists can play an important role in surgery; the World Health Organization (WHO) released its surgical safety checklist in June 2008 and demonstrated its impact in a 2009 study of eight international hospitals. The rate of death was 1.5 % before the checklist was introduced and declined to 0.8 % afterward; inpatient complications occurred in 11 % of patients at baseline and in 7 % after the introduction of the checklist. However, each transplant center has its own systems and checklists for procedures, because at this point there are no international standard guidelines specific to transplantation [7].
1.3 High Infectious Risk Donors
High infectious risk donors (HRDs) are non-ideal donors that are considered suboptimal because they are thought to carry an increased risk of infectious transmission. The definition of HRDs was introduced in 1994 when the Centers for Disease Control and Prevention (CDC) developed guidelines to identify persons at risk of transmitting infectious disease through transplantation. HRDs are defined as persons meeting one of the following behavioral criteria:
-
Men who had sex with other men (MSM) in the preceding 5 years
-
Persons who report the nonmedical intravenous, intramuscular, or subcutaneous injection of drugs in the preceding 5 years
-
Persons with hemophilia or related clotting disorders who have received clotting factor concentrates
-
Commercial sex workers (CSWs) who have engaged in sex in exchange for money or drugs in the preceding 5 years
-
Persons who have had sex in the preceding 12 months with any person described above or with a person known or suspected to have HIV infection
-
Persons exposed in the preceding 12 months to known or suspected HIV-infected blood
-
Inmates of correctional systems (incarcerated donors)
All donors are tested for infectious disease; thus, the HRD designation is currently used to identify those donors at risk of acquiring an infection in the weeks or months before death and those likely to have false-negative serologic tests due to the associated window period. Despite controversy surrounding their use, organs from HRDs benefit the transplant community as a whole by expanding the supply of available organs and decreasing waiting times for patients with high waitlist mortality. Special informed consent use can mitigate legal risk. Communicating infectious risk to patients is extremely difficult because the true infection risk is unknown and varies widely depending on individual donor risk factors. Putting the issue in terms the patients can understand is also challenging. Nucleic acid testing (NAT) mitigates infectious risk by decreasing the window period, and it is therefore recommended for HRDs to reduce the risk of infectious transmission [8].
1.4 Surgical Technique: Main Principles
The surgical technique for multiple organ procurement will be extensively discussed in the following dedicated chapters. Here we only focus on some essential general aspects of the procedure. Regardless of the technique adopted, the first operative step must include a careful exploration of the thoracic and abdominal cavities to exclude gross pathological conditions that contraindicate the procedure. This inspection completes the preoperative evaluation of the donor and helps minimize the risk of disease transmission to the recipient.
The guiding principle of all procurement techniques is the avoidance of warm ischemia. This can be achieved with in situ organ cooling by the carefully timed and controlled intravascular infusion of cold preservation solutions at the time of circulatory arrest [4]. Therefore, the organ procurement procedure is generally divided into two parts (called “warm” and “cold” phases), depending on which comes before or after in situ flushing.
The surgical techniques for multiple organ procurement have undergone progressive changes over time. The first techniques used during the 1970s and early 1980s required the exposure and complete dissection of each organ while the heart was still beating, during the warm phase. This was obviously time consuming, particularly as less experienced surgeons were increasingly involved in the procedure and become a deterrent to collaboration between abdominal and cardiac teams [9, 10]. Starzl and colleagues described the “flexible technique” for organ procurement in 1984, in which all donor organs to be procured were rapidly cooled in situ, simultaneously removed in a bloodless field, and further dissected on a back table [4, 9, 11]. Several teams subsequently introduced the in situ flush and cold dissection technique, initially recommended for instable donors, as a rapid procurement method for all multiorgan retrievals [9]. Thus, surgical techniques for abdominal organ procurement can be roughly divided in two main categories: the warm dissection technique and the rapid retrieval technique (“dissection in the cold”). In the warm dissection technique, organ dissection takes place before the cannulation of the aorta and cold perfusion. Conversely, the rapid retrieval technique aims first to achieve control of the aorta and its cannulation to rapidly start cold perfusion. Sometimes, isolated kidney retrieval needs to be performed if no other organ is suitable for transplantation [3].
We think that donor surgeons must be familiar with both the cold and warm dissection techniques, because they have complementary roles. We recommend the rapid retrieval technique only if the donor becomes unstable, because this procedure minimizes warm ischemic times during hemodynamic instability and can be safely performed in critical situations by non-expert surgeons. However, one well-known disadvantage of this rapid technique is the difficulty in recognizing any possible accessory or replacing blood vessels, which during the cold phase turn into non-pulsating, non-bleeding structures. Thus, a rapid perfusion is usually obtained at the expense of the subsequent cold phase and back table surgery, which consequently requires more time and increases the risk of injury to cooled grafts [2, 10]. In stable donors, we recommend instead a combined technique, which first involves gaining access to the retroperitoneal space and the control of the abdominal aorta (to easily convert to the rapid technique if the donor suddenly becomes unstable during the procedure) and is followed by an accurate dissection of the hepatic hilum and the recognition of any vascular abnormalities before in situ cooling. In our experience, this technique is instructive for the surgeon in training and minimizes the risk of not recognizing vascular abnormalities in exchange for a negligible number of graft injuries during the warm phase if a well-trained surgeon assists the procedure. Moreover, additional time for warm dissection does not hamper collaboration between different transplantation teams if the donor remains stable. There is little evidence that manipulation and dissection prior to perfusion can cause vasospasm and increase oxygen consumption of the abdominal organs (especially the liver), leading to poor preservation [3, 9]. In our opinion, this effect is insignificant and may be overcome by the faster cold phase and back table surgery, which expose the graft for a shorter time to a temperature that is greater than the optimum for proper preservation.
2 Organ Preservation
2.1 Ischemia-Reperfusion Injury Physiopathology
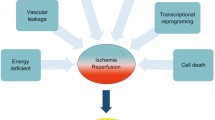
The exact underlying mechanisms of both primary nonfunction and delayed graft function remain unclear but most likely involve ischemia-reperfusion injury, which develops soon after transplantation [12]. Once removed from the donor, the organ undergoes a period of ischemia and incurs subsequent damage. The longer the organ is removed from blood, oxygen, and nutrients, the less likely it will be able to return to normal function after transplantation. The injury process that begun during hypothermia is exacerbated by the rewarming as the organ is being implanted [13]. A wide range of pathological processes contributes to ischemia and reperfusion-associated tissue injury.
The ischemic period in particular is associated with significant alterations in the control of gene expression. For example, ischemia is associated with the inhibition of oxygen-sensing prolylhydroxylase (PHD) enzymes, because they require oxygen as a cofactor. The hypoxia-associated inhibition of PHD enzymes leads to the posttranslational activation of hypoxia and inflammatory signaling cascades, which control the stability of the transcription factors, hypoxia-inducible factor (HIF) and nuclear factor-κB (NF-κB), respectively [14]. Changes in gene expression seem to be the earliest indicators of ischemia-reperfusion-related injury that are measurable in the graft, and quantitative gene expression analysis in postreperfusion biopsies may be a valuable tool to postoperatively identify patients at risk of early clinical allograft dysfunction after transplantation [12]. Several studies have suggested a functional role for microRNAs in ischemia and reperfusion. Pharmacological approaches to inhibit microRNAs seem likely to become treatment modalities for patients in the near future [14].
Once the oxygen supply has been exhausted, the cells transfer from aerobic to anaerobic metabolism, which quickly becomes self-limiting due to the production of lactate and protons. Membrane ion transport begins to shut down without excess adenosine triphosphate (ATP) to fuel the pumps, and passive redistribution occurs, resulting in cellular swelling as water osmotically corrects the imbalance. Taken together, these and other changes lead to the activation of cell death programs, including apoptosis and necrosis [13].
Necrotic cells are highly immunostimulatory and lead to inflammatory cell infiltration and cytokine production. Ischemia and reperfusion activate a host immune response in a sterile environment. This sterile immune response involves signaling events through pattern recognition molecules such as Toll-like receptors (TLRs), which can be activated by endogenous molecules in the absence of microbial compounds, particularly in the context of cell damage or death. Many studies suggest that inhibitors of TLR signaling could be effective for the treatment of sterile inflammation induced by ischemia and reperfusion. Ischemia and reperfusion also elicit a robust adaptive immune response that involves, among other cell types, T lymphocytes. In contrast, regulatory T cells appear to have a protective role in ischemia and reperfusion injury. A series of studies have shown that neo-epitopes expressed on ischemic tissues are targets for natural antibody binding during the reperfusion phase with subsequent complement activation, neutrophil recruitment, and tissue injury. The production of reactive oxygen species (ROS) at sites of sterile inflammation alters cellular proteins, lipids, and ribonucleic acids, leading to cell dysfunction and death [14].
Oxidative stress associated with reperfusion results in further free radical damage to the organs [13]. Xanthine oxidase may serve as the initial source of free radical generation in ischemia-reperfusion injury. With the onset of ischemia, ATP is degraded to hypoxanthine. Simultaneously, xanthine dehydrogenase is converted by ischemia to xanthine oxidase. Although the concentrations of substrate and enzyme are high during ischemia, the absence of oxygen prevents purine oxidation until reperfusion. During reperfusion, oxygen becomes available, suddenly and in excess, and the oxidation of hypoxanthine proceeds rapidly, generating a burst of superoxide radical by-products [2]. In this way, reperfusion-dependent events further aggravate ischemia-induced parenchymal injury either by prolonging focal ischemia (due to direct free radical damage on pericytes) or from the release of proinflammatory mediators that promote leukocyte infiltration and local activation [13]. Attenuated vascular relaxation after reperfusion due to injured pericytes can result in a no-reflow phenomenon characterized by the increased impedance of microvascular blood flow after reperfusion [14].
2.2 Strategies for Organ Preservation
The transplant process requires the retrieval of an organ from the donor and the preservation of it throughout its implantation in the recipient. Preservation is logistically essential for organ transplantation because it buys time to organize staff and facilities, transport organs, and perform necessary laboratory tests. The purpose of organ preservation is to slow extracorporeal biological deterioration that occurs in organs during the time between harvesting and reperfusion, providing a viable graft with primary function after transplantation [11, 15]. Good organ preservation has proven to be a major determinant of graft outcome after revascularization [12].
Clinical organ transplantation has moved forward from an experimental procedure in the early 1950s to the current treatment of choice for patients with end-stage organ disease, and the development of organ preservation techniques has flanked and supported the success of this procedure. With the shortage of organs available for transplant, “marginal donors” have become an important source to expand the donor pool. For these organs, preservation is of even greater importance and remains a subject of ongoing research [11].
There are currently two approaches of preservation for most transplantable organs: static or dynamic. Both preservation modalities are preceded by the donor procurement phase, in which access to the required organs is surgically achieved by introducing chilled solutions in sufficient volumes into the major vascular channels to wash out the blood and achieve moderate cooling before final removal from the body [11].
Simple cold storage (SCS) is the easiest and most widely used preservation method in organ transplantation. SCS relies on the effects of hypothermia supplemented by the use of special preservation solutions that are aimed at inhibiting the unavoidable deterioration. Hypothermia represents a compromise between the benefits and detriments of cooling. The standard recommended temperature for SCS is 4 °C. Below this point, organs can freeze, which will result in coagulative necrosis upon reperfusion. Temperatures above 4 °C are associated with increased metabolic activity, ATP depletion, lactic acid buildup, and mitochondrial disturbances, resulting in severe parenchymal and endothelial injury. Clinical hypothermia slows total cellular metabolism, reduces the requirements for oxygen, and inhibits the activity of hydrolytic enzymes to prevent tissue injury [11, 13].
In contrast, machine perfusion constitutes a method for dynamic preservation in which the organ, after an initial washout of blood, is connected to a perfusion device, and a solution is pumped through its vasculature [16]. This continuous perfusion permits the delivery of oxygen and nutrients to the parenchyma and the removal of toxic metabolites. Various temperatures have been used from hypothermic perfusion at 4 °C to normothermic perfusion at 37 °C; the latter maintains the organ in a more physiological and metabolically active state. With normothermic perfusion, organs resume their function. Solutions used as perfusate also vary from low potassium crystalloid solutions to blood-based solutions. The flow can be continuous or pulsatile, mimicking the physiological variation in systolic and diastolic pressure [17].
Although machine perfusion was the original preservation technique used for organ transplantation, the early perfusion devices required significant resources and customized vans to transport them between donor and recipient hospitals. The introduction of conventional preservation solutions for SCS overshadowed for years the more complicated use of machine perfusion in clinics [13]. In an era of donor shortage and the increased use of suboptimal grafts and organ exchange across sometimes distant geographical areas, SCS has reached its limits. Machine perfusion has reemerged, and new portable devices have been developed, with the largest clinical experience acquired in kidney and lung transplantation [17]. In kidney transplantation, several studies including an international randomized controlled trial have shown a reduced rate of delayed graft function and better graft survival after hypothermic machine perfusion versus static cold perfusion [16, 18]. One clinical study has been published comparing 20 adult liver recipients after hypothermic machine perfusion with a historically matched group of recipients after SCS with a reduction in early allograft dysfunction [19]. The results of an international multicenter trial randomizing standard donor lungs for preservation with SCS versus machine perfusion are awaited [20].
2.3 Most Common Cold Static Preservation Solutions
The most common solutions used for cold static preservation are compared in Table 8.3. When cold ischemic times are limited, most studies in the liver, kidney, and pancreas transplantation found equivalent outcomes for histidine-tryptophan-ketoglutarate and Celsior versus University of Wisconsin solution [21].
2.3.1 Collins’ Solution
Developed in 1969, Collins’ solution was based on a combination of high-potassium ion content, to mimic intracellular composition, and osmotic barrier supported by glucose, to suppress cell swelling [11]. Magnesium was added to act as a membrane stabilizer, but in the presence of phosphate, the magnesium phosphate formed crystal precipitates, which was reported when using the original solution. To eliminate this problem, a modified Collins’ solution was developed in Europe (named Euro-Collins) that omitted the magnesium and used mannitol in place of glucose [13]. Collins’ solution and its more recent variant Euro-Collins were widely distributed for organ preservation until the advent of the University of Wisconsin solution in the late 1980s [11, 21].
2.3.2 University of Wisconsin (UW) or Belzer Solution
Originally developed by Belzer and Southard for pancreas preservation in 1987, the University of Wisconsin (UW) solution is currently the most commonly used static cold preservation solution for abdominal organs [21]. It is a potassium-rich, sodium-depleted, osmotically active solution that is supplemented with a precursor of ATP (adenosine) and antioxidant agents (allopurinol, reduced glutathione). Osmotically active substances (raffinose and lactobionate) prevent cellular swelling but generate high viscosity. The colloid hydroxyethyl starch permits more effective flushing [11, 21]. Potential risks include hyperkalemic cardiac arrest at reperfusion, ischemic-type biliary complications, and microcirculatory disturbances as a result of particle formation. These disadvantages are not clinically relevant in most cases because UW solution yields prolonged safe preservation for abdominal organs [21].
2.3.3 Histidine-Tryptophan-Ketoglutarate (HTK) Solution
Originally designed as a cardioplegic solution in 1980 by Bretschneider, histidine-tryptophan-ketoglutarate (HTK) solution represents an alternative to UW solution. It is a crystalloid solution with an osmolarity slightly higher than that of the intracellular space [12, 21]. Its major components are a strong buffer (histidine), osmotic barrier (mannitol), and low-permeability amino acids (tryptophan and alpha-ketoglutaric acid), which help to stabilize cell membranes and may be substrates for anaerobic metabolism. The electrolyte composition is characterized by low concentrations of potassium, which therefore allow a safe, direct release into the recipient’s blood circulation. The lower viscosity of HTK solution provides more effective flushing and the rapid cooling of organs. Because HTK is less expensive and lower preservation costs per donor can be obtained, it has become increasingly popular over the last 20 years, especially in developing countries [11]. HTK solution has been reported to have less biliary complications than UV solution, but contrary to most clinical trials, US national registry data in the kidney, pancreas, and liver transplantation demonstrate more detrimental effects and earlier graft loss after preservation with HTK versus UW solution [21, 22].
2.3.4 Celsior Solution
Initially formulated specifically for heart preservation in 1995, Celsior solution is now widely utilized for abdominal and thoracic organ storage [21]. It adopts many of the principles of UW solution (impermeants lactobionate and mannitol) and the strong buffer of HTK solution (histidine), but in contrast with UW solution, reduced glutathione is the only antioxidant agent included. Celsior solution contains relatively lower potassium levels compared with UW solution and Euro-Collins’ solution. It has the advantage of being less viscous than UW solution and can rapidly perfuse large parenchymal volumes, such as the liver and lungs [2, 11].
2.4 Some Special Considerations on Perfusion Preservation of the Heart Graft
Perfusion preservation provides oxygen and metabolic substances to harvested donor organs, thus improving their reperfusion function and the survival of the transplanted patient. If we consider the heart graft, the transplant procedure remains consistently feasible for only 4–6 h. In fact, even beyond 3 h of storage, recipient mortality has been demonstrated to increase exponentially [23]. Therefore, current research is directed to improve the existing perfusion solutions by adding or omitting various components. The composition of the preservation solution is intended to represent some important variables that affect graft survival. Preservation solutions are frequently classified into two broad categories on the basis of electrolyte content:
-
Intracellular type, characterized by high potassium and low sodium (Celsior solution, Euro-Collins’ solution)
-
Extracellular type, low potassium and high sodium (UW solution, HTK solution, Belzer-Machine Perfusion solution)
Intracellular-type storage solutions may decrease the ATP requirements of the preserved organ by reducing the energy production needed to maintain membrane Na+/K+ ATPase activity [24]. However, the mere fact that more than 150 different organ preservation solutions are used in the United States alone explains that the optimal heart preservation solution remains to be defined [25]. As a matter of fact, the initial cellular and functional preservation of the myocardial tissue is achieved with hypothermia and mechanical arrest. ATP is consumed at a minor level, even during mechanical cardiac arrest, allowing the breakdown of ATP, which is produced by anaerobic glycolysis during the ischemic time. If the ATP reserve is depleted irreversibly, myofiber contracture may occur [26]. The perfusion solutions should also maintain ion homeostasis, and even though Na+/K+ ATPase activity is significantly reduced by hypothermia, ions flow down their concentration gradient; intracellular hydrogen ions are exchanged for extracellular sodium ions that, in turn, are replaced by calcium ions. This process increases the concentration of calcium ions inside the sarcolemma, thus damaging cardiac myofibers during reperfusion.
3 New Strategies
3.1 Machine Perfusion and Ex Vivo Graft Conditioning
Machine perfusion creates a window between procurement and transplantation during which functional performance and the viability of the organ can be evaluated prior to transplantation. Different physiological parameters can be measured, and various biochemical markers released in the perfusate can be analyzed, but the exact value of these markers to predict functional performance after transplantation is not clear and needs to be further investigated [17]. For the kidneys, vascular resistance correlates with delayed graft function and 1-year graft failure, but the predictive value is low, making this information inadequate as a stand-alone viability parameter to accept or discard a given kidney. More accurate prediction of graft outcome will require the integration of perfusion parameters and biomarker concentrations into multifactorial graft quality scoring systems [27]. Machine perfusion provides a unique opportunity to administer innovative pharmacological agents for ex vivo repair and the improvement of graft quality prior to transplantation. Few papers have been published on organ therapy during machine perfusion, and they are generally limited to preclinical large animal models; however, the potential for this approach is great because important graft improvements can be accomplished through the relatively simple addition of a specific pharmaceutical agent to the preservation solution [28]. The use of mesenchymal stem cells (MSCs) has gained attention in the field of organ transplantation because of their pro-regenerative, anti-inflammatory, and immunomodulatory properties. The administration of MSCs has been shown to enhance recovery from ischemia-reperfusion-induced acute renal failure in rats. The role of autologous MSCs as an induction therapy to promote graft acceptance has also been studied in a randomized controlled trial after living-related kidney transplantation. Machine perfusion offers a unique platform to selectively administer these MSCs directly into the donor organ, overcoming the issues of homing, trafficking, and safety [17].
3.2 Donation After Cardiac Death
The use of non-heart-beating donors, today better known as donation after cardiac death (DCD), has been extensively discussed elsewhere in a dedicated chapter. Here we only focus on some essential aspects. The potential of DCD has been recognized since the early days of kidney transplantation and has recently undergone a resurgence of interest to expand the donor pool. In DCD, the cessation of circulatory and respiratory function happens first, leading to warm ischemia during the period between circulatory dysfunction and subsequent cold perfusion by the procurement team. This is in contrast with the heart-beating cadaver donor, defined by the irreversible cessation of all brain functions but with full circulatory and respiratory functions until cold perfusion, resulting in minimal organ ischemia or preservation injury. Thus, in DCD, the effects of injury sustained during warm ischemia are superimposed on subsequent cold preservation injury [29]. DCD can be controlled or uncontrolled. In the uncontrolled situation, the donor is declared dead on the arrival at the hospital or fails to respond to cardiopulmonary resuscitation after circulatory arrest. This is an unplanned situation, and there is no opportunity to organize the process of organ donation in advance; true organ ischemia and damage occur before procurement. In controlled DCD, the donor experiences circulatory arrest after a process of planned withdrawal of support when further treatment is deemed futile. There is usually an opportunity to obtain family consent and mobilize the retrieval team prior to the withdrawal of support, and for this reason, the warm ischemia time is usually shorter [29, 30]. The outcomes for organs transplanted after cardiac death are similar to those for organs transplanted after brain death. However, the length of time that organs can be deprived of oxygen and still be transplanted successfully varies; it is best to retrieve the liver less than 30 min after the withdrawal of life-sustaining measures, whereas the kidneys and pancreas can often be recovered up to 60 min after this withdrawal [31]. A femoral cannula can be placed after or before cardiac arrest; organs are cold flushed after the declaration of death and retrieved as in the aforementioned rapid technique [29].
Notes
- 1.
Written by A.M. Grande.
References
Baranski A (2009) Surgical technique of abdominal organ procurement: step by step. Springer, London
Aseni P, De Carlis L (2011) Prelievomultiorgano a scopo di trapianto. Ghedimedia, Milano
Wunderlich H, Brockmann JG, Voigt R et al (2011) DTG procurement guidelines in heart beating donors. Transpl Int 24(7):733–757
Starzl TE, Hakala TR, Shaw BW Jr et al (1984) A flexible procedure for multiple cadaveric organ procurement. Surg Gynecol Obstet 158(3):223–230
Bellandi T, Albolino S, Tartaglia R et al (2010) Unintended transplantation of three organs from an HIV-positive donor: report of the analysis of an adverse event in a regional health care service in Italy. Transplant Proc 42(6):2187–2189
Ison MG, Holl JL, Ladner D (2012) Preventable errors in organ transplantation: an emerging patient safety issue? Am J Transplant 12(9):2307–2312
Pondrom S (2011) The AJT report: news and issues that affect organ and tissue transplantation. Am J Transplant 11(7):1345–1346
Kucirka LM, Singer AL, Segev DL (2011) High infectious risk donors: what are the risks and when are they too high? Curr Opin Organ Transplant 16(2):256–261
Brockmann JG, Vaidya A, Reddy S et al (2006) Retrieval of abdominal organs for transplantation. Br J Surg 93(2):133–146
Vegeto A, Berardinelli L (1999) Il trapianto di rene. UTET, Milano
Guibert EE, Petrenko AY, Balaban CL et al (2011) Organ preservation: current concepts and new strategies for the next decade. Transfus Med Hemother 38(2):125–142
Anaya-Prado R, Delgado-Vázquez JA (2008) Scientific basis of organ preservation. Curr Opin Organ Transplant 13(2):129–134
Henry SD, Guarrera JV (2012) Protective effects of hypothermic ex vivo perfusion on ischemia/reperfusion injury and transplant outcomes. Transplant Rev (Orlando) 26(2):163–175
Eltzschig HK, Eckle T (2011) Ischemia and reperfusion – from mechanism to translation. Nat Med 17(11):1391–1401
McLaren AJ, Friend PJ (2003) Trends in organ preservation. Transpl Int 16(10):701–708
Moers C, Smits JM, Maathuis MH et al (2009) Machine perfusion or cold storage in deceased-donor kidney transplantation. N Engl J Med 360(1):7–19
Van Raemdonck D, Neyrinck A, Rega F et al (2013) Machine perfusion in organ transplantation: a tool for ex-vivo graft conditioning with mesenchymal stem cells? Curr Opin Organ Transplant 18(1):24–33
Moers C, Pirenne J, Paul A et al (2012) Machine perfusion or cold storage in deceased-donor kidney transplantation. N Engl J Med 366(8):770–771
Guarrera JV, Henry SD, Samstein B et al (2010) Hypothermic machine preservation in human liver transplantation: the first clinical series. Am J Transplant 10(2):372–381
Warnecke G, Moradiellos J, Tudorache I et al (2012) Normothermic perfusion of donor lungs for preservation and assessment with the organ care system lung before bilateral transplantation: a pilot study of 12 patients. Lancet 380(9856):1851–1858
Parsons RF, Guarrera JV (2014) Preservation solutions for static cold storage of abdominal allografts: which is best? Curr Opin Organ Transplant 19(2):100–107
Stewart ZA, Cameron AM, Singer AL et al (2009) Histidine-Tryptophan-Ketoglutarate (HTK) is associated with reduced graft survival in deceased donor livers, especially those donated after cardiac death. Am J Transplant 9(2):286–293
Taylor DO, Edwards LB, Mohacsi PJ et al (2003) The registry of the International Society for Heart and Lung Transplantation: twentieth official adult heart transplant report – 2003. J Heart Lung Transplant 22(6):616–624
Belzer FO, Southard JH (1988) Principles of solid-organ preservation by cold storage. Transplantation 45(4):673–676
Demmy T, Biddle JS, Bennett LE et al (1997) Organ preservation solutions in heart transplantation – patterns of usage and related survival. Transplantation 63(2):262–269
Costanzo MR, Dipchand A, Starling R et al (2010) The International Society of Heart and Lung Transplantation Guidelines for the care of heart transplant recipients. J Heart Lung Transplant 29(8):914–956
Jochmans I, Moers C, Smits JM et al (2011) The prognostic value of renal resistance during hypothermic machine perfusion of deceased donor kidneys. Am J Transplant 11(10):2214–2220
Bon D, Chatauret N, Giraud S et al (2012) New strategies to optimize kidney recovery and preservation in transplantation. Nat Rev Nephrol 8(6):339–347
Reddy S, Friend P (2005) Liver transplantation outcomes using donors after cardiac death. Curr Opin Organ Transplant 10:95–100
Manzarbeitia C, Reich DJ (2005) Non-heartbeating donors: special considerations for procurement and preservation in liver transplantation. Curr Opin Organ Transplant 10:101–104
Steinbrook R (2007) Organ donation after cardiac death. N Engl J Med 357(3):209–213
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2016 Springer International Publishing Switzerland
About this chapter
Cite this chapter
De Carlis, R., Sguinzi, R., Grande, A.M., Aseni, P. (2016). Multiple Organ Retrieval: General Principles, Organ Preservation, and New Strategies. In: Aseni, P., Grande, A., De Carlis, L. (eds) Multiorgan Procurement for Transplantation. Springer, Cham. https://doi.org/10.1007/978-3-319-28416-3_8
Download citation
DOI: https://doi.org/10.1007/978-3-319-28416-3_8
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-28414-9
Online ISBN: 978-3-319-28416-3
eBook Packages: MedicineMedicine (R0)