Abstract
Ankle fractures are so commonly seen and routinely treated that we fail to remember that the diabetic patient also presents with impaired healing of the wound and bone, vascular insufficiency, and neuropathy. This is because chronic hyperglycemia increases osteoclastic activity, decreases osteoblastic activity, impairs the ability of the red blood cell to deliver oxygen, and decreases the ability of fibroblasts from migrating and attaching to wounds. Overall, the incidence of adult ankle fractures is 100.8/100,000/per year with approximately 260,000 Americans per year sustaining an ankle, six percent of which are diabetics. Preoperatively, patients should have good neurovascular examinations along with an evaluation of their hemoglobin A1c. Preoperative evaluation combined with surgical techniques, specific for the diabetic pathology, is presented to provide optimal patient outcome for this patient population. Non-operative treatment is indicated for non-displaced fractures. All displaced fractures should be managed surgically with four options available: standard fixation, trans-syndesmotic, trans-articular or a combined technique. Major complications associated with managing these patients consist of failure of fixation, skin and wound problems, infections and the development of Charcot neuroarthropathy.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
Ankle fractures are common skeletal injuries and are one of the most commonly managed joint injuries in orthopedic surgery. Surgical fixation is well-established as the treatment of choice for displaced fractures. This produces an anatomic reduction of the mortise, decreases instability, and lessens the development of posttraumatic arthrosis of the ankle. Although the use of non-operative care for some fractures have demonstrated good outcomes, nonsurgical treatment is currently reserved for patients presenting with non-displaced fractures, those whose medical co-morbidities preclude any surgical intervention, patients who refuse surgery or most often as an intermediate step until the soft-tissue envelope has sufficiently stabilized to allow surgery. These fractures are so routinely treated that there is often a certain disregard for their seriousness and their potential complications, especially in the diabetic patient. At times we fail to remember that the diabetic patient can also present with impaired healing of the wound and bone, along with some vascular insufficiency and neuropathy.
In the diabetic , chronic hyperglycemia results in high levels of blood viscosity, it impairs the ability of the red blood cell to deliver oxygen, it affects nitric oxide, which functions as an antioxidant and neurotransmitter, and it leads to microvascular compromise. The last of which results in coronary artery disease, stroke, peripheral artery disease and produces nerve ischemia [1, 2]. In addition, hyperglycemia also decreases the ability of immune cells, specifically fibroblasts, from migrating and attaching to wounds ultimately resulting in healing stagnation that may last for up to 8 weeks [3].
In bone physiology , chronic hyperglycemia increases osteoclastic activity, leading to osteoporosis and demineralization, and decreases osteoblastic activity, resulting in a decrease in osteon formation and the ability of the bone to remodel. This impairs proliferation and migration of the osteocytes which results in a decrease in callus formation, tensile strength, and bone stiffness [4]. Ultimately it is a combination of all of these changes that results in a significant delay in bone healing [5], with studies reporting union times increasing to 163 % to that of non-diabetic patients, which is further increased to 187 % of non-diabetics when the fractures are displaced [6, 7]. These bony changes also raise their chances of sustaining a more severe ankle fracture, along with increasing their mortality rates, postoperative complications, lengths of hospital stays and costs, than in the non-diabetic patient [8–11]. It is, perhaps, for all of these reasons that the use of non-operative care is more often considered for management of the diabetic patient who presents with an ankle fracture.
How then, do we manage the acute diabetic ankle fracture? Do we withhold certain treatments because they will be too expensive? Or do we withhold treatments, due to expectations that they will have poorer outcomes than the non-diabetic patient? This comes with the understanding that withholding treatment can produce avoidable complications, result in significant disabilities, and create chronic conditions that can lead to socioeconomic burdens to patients, their families, and to payer systems. The decision driving treatment should be based on the injury pattern and the patient’s physiology. If surgery is anticipated a discussion with the patient should include the need for preoperative medical evaluations and whether any adjunctive fixation will be needed to augment the reduction. Additionally, and regardless of whether the patient is treated operatively or non-operatively, a long discussion should be held to discuss prolonged immobilization and non-weight-bearing of the patient. Given the advancements in techniques and implants, this chapter will hopefully provide a rational approach for the physician tasked with managing acute ankle fractures in the diabetic patient.
Epidemiology
The 2014 National Diabetes Statistics reported that 29.1 million people (9.3 % of the US population) have diabetes of which 8.1 million (27.8 % of people) are undiagnosed [12]. Approximately 89 % have one additional co-morbidity and 15 % have four or more [13]. Patients, presenting with neuropathy and at least one other co-morbidity, have higher rates of complications (47 % vs. 14 %) compared to diabetics without neuropathy or another co-morbidity [11]. Although complications are often related to poor glucose control, hypertension, and dyslipidemia, only 36–57 % of patients achieve adequate glycemic or blood pressure levels, while only 13.2 % of all patients achieve all three target levels [14].
The incidence of adult ankle fractures has been shown to be 100.8/100,000/per year. The ratio of men to women is 47:53, with bi- and trimalleolar fractures increasing in incidence, more so in women, as patients get older [15]. In the United States it has been estimated that approximately 260,000 Americans per year sustain an ankle fracture, with about 25 % undergoing surgical management [9]. Within this population nearly 6 %, or almost 16,000 patients per year, are diabetics who sustain an ankle fracture [8]. If the 25 % needing surgery is extrapolated into the diabetic population, it would mean that one would expect that annually approximately 4000 diabetics sustain an ankle fracture, or less than 2 % of all diabetic ankle fractures in the United States, are going to be managed surgically for their injury.
Preoperative Evaluations
Unless the patient presents with an open fracture or an irreducible dislocation, there is no emergency for surgery. It is important that one understands that both medical and surgical treatment will be needed to manage these patients, rather than placing conveniently into the surgical schedule
History
The management begins with a thorough history, specifically asking about the mechanisms and the timing of the injury. Up to 74 % of diabetic patients have scores less than the threshold for osteopenia and 39 % below the threshold for osteoporosis [16]. Therefore, a low (ground level fall) mechanism of energy resulting in a complex fracture pattern may indicate poor bone quality. Additionally, questions about when the injury occurred are also important. Because neuropathy is present in 10 % of diabetics [17] it can be inferred for any patient continuing to ambulate on that extremity and presenting more than 24 h after the injury occurred.
The history should also include questions about the presence of comorbidities since they have been shown to increase the rates of complications [11]. With approximately 89 % of diabetics presenting with one additional comorbidity and 15 % have four or more [2], this means that all medical and vascular evaluations should be performed prior to any surgical intervention. Additional questions should include whether ambulatory aids were used prior to their injury, whether or not they smoke, their use of insulin or other medicines, and whether they have a history of previous ulcers or infections.
Physical Examination
The examination should begin by inspecting the soft-tissue envelope and evaluating the neurovascular structures of the limb. Any wounds or lacerations should be evaluated for an open fracture. Look and palpate for changes in skin color, temperature changes, or any bony prominences, all of which may be an indication of impending skin necrosis. Additionally, fracture blisters or the presence of any tense compartments may indicate that the extremity is not ready for operative fixation.
The neurologic examination should also begin with an observation of the extremity. Motor dysfunction , indicating intrinsic atrophy, is often manifested as clawing of the toes and neuropathic autonomic dysfunction is suspected in patients presenting with dry, cracking, hyperemic skin. Of greatest concern is the loss of protective sensation due to neuropathy. Loss of vibratory sensation, pinprick, sense of position or absence of deep tendon reflexes at the ankle (difficult to perform in the presence of a fracture) may indicate neuropathy but have only a fair agreement amongst evaluators [18]. Although the gold standard for identifying peripheral neuropathy is a nerve conduction study, the accepted method for detecting the loss of protective sensation is the use of a 5.07 (10-g) Semmes-Weinstein monofilament. This simple exam has a sensitivity and specificity of 91 % and 86 %, respectively [19], which increases with a minimum testing of four plantar sites (great toe, first, third and fifth metatarsals) [18]. Detecting peripheral neuropathy is important since it increases both the risk of non-compliance and postoperative infections by a factor of four [20].
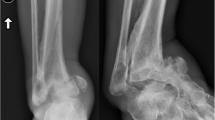
The last part of the physical exam should include a vascular evaluation. This is important since more than 40 % of diabetics present with peripheral arterial disease [21]. The popliteal trifurcation is most often affected however, vessel calcification in the ankle and the foot are suggestive of vascular compromise (Fig. 8.1). Visual signs suggestive of peripheral artery disease include dependant rubor, pallor with elevation of the extremity, dystrophic toenails, and hair loss [22]. The evaluation should continue with an attempt to palpate pulses and comparing it with the contralateral extremity. If pulses are still absent or diminished, after reducing the dislocation or improving the fracture alignment, the aid of Doppler ultrasound can be used to identify the vessels. However, the use of the ankle-brachial (ABI) index is often described as a more sensitive, noninvasive test for evaluating the patient’s vascular status. A value of 0.91–1.3 is considered normal. However, in the diabetic, an ABI ≥ 1.1 can be suggestive of arterial calcinosis and an ABI > 1.3 indicates poor compressibility of the vessel [22]. In patients with acute ankle fractures an ABI may be difficult to perform, so for these patients one should pursue additional testing.
Currently, three additional, noninvasive tests are available. The first measures the transcutaneous oxygen pressure (TcPO2) of the skin. Pressures >30 mmHg are the minimum value needed to heal surgical wounds [22]. The second test places small blood pressure cuffs around each toe and measures the systolic pressure of each toe. A toe pressure >40 mmHg is predictive of good wound healing [22]. If there is any question, however, they should be referred to a vascular surgeon for further work-up. The third test is the toe-brachial index (TBI) and is calculated by dividing the toe pressure by the highest obtained ankle pressure. Currently a value >0.7 has been reported as the cutoff for a normal value [23]. Again, the problem with the TBI is that in the presence of a fracture the patient may not tolerate a cuff placed around the ankle. Currently, the authors’ preferred method of evaluation is measuring the patient’s toe pressures. Further discussions can be found in the chapter on the Vascular Evaluation and Management of Vascular Disease in the Diabetic Patient.
Laboratory Evaluations
As discussed, uncontrolled hyperglycemia results in pathophysiologic dysfunctions [3, 4, 6–11]. Therefore, in addition to standard preoperative laboratory studies, all patients should also have their hemoglobin A1c (HgA1c) evaluated. Levels > 6.5 increase the risk of complications, produce longer hospital stays, and result in poor radiologic outcomes [24]. Those with values >8 have a 2.5 times greater risk of developing an infection [25]. It should be noted that for every 1 % reduction in HgA1c, there is approximately a 25–30 % reduction in the rate of complications [26]. Patients may not necessarily be excluded from surgery, due to an elevated HgA1c, but this information may help manage their diabetes during their postoperative care.
Fracture Management
Whether managed operatively or non-operatively, the goals of treatment are to achieve a stable and congruent joint, restore function, and to prevent complications from occurring. Unfortunately, there is no clear algorithm to guide the treatment, based on fracture displacement, for this population.
Non-operative Treatment
The nonsurgical management can be controversial because of the concern for displacement; however, these patients can be treated to completion successfully. Nonsurgical care is offered to patients presenting with non-displaced fractures, with a good rule of thumb being to double or triple the treatment offered to non-diabetic patients. Therefore, the authors’ preferred method for non-operative treatment consists of placing patients into a short leg, non-weight-bearing cast for 10–12 weeks. Weekly or biweekly radiographs and inspection of the soft-tissue envelope should be performed to ensure that there has been no displacement of the mortise and no problems to the soft tissue envelope have developed (Fig. 8.2). After the casting period, patients are placed into a period of protective weight-bearing, using a brace or boot, for an additional 2–3 months.
Significant necrosis on the medial surface of the ankle and foot in a neuropathic, non-compliant, type I diabetic treated with a short leg, non-weight-bearing cast for a non-displaced fracture. Patient did not return to clinic for 8 weeks after initial cast application. An amputation was ultimately performed
Very few studies discuss the nonsurgical management of diabetic ankle fractures. Most contain very small numbers of patients and are often discussed as one of the arms of treatment, in-lieu of surgical management [9, 11, 27–29]. The complications reported in these studies have included malunions, due to a loss in the initial reduction; non-unions; the development of Charcot neuroarthropathy; infections; and the development of ulcers. Risk factors for developing a complication include seeing patients infrequently, early weight-bearing or non-compliance, having a long duration of diabetes, the presence of neuropathy, insulin dependence, and those with a history of Charcot neuroarthropathy. Risk factors not associated with complications include age, gender, and type of fracture [9, 11].
Operative Treatment
Preoperative Care and Planning
The indication for surgical management is an unstable ankle fracture. However, before fixation is performed it is important to stabilize the soft-tissue envelope. This includes a prompt reduction and splinting of the extremity, especially if fracture blisters have occurred. Immobilization can be achieved using a well-padded, non-removable splint or with the use of an external fixator, if the reduction cannot be maintained by using the splint alone. The patient is instructed to keep the leg elevated as much as possible and is evaluated at weekly intervals. The ability to wrinkle the skin and a re-epithelialization of the skin, after fracture blisters have resolved, indicates that the soft tissues have stabilized and are ready for surgical management. This may take anywhere between 10 and 21 days and during this period the preoperative evaluations and planning should be performed.
The preoperative planning is undertaken to ensure that all the equipment and implants needed for surgery will be present. This includes small, large, and periarticular bone clamps, extra-long drill bits, extra-long screws, with lengths reaching 90–110 mm in length and in sizes ranging from 2.7 to 4.5 mm, Steinman pins, and extra-long k-wires. In addition, locking mini, small, and large extra-long locking plates, and their corresponding locking screws, should also be readily accessible. A 3.5- or 4.5-mm locking plate, at least ten holes in length, for fixation of the fibula should be utilized while avoiding semi-tubular or easily deformable (malleable) plates. Lamina spreaders or distractors should also be on hand if distraction of the fractures, especially in the fibula, is anticipated. Lastly, an external fixator should also be on hand if the anticipation is that the ankle construct will need to be augmented with external fixation.
Operative Management
There are four approaches that can be used to manage the diabetic ankle fracture: standard fixation, trans-syndesmotic, trans-articular, and a combination of these techniques. Standard fixation, with expected good outcomes, can be considered for any patient presenting with an HbA1c less than 7.0, a body-mass index (BMI) less than 25, able to sense a 5.07 or smaller Semmes-Weinstein monofilament, the presence of palpable pulses, non-osteoporotic bone, and those without any manifestations of autonomic dysfunction. Postoperatively, patients can be managed similar to non-diabetic patients.
For patients who do not meet these criteria, three methods of fixation are available. These three techniques are much different than standard methods of ankle fixation but have been developed to maintain an anatomic mortise and decrease the risk that failure of fixation will occur prior to adequate healing. In addition to prolonged immobilization and non-weight bearing, the operative principles for these three techniques include the use of long, rigid, locking fixation, using some kind of adjunctive fixation, considering adding a bone graft, and contemplating the use of a bone stimulator (Table 8.1). Because of the patient’s abnormal bony metabolism, the authors’ current treatment of choice is to add a bone stimulator to all patients when using one of these three alternative techniques.
The trans-syndesmotic fixation technique uses the tibia to help stabilize the fibular fixation. Described using tetracortical screws (crossing four cortices), this method consists of getting the fibula out to length, reducing the fracture, applying at least a 10-hole 3.5 mm or larger locking plate onto the fibula, and then inserting as many locking screws as possible through the fibula and into the tibia [30]. The advantage of using a locking plate is that it provides angular stability, which increases its load-carrying capacity, which allows locking plates to be four times stronger than load-sharing constructs. This means that for failure of fixation to occur it requires that all points of fixation fail as opposed to the loosening of individual screws, as seen with traditional compression plating techniques. To complete the fixation of the ankle, long, 4.0-mm bicortical screws should be used to stabilize the medial and posterior malleolar fractures (Fig. 8.3a–d). This construct improves fixation stiffness without relying solely on the screw’s purchase in the fibula. Although there is some concern that this technique may alter the biomechanics of the syndesmosis, this has not been demonstrated clinically. For postoperative care see Table 8.1.
Mortise (a) and lateral (b) views of a displaced, right trimalleolar ankle fracture in a neuropathic male. Using a trans-syndesmotic technique , a good reduction is noted in the postoperative mortise (c) and lateral (d) views. Note the use of bicortical screws for the medial and posterior malleolar fractures
The second alternative technique is a trans-articular (non-fusion) method of fixation , and can be approached in one of two ways. The first is to treat the patient using standard reduction techniques, which is then augmented using two or three large, smooth, retrograde tibio-talar-calcaneal Steinmann pins [30] (Fig. 8.4a–c). This produces some stiffness of ankle and the hindfoot but does not rely solely on standard fixation techniques to main the reduction. The second approach is the use of a retrograde tibial-talar-calcaneal intramedullary nail. Although some calcification or arthrodesis of the ankle or subtalar joints is possible, the difference between this method and an arthrodesis technique is that neither the subtalar nor the ankle joint is exposed and prepared as when performing a formal arthrodesis (Fig. 8.5a–c). This approach works well in patients presenting with pilon fractures but can also be used in certain unstable bi- or trimalleolar ankle fractures, especially in patients with morbid obesity. Once the fracture is healed a decision regarding nail removal can be made. For postoperative care of both approaches see Table 8.1. To complete the discussion of trans-articular methods of fixation, immediate arthrodesis of the ankle has been described for non-reconstructable fractures [31] but has rarely been performed for an acute diabetic ankle fracture. However, in the setting of poor bone quality, a poorly controlled diabetic with neuropathy, autonomic changes and poor potential to heal the fracture, an immediate arthrodesis may be considered to improve the outcome of that patient.
Anteroposterior (AP) view (a) of a displaced pilon fracture sustained in an insulin-dependent, neuropathic male with peripheral artery disease and a 3 pack/day smoking history. Patient required revascularization and fixation consisted of a retrograde nail. Improved alignment and healing are noted in the AP (b) and lateral (c) views
The third technique is described as a combined technique, with the surgical tactic described in Table 8.2. In this approach the trans-syndesmotic technique is augmented using two or three large, smooth, retrograde tibio-talar-calcaneal Steinmann pins (Fig. 8.6a–d). This approach provides significant stiffness to the construct and is currently the authors’ treatment of choice for the management of acute diabetic ankle fractures that are unable to be managed with standard fixation. Similar to the other two methods described, the stiffness acquired with this approach does not seem to be a problem clinically because ambulation progressively restores motion between the tibia and fibula. The postoperative care is described in Table 8.1 .
Mortise view (a) of a fracture dislocation in a morbidly obese, neuropathic male. Note the displacement (arrow) of Chaput’s tubercle. Using a combined technique, the fibular was lengthened using a push–pull technique (b) and once out to length the syndesmosis was reduced with a periarticular clamp (c). Postoperative reduction (d) shows improved alignment of the fracture. See Table 8.2 for the surgical tactic
Complications and Salvage
The four major complications associated with managing these patients consist of failure of fixation, skin and wound problems, infections, and the development of Charcot arthropathy. Complications range from 3.6 to 43 % [8, 20, 25, 27, 29, 32, 33] and can occur individually or in any combination. It is of no surprise that the rates of complications are higher for the diabetic than in the non-diabetic population, with the highest risk occurring in poorly controlled diabetics [25]. Therefore, the question is, after operating on these high-risk patients can their complication(s) be treated without necessitating an amputation as the only salvageable option?
Failure of Fixation
In this context, failure of fixation is defined as a loss of the reduction early in the postoperative period, without the development of a Charcot joint (Fig. 8.7a–c). The most common reasons for this complication are often a combination of the patient’s neuropathy, their inability to avoid weight-bearing on the extremity, and inadequate fixation performed at the index procedure. By far the biggest mistake is in managing these patients like a well-controlled or non-diabetic patient. Because a significant number of patients have little or no upper body strength, patients will often begin full weight bearing within hours after their surgery. In an attempt to decrease this complication, patients should be placed into wheelchairs to help them maintain a non-weight-bearing attitude, a discussion should be made with their caregivers about the importance of keeping them off their foot, and weekly visits may be necessary if non-compliance persists to make sure that displacement has not occurred.
An AP view (a) of an unstable bimalleolar left ankle. Immediate post-fixation in a splint (b) demonstrates a good reduction of the ankle. Short leg cast applied and patient returned to clinic 10 days later demonstrating a broken plate (c) and displacement of the fracture. Courtesy of Robert Probe, MD
The salvage of a failed fixation is via one of the three previously discussed alternative approaches, with the timing dependant on the health of the soft-tissue envelope. Continued conservative treatment of the malaligned extremity will result in malunions, non-unions, the development of contractures, and possible skin breakdown and/or ulcerations (Fig. 8.8a–b). It is possible that the addition of trans-articular external fixation can improve the overall alignment of the extremity but it may not produce an anatomic reduction of the mortise. If a revision fixation is unable to be performed then a salvage using an ankle or double hindfoot arthrodesis (ankle and subtalar joint), may be necessary to salvage the extremity (Fig. 8.9a–g).
Minimally displaced bimalleolar ankle fracture (a) managed with percutaneous fixation of the fibula and medial malleolus (b). Failure of fixation (c) identified at first office visit. Revision fixation (d) was performed with failure of second fixation (e) identified at that initial postoperative visit. Patient had significant medical comorbidities and was ultimately salvaged using a double hindfoot arthrodesis, with improved alignment noted in the AP (f) and lateral (g) views
Skin and Wound Problems
Wound edge necrosis and dehiscence, without the presence of infection, are constant concerns when managing these patients. Even without the presence of a fracture or surgery, there is a considerable challenge in trying to get things to heal in this population [27]. As has already been noted hyperglycemia decreases blood flow to both small and large vessels [22], increases blood viscosity, impairs the ability of the red blood cell to adequately flow, and decreases the amount of oxygen reaching the tissues. This resulting hypoxia inhibits fibroblasts from migrating to the wound and causes them to lose their ability to proliferate, which may last for up to 8 weeks [3]. This is in addition to smoking, hypertension, dyslipidemia, increased body-mass index, and advanced age, which have also been shown to have a negative effect on healing [3, 8–11, 14, 20].
Given the combination of fracture edema, hypoxia, and hyperglycemia one can envision a poor environment for diabetic wound healing [29] even in at-risk patients managed non-operatively. Early salvage requires frequent (often weekly) clinic visits since these problems are usually identified during routine cast changes. During these visits, encouraging good control of their diabetes, discussing the need for elevating the extremity, and placing them into wheelchairs may all help with healing, compliance, and edema. In addition, reapplying a well-padded splint, in-lieu of the cast, may help avoid pressure to the compromised skin. When skin or wound problems are identified, a systematic approach should be used to manage these patients. For the first 3–4 weeks, after the wound has been identified, initial treatment includes local, daily wound care, through a windowed cast, and the empiric use of a broad spectrum oral antibiotic. If the wound fails to improve, irrigation and debridement and the use of negative pressure wound therapy may be necessary. If after 4–6 weeks of negative pressure therapy, worsening or no improvement is noted, a plastic surgery consultation may be necessary.
Infection
The biggest concern in managing these patients is the development of an infection. Both superficial and deep infections can occur with rates ranging from 3.6 to 43 % [32, 33]. Due to neuropathy, they lose their ability to sense an infection, which is why even patients treated non-operatively have been identified with an infection [9]. Risk factors for the development of an infection include, the presence of peripheral arterial disease, neuropathy, diabetes of long duration, poor glucose control (especially a HgA1c >8), the presence of a Charcot joint, the presence of edema and ecchymosis, older patients, obesity, a history of rheumatoid arthritis, a history of a previous ulcer, and in patients presenting with an open fracture [9, 11, 20, 25, 33]. Factors that do not increase the risk of infection include tobacco use, gender, type of fracture, American Society of Anesthesiologists (ASA) classification, and whether the surgery was performed as an inpatient or an outpatient [11, 20].
Frequent visits may not decrease this complication from occurring but can offer earlier treatment when they are identified. As with wound complications, the infection is often identified during a routine change of the patient’s cast. For superficial infections, windowing the cast, to allow local, daily wound care, providing oral antibiotics, and weekly office visits may be sufficient to manage the problem. In contrast, all deep infections should be managed with irrigation and debridement, a minimum 6-week course of intravenous antibiotics, and removal of all loose implants. Avoid the urge to perform a local swab of the area. Rather, deep cultures or even a bone biopsy may be necessary to identify the organism(s) if osteomyelitis is suspected. Once the infection has been controlled, the use of a local flap or a free tissue transfer may be necessary to address the wound. If after bony debridement significant bone has been removed or the articular surfaces have been lost then an ankle or double hindfoot arthrodesis may be needed to salvage the extremity. If the extremity is not salvageable then an amputation may be necessary. Further discussions on reconstructions can be found in the chapter on the Management of Infections and Osteomyelitis in the Diabetic Patient.
Charcot Neuroarthropathy
Its incidence, in diabetic ankle fractures, has been reported to occur between 6 and 47 % [11, 25, 28, 33]. It is challenging to manage, especially when it presents after the surgical care of an ankle fracture, because it is often confused with infection. On initial presentation, patients often present with erythema, edema, and some warmth to palpation. The differential diagnosis can include gout, cellulitis, abscess, and osteomyelitis. However, the diagnosis of a Charcot joint should be considered in any compliant patient, who had an anatomic reduction of the mortise and presents with failure of fixation. Careful physical, laboratory, and radiographic examinations will identify whether the patient has developed a Charcot neuroarthropathy or has a postoperative infection (See Appendix, Table 1).
The salvage of these patients can be difficult because they often present late with malunions, non-unions and contractures of the extremities. Reconstructions should be considered when the extremity is in the subacute or chronic stages. Indications for surgery should include failure of conservative care, chronic deformity, instability not amenable to bracing, and evidence of abnormal plantar pressures, despite the use of an orthoses and special shoes. Reconstructions often involve bony and soft-tissue procedures in order to improve the alignment and obtain a viable extremity. Further discussions on reconstructions can be found in the chapter on the Management of the Charcot Ankle.
In conclusion, avoid managing the acute diabetic ankle fracture similar to those treated in the non-diabetic population. These patients have increased rates of complications and infections and are usually non-compliant due to their neuropathy. Careful preoperative evaluations and postoperative vigilance can improve outcomes. These patients require very rigid repair, often with some kind of adjunctive fixation, with long periods of immobilization and protective weight bearing. Significant deformities can produce abnormal plantar pressure, irritability with shoewear and malalignment of the extremity. However, good outcomes can be expected with alternative techniques and even some residual deformity does not seem to produce much disability.
References
Vincent AM, Russell JW, Low P, Feldman EL. Oxidative stress in the pathogenesis of diabetic neuropathy. Endocr Rev. 2004;25:612–28.
Paraskevas KI, Baker DM, Pompella A, Mikhailidis DP. Does diabetes mellitus play a role in restenosis and patency rates following lower extremity peripheral arterial revascularization? A critical overview. Ann Vasc Surg. 2008;22:481–91.
Stadelmann WK, Digenis AG, Tobin GR. Impediments to wound healing. Am J Surg. 1998;176(2A Suppl):39S–47.
Macey LR, Kana SM, Jingushi S, Terek RM, Borretos J, Bolander ME. Defects of early fracture-healing in experimental diabetes. J Bone Joint Surg Am. 1989;71:722–33.
Weinberg E, Maymon T, Moses O, Weinreb M. Streptozotocin-induced diabetes in rats diminishes the size of the osteoprogenitor pool in bone marrow. Diabetes Res Clin Pract. 2014;103:35–41.
Loder RT. The influence of diabetes mellitus on the healing of closed fractures. Clin Orthop Relat Res. 1988;232:210–6.
Boddenberg U. Healing time of foot and ankle fractures in patients with diabetes mellitus: literature review and report on own cases. Zentralbl Chir. 2004;129:453–9.
Ganesh SP, Pietrobon R, Cecílio WAC, Pan D, LightdaleN NJA. The impact of diabetes on patient outcomes after ankle fracture. J Bone Joint Surg Am. 2005;87:1712–8.
Flynn JM, Rodriguez-del Río F, Pizá PA. Closed ankle fractures in the diabetic patient. Foot Ankle Int. 2000;21:311–9.
Carnevale V, Romagnoli E, D’Erasmo E. Skeletal involvement in patients with diabetes mellitus. Diabetes Metab Res Rev. 2004;20:196–204.
Jones KB, Maiers-Yeldon KA, Marsh JL, Zimmerman MB, Estin M, Saltzman CL. Ankle fractures in patients with diabetes mellitus. J Bone Joint Surg Br. 2005;87:489–95.
Centers for Disease Control and Prevention. National Diabetes Statistics Report: Estimates of Diabetes and Its Burden in the United States. Atlanta, GA: U.S. Department of Health and Human Services; 2014. http://www.cdc.gov/diabetes/pubs/statsreport14/national-diabetes-report-web.pdf.
Clark JL, Meiris DC. Building bridges: integrative solutions for managing complex comorbid conditions. Am J Med Qual. 2007;22:5–15.
Ong KL, Cheung BM, Wong LY, Wat NM, Tan KC, Lam KS. Prevalence, treatment, and control of diagnosed diabetes in the U.S. National Health and Nutrition Examination Survey 199-2004. Ann Epidemiol. 2008;18:222–9.
Court-Brown CM, Caesar B. Epidemiology of adult fractures: a review. Injury. 2006;37:691–7.
Herbst SA, Jones KB, Saltzman CL. Pattern of diabetic neuropathic arthropathy associated with peripheral bone mineral density. J Bone Joint Surg Br. 2004;86:378–83.
Prisk VR, Wukich DK. Ankle fractures in diabetics. Foot Ankle Clin N Am. 2006;11:849863.
Smieja M, Hunt DL, Edelman D, Etchells E, Cornuz J, Simel DL. Clinical examination for the detection of protective sensation in the feet of diabetic patients. International Cooperative Group for Clinical Examination Research. J Gen Intern Med. 1999;14:418–24.
Pham H, Armstrong DG, Harvey C, Harkless LB, Giurini JM, Veves A. Screening techniques to identify people at high risk for diabetic foot ulceration: a prospective multicenter trial. Diabetes Care. 2000;23:606–11.
Wukich DK, Lowery NJ, McMillen RI, Fryberg RG. Postoperative infection rates in foot and ankle surgery: a comparison of patients with and without diabetes mellitus. J Bone Joint Surg Am. 2010;92:287–95.
Novo S. Classification, epidemiology, risk factors, and natural history of peripheral artery disease. Diabetes Obes Metab. 2002;4 Suppl 2:S1–6.
American Diabetes Association. Peripheral arterial disease in people with diabetes. Diabet Care. 2003;26:3333–41.
Høyer C, Sandermann J, Petersen LJ. The toe-brachial index in the diagnosis of peripheral artery disease. J Vasc Surg. 2013;58:231–8.
Liu J, Ludwig T, Ebraheim NA. Effect of the blood HbA1c level on surgical treatment outcomes of diabetics with ankle fractures. Orthop Surg. 2013;5:203–8.
Wukich DK, Crim BE, Frykberg RG, Rosario BL. Neuropathy and poorly controlled diabetes increase the rate of surgical site infection after foot and ankle surgery. J Bone Joint Surg Am. 2014;96:832–9.
Hoogwerf BJ, Sferra J, Bonley BG. Diabetes mellitus—overview. Foot Ankle Clin N Am. 2006;11:703–15.
Chaudhary SB, Liporace FA, Gandhi A, Donley BG, Pinzur MS, Lin SS. Complications of ankle fractures in patients with diabetes. J Am Acad Orthop Surg. 2008;16:159–70.
Wukich DK, Kline AJ. Current concepts review. The management of ankle fractures in patients with diabetes. J Bone Joint Surg Am. 2008;90:1570–8.
McCormack RG, Leith JM. Ankle fractures in diabetics. Complications of surgical management. J Bone Joint Surg Br. 1998;80:689–92.
Jani MM, Ricci WM, Borrelli Jr J, Barrett SE, Johnson JE. A protocol for treatment of unstable ankle fractures using transarticular fixation in patients with diabetes mellitus and loss of protective sensation. Foot Ankle Int. 2003;24:838–44.
Bozic V, Thordarson DB, Hertz J. Ankle fusion for definitive management of non-reconstructable pilon fractures. Foot Ankle Int. 2008;29:914–8.
SooHoo NF, Krenek L, Eagen MJ, Gurbani B, Ko CY, Zingmond DS. Complication rates following open reduction and internal fixation of ankle fractures. J Bone Joint Surg Am. 2009;91:1042–9.
Blotter RH, Connolly E, Wasan A, Chapman MW. Acute complications in the operative treatment of isolated ankle fractures in patients with diabetes mellitus. Foot Ankle Int. 1999;20:687–94.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2016 Springer International Publishing Switzerland
About this chapter
Cite this chapter
Herscovici, D., Scaduto, J.M. (2016). Management of Acute Diabetic Fractures of the Ankle. In: Herscovici, Jr., D. (eds) The Surgical Management of the Diabetic Foot and Ankle. Springer, Cham. https://doi.org/10.1007/978-3-319-27623-6_8
Download citation
DOI: https://doi.org/10.1007/978-3-319-27623-6_8
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-27621-2
Online ISBN: 978-3-319-27623-6
eBook Packages: MedicineMedicine (R0)