Abstract
Eukaryotic cells are characterized by compartmentalization and specialization of metabolism within membrane-bound organelles. Nevertheless, many fundamental processes extend across multiple subcellular compartments. Here, we describe and assess the pathways and cellular organization of triacylglycerol biosynthesis in microalgae. In particular, we emphases the dynamic interplay among the endoplasmic reticulum, lipid droplets and chloroplasts in acyl remodeling and triacylglycerol accumulation under nitrogen starvation in the model alga Chlamydomonas reinhardtii.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
One of the defining features of photosynthetic eukaryotic organisms is the presence of the chloroplast. Beyond their role in photosynthesis, chloroplasts contribute to a wide array of fundamental functions that include the de novo synthesis of fatty acids, the building blocks of membrane lipids and storage triacylglycerol. Under normal growth conditions, the vast majority of de novo-synthesized fatty acids are used for membrane lipid assembly to support cell growth, organelle biogenesis and membrane proliferation. Under stress conditions such as nutrient deprivation, many microalgae can accumulate large amounts of triacylglycerol by diverting fatty acids from membrane lipid synthesis to the synthesis of TAG and/or by converting preformed membrane lipids to TAG. In most eukaryotic cells, storage lipids are packaged in simple structures termed lipid droplets (also referred to as oil bodies), which consist of a central core of TAG within a monolayer of membrane lipids with a small amount of embedded specific proteins. The current model of lipid body biogenesis favors formation of lipid droplets through budding from the ER (Thiele and Spandl 2008; Walther and Farese 2009; Chapman et al. 2012; Wilfling et al. 2014). According to this hypothesis, lipid droplets originate from specialized ER subdomains enriched with enzymes involved in TAG biosynthesis. Because the newly formed TAGs in these ER domains are unable to integrate into membrane bilayers due to lack of polar head groups, they accumulate in the hydrophobic region between the two leaflets of the ER membrane which leads to swelling of the membrane bilayer and eventually the budding of growing lipid droplets from ER into the cytosol. Recent years have seen rapid progress in identifying and characterizing molecular components of lipid droplets, revealing the genes and enzymes in lipid metabolic pathways and deciphering the regulatory networks controlling carbon metabolism and storage reserve accumulation in microalgae, particularly in the model alga Chlamydomonas. Here we review data on the pathways and cellular compartmentalization of glycerolipid biosynthesis in microalgae, focusing on interconnections among the endoplasmic reticulum, chloroplasts and lipid droplets in lipid remodeling and TAG biosynthesis in Chlamydomonas. Where appropriate, knowledge gained from higher plants, yeast and mammals will be discussed to highlight similarities and differences between these experimental systems.
Diacylglycerol and Glycerolipid Synthesis
Biosynthesis of diacylglycerol (DAG) and glycerolipids in plants and algae encompasses two parallel pathways involving multiple subcellular compartments. Fatty acid synthesis occurs almost exclusively in the plastid and is catalyzed by two large, evolutionarily conserved enzyme complexes: acetyl-CoA carboxylase (ACCase) and fatty acid synthase (Ohlrogge and Jaworski 1997). In most photosynthetic organisms, the major end products of fatty acid synthesis are 16:0- and 18:0 fatty acids esterified to acyl carrier protein (ACP), which are subsequently processed by stearoyl-ACP desaturase to generate 18:1-ACP and/or by acyl-ACP thioesterases to release fatty acids and ACP. A fraction of newly synthesized acyl-ACPs are used in plastids for the sequential acylation of glycerol-3-phosphate (G-3-P) catalyzed by plastidic acyltransferases, leading to the generation of phosphatidic acid (PA). PA is a key intermediate in the formation of the thylakoid membrane phospholipid phosphatidylglycerol. In addition, PA can be dephosphorylated by PA phosphohydrolases (PAHs) to generate DAG, which is normally used for assembly of thylakoid glycolipids including galactolipids, monogalactosyldiacylglycerol (MGDG), digalactosyldiacylglycerol (DGDG) and the sulfolipid sulfoquino vosyldiacylglycerol (SQDG) in the plastid envelope membranes. This sequence of reactions is commonly referred to as the prokaryotic pathway (Browse et al. 1986; Browse and Somerville 1991). In photosynthetic cells, two galactolipids MGDG and DGDG account for the bulk (i.e., up to 80 %) of thylakoid lipids (Dörmann et al. 1999).
Alternatively, fatty acids can be exported from the plastid to enter into glycerolipid biosynthetic pathways in the endoplasmic reticulum (ER), leading to the formation of DAG by sequential G-3-P acylation and PA dephosphorylation catalyzed by ER-resident acyltransferases and PAHs, respectively (Browse and Somerville 1991). The resulting DAG can be used to synthesize extraplastidic membrane lipids including phosphatidylcholine (PC), phosphatidylethanolamine (PE) and a non-phosporus-containing polar lipid diacylglycerol-N,N,N-trimethylhomoserine (DGTS) (Thompson 1996; Harwood and Guschina 2009). Some of the phospholipids assembled in the ER return to the plastid, thus providing the DAG moieties for the synthesis of thylakoid glycolipids in the plastid (Roughan and Slack 1982; Browse et al. 1986). This sequence of events is referred to as the eukaryotic pathway of thylakoid lipid synthesis. Because of the different substrate specificity of acyltransferases present in the plastid and endoplasmic reticulum, glycerolipids made via the prokaryotic and eukaryotic pathway are characterized by the presence of a 16-carbon (C16) or 18-carbon (C18) fatty acid at the sn-2 position of the glycerol backbone, respectively (Frentzen 1998).
Chlamydomonas chloroplast lipid biosynthesis is thought to be almost completely autonomous because it lacks thylakoid lipids derived from the eukaryotic pathway characterized by the presence of C18 acyl chains at the sn-2 position (Giroud et al. 1988; Giroud and Eichenberger 1989; Harwood and Guschina 2009). On the other hand, the galactolipids in the marine brown alga Dictyopteris membranacea (Hofmann and Eichenberger 1997) and several other brown algal species (Jones and Harwood 1992) are almost completely of the eukaryotic type. A few green algae such as Chlorella kessleri (Sato et al. 2003) and Acetabularia mediterranea (Thompson 1996) and some red and brown algae (Hofmann and Eichenberger 1997; Makewicz et al. 1997; Sato and Moriyama 2007) contain substantial amounts of ER-derived eukaryotic lipids in photosynthetic membranes and therefore employ two parallel pathways for chloroplast lipid assembly.
A characteristic feature of lipid metabolism in Chlamydomonas is the absence of PC (Sakurai et al. 2014). In higher plants, this phospholipid is known to play a critical role in supply of DAG moieties for the synthesis of eukaryotic thylakoid (Benning 2008, 2009). Therefore, the absence of the eukaryotic thylakoid lipid synthesis in Chlamydomonas has been assumed to be due to the lack of PC (Moellering et al. 2009; Riekhof and Benning 2009). However, radiotracer labeling studies in the marine brown alga Dictyopteris membranacea have shown that the absence of PC does not compromise the eukaryotic pathway of galactolipid biosynthesis (Hofmann and Eichenberger 1997). In addition, several brown algae contain almost exclusively eukaryotic thylakoid glycolipids despite the lack of PC (Jones and Harwood 1992). Together, these results suggest that PC is not absolutely required for the eukaryotic pathway of chloroplast lipid biosynthesis.
TAG synthesis shares the common precursor DAG produced by sequential G-3-P acylation and PA dephosphorylation reactions as described above with the synthesis of membrane lipids. Results from yeast, plant and mammals indicate that DAG partitioning between membrane lipids and TAG is, to a major extent, modulated by lipin family of PAHs (Harris and Finck 2011; Pascual and Carman 2013; Siniossoglou 2013; Fan et al. 2014). Disruption of the yeast lipin homolog impairs TAG synthesis (Han et al. 2006) and lipid droplet formation (Adeyo et al. 2011), causing an increase in phospholipid synthesis and a massive proliferation of ER and nuclear membranes (Santos-Rosa et al. 2005). Similarly, Arabidopsis lipins have been implicated in the regulation of TAG accumulation (Fan et al. 2014), phospholipid synthesis and ER membrane expansion (Eastmond et al. 2010). Mammalian lipins are bifunctional intracellular proteins, acting as regulators of DNA-bound transcription factors, in addition to catalyzing the dephosphorylation of PA (Harris and Finck 2011). A recent study showed that genetic modifications of PAHs result in altered TAG content in Chlamydomonas (Deng et al. 2013), suggesting that the role of lipin family of PAHs in TAG synthesis and regulation may be evolutionally conserved in eukaryotes ranging from yeast, algae to humans.
Acyl-CoA-Dependent and -Independent Pathways of TAG Synthesis
Microsomal membranes have long been recognized as the primary site of TAG assembly in yeast, plants and mammals. In support of the ER-localization of TAG biosynthesis, key enzymes involved in the final step of TAG synthesis are also associated with the ER network (Wakimoto et al. 2003; Shockey et al. 2006; Cao et al. 2007) and TAGs are packaged in oil droplets in the cytosol. Both acyl-CoA-dependent and –independent pathways contribute to the last step of TAG synthesis in yeast and higher plants and a similar situation may also apply to algae. The acyl-CoA-dependent TAG synthesis is catalyzed by diacylglycerol:acyl-CoA acyltransferases (DGATs) using acyl-CoA and DAG as substrates to form TAG. Two types of membrane-bound DGATs with distinct protein sequences, functionality, expression patterns and subcellular localization are known. The acyl-CoA-independent reaction is catalyzed by phospholipid:diacylglycerol acyltransferase (PDAT), which use phospholipids as acyl donor and DAG as acyl acceptor to produce TAG and lysophospholipids. The Chlamydomonas genome codes for five type 2 DGATs (DGTT1–DGTT5), one type 1 DGAT (DGAT1) and one PDAT (PDAT1). Among them, the functionality of DGTT1-DGTT3 and PDAT1 has been validated by heterologous complementation of a yeast ∆dga1∆lro1 mutant lacking both DGAT and PDAT activity (Sanjaya et al. 2013; Boyle et al. 2012; Yoon et al. 2012). In addition, DGTT4 has also been demonstrated to possess DGAT activity in vitro assays using recombinant proteins, while DGTT5 is likely to be an inactive pseudogene (Sanjaya et al. 2013).
Studies in both yeast (Oelkers et al. 2000, 2002) and plants (Fan et al. 2013a, b) have indicated that PDAT plays a major role in TAG synthesis during stages of active cell growth and division, while DGATs appear to be more important in non-growing cells (Oelkers et al. 2002; Fan et al. 2013a, b). Similarly, A recent study has shown that artificial microRNA-mediated silencing of PDAT1 specifically compromises cell growth and TAG synthesis under favorable growth conditions in Chlamydomonas (Yoon et al. 2012). Taken together, these results point to evolutionarily conserved PDAT functions associated with rapid cell growth and membrane proliferation in yeast, microalgae and plants, and may suggest a role for TAG metabolism in membrane lipid homeostasis during cell growth.
The Origin of DAG for TAG Assembly
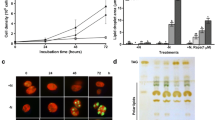
In plants, DAG used for TAG synthesis is mostly derived from PC (Bates et al. 2009) and TAG assembled in the ER is characterized by the exclusive presence of C18 fatty acids at the sn-2 position of the glycerol backbone in many plants (Mattson and Volpenhein 1963). In nitrogen-starved Chlamydomonas cells, however, as much as 90 % DAG used for TAG synthesis is of the prokaryotic type characterized by the presence of C16 fatty acids at the sn-2 position (Fan et al. 2011; Li et al. 2012; Urzica et al. 2013). In addition, while TAG is stored in cytosolic oil droplets in wild-type strains (Goodson et al. 2011), it is deposited in both the cytosol and the chloroplast in TAG hyperaccumulating Chlamydomonas starchless mutants (Fan et al. 2011; Goodson 2011). The use of the prokaryotic type of DAG for TAG assembly appears not to be restricted to Chlamydomonas cells, as TAGs from a wide variety of other algae were reported to be also highly enriched in C16 fatty acids (Suen et al. 1987; Yongmanitchai and Ward 1993; Davidi et al. 2014b). Our analysis of the fatty acid distribution of TAG isolated from N-starved Nannochloropsis sp. revealed that the sn-2 position of TAG is mostly occupied by 16:0 with less than 10 % of 14:0 and 18:0 (Fig. 9.1), whereas more than 20 % of acyl chains at the sn-1+3 positions are C18 fatty acids. Very similar results were obtained in the diatom Phaeodactylum tricornutum. TAG isolated from the N-starved, fresh water alga Chlorella vulgaris contains more than 70 % of C16 fatty acids at the sn-2 position, whereas the C16 and C18 fatty acids are equally present at the sn-1+3 positions, suggesting that the prokaryotic type of DAG may be the major precursor used for TAG assembly in many algal species.
Enrichment of 16-carbon fatty acids at the sn-2 position of TAG in algae. Fatty acid composition exclusively at the sn-2 (a) or sn-1+3 (b) positions of TAGs isolated from C. vulgaris (UTEX 259) P. tricornutm (UTEX 640) or Nannochloropsis sp. (CCMP 1779) grown in media lacking N for 1 day is shown. TAG positional analysis was done as described by Fan et al. (2011). Data are presented as the means and standard deviation of three or four replicates
We envision three pathways for accumulating the prokaryotic type of TAG in the cytosol in algae. In the first pathway, this prokaryotic type of TAG is assembled at the chloroplast envelope using DAG derived from the prokaryotic pathway and TAG is deposited in lipid droplets originating from the chloroplast envelope membranes. This hypothesis is supported by the presence of chloroplast envelope proteins such as TGD1, TGD2 and TGD3 in lipid droplets isolated from nitrogen-starved Chlamydomonas cells (Nguyen et al. 2011) and the association of TAG synthetic activities with chloroplast envelope membranes in plants (Martin and Wilson 1984; Kaup et al. 2002). A recent study has showed that phytyl ester synthases (PESs) from chloroplasts of Arabidopsis process diacylglycerol acyltransferase activities in addition to catalyzing the synthesis of fatty acid phytyl esters (Lippold et al. 2012). Homologs of Arabidopsis PESs were identified in Chlamydomonas (Moellering and Benning 2010; Nguyen et al. 2011) and Dunaliella bardawil (Davidi et al. 2014a) and these putative PES enzymes may be involved in TAG assembly in algal chloroplasts (Davidi et al. 2014a). Interestingly, a recent study has shown that PDAT1 from Chlamydomonas can catalyze transacylation reactions between galactolipids and the prokaryotic type of DAG, leading to generation of TAG and lysogalactolipids (Yoon et al. 2012), suggesting that PDAT1 may be important in generating the prokaryotic type of TAG in Chlamydomonas.
In the second pathway, TAG is assembled in the ER using DAG pools exported from the chloroplast and lipid droplets originate from the ER. In the third pathway, both DAG and TAG are assembled in the ER. This hypothesis requires the existence of acyltransferases in the ER that specifically incorporate C16 acyl chains into the sn-2 position of DAG used for TAG synthesis or selective channeling of C16 fatty acids toward the sn-2 position esterification catalyzed by ER-resident acyltransferases to generate “the prokaryotic form” of DAG in the ER. All three pathways are distinct from the relatively well known pathways present in higher plants and yeast. Testing these hypotheses awaits the identification and detailed characterization of acyltransferases from algae.
TAG Synthesis and Acyl Remodeling
In plants, fatty acids exported from the plastid are first incorporated into PC through acyl editing (remodeling) involving PC deacylation and reacylation reactions and acyl groups released from PC remodeling, rather than nascent fatty acids exported from the plastid, are used for the de novo-synthesis of membrane lipids and storage TAG in the ER (Bates and Browse 2011). In nitrogen-starved Chlamydomonas cells, a major fraction of fatty acids stored in TAG are derived from MGDG (Li et al. 2012). Both PDAT1 (Yoon et al. 2012) and a galactoglycerolipid lipase named PGD1 (Li et al. 2012) are implicated in the deacylation of MGDG, generating free fatty acids and lysoMGDG. Disruption of PDAT1 or PGD1 leads to a 20 or 50 % reduction in TAG content, respectively, but the exact subcellular localization of PDAT1- and PGD1-mediated deacylation reactions and the fate of resulting lyso MGDG remain uncertain.
Because the similarities in structure and biophysical properties between PC and DGTS (Sato and Murata 1991), it has been widely assumed that the betaine lipid DGTS may play some of the metabolic roles of PC in algae. Indeed, the presence of DGTS is often correlated with a decrease in the level of PC (Thompson 1996). Like PC, the betaine lipid DGTS was also found to be involved in lipid-linked fatty acid desaturation (Giroud and Eichenberger 1989), acyl remodeling and distribution in algal species Dictyopteris membranacea (Hofmann and Eichenberger 1997), Fucus serratus (Smith and Harwood 1984) and Ascophyllum nodosum and Fucus vesiculosus (Jones and Harwood 1993).
Consistent with a role of DGTS and other extraplastidic membrane lipids in supplying fatty acids for TAG synthesis, TAG isolated from nitrogen-starved cells also contains significant amounts of C18 polyunsaturated fatty acids specific to extraplastidic membrane lipids such as DGTS and PE at the sn-1 and sn-3 positions (Fan et al. 2011; Li et al. 2012; Sakurai et al. 2014). Additional evidence supporting the role of extraplasitidic membrane lipid turnover in TAG accumulation comes from pulse-chase labeling studies using radiolabeled free fatty acids. In these experiments, Chlamydomonas cells in the logarithmic growth phase were pulse-labeled with [1-14C] labeled 16:0 or 18:1 for 16 h in complete growth medium and the movement of label was subsequently chased for 3 days. Because of the acyl asymmetrical distribution of fatty acids in glycerolipids, [1-14C] 16:0 was reported to label mainly the sn-2 position of chloroplast lipids such as MGDG and DGDG, but the sn-1 position of extraplastidic lipids such as DGTS in Chlamydomonas (Giroud and Eichenberger 1989). As shown in Fig. 9.2a, immediately after the pulse, DGTS and SQDG/PI were the most strongly labeled lipids. During the chase in complete growth medium, DGTS, SQDG/PI and PG lost label over time. TAG contained minor amounts of label and stayed largely unchanged during the chase (Fig. 9.2c), whereas label in MGDG and DGDG increased significantly, suggesting that chloroplast membrane proliferation is the major sink for lipid intermediates derived from membrane lipid turnover in cells grown in nitrogen-replete medium. Under nitrogen starvation conditions, the labeled 16:0 fatty acid was rapidly decreased in DGTS, SL/PI, PE and PG, but slowly in MGDG and DGDG (Fig. 9.2b), while the labeled TAG increased. At 2 days of chase, more than 60 % of total label was accumulated in TAG (Fig. 9.2c). Very similar results were obtained in pulse-chase experiments with [1-14C] 18:1 (Fig. 9.3), which was previously shown to label mainly the sn-1 position of MGDG and DGDG, but the sn-2 position of DGTS (Giroud and Eichenberger 1989). Together, these results suggest an enhanced turnover of acyl groups at both the sn-1 and sn-2 positions of membrane lipids and the released fatty acids and/or other lipid intermediates are recycled into the TAG biosynthetic pathway under nitrogen starvation conditions. Because the losses in radioactivity in DGTS and PE (Figs. 9.2 and 9.3) are not accompanied by decreases in their mass under our growth conditions (Fan et al. 2011), the turnover of these membrane lipids is unlikely to be merely a consequence of net catabolism of membrane lipids but rather it may reflect the process of lipid remodeling involving deacylation and reacylation, a common mechanism widely conserved in yeasts (de Kroon 2007), plants (Bates et al. 2007, 2009) and mammals (Schmid et al. 1991). Thus, like PC in plants (Bates et al. 2007, 2009), the acyl remodeling of DGTS represents an important mechanism mediating the flux of fatty acids to TAG in Chlamydomonas under nitrogen starvation conditions.
Membrane lipid turnover contributes to TAG accumulation in response to N starvation. Cells were labeled for 16 h with [1-14C] 16:0 in complete medium, thereafter shifted to unlabeled medium with (+N) or without (−N) N. Lipids were then extracted and separated by TLC at the indicated time points and radioactivity in individual polar lipids (a and b) and TAG (c) were determined by phosphor imaging. The experiment was repeated twice with similar results and a representative experiment is shown
Membrane lipid turnover contributes to TAG accumulation in response to N starvation. Cells were labeled for 16 h with [1-14C] 18:1 in complete medium, thereafter shifted to unlabeled medium with (+N) or without (−N) N. Lipids were then extracted and separated by TLC at the indicated time points and radioactivity in individual polar lipids (a and b) and TAG (c) were determined by phosphor imaging. The experiment was repeated twice with similar results and a representative experiment is shown
The Role of Lipid Droplets in TAG Synthesis
Research in the past couple of decades has completely changed our perception of lipid droplets. It is now well recognized that rather than serving as inert globules of fats, lipid droplets are dynamic subcellular organelles that play vital roles in lipid metabolism, homeostasis and trafficking (Martin and Parton 2006; Beller et al. 2008; Guo et al. 2008; Olofsson et al. 2009; Chapman et al. 2012; Kohlwein et al. 2013; Wilfling et al. 2014). Thus, it is not surprising that many enzymes in lipid metabolic pathways are often found to be associated with lipid droplets (Sorger and Daum 2002; Rajakumari et al. 2008; Kohlwein 2010; Moessinger et al. 2011). Intriguingly, recent studies in mammalian cells have showed that the core machinery for TAG synthesis re-localizes from the ER to lipid droplets via membrane bridges between the organelles under conditions of fatty acid overload (Xu et al. 2012; Wilfling et al. 2013) and that such relocalization of TAG synthesis enzymes is essential for lipid droplet expansion (Wilfling et al. 2013). Similarly, although yeast DGAT2 is an integral ER membrane protein, it relocates to lipid droplets during stationary phase of growth, when rapid neutral lipid accumulation is occurring (Jacquier et al. 2011; Markgraf et al. 2014). These results suggest a coupling of TAG synthesis with lipid droplet expansion under conditions of lipid excess.
Recent functional genomic screens have identified as much as 1.5 % of all known genes functioning in oil-droplet formation and regulation in Drosophila (Guo et al. 2008) and 1.2 % in yeast (Szymanski et al. 2007). In plants, 25 oil droplet-associated proteins from mature Brassica napus seeds were identified in recent proteomic analysis (Jolivet et al. 2009), with oleosins being the most abundant proteins of seed oil droplets. Genetic studies showed that oleosins play an important role in regulating the size of lipid droplets in Arabidopsis seeds (Siloto et al. 2006). In addition, an OLEOSIN3 isoform from peanut cotyledons has been demonstrated to exhibit both a monoacylglycerol acyltransferase and a phospholipase activity, and overexpression of peanut OLEOSIN3 results in increased accumulation of DAG and TAG in yeast (Parthibane et al. 2012). Interestingly, a Nannochloropsis lipid droplet protein was recently shown to partially complement the OLEOSIN-deficiency with respect to lipid droplet size in Arabidopsis seeds (Vieler et al. 2012). Proteomic analysis yielded over 600 lipid droplet-associated proteins in Chlamydomonas, and many of them are likely involved in lipid metabolism (Moellering and Benning 2010; Nguyen et al. 2011). Notably, key enzymes in the TAG synthesis pathway including a glycerol-3-phosphate acyltransferase, a lysophosphatidic acid acyltransferase and a PDAT, along with proteins putatively involved in acyl remodeling, sterol synthesis and lipid trafficking were found in the recently reported Chlamydomonas oil droplets (Nguyen et al. 2011). Taken together, these results suggest that lipid droplets may play an evolutionary conserved role in many aspects of lipid metabolism including TAG synthesis in wide variety of organisms, ranging from microalgae to mammals and higher plants.
Conclusion
The study of biochemistry and cell biology of TAG metabolism in algae is still in its infancy but recent advances have been rapid, due primarily to a recent surge of interest in developing renewable fuels from microalgae. In particular, the discovery that TAG derived from the prokaryotic pathway accumulates in cytosolic lipid droplets in Chlamydomonas offers a new paradigm for interpreting TAG synthesis pathways and their organization in microalgae. In addition, the number of proteins involved in TAG synthesis, remodeling and regulation that are associated with lipid droplets has increased substantially and offers insights into the cellular compartmentalization of TAG metabolism. Future studies to exploit the molecular identity, biochemical properties, subcellular localization and dynamics of acyltransferases involved in DAG synthesis and the mechanisms underlying lipid droplet biogenesis and growth will be fundamental to our understanding of TAG metabolism and storage in microalgae and other organisms.
References
Adeyo O, Horn PJ, Lee SK, Binns DD, Chandrahas A, Chapman KD, Goodman JM (2011) The yeast lipin orthologue Pah1p is important for biogenesis of lipid droplets. J Cell Biol 192:1043–1055. doi:10.1083/jcb.201010111
Bates PD, Browse J (2011) The pathway of triacylglycerol synthesis through phosphatidylcholine in Arabidopsis produces a bottleneck for the accumulation of unusual fatty acids in transgenic seeds. Plant J 68:387–399. doi:10.1111/j.1365-313X.2011.04693
Bates PD, Ohlrogge JB, Pollard M (2007) Incorporation of newly synthesized fatty acids into cytosolic glycerolipids in pea leaves occurs via acyl editing. J Biol Chem 282:31206–31216
Bates PD, Durrett TP, Ohlrogge JB, Pollard M (2009) Analysis of acyl fluxes through multiple pathways of triacylglycerol synthesis in developing soybean embryos. Plant Physiol 150:55–72. doi:10.1104/pp. 109.137737
Beller M, Sztalryd C, Southall N, Bell M, Jackle H, Auld DS, Oliver B (2008) COPI complex is a regulator of lipid homeostasis. PLoS Biol 6:e292. doi:10.1371/journal.pbio.0060292
Benning C (2008) A role for lipid trafficking in chloroplast biogenesis. Prog Lipid Res 47:381–389. doi:10.1016/j.plipres.2008.04.001
Benning C (2009) Mechanisms of lipid transport involved in organelle biogenesis in plant cells. Ann Rev Cell Dev Biol 25:71–91. doi:10.1146/annurev.cellbio.042308.113414
Boyle NR, Page MD, Liu BS, Blaby IK, Casero D, Kropat J, Cokus SJ, Hong-Hermesdorf A, Shaw J, Karpowicz SJ, Gallaher SD, Johnson S, Benning C, Pellegrini M, Grossman A, Merchant SS (2012) Three acyltransferases and nitrogen-responsive regulator are implicated in nitrogen starvation-induced triacylglycerol accumulation in Chlamydomonas. J Biol Chem 287:15811–15825. doi:10.1074/jbc.M111.334052
Browse J, Somerville C (1991) Glycerolipid synthesis – biochemistry and regulation. Annu Rev Plant Physiol Plant Mol Biol 42:467–506
Browse J, Warwick N, Somerville CR, Slack CR (1986) Fluxes through the prokaryotic and eukaryotic pathways of lipid synthesis in the ‘16:3’ plant Arabidopsis thaliana. Biochem J 235:25–31
Cao JS, Cheng L, Shi YG (2007) Catalytic properties of MGAT3, a putative triacylglycerol synthase. J Lipid Res 48:583–591
Chapman KD, Dyer JM, Mullen RT (2012) Biogenesis and functions of lipid droplets in plants. J Lipid Res 53:215–226. doi:10.1194/jlr.R021436
Davidi L, Levin Y, Ben-Dor S, Pick U (2014a) Proteome analysis of cytoplasmatic and of plastidic beta-carotene lipid droplets in Dunaliella bardawil. Plant Physiol 167:60–79. doi:10.1104/pp. 114.248450
Davidi L, Shimoni E, Khozin-Goldberg I, Zamir A, Pick U (2014b) Origin of beta-carotene-rich plastoglobuli in Dunaliella bardawil. Plant Physiol 164:2139–2156. doi:10.1104/pp. 113.235119
de Kroon AI (2007) Metabolism of phosphatidylcholine and its implications for lipid acyl chain composition in Saccharomyces cerevisiae. Biochim Biophys Acta 1771:343–352
Deng XD, Cai JJ, Fei XW (2013) Involvement of phosphatidate phosphatase in the biosynthesis of triacylglycerols in Chlamydomonas reinhardtii. J Zhejiang Univ Sci B 14:1121–1131. doi:10.1631/jzus.B1300180
Dörmann P, Balbo I, Benning C (1999) Arabidopsis galactolipid biosynthesis and lipid trafficking mediated by DGD1. Science 284:2181–2184
Eastmond PJ, Quettier AL, Kroon JTM, Craddock C, Adams N, Slabas AR (2010) PHOSPHATIDIC ACID PHOSPHOHYDROLASE1 and 2 regulate phospholipid synthesis at the endoplasmic reticulum in Arabidopsis. Plant Cell 22:4216–4216. doi:10.1105/tpc.109.071423
Fan JL, Andre C, Xu CC (2011) A chloroplast pathway for the de novo biosynthesis of triacylglycerol in Chlamydomonas reinhardtii. FEBS Lett 585:1985–1991. doi:10.1016/j.febslet.2011.05.018
Fan JL, Yan CS, Xu CC (2013a) Phospholipid: diacylglycerol acyltransferase-mediated triacylglycerol biosynthesis is crucial for protection against fatty acid-induced cell death in growing tissues of Arabidopsis. Plant J 76:930–942. doi:10.1111/tpj.12343
Fan JL, Yan CS, Zhang XB, Xu CC (2013b) Dual role for phospholipid: diacylglycerol acyltransferase: enhancing fatty acid synthesis and diverting fatty acids from membrane lipids to triacylglycerol in Arabidopsis leaves. Plant Cell 25:3506–3518. doi:10.1105/tpc.113.117358
Fan J, Yan C, Roston R, Shanklin J, Xu C (2014) Arabidopsis lipins, PDAT1 acyltransferase, and SDP1 triacylglycerol lipase synergistically direct fatty acids toward beta-oxidation, thereby maintaining membrane lipid homeostasis. Plant Cell 26:4119–4134. doi:10.1105/tpc.114.130377
Frentzen M (1998) Acyltransferases from basic science to modified seed oils. Fett Lipid 100:161–166
Giroud C, Eichenberger W (1989) Lipids of Chlamydomonas reinhardtii – incorporation of [C-14] acetate, [C-14] palmitate and [C-14] oleate into different lipids and evidence for lipid-linked desaturation of fatty acids. Plant Cell Physiol 30:121–128
Giroud C, Gerber A, Eichenberger W (1988) Lipids of Chlamydomonas reinhardtii – analysis of molecular species and intracellular sites of biosynthesis. Plant Cell Physiol 29:587–595
Goodson C, Roth R, Wang ZT, Goodenough U (2011) Structural correlates of cytoplasmic and chloroplast lipid body synthesis in Chlamydomonas reinhardtii and stimulation of lipid-body production with acetate-boost. Eukaryot Cell 10:1592–1606. doi:10.1128/EC.05242-11
Guo Y, Walther TC, Rao M, Stuurman N, Goshima G, Terayama K, Wong JS, Vale RD, Walter P, Farese RV (2008) Functional genomic screen reveals genes involved in lipid-droplet formation and utilization. Nature 453:657–661
Han GS, Wu WI, Carman GM (2006) The Saccharomyces cerevisiae lipin homolog is a Mg2+-dependent phosphatidate phosphatase enzyme. J Biol Chem 281:9210–9218
Harris TE, Finck BN (2011) Dual function lipin proteins and glycerolipid metabolism. Trends Endocrinol Metab 22:226–233. doi:10.1016/j.tem.2011.02.006
Harwood JL, Guschina IA (2009) The versatility of algae and their lipid metabolism. Biochimie 91:679–684. doi:10.1016/j.biochi.2008.11.004
Hofmann M, Eichenberger W (1997) Lipid and fatty acid composition of the marine brown alga Dictyopteris membranacea. Plant Cell Physiol 38:1046–1052
Jacquier N, Choudhary V, Mari M, Toulmay A, Reggiori F, Schneiter R (2011) Lipid droplets are functionally connected to the endoplasmic reticulum in Saccharomyces cerevisiae. J Cell Sci 124:2424–2437. doi:10.1242/jcs.076836
Jolivet P, Boulard C, Bellamy A, Larre C, Barre M, Rogniaux H, d’Andrea S, Chardot T, Nesi N (2009) Protein composition of oil bodies from mature Brassica napus seeds. Proteomics 9:3268–3284. doi:10.1002/pmic.200800449
Jones AL, Harwood JL (1992) Lipid composition of the brown algae Fucus vesiculosus and Ascophyllum nodosum. Phytochemistry 31:3397–3403
Jones AL, Harwood JL (1993) Lipid metabolism in the brown marine algae Fucus vesiculosus and Ascophyllum nodosum. J Exp Bot 44:1203–1210
Kaup MT, Froese CD, Thompson JE (2002) A role for diacylglycerol acyltransferase during leaf senescence. Plant Physiol 129:1616–1626
Kohlwein SD (2010) Triacylglycerol homeostasis: insights from yeast. J Biol Chem 285:15663–15667. doi:10.1074/jbc.R110.118356
Kohlwein SD, Veenhuis M, van der Klei IJ (2013) Lipid droplets and peroxisomes: key players in cellular lipid homeostasis or a matter of fat-store ‘em up or burn ’em down. Genetics 193:1–50. doi:10.1534/genetics.112.143362
Li XB, Moellering ER, Liu BS, Johnny C, Fedewa M, Sears BB, Kuo MH, Benning C (2012) A galactoglycerolipid lipase is required for triacylglycerol accumulation and survival following nitrogen deprivation in Chlamydomonas reinhardtii. Plant Cell 24:4670–4686. doi:10.1105/tpc.112.105106
Lippold F, vom Dorp K, Abraham M, Holzl G, Wewer V, Yilmaz JL, Lager I, Montandon C, Besagni C, Kessler F, Stymne S, Dormann P (2012) Fatty acid phytyl ester synthesis in chloroplasts of Arabidopsis. Plant Cell 24:2001–2014. doi:10.1105/tpc.112.095588
Makewicz A, Gribi C, Eichenberger W (1997) Lipids of Ectocarpus fasciculatus (Phaeophyceae). Incorporation of [1-C-14] oleate and the role of TAG and MGDG in lipid metabolism. Plant Cell Physiol 38:952–960
Markgraf DF, Klemm RW, Junker M, Hannibal-Bach HK, Ejsing CS, Rapoport TA (2014) An ER protein functionally couples neutral lipid metabolism on lipid droplets to membrane lipid synthesis in the ER. Cell Rep 6:44–55. doi:10.1016/j.celrep.2013.11.046
Martin S, Parton RG (2006) Lipid droplets: a unified view of a dynamic organelle. Nat Rev Mol Cell Biol 7:373–378
Martin BA, Wilson RF (1984) Subcellular localization of triacylglycerol synthesis in Spinach leaves. Lipids 19:117–121
Mattson FH, Volpenhein RA (1963) Specific distribution of unsaturated fatty acids in triglycerides of plants. J Lipid Res 4:392–398
Moellering ER, Benning C (2010) RNA interference silencing of a major lipid droplet protein affects lipid droplet size in Chlamydomonas reinhardtii. Eukaryot Cell 9:97–106. doi:10.1128/EC.00203-09
Moellering ER, Miller R, Benning C (2009) Molecular genetics of lipid metabolism in the model green alga Chlamydomonas reinhardtii. In: Wada H, Murata M (eds) Lipids in photosynthesis: essential and regulatory functions. Springer, Dordrecht, pp 139–150
Moessinger C, Kuerschner L, Spandl J, Shevchenko A, Thiele C (2011) Human lysophosphatidylcholine acyltransferases 1 and 2 are located in lipid droplets where they catalyze the formation of phosphatidylcholine. J Biol Chem 286:21330–21339. doi:10.1074/jbc.M110.202424
Nguyen HM, Baudet M, Cuine S, Adriano JM, Barthe D, Billon E, Bruley C, Beisson F, Peltier G, Ferro M, Li-Beisson Y (2011) Proteomic profiling of oil bodies isolated from the unicellular green microalga Chlamydomonas reinhardtii: with focus on proteins involved in lipid metabolism. Proteomics 11:4266–4273. doi:10.1002/pmic.201100114
Oelkers P, Tinkelenberg A, Erdeniz N, Cromley D, Billheimer JT, Sturley SL (2000) A lecithin cholesterol acyltransferase-like gene mediates diacylglycerol esterification in yeast. J Biol Chem 275:15609–15612
Oelkers P, Cromley D, Padamsee M, Billheimer JT, Sturley SL (2002) The DGA1 gene determines a second triglyceride synthetic pathway in yeast. J Biol Chem 277:8877–8881
Ohlrogge JB, Jaworski JG (1997) Regulation of fatty acid synthesis. Annu Rev Plant Physiol Plant Mol Biol 48:109–136
Olofsson SO, Bostrom P, Andersson L, Rutberg M, Perman J, Boren J (2009) Lipid droplets as dynamic organelles connecting storage and efflux of lipids. Biochim Biophys Acta 1791:448–458. doi:10.1016/j.bbalip.2008.08.001
Parthibane V, Rajakumari S, Venkateshwari V, Iyappan R, Rajasekharan R (2012) Oleosin is bifunctional enzyme that has both monoacylglycerol acyltransferase and phospholipase activities. J Biol Chem 287:1946–1954. doi:10.1074/jbc.M111.309955
Pascual F, Carman GM (2013) Phosphatidate phosphatase, a key regulator of lipid homeostasis. Biochim Biophys Acta 1831:514–522. doi:10.1016/j.bbalip.2012.08.006
Rajakumari S, Grillitsch K, Daum G (2008) Synthesis and turnover of non-polar lipids in yeast. Prog Lipid Res 47:157–171
Riekhof W, Benning C (2009) Glycerolipid biosynthesis. In: Stern DB (ed) The chlamydomonas sourcebook: organellar and metabolic processes, 2nd edn. Academic, Oxford
Roughan PG, Slack CR (1982) Cellular organization of glycerolipid metabolism. Ann Rev Plant Physiol 33:97–132
Sakurai K, Moriyama T, Sato N (2014) Detailed identification of fatty acid isomers sheds light on the probable precursors of triacylglycerol accumulation in photoautotrophically grown Chlamydomonas reinhardtii. Eukaryot Cell 13:256–266. doi:10.1128/EC.00280-13
Sanjaya MR, Durrett TP, Kosma DK, Lydic TA, Muthan B, Koo AJ, Bukhman YV, Reid GE, Howe GA, Ohlrogge J, Benning C (2013) Altered lipid composition and enhance notional value of Arabidopsis leaves following the introduction of an algal diacylglycerol acyltransferase 2. Plant Cell 25:677–693. doi:10.1105/tpc.112.104752
Santos-Rosa H, Leung J, Grimsey N, Peak-Chew S, Siniossoglou S (2005) The yeast lipin Smp2 couples phospholipid biosynthesis to nuclear membrane growth. EMBO J 24:1931–1941
Sato N, Moriyama T (2007) Genomic and biochemical analysis of lipid biosynthesis in the unicellular rhodophyte Cyanidioschyzon merolae: lack of a plastidic desaturation pathway results in the coupled pathway of galactolipid synthesis. Eukaryot Cell 6:1006–1017
Sato N, Murata N (1991) Transition of lipid phase in aqueous dispersions of diacylglyceryltrimethylhomoserine. Biochim Biophys Acta 1082:108–111
Sato N, Tsuzuki M, Kawaguchi A (2003) Glycerolipid synthesis in Chlorella kessleri 11 h – I. Existence of a eukaryotic pathway. Biochim Biophys Acta 1633:27–34
Schmid PC, Johnson SB, Schmid HH (1991) Remodeling of rat hepatocyte phospholipids by selective acyl turnover. J Biol Chem 266:13690–13697
Shockey JM, Gidda SK, Chapital DC, Kuan JC, Dhanoa PK, Bland JM, Rothstein SJ, Mullen RT, Dyer JM (2006) Tung tree DGAT1 and DGAT2 have nonredundant functions in triacylglycerol biosynthesis and are localized to different subdomains of the endoplasmic reticulum. Plant Cell 18:2294–2313
Siloto RMP, Findlay K, Lopez-Villalobos A, Yeung EC, Nykiforuk CL, Moloney MM (2006) The accumulation of oleosins determines the size of seed oilbodies in Arabidopsis. Plant Cell 18:1961–1974
Siniossoglou S (2013) Phospholipid metabolism and nuclear function: roles of the lipin family of phosphatidic acid phosphatases. Biochim Biophys Acta 1831:575–581. doi:10.1016/j.bbalip.2012.09.014
Smith KL, Harwood JL (1984) Lipids and lipid metabolism in the brown alga, Fucus serratus. Phytochemistry 23:2469–2473
Sorger D, Daum G (2002) Synthesis of triacylglycerols by the acyl-coenzyme A: diacyl-glycerol acyltransferase Dga1p in lipid particles of the yeast Saccharomyces cerevisiae. J Bacteriol 184:519–524
Suen Y, Hubbard JS, Holzer G, Tornabene TG (1987) Total lipid production of the green-alga Nannochloropsis Sp Qii under different nitrogen regimes. J Phycol 23:289–296
Szymanski KM, Binns D, Bartz R, Grishin NV, Li WP, Agarwal AK, Garg A, Anderson RG, Goodman JM (2007) The lipodystrophy protein seipin is found at endoplasmic reticulum lipid droplet junctions and is important for droplet morphology. Proc Natl Acad Sci U S A 104:20890–20895
Thiele C, Spandl J (2008) Cell biology of lipid droplets. Curr Opin Cell Biol 20:378–385
Thompson GA (1996) Lipids and membrane function in green algae. Biochim Biophys Acta 1302:17–45
Urzica EI, Vieler A, Hong-Hermesdorf A, Page MD, Casero D, Gallaher SD, Kropat J, Pellegrini M, Benning C, Merchant SS (2013) Remodeling of membrane lipids in iron-starved Chlamydomonas. J Biol Chem 288:30246–30258. doi:10.1074/jbc.M113.490425
Vieler A, Brubaker SB, Vick B, Benning C (2012) A lipid droplet protein of Nannochloropsis with functions partially analogous to plant oleosins. Plant Physiol 158:1562–1569. doi:10.1104/pp. 111.193029
Wakimoto K, Chiba H, Michibata H, Seishima M, Kawasaki S, Okubo K, Mitsui H, Torii H, Imai Y (2003) A novel diacylglycerol acyltransferase (DGAT2) is decreased in human psoriatic skin and increased in diabetic mice. Biochem Biophys Res Commun 310:296–302
Walther TC, Farese RV Jr (2009) The life of lipid droplets. Biochim Biophys Acta 1791:459–466. doi:10.1016/j.bbalip.2008
Wilfling F, Wang HJ, Haas JT, Krahmer N, Gould TJ, Uchida A, Cheng JX, Graham M, Christiano R, Frohlich F, Liu XR, Buhman KK, Coleman RA, Bewersdorf J, Farese RV, Walther TC (2013) Triacylglycerol synthesis enzymes mediate lipid droplet growth by relocalizing from the ER to lipid droplets. Dev Cell 24:384–399. doi:10.1016/j.devcel.2013.01.013
Wilfling F, Haas JT, Walther TC, Farese RV (2014) Lipid droplet biogenesis. Curr Opin Cell Biol 29:39–45. doi:10.1016/j.ceb.2014.03.008
Xu NY, Zhang SBO, Cole RA, McKinney SA, Guo FL, Haas JT, Bobba S, Farese RV, Mak HY (2012) The FATP1-DGAT2 complex facilitates lipid droplet expansion at the ER-lipid droplet interface. J Cell Biol 198:895–911. doi:10.1083/jcb.201201139
Yongmanitchai W, Ward OP (1993) Molecular species of triacylglycerols from the fresh-water diatom, Phaeodactylum tricornutum. Phytochemistry 32:1137–1139
Yoon K, Han DX, Li YT, Sommerfeld M, Hu Q (2012) Phospholipid:diacylglycerol acyltransferase is a multifunctional enzyme involved in membrane lipid turnover and degradation while synthesizing triacylglycerol in the unicellular green microalga Chlamydomonas reinhardtii. Plant Cell 24:3708–3724. doi:10.1105/tpc.112.100701
Acknowledgements
We acknowledge the support of the Division of Chemical Sciences, Geosciences, and Biosciences, Office of Basic Energy Sciences, US Department of Energy (grant DOE KC0304000)
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2016 Springer International Publishing Switzerland
About this chapter
Cite this chapter
Xu, C., Andre, C., Fan, J., Shanklin, J. (2016). Cellular Organization of Triacylglycerol Biosynthesis in Microalgae. In: Nakamura, Y., Li-Beisson, Y. (eds) Lipids in Plant and Algae Development. Subcellular Biochemistry, vol 86. Springer, Cham. https://doi.org/10.1007/978-3-319-25979-6_9
Download citation
DOI: https://doi.org/10.1007/978-3-319-25979-6_9
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-25977-2
Online ISBN: 978-3-319-25979-6
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)