Abstract
Most ‘green’ plants form green leaf volatiles (GLVs). GLVs are a familiar plant secondary metabolite, but knowledge of their physiological and ecological functions is limited. GLV formation is tightly suppressed when plant tissues are intact, but upon mechanical wounding, herbivore attack, or abiotic stresses, GLVs are formed rapidly, within seconds or minutes. Thus, this may be an important system for defense responses, allowing plants to protect themselves from damage as soon as possible. Because GLV formation in the natural environment is roughly related to the degree of stress in the plant life, sensing the amount of GLVs in the atmosphere might allow plants to recognize their surroundings. Because some plants respond to GLVs, they may communicate with GLVs. GLVs that contain α,β-unsaturated carbonyl groups might activate signaling systems regulated under the redox state of plant cells. Plasma membranes would also be targets of interactions with GLVs. Additionally, the metabolism of GLVs in plant cells after absorption from the atmosphere could also be classified as a plant–plant interaction.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
Introduction
Lipids, in general, have three important roles in living organisms. First, they are essential components of the cell membrane, which can separate the cells from an abiotic environment. Lipids are also important nutrients for many organisms, and the catabolism through β-oxidation of lipid constituents yields large amounts of energy. Lipids can also be used as signal molecules. Lipid mediators in mammalian cells, such as prostaglandins, leukotrienes, or platelet-activating factors, have various biological functions in inflammation, blood clotting, and immune systems (Murakami 2011). This is also the case with plants, even though the structures and functions of the lipid mediators found in plants are usually different from those in mammals.
Jasmonates are typical and important lipid mediators in plants, as described in detail elsewhere of this book. Volatile compounds with six carbon (C6) backbones, which are collectively called green leaf volatiles (GLVs) (Fig. 17.1), are also formed in plants. They use a biosynthetic pathway similar to that for jasmonates, i.e., using lipoxygenases to form fatty acid hydroperoxides, followed by the homolytic cleavage of the hydroperoxide by cytochrome P450s (Grechkin et al. 2006). GLVs consist of C6 volatile compounds containing aldehyde, alcohol, and esters. Because almost all green leaves on the Earth form GLVs, human beings correlate the olfactory sensation emitted by GLVs with green leaves, and thus, we usually sense the volatiles as a green note.
One GLV, (E)-2-hexenal (leaf aldehyde), was isolated in 1912 by Curtius and Franzen as a component in the essential oil prepared from 600 kg of hornbeam leaves by steam distillation (Curtius and Franzen 1912). Since then, because of their peculiar aromatic properties and common occurrence in plants, GLVs have been studied in flavor chemistry. However, their functions in ecological systems have also been noticed, especially because plants usually accumulate only small amounts of GLVs but quickly form them after mechanical wounding or herbivore attack (Scala et al. 2013).
In this chapter we discuss novel knowledge regarding the biosynthesis of GLVs and their evolution. Thereafter, we give up-to-date reviews of signaling and plant responses elicited by GLVs.
Biosynthesis of GLVs
GLVs are formed from fatty acids through a dioxygenation reaction catalyzed by lipoxygenases (LOXs) to yield fatty acid hydroperoxide, and a subsequent rearrangement reaction to cleave fatty acid hydroperoxides by hydroperoxide lyase (HPL) to form C6 aldehydes and 12 carbon oxo acids as counterparts (Fig. 17.2). The general outline of the GLV-forming pathway shares similarities with those for other oxylipin products, including jasmonates, and each branch in the oxylipin pathway could have diverged from one ancestral pathway. Based on the structure of allene oxide synthase (AOS) and HPL, and also on the results of the interconversion of AOS to HPL, Lee et al. (2008) proposed that the HPL pathway was established first and that the AOS pathway diverged thereafter. This view might be changed through the accumulation of knowledge regarding the distribution of HPL and AOS among algae, bryophytes, ferns, or gymnosperms as described in section “Evolution of genes involved in GLV formation”.
Biosynthetic pathway to form green leaf volatiles (GLVs). It has been believed that a lipase is essential in forming free fatty acids from esterified lipids in the first committed step to form GLVs; however, recently we found that GLVs are formed even without the lipase reaction. In that case, lipoxygenase is the first enzyme that acts on the esterified lipids to form lipid hydroperoxides, which in turn is subsequently cleaved by hydroperoxide lyase to form the 6 carbon volatile aldehyde and 12 carbon oxo acid esterified to the glycerol backbone
The HPL and AOS pathways share the first part of a metabolic pathway, and they also use the same substrate. HPL and AOS should catalyze their reactions without competition to meet the demands for GLVs and JAs, respectively, under certain growth conditions because they have distinct physiological functions. Most HPL and AOS enzymes identified so far have chloroplast transit peptides. Their chloroplast localizations have been established through investigations using marker proteins, such as green fluorescence protein (GFP) , and the fractionation of chloroplasts. In some plants, HPLs are localized to the lipid bodies (Mita et al. 2005), outer envelopes of chloroplasts (Froehlich et al. 2001), stroma (Bonaventure 2014), and, in some cases, no specific localization is observed (Phillips and Galliard 1978; Shibata et al. 1995; Noordermeer et al. 2000). Rice HPL3 (OsHPL3), which has the shortest extension on its N-terminal among the three rice HPLs, was transported to chloroplasts when a fusion protein of the transit peptide of OsHPL3 with GFP was expressed in Arabidopsis leaves (Savchenko et al. 2014). This is also the case with Arabidopsis, and Arabidopsis HPL fused with GFP was transported to the plastids (Mwenda et al. 2015). Although it is still possible for the two CYP74 enzymes to be segregated at the level of sub-chloroplast membrane, or even within the same membrane (Mita et al. 2005), the close localization of two enzymes sharing the same substrate would cause disordered competition, especially when the enzymes form their products in the disrupted tissues during rapid oxylipin bursts (Matsui 2006; Glauser et al. 2008, 2009). To avoid such competition, they should have different spatiotemporal expression patterns.
With a transgenic Arabidopsis harboring the GUS gene downstream of Arabidopsis HPL promoter, an intense expression of GUS activity was found in floral organs in a different manner than when the GUS activity was controlled by the Arabidopsis AOS promoter (Mwenda et al. 2015). The GUS activity under the control of the HPL promoter in intact cotyledons was low, but extensively enhanced after mechanical wounding, especially at their rims. However, high GUS activity under the control of the AOS promoter was detected in the vascular tissues of cotyledons (Kubigsteltig et al. 1999). Therefore, the promoter activities of HPL and AOS are distinctly spatially regulated after mechanical wounding, which may allow them to avoid competition. The distribution of the ability to form C6 volatiles roughly correlated with the profile of HPL promoter activity; however, the abilities showed little change after mechanical wounding. An inconsistency between the AOS promoter activity and JA levels was also evident. Accordingly, an additional factor, other than spatial segregation of HPL and AOS, also controls the GLV- and JA-forming abilities.
The most prominent feature of the HPL reaction is the rapid ‘burst’ of GLV formation after mechanical wounding. In intact leaf tissues, the amounts of GLVs are usually low, but after tissue disruption, the extensive formation of GLVs is induced. In Arabidopsis leaves, the amount of (Z)-3-hexenal went up to 1.5 μmol g FW−1 within 5 min after disruption (Matsui et al. 2012). This value corresponded to as much as ~30 % of the total amount of trienoic fatty acids in the leaf tissues. Because of the short period needed for the burst and the cells disruption, which made it impossible to activate the gene expression operating in intact cells, this burst should depend on enzymes and substrates that already exist in the cells. One possible explanation for the burst is a rapid mixing of enzymes and substrates; however, the situation is not that simple because some of the enzymes involved in GLV formation, such as lipoxygenases and HPLs, are usually localized in chloroplasts where their substrates, such as galactolipids, are abundant. A lipase that liberates free fatty acids from lipids might play a role in GLV formation by turning on the burst, but the fact that GLVs are formed even without free fatty acids (Nakashima et al. 2013) makes the possibility less likely. Another possibility is the segregation of enzymes and substrates at the cellular level, as found for glucosinolates and myrosinase in Brassicaceae plants (Kissen et al. 2009), but the distributions of LOX and HPL appear rather uniform in tissues when observed using their promoter::GUS constructs in reporter plants (Mwenda et al. 2015). Therefore, there must be a system to activate enzymes after tissue disruption. Such a regulatory mechanism is known for mammalian phospholipase A2 and lipoxygenases, and it has been shown that calcium ions play a significant role in the regulation (Murakami 2011). However, again, the disruption of leaf tissues in the presence of a calcium ion-chelating reagent, such as EGTA, showed no effect on the GLV formation rate. Apparently, the system to turn on the GLV burst is still unknown, and further studies are needed.
GLV formation is induced in occasions other than tissue disruption. When tomato plants were exposed to high temperatures (>46 °C), GLVs were extensively formed (Copolovici et al. 2012). Because several GLVs and their related carbonyl species, harboring α,β-unsaturated carbonyl groups, induce resistance to high temperature stress by inducing several genes involved in responses against abiotic stresses (Yamauchi et al. 2015), the formation of GLVs after heat stress may be a plant defense response. Under heat stress, plant cells generally encounter oxidative stress, which in turn, causes the deterioration of the membrane organization through oxidation of membrane components (Suzuki et al. 2012). This might induce GLV formation. GLV bursts following light-dark transitions have also been reported (Jardine et al. 2012). With this GLV burst, which was enhanced by darkening, there was a positive relationship between the amount of GLVs formed and the photosynthetic activity prior to darkening. This implies the involvement of a photosynthetic electron transport system in the GLV burst; however, details have not been elucidated. By investigating the mechanism behind the GLV burst that is elicited after darkening, insights into the regulatory mechanisms of GLV formation could be revealed.
Evolution of Genes Involved in GLV Formation
The CYP74 family is a class of enzymes, including HPL and AOS, that uses unsaturated fatty acid hydroperoxides derived from linoleic acid or α-linolenic acid as substrates. The CYP74B subfamily contains HPLs, which are widely distributed in higher plants (Grechkin 2002; Matsui 2006). Bell pepper HPL was the first CYP74B isolated in the 1990s, and since then an increasing number of HPL isoforms have been identified in the complete genome sequences of Arabidopsis thaliana, rice, and other plant species (Matsui et al. 1996; Bate et al. 1998). Recently, it has been reported that the moss Physcomitrella patens contains three CYP74 orthologs (Stumpe et al. 2006; Scholz et al. 2012). One of the three orthologs in moss has been identified as bona fide HPL and the others as AOSs. A database search revealed that liverwort Marchantia and Charophyta Klebsormidium encode two and one CYP74 genes, respectively (Table 17.1). However, they are AOSs, not HPLs (Koeduka et al. 2015). These observations raise the important questions of when and how an ancestral CYP74 may have functioned as an HPL. The investigation of the genetic context of CYP74 uncovered that the Selaginella genome includes ten CYP74 orthologs, whereas Chlamydomonas and Nostoc do not contain CYP74 genes. Thus, the acquisition of CYP74 genes may have occurred during the evolutionary process from Chlamydomonas to Klebsormidium, and then a CYP74 gene duplication event and the functional divergence of AOS to HPL, or vice versa, arose multiple times during plant evolution. This is a totally distinct view of the evolution of CYP74s from that proposed by Lee et al. (2008).
Physiological and Ecological Function of GLVs
Do Plants Sense GLVs?
Since the confirmation of GLVs as aroma constituents in green leaves, scientists have been considering their physiological functions. At the beginning, the effect of GLVs on insect behavior was investigated. For example, in 1967, Riddiford (1967) found that (E)-2-hexenal was an essential mating stimulant for polyphemus moths (Antheraea polyphemus). At present, the effects of just one GLV, (E)-2-hexenal, for example, acts as an attractant, allomone, kairomone, or pheromone on 186 different species of arthropods, which are listed in The Pherobase (www.pherobase.com). A review on the involvement of GLVs in plant–herbivore and plant–pathogen interactions was published recently (Scala et al. 2013). GLVs also exert several kinds of physiological effects on mammals. For example, n-hexanal increases the dopamine release from rat brains (Kako et al. 2011).
It has been repeatedly reported that the volatile organic chemicals formed by stress-treated plants induce several defense responses in neighboring plants (Dicke and Baldwin 2010). Because GLVs are common and abundant volatiles emitted from stressed plants, the effects of GLVs on plants were examined. Vapors from a series of alkenals and alkanals, including (E)-2-hexenal, induced the formation of phytoalexins, such as cadalene and scopoletin, in developing cotton bolls (Zeringue 1992). Among the aldehydes used in his study, (E)-2-alkenal induced a higher production of phytoalexins than saturated alkanals. Therefore, the structure of compounds might be important in eliciting the phytoalexin formation in cotton bolls. Aerial treatments of Arabidopsis seedlings with (E)-2-hexenal at 10 μM induced a subset of defense-related genes, including chalcone synthase, lipoxygenase, and AOS, all of which are somehow involved in the biosynthesis of secondary metabolites (Bate and Rothstein 1998). (E)-2-Hexenal and the other (E)-2-alkenals used to treat cotton bolls are reactive carbonyl species (RES) because they contain α,β-unsaturated carbonyl moieties that have the potential to inactivate biological molecules through a spontaneous reaction (Michael addition reaction) with nucleophilic substances containing amino or sulfhydryl groups. Therefore, it is assumed that (E)-2-alkenal induced defense responses because of the stress response elicited by the toxicity of (E)-2-alkenal. In the case of Arabidopsis, the response was observed in the jar1-1 mutant that had a deficiency in JA signaling (Bate and Rothstein 1998), and thus, the response was thought to use a signaling system different from that used by JA. Even though α,β-unsaturated carbonyl moieties are important factors in eliciting defense responses in plants, (Z)-3-hexen-1-yl acetate, which is a much less harmful compound because of the absence of the α,β-unsaturated carbonyl moiety, was also effective to inducing the lipoxygenase gene in Arabidopsis seedlings. This implies that plants also sense some GLVs in a way other than as a response to toxic compounds containing α,β-unsaturated carbonyl groups.
We also found that treating with GLVs or an isoprenoid at 10 μM in the vapor phase elicited the induction of chalcone synthase, caffeic acid-O-methyltransferase, diacylglycerol kinase1, glutathione-S-transferase1, and lipoxygenase2 in Arabidopsis (Kishimoto et al. 2005). As a result of the induction of these defense-related genes, Arabidopsis acquired a higher resistance to the necrotrophic fungal pathogen, Botrytis cinerea. We also noticed that (E)-2-hexenal was a powerful elicitor, but at the same time, (Z)-3-hexenal, (Z)-3-hexen-1-ol, and allo-ocimene, also induced defense genes and resistance against B. cinerea to a level similar to or even higher than (E)-2-hexenal. The responses of Arabidopsis to these volatile compounds were partially suppressed in jar1-1 and etr1 mutants, thus, the involvement of JA signaling and ethylene signaling was hypothesized. The pre-treatment of Arabidopsis with okadaic acid also suppressed the response, which indicated the involvement of protein phosphatases in the system that sensed the volatiles. Treating with aerial GLVs also induced defense responses in lima bean (Arimura et al. 2000) and corn (Engelberth et al. 2004; Farag et al. 2005). In corn seedlings, GLVs primed the plants for the higher production of JAs and sesquiterpene volatiles after subsequent herbivore attacks (Engelberth et al. 2004).
Some claimed that the concentrations of volatiles used in these studies (10 μM in vapor phase, which corresponds to 224 ppmV) was unrealistically high and that the responses observed were not physiologically and ecologically relevant (Dicke et al. 2003). However, most green plants have the ability to form massive amounts of GLVs after mechanical wounding, and, in the case of Arabidopsis leaves, the local concentration in the disrupted leaf tissue could go up to 1 mM (in the aqueous phase in plant tissues) (Matsui et al. 2012). Also, volatiles usually diffuse into the atmosphere in a non-concentric manner as fragmented plumes directed by a turbulent flow (Baldwin et al. 2006). Therefore, it is possible for the plants to encounter relatively high concentrations of GLVs. Because it is difficult to simulate the diffusion or fate of volatiles once they are released into the atmosphere, the best way to know if the response is physiologically or ecologically relevant is to observe the effects of volatiles on plants in a natural environment or in an environment equivalent to nature.
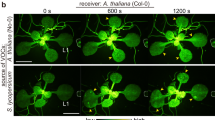
When volatiles released by hybrid poplar (Populus deltoids × P. nigra) after herbivore damage were introduced into undamaged adjacent leaves, the exposed leaves had elevated defensive responses against feeding by gypsy moth larvae (Lymantria dispar L.) (Frost et al. 2008). Even though a detailed analysis of volatiles emitted by herbivore-damaged leaves was not performed in this study, it should contain substantial amounts of GLVs (Frost et al. 2008). Lima bean plants (Phaseolus lunatus) secrete extrafloral nectar to recruit carnivorous ants that feed on herbivores. Exposing intact lima bean plants to volatiles released from herbivore-damaged conspecifics induced the secretion of the extrafloral nectar (Kost and Heil 2006). For an interaction mediated by herbivore-induced volatiles, (Z)-3-hexen-1-yl acetate being the most abundant, in lima beans, their effects were reproduced using a low concentration of synthetic (Z)-3-hexen-1-yl acetate. Also, in an open chamber experiment conducted using transgenic Nicotiana attenuata that had a lowered HPL activity through an antisense technique, it was indicated that GLVs were responsible for the induction of some defense genes in plant–plant interactions (Paschold et al. 2006). We also observed that intact tomato plants acquired higher defense responses against herbivores after receiving volatiles released from damaged plants (Sugimoto et al. 2014). In the volatiles emitted from the herbivore-damaged tomato plants, (Z)-3-hexen-1-ol was the most abundant GLV. Because of these results, many scientists started to consider that plants perceive GLVs even under non-stressed conditions, and that, in some instances, the plants that sensed GLVs in their surrounding atmosphere changed their behavior to achieve a higher fitness in their environment.
How Do Plants Sense GLVs?
Perception as Toxic Xenobiotics
Even though the involvement of GLVs in plant–plant communication has been mostly established in section “Do plants sense GLVs?”, we still do not know the mechanism behind how plants sense GLVs. To dissect the mechanism, it is better to separate members of the GLVs into two groups based on their chemical reactivity, RES and the others (Fig. 17.3). (E)-2-Hexenal is a representative RES-type GLV because it contains an α,β-unsaturated carbonyl group. (E)-2-Hexenal is formed by the isomerization of (Z)-3-hexenal, which is the first product of HPL. The ability to isomerize (Z)-3-hexenal to (E)-2-hexenal varies among plant species, and as a result, the ratio between the two hexenals is also different. The isomerization might have physiological and ecological relevance because the herbivore Manduca sexta has the isomerase in its oral secretion, and it decreases the (Z)/(E) ratio of GLVs formed by Nicotiana attenuata (Allmann and Baldwin 2010). The parasite of M. sexta is recruited by monitoring the (Z)/(E) ratio.
(Z)-3-Hexenal is a ‘potential’ reactive electrophile species (RES) -type green leaf volatile (GLV). Because (Z)-3-hexenal does not contain a α,β-unsaturated carbonyl group, it is not a member of the RES-type GLVs. However, spontaneous and enzymatic isomerization, or oxygenation, results in the formation of RES-type structures as shown in the chemical structures with the gray background
Because (E)-2-hexenal is a RES, its reactivity against biological substances is higher than the other GLVs, such as (Z)-3-hexenal, (Z)-3-hexen-1-ol, and (Z)-3-hexen-1-yl acetate. However, (Z)-3-hexenal is sensitive to enzymatic and spontaneous oxygenation to form 4-hydroperoxy-(E)-2-hexenal, which in turn, is converted into 4-hydroxy-(E)-2-hexenal or 4-oxo-(E)-2-hexenal (Matsui et al. 2012; Bonaventure et al. 2011) (Fig. 17.3). Because of this, (Z)-3-hexenal should also be considered as a ‘potential’ RES-type GLV.
12-Oxophytodienoic acid (OPDA) functions not only as a precursor of JA, but also as a signal molecule per se, inducing a distinct set of genes other than those induced by the JA (Taki et al. 2005). Phytoprostanes, which are formed non-enzymatically by the reaction of polyunsaturated fatty acid with reactive oxygen species (ROS) , are structurally related to the OPDA group, especially because of the presence of the α,β-unsaturated carbonyl group found in the conjugated cyclopentenone group. Phytoprostanes also induce a subset of genes in plants, and it is assumed that this ability is largely because of their α,β-unsaturated carbonyl group. In Arabidopsis, TGA transcription factors are essential factors in mediating the response to OPDA and phytoprostanes (Mueller et al. 2008). Additionally, cyclophilin 20-3 in plastids was identified as a protein capable of binding OPDA, relaying the signal to induce gene expression (Park et al. 2013). Because the α,β-unsaturated carbonyl group is the essential structural requirement for the induction of this distinct signal transduction pathway, it is probable that GLVs having α,β-unsaturated carbonyl groups also activate the same signaling pathway.
Another important (bio)chemical aspect of RES is its high reactivity with nucleophiles in cells. Glutathione (GSH) reacts with (E)-2-hexenal either enzymatically using glutathione S-transferases or non-enzymatically at a physiological pH like 7.0. In tobacco leaves where hypersensitive responses were elicited by treating with cryptogein, several species of GSH adducts, such as OPDA-GSH, ketooctadecadienoic acid-GSH, 4-hydroxy-(E)-2-nonenal-GSH, and hexenal-GSH, are formed, probably as a detoxification reaction, to cope with potentially active and even toxic substances (Davoine et al. 2006). Among these adducts, the GSH adduct with (E)-2-hexenal accumulated to the highest level, as much as 100 nmol g−1 dry weight. When plants were exposed to volatile compounds having α,β-unsaturated carbonyl species, the volatiles would be partitioned into the plant tissues under the gas phase/water phase (inside of tissues) equilibrium determined by the Henry’s law. Even though the partition would be much more complicated because volatiles could cross cell walls in apoplasts and plasma membranes, this indicated that it is inevitable for plants to accumulate such reactive xenobiotics in cells during normal gas-exchanges in photosynthesis through stomata. If the compounds were not appropriately detoxified, they would react with GSH to form adducts. Accordingly, endogenous GSH would be consumed, and the consumption of GSH would lead to an imbalance in the redox state (GSH/GSSG), which might cause the induction of several genes under redox regulation.
The involvement of GSH in responding to (E)-2-hexenal and (Z)-3-hexenal was confirmed using the pad2-1 Arabidopsis mutant that had a lower level of GSH because of a defect in GSH biosynthesis. The expression of PDF1.2 elicited by hexenals was not affected by the pad2-1 mutation, but that of VSP1 was totally eliminated in the mutant (Kishimoto et al. 2006). This, again, indicated that redox regulation, mediated by GSH, should play a crucial role in responding to GLVs containing α,β-unsaturated carbonyl groups.
Because the reactivity of RES is based on a simple chemical reaction, it is also possible for RES to react with the other biological components, such as proteins, nucleotides, and membrane components. Of course, there might be a distinct set of molecules that show a specificity, depending on the chemical nature of RES, and nucleophiles that might be involved in the reaction (Mano 2012; Mano et al. 2014). For GLVs containing α,β-unsaturated carbonyl groups, their moderate hydrophobicity allows them accumulate in a hydrophobic environment, such as a biological membrane. If this was the case, then a protein in the membrane would be a primary target for the GLVs. Also, the nucleophilicity of residues on the surface of biological molecules would be a determinant for the target. Signaling mediated by covalent binding is well studied in mammalian systems, and the Nrf/Keap1 system plays a central role (Higdon et al. 2012). There has been no report of Nrf/Keap1 homologs in plants and our preliminary BLAST search also failed to find the counterparts in plants, although it is still possible for plants to use a similar, covalent binding-mediated signaling pathway.
Interactions with Membranes
When volatiles released from herbivore-damaged tomato plants were blown across the surface of intact tomato leaves, their plasma membrane potential jumped to a depolarized value (from −114 to −76 mV) within 10 or 20 s (Zebelo et al. 2012). GLVs were the most abundant volatiles released by the herbivore-infested tomato. As expected, a similar membrane potential depolarization was observed by blowing vapors of (Z)-3-hexenal, (E)-2-hexenal, or (Z)-3-hexen-1-yl acetate. (Z)-3-Hexen-1-yl acetate, even though it is not the RES-type, causes depolarization as much as, or even to higher degree than, (E)-2-hexenal, a RES, or (Z)-3-hexenal, a potential RES. The reactivity caused by the α,β-unsaturated carbonyl group should not be the prerequisite of depolarization.
It was expected that the volatile affected the nature of ion channels located in the plasma membrane. The α,β-unsaturated carbonyl group might be partially involved in the modulation of ion channels, but volatiles without the reactive moiety could induce almost the same depolarization, which indicates that the moiety does not play the critical role. These GLVs also induced a calcium influx into the cytosols of epidermal cells (Zebelo et al. 2012). The ability to promote calcium influx apparently varied depending on the molecular species used, and (Z)-3-hexen-1-yl acetate was the most potent. Using fluorescence microscopy, the integrity of chloroplasts in parenchymal cells could also be examined because of the autofluorescence of chlorophyll. Interestingly, exposure to a gas containing (E)-2-hexenal or (Z)-3-hexenal seemed to be toxic to the cells, and the integrity of the chloroplast was extensively damaged by the treatment. However, the (Z)-3-hexen-1-yl acetate treatment resulted in no apparent change in the chloroplasts’ integrity. From this observation, again, it is proposed that the reactivity belonging to α,β-unsaturated carbonyl group does not always correlated to the ability to induce a calcium influx into the cytoplasm.
Heil et al. (2008) investigated the structure-activity relationship of a series of alka(e)nyl acetates of different chain lengths, and different degrees and positions of unsaturation by monitoring the amount of extrafloral nectar secreted by lima bean plants after exposure to the vapor. They found a relatively broad spectrum of activity for each compound, and apparently there was no essential structural requirement to exert the effect. Accordingly, they proposed that a physicochemical processes based on the amphiphilic nature of the compound was the important factor for the effect. Because plasma membranes should be the first site of penetration for exogenously supplied volatiles, the deposition of volatiles in the plasma membrane would cause the effect by distorting the membrane’s organization. Alternatively, it is possible that some proteins, such as odorant binding proteins or lipid transfer proteins, are involved in the system.
Metabolism
Most plants also have the ability to detoxify reactive xenobiotics such as RES. Cucumber plants and Arabidopsis contain several reductases that detoxify them. An alkenal/one oxidoreductase (AOR) catalyzes the reduction of the α,β-unsaturated bond in RES (Yamauchi et al. 2011). Aldo-keto reductase (AKR) and aldehyde reductase catalyze the reduction of aldehyde to alcohol. In cucumber, acrolein was efficiently reduced to form propionaldehyde by two distinct AORs localized in the chloroplasts and cytosol, respectively. Yamauchi et al. (2011) also detected the reducing activity to form alcohol from aldehyde. For example, Arabidopsis has both AKR and aldehyde reductase in its chloroplasts. When intact Arabidopsis was exposed to a vapor containing (Z)-3-hexenal, the aldehyde was taken up by tissues, efficiently converted into (Z)-3-hexen-1-ol in a NADPH-dependent manner, and released in its alcohol form into the atmosphere (Matsui et al. 2012). This reduction is also an important detoxification system to Arabidopsis, where the excess amount of (Z)-3-hexenal above the reducing capacity of Arabidopsis resulted in the suppression of PSII activity estimated by PAM. This implies that exposing plant tissues to (Z)-3-hexenal at a level where the tissues employ a reductive detoxification system as much as they can would cause an imbalance in the redox state maintained by the NADPH/NADP+ ratio. The imbalance caused by the volatile might result in the modulation of gene expression levels in tissues. This might be a scenario caused by GLVs during plant–plant interactions.
Even though the alcohol, (Z)-3-hexen-1-ol, is a rather inert compound, it is sometimes converted into (Z)-3-hexen-1-yl acetate, which is more volatile than (Z)-3-hexen-1-ol according to their Henry’s law constants (25 and 1 M atm−1, respectively). Because this conversion requires acetyl-CoA, which would otherwise be used in growth, this definitely is an active process. This suggests that (Z)-3-hexen-1-ol is also an active compound, and a surplus supply during the reduction of (Z)-3-hexenal or from the atmosphere would cause an imbalance in the homeostasis of plant cells (Farag et al. 2005). When tomato plants were exposed to volatile compounds released from herbivore-damaged conspecifics, the receiver plants adsorb (Z)-3-hexen-1-ol, and converted it to a glycoside, (Z)-3-hexenyl vicianocide (Fig. 17.4). Because the glycoside has a slight but distinct activity to suppress the growth of herbivore, the glycosidation and subsequent accumulation of the compound from the atmosphere benefitted the receiver plants. Therefore, glycosidation could be one way for tomato plants to carry out plant–plant communications. All flowering plants examined so far accumulated glycosides after exposure to vapor containing (Z)-3-hexen-1-ol. Therefore, the perception of volatiles through glycosidation is a general property of plants.
Glycosidation is one way to ‘sense’ green leaf volatiles (GLVs). A herbivore-damaged tomato plant releases (Z)-3-hexen-1-ol into atmosphere. The neighboring plant takes up the GLV and converts it to (Z)-3-hexenyl vicianoside. Because the glycoside has an insecticidal activity, the ‘receiver’ plant acquires a higher resistance against the herbivore. Even though this is the metabolism of the plant responding to an exogenous compound, as a whole, this metabolism can be considered as an example of plant–plant interaction
Concluding Remarks
Because of their abundance in nature, GLVs are familiar plant secondary metabolites. We enjoy their smells when we eat foods made from plants or when we walk through forests. Of course, plants form GLVs because it is beneficial. Since the discovery of the volatile sex pheromones of silkworms, we have been accumulating examples of the physiological and ecological functions of volatiles. GLVs are largely involved in the defense responses of plants against herbivores and pathogens, or even abiotic stresses. We are interested in when plants first acquired the ability to form GLVs because this knowledge would give us insights into how plants survive in environments where they interact with the other organisms. Also, the recent finding that plants respond to GLVs opens many areas to further study. Because they lack the nervous and olfactory systems of animals, plants may employ their own original systems to ‘sense’ GLVs. Chemical reactivity, physicochemical nature, and the nature of metabolization are the ways plants detect GLVs, but these fail to explain the phenomena found in nature completely. We are still beginning to decipher how plants ‘sense’ volatiles, and further extensive studies await.
References
Allmann S, Baldwin IT (2010) Insects betray themselves in nature to predators by rapid isomerization of green leaf volatiles. Science 329:1075–1078
Arimura G, Ozawa R, Shimoda T, Nishioka T, Boland W, Takabayashi J (2000) Herbivory-induced volatiles elicit defense gene in lima bean leaves. Nature 406:512–515
Baldwin IT, Halitschke R, Paschold A, von Dahl CC, Preston CA (2006) Volatile signaling in plant-plant interactions: “Talking trees” in the genomics era. Science 311:812–815
Bate NJ, Rothstein SJ (1998) C6-volatiles derived from the lipoxygenase pathway induce a subset of defense-related genes. Plant J 16:561–569
Bate NJ, Sivasankar S, Moxon C, Riley JM, Thompson JE, Rothstein SJ (1998) Molecular characterization of an Arabidopsis gene encoding hydroperoxide lyase, a cytochrome P-450 that is wound inducible. Plant Physiol 117:1393–1400
Bonaventure G (2014) Lipases and the biosynthesis of free oxylipins in plants. Plant Signal Behav 3:9
Bonaventure G, Schuck S, Baldwin IT (2011) Revealing complexity and specificity in the activation of lipase-mediated oxylipin biosynthesis: a specific role of the Nicotiana attenuata GLA1 lipase in the activation of jasmonic acid biosynthesis in leaves and roots. Plant Cell Environ 34:1507–1520
Copolovici L, Kännaste A, Pazouki L, Niinemets Ü (2012) Emissions of green leaf volatiles and terpenoids from Solanum lycopersicum are quantitatively related to the severity of cold and heat shock treatments. J Plant Physiol 169:664–672
Curtius T, Franzen H (1912) Über die chemischen Bestandteile grüner Pflanzen. Über den Blätteraldehyd. Justus Liebigs Annalen der Chemie 390:89–121
Davoine C, Falletti O, Douki T, Iacazio G, Ennar N, Montillet JL, Triantaphylides C (2006) Adducts of oxylipin electrophiles to glutathione reflect a 13 specificity of the downstream lipoxygenase pathway in the tobacco hypersensitive response. Plant Physiol 140:1484–1493
Dicke M, Baldwin IT (2010) The evolutionary context for herbivore-induced plant volatiles: beyond the ‘cry for help’. Trends Plant Sci 15:167–175
Dicke M, Agrawal AA, Bruin J (2003) Plants talk, but are they deaf? Trends Plant Sci 9:403–405
Engelberth J, Alborn HT, Schmelz EA, Tumlinson JH (2004) Airborne signals prime plants against insect herbivore attack. Proc Natl Acad Sci U S A 101:1781–1785
Farag MA, Fokar M, Abd H, Zhang H, Allen RD, Paré PW (2005) (Z)-3-Hexenol induces defense genes and downstream metabolites in maize. Planta 220:900–909
Froehlich JE, Itoh A, Howe GA (2001) Tomato allene oxide synthase and fatty acid hydroperoxide lyase, two cytochrome P450s involved in oxylipin metabolism, are targeted to different membranes of chloroplast envelope. Plant Physiol 125:306–317
Frost CJ, Mescher MC, Dervinis C, Davis JM, Carlson JE, De Moraes CM (2008) Priming defense genes and metabolites in hybrid poplar by the green leaf volatile cis-3-hexenyl acetate. New Phytol 180:722–734
Glauser G, Grata E, Dubugnon L, Rudaz S, Farmer EE, Wolfender J-L (2008) Spatial and temporal dynamics of jasmonate synthesis and accumulation in Arabidopsis in response to wounding. J Biol Chem 283:16400–16407
Glauser G, Dubugnon L, Mousavi SA, Rudaz S, Wolfender J-L, Farmer EE (2009) Velocity estimates for signal propagation leading to systemic jasmonic acid accumulation in wounded Arabidopsis. J Biol Chem 284:34506–34513
Grechkin AN (2002) Hydroperoxide lyase and divinyl ether synthase. Prostaglandins Other Lipid Mediat 68:457–470
Grechkin AN, Brühlmann F, Mukhtarova LS, Gogolev YV, Hamberg M (2006) Hydroperoxide lyases (CYP74C and CYP74B) catalyze the hemolytic isomerization of fatty acid hydroperoxides into hemiacetals. Biochim Biophys Acta 1761:1419–1428
Heil M, Lion U, Boland W (2008) Defense-inducing volatiles: in search of the active motif. J Chem Ecol 34:601–604
Higdon A, Diers AR, Oh JY, Landar A, Darley-Usmar VM (2012) Cell signaling by reactive lipid species: new concepts and molecular mechanisms. Biochem J 442:453–464
Jardine K, Barron-Gafford GA, Norman JP, Abrell L, Monson RK, Meyers KT, Pavao-Zuckerman M, Dontsova K, Kleist E, Werner C, Huxman TE (2012) Green leaf volatiles and oxygenate metabolite emission bursts from mesquite branches following light-dark transitions. Photosynth Res 113:321–333
Kako H, Kobayashi Y, Yokogoshi H (2011) Effects of n-hexanal on dopamine release in the striatum of living rats. Eur J Pharmacol 651:77–82
Kishimoto K, Matsui K, Ozawa R, Takabayashi J (2005) Volatile C6-aldehydes and allo-ocimene activate defense genes and induce resistance against Botrytis cinerea in Arabidopsis thaliana. Plant Cell Physiol 46:1093–1102
Kishimoto K, Matsui K, Ozawa R, Takabayashi J (2006) ETR1-, JAR1- ad PAD2-dependent signaling pathways are involved in C6-aldehyde-induced defense responses of Arabidopsis. Plant Sci 171:415–423
Kissen R, Rossiter JT, Bones AM (2009) The ‘mustard oil bomb’: not so easy to assemble?! Localization, expression and distribution of the components of the myrosinase enzyme system. Phytochem Rev 8:69–86
Koeduka T, Ishizaki K, Mwenda CM, Hori K, Sasaki-Sekimoto Y, Ohta H, Kohchi T, Matsui K (2015) Biochemical characterization of allene oxide synthases from the liverwort Marchantia polymorpha and green microalgae Klebsormidium flaccidum procides insight into the evolutionary divergence of the plant CYP74 family. Planta 242:1175–1186
Kost C, Heil M (2006) Herbivore-induced plant volatiles induce an indirect defence in neighbouring plants. J Ecol 94:619–628
Kubigsteltig I, Laudert D, Weiler E (1999) Structure and regulation of Arabidopsis thaliana allene oxide synthase gene. Planta 208:463–471
Lee DS, Nioche P, Hamberg M, Raman CS (2008) Structural insights into the evolutionary paths of oxylipin biosynthetic enzymes. Nature 455:363–368
Mano J (2012) Reactive carbonyl species: their production from lipid peroxides, action in environmental stress, and the detoxification mechanism. Plant Physiol Biochem 59:90–97
Mano J, Nagata M, Okamura S, Shiraya T, Mitsui T (2014) Identification of oxidatively modified proteins in salt-stressed Arabidopsis: a carbonyl-targeted proteomics approach. Plant Cell Physiol 55:1233–1244
Matsui K (2006) Green leaf volatiles. Hydroperoxide lyase pathway of oxylipin metabolism. Curr Opin Plant Biol 9:274–280
Matsui K, Shibutani M, Hase T, Kajiwara T (1996) Bell pepper fruit fatty acid hydroperoxide lyase is a cytochrome P450 (CYP74B). FEBS Lett 394:21–24
Matsui K, Sugimoto K, Mano J, Ozawa R, Takabayashi J (2012) Differential metabolisms of green leaf volatiles in injured and intact parts of a wounded leaf meet distinct ecophysiological requirements. PLoS ONE 7:e36433. doi:10.1371/journal.pone.0036433
Mita G, Quarta A, Fasano P, De Paolis A, Di Sansebastiano GP, Perrotta C, Iannacone R, Belfield E, Hughes R, Tsesmetzis N et al (2005) Molecular cloning and characterization of an almond 9-hydroperoxide lyase, a new CYP74 targeted to lipid bodies. J Exp Bot 56:2321–2333
Mueller S, Hilbert B, Dueckersjhoff K, Roitsch T, Krischke M, Mueller M, Berger S (2008) General detoxification and stress responses are mediated by oxidized lipids through TGA transcription factors in Arabidopsis. Plant Cell 20:768–785
Murakami M (2011) Lipid mediators in life science. Exp Anim 60:7–20
Mwenda CM, Matsuki A, Nishimura K, Koeduka T, Matsui K (2015) Spatial expression of the Arabidopsis hydroperoxide lyase is controlled differently from that of the allene oxide synthase gene. J Plant Interact 10:1–10
Nakashima A, von Reuss SH, Tasaka H, Nomura M, Mochizuki S, Iijima Y, Aoki K, Shibata D, Boland W, Takabayashi J, Matsui K (2013) Traumatin- and dinortraumatin-containing galactolipids in Arabidopsis: their formation in tissue-disrupted leaves as counterparts of green leaf volatiles. J Biol Chem 288:26078–26088
Noordermeer MA, Van Dijken AJ, Smeekens SC, Veldink GA, Vliegenthart JF (2000) Characterization of three cloned and expressed 13-hydroperoxide lyase isoenzymes from alfalfa with unusual N-terminal sequences and different enzyme kinetics. Eur J Biochem 267:2473–2482
Park SW, Li W, Viehhauser A, He B, Kim S, Nilsson AK, Andersson MX, Kittle JD, Ambavaram MMR, Luan S, Esker AR, Tholl D, Cimini D, Ellerström M, Coaker G, Mitchell TK, Pereira A, Dietz KJ, Lawrence CB (2013) Cyclophilin 20-3 relays a 12-oxo-phytodienoic acid signal during stress responsive regulation of cellular redox homeostasis. Proc Natl Acad Sci U S A 110:9559–9564
Paschold A, Halitschke R, Baldwin IT (2006) Using ‘mute’ plants to translate volatile signals. Plant J 45:275–291
Phillips DR, Galliard T (1978) Flavor biogenesis: partial purification and properties of a fatty acid hydroperoxide cleaving enzyme from fruits of cucumber. Phytochemistry 17:355–358
Riddiford LM (1967) Trans-2-hexenal: mating stimulant for polyphemus moths. Science 157:139–141
Savchenko T, Kolla VA, Wang CQ, Nasafi Z, Hicks DR, Phadungchob B, Chehab WE, Brandizzi F, Froehlich J, Dehesh K (2014) Functional convergence of oxylipin and abscisic acid pathways controls stomatal closure in response to drought. Plant Physiol 164:1151–1160
Scala A, Allman S, Mirabella R, Haring MA, Schuurink RC (2013) Green leaf volatiles: a plant’s multifunctional weapon against herbivores and pathogens. Int J Mol Sci 14:17781–17911
Scholz J, Brodhun F, Hornung E, Herrfurth C, Stumpe M, Beike AK, Faltin B, Frank W, Reski R, Feussner I (2012) Biosynthesis of allene oxides in Physcomitrella patens. BMC Plant Biol 12:228
Shibata Y, Matsui K, Kajiwara T, Hatanaka A (1995) Purification and properties of fatty acid hydroperoxide lyase from green bell pepper fruits. Plant Cell Physiol 36:147–156
Stumpe M, Bode J, Göbel C, Wichard T, Schaaf A, Frank W, Frank M, Reski R, Pohnert G, Feussner I (2006) Biosynthesis of C9-aldehydes in the moss Physcomitrella patens. Biochim Biophys Acta 1761:301–312
Sugimoto K, Matsui K, Iijima Y, Akakabe Y, Muramoto S, Ozawa R, Uefune M, Sasaki R, Alamgir KM, Akitake S et al (2014) Intake and transformation to a glycoside of (Z)-3-hexenol from infested neighbors reveals a mode of plant odor reception and defense. Proc Natl Acad Sci U S A 111:7144–7149
Suzuki N, Koussevitzky S, Mittler R, Miller G (2012) ROS and redox signaling in the response of plants to abiotic stress. Plant Cell Environ 35:259–270
Taki N, Sasaki-Sekimoto Y, Obayashi T, Kikuta A, Kobayashi K, Ainai T, Yagi K, Sakurai N, Suzuki H, Masuda T, Takamiya K, Shibata D, Kobayashi Y, Ohta H (2005) 12-Oxo-phytodienoic acid triggers expression of a distinct set of genes and plays a role in wound-induced gene expression in Arabidopsis. Plant Physiol 139:1268–1283
Yamauchi Y, Hasegawa A, Taninaka A, Mizutani M, Sugimoto Y (2011) NADPH-dependent reductases involved in the detoxification of reactive carbonyls in plants. J Biol Chem 286:6999–7009
Yamauchi Y, Kunishima M, Mizutani M, Sugimoto Y (2015) Reactive short-chain leaf volatiles act as powerful inducers of abiotic stress-related gene expression. Sci Rep 5:8030. doi:10.1038/srep08030
Zebelo A, Matsui K, Ozawa R, Maffei ME (2012) Plasma membrane potential depolarization and cytosolic calcium flux are early events involved in tomato (Solanum lycopersicon) plant-to-plant communication. Plant Sci 196:93–100
Zeringue HJ Jr (1992) Effects of C6–C10 alkenals and alkanals on eliciting a defence response in the developing cotton boll. Phytochemistry 31:2305–2308
Acknowledgement
This study was partly supported by a Grant-in-aid for Scientific Research (K.M., 25282234 and 90199729) from the Ministry of Education, Culture, Sports, Science and Technology, Japan.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2016 Springer International Publishing Switzerland
About this chapter
Cite this chapter
Matsui, K., Koeduka, T. (2016). Green Leaf Volatiles in Plant Signaling and Response. In: Nakamura, Y., Li-Beisson, Y. (eds) Lipids in Plant and Algae Development. Subcellular Biochemistry, vol 86. Springer, Cham. https://doi.org/10.1007/978-3-319-25979-6_17
Download citation
DOI: https://doi.org/10.1007/978-3-319-25979-6_17
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-25977-2
Online ISBN: 978-3-319-25979-6
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)