Abstract
During the 1960s to 1980s a human mandible, together with fossils of other animals and a lithic industry, were recovered from Units I to VI of Azokh Cave. After the year 2002, new excavations in Units I to V were undertaken. The new large mammal fossils are described and the fauna is revised, using part of the older collections. The only clear break in the sequence is the appearance of domestic mammals in Unit I. The following taxa recovered from Pleistocenic sediments were identified: Ursus spelaeus (the most abundant), Ursus sp. (U. aff. arctos/thibetanus), Vulpes vulpes, Canis aureus, Canis lupus, Meles meles, Martes cf. foina, Crocuta crocuta, Felis chaus, Panthera pardus, Equus hydruntinus, Equus ferus, Stephanorhinus hemitoechus, Stephanorhinus kirchbergensis, Sus scrofa, Capreolus pygargus, Dama aff. peleponesiaca, Dama sp., Megaloceros solilhacus, Cervus elaphus, Bison schoetensacki, Ovis ammon, Capra aegagrus and Saiga. Most species present are common in western Eurasia. All fossiliferous Units have taxa that in mid-latitude Europe are considered to be “interglacial” elements, while there are no clear “glacial” elements, which suggests temperate conditions despite the altitude of the cave. The evolutionary levels of various species suggest ages of about 300 ka for Units VI–IV, while Units III–II are slightly younger. Domestic mammals indicate a Holocene age for Unit I. Most sediments represent a normal transition between units. Processes of erosion, however, affected the top of the Pleistocene sediments recorded in the cave. Therefore, Unit I (Holocene sediments containing domestic animals) lies disconformably over Unit II (Late Pleistocene).
Резюме
За период с 1960-х по 1980-е гг. в уровнях I–VI азохской пещеры были обнаружены фрагмент нижней челюсти человека, окаменелости других животных и каменные орудия. После 2000 г. раскопки были возобновлены на уровнях I–V. В данной главе описаны находки новых крупных млекопитающих, полностью пересмотрена коллекция фауны с включением в нее части более ранних собраний.
Единственный отчетливый перерыв в последовательности находок связан с появлением домашних животных в подразделении 1. В ходе исследования удалось идентифицировать следующие виды, обнаруженные в плейстоценовых отложениях: Ursus spelaeus (наиболее богато представленный), Ursus sp. (U. aff. arctos/thibetanus), Vulpes vulpes, Canis aureus, Canis lupus, Meles meles, Martes cf. foina, Crocuta crocuta, Felis chaus, Panthera pardus, Equus hydruntinus, Equus ferus, Stephanorhinus hemitoechus, Stephanorhinus kirchbergensis, Sus scrofa, Capreolus pygargus, Dama aff. peleponesiaca, Dama sp., Megaloceros solilhacus, Cervus elaphus, Bison schoetensacki, Ovis ammon, Capra aegagrus и Saiga. Останки плотоядных животных были раскопаны главным образом из подразделения 1.
Dama aff. Peleponesiaca интересна тем, что сочетает в себе примитивное качество сильного разветвления лобного отростка и ствола рога с прогрессивной характеристикой хорошо развитой лапчатости. Эта особенность приписана боковой ветви таксона Dama в том же регионе, существовавшей до появления вида D. mesopotamica. Megaloceros solilhacus примечателен тем, что его находка в Азохе является самой молодой из всех известных нам. Этот вид широко представлен в Европе и юго-западной Азии (Убейдия, Латамна), он является наиболее вероятным предком M. algericus, который намного позднее появился в Северной Африке. Находки свидетельствуют о том, что данный род выжил в пределах юго-западной Азии после полного вымирания в Европе и до его распространения в Северную Африку.
Большинство видов, представленных в Азохе, являются или были обычными формами в западной Евразии, но некоторые из них имеют или в прошлом имели область распространения вплоть до Дальнего Востока. Основной ареал других видов был представлен юго-восточной, южной или центральной Азией, или Северной Африкой. Все горизонты с ископаемыми организмами включают в себя таксоны, которые в средних широтах Европы квалифицируются как “межледниковые”, в то время как в этих же слоях отсутствуют явные “ледниковые” артефакты, что указывает на умеренные климатические условия несмотря на высоту расположения пещеры.
Многие из обнаруженных видов живут и сегодня, однако Ursus spelaeus, Equus hydruntinus, два вида Stephanorhinus и Bison schoetensacki вымерли в эпоху позднего плейстоцена, в то время как M. solilhacus и Dama aff. pelopenesiaca, должно быть, вымерли или эволюиционировали в другие виды значительно ранее.
Поскольку большинство видов дожило до наших дней, многие из них характеризуют предельные возраста для стратиграфических подразделений: Stephanorhinus hemitoechus, Ursus spelaeus и Canis lupus свидетельствуют в пользу более молодого возраста некоторых слоев, чем это предполагалось ранее. Эволюционное положение Cervus elaphus и различных видов рода Dama предоставляет дополнительную информацию о возрасте подразделений. Биохронологические данные указывают на возраст около 300 тыс. лет для подразделений VI–IV, в то время как подразделения III–II немного моложе. Наличие останков домашних животных свидетельствует о голоценовом возрасте подразделения I. Отложения указывают на нормальный переход между большинством из подразделений. Процессы эрозии, однако, повлияли на поверхность плейстоценовых отложений в пещере. По этой причине подразделение I (голоценовые отложения, содержащие домашних животных) находится в явном несоответствии с подразделением II (поздний плейстоцен).
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
Introduction
The site of Azokh (also known as Azykh or Azikh), in the Lesser Caucasus (Fig. 6.1), has provided an extensive large mammal assemblage recovered from excavations from 1963 to 1988 and from 2002 to present. The largest mammal fossil collection was recovered during the 25 years of the former excavations lead by M. Huseinov (see Fernández-Jalvo et al. 2016) from Units VI to II. This collection is currently hosted at the Medical University of Baku in Azerbaijan. Excavations from 2002 to the present have been carried out at the back of the cave. Fossils have been referred to units following the same nomenclature and stratigraphy established by Huseinov from Units V to I. The top of the sequence (I) Holocene (Appendix, radiocarbon) was not palaeontologically studied by previous authors. The bottom of the sequence (Unit Vm) comes from an excavation surface left by Huseinov’s team that is located at about a metre above the bottom of this unit. Sediments from Unit VI are recorded at the cave entrance (at present on the sides of the cave walls), but it loses thickness towards the back of the cave and has no identifiable record in the area where excavations were performed from 2002 to present (Murray et al. 2016). Fossils from this unit were excavated during the previous seasons lead by Huseinov, and taxonomic identifications refer to the fossil collection currently hosted in Baku.
Excavations performed from 2002, located at the back of the cave, yielded fossils that show differences in the mammalian faunal composition compared to excavations performed by Huseinov (1960–1980) closer to the open-air connection. The new material has a larger and better representation of bears, probably as result of relatively prolonged hibernation and occupation periods of cave bears. Humans have entered the cave and transported in some animals inhabiting the area in the vicinity of the cave (Marin-Monfort et al. 2016). We are here describing the large mammal taxonomy, comparing results with previous identifications, and discussing the meaning of these groups and their geographic distribution across the area that gave access from and to Eurasia from and to Africa.
An interesting aspect of the study area is its geographical and biogeographical position. Situated on the southern flanks of the Lesser Caucasus, the area is west Eurasian in its biogeographic affinities. Many “typically European” species range far into Asia, as did Neanderthals. With increasing distance, such species may show morphological change or be replaced by other species eastward, but also southward. Towards the south, species adapted to more dry or open environments replace the species with European affinities. To the east, there may be gradual or abrupt morphological or metrical changes within a species. Such changes are probably related to periods of isolation during the cold phases and thus these phenomena contain information on past environmental conditions, conditions in which the Neanderthals also lived. Ideally, long detailed records of faunal composition and of morphological and metrical evolution of the different species should be compared with the European records. At present this is not possible, but it is possible to compare the fauna of a single or few localities with the European record.
The fauna from Azokh was formerly studied by Aliev (1969). Lioubine (2002) gave faunal lists per unit based on Aliev (1969, 1989, 1990), Gadziev et al. (1979), Velichko et al. (1980), Markova (1982) and Burchak-Abramovitch and Aliev (1989, 1990) and mentioned later additions or modifications by Guérin and Barychnikov (1987) and Barychnikov (1991), who identified the presence of Dicerorhinus etruscus brachycephalus (presently mostly Stephanorhinus hundsheimensis) and Ursus mediterraneus. Rivals (2004) gave the composite list of large mammals according to Aliev (1969). There are small differences between the two lists, which probably reflect the work done between 1969 and 2002, such as the elimination of several “cf.” citations, the assignment to Equus suessenbornensis instead of to Equus caballus, the omision of Gazella aff. subgutturosa, etc. Table 6.1 shows the large mammal taxonomic identification cited by previous authors.
The faunal material from the previous excavations is kept in the Medical University of Baku (Azerbaijan). One of us (JvdM) had the opportunity to study the Artiodactyla and Rhinocerotidae of this collection. It is the aim of this chapter to describe the new material, to discuss the older collections, present an updated faunal list and make comparisons with the European faunal record.
Materials and Methods
Conventional methods were used in the morphological studies, based on visual comparisons and simple morphometrics. The measurements of the Equidae are taken as indicated by Eisenmann et al. (1988), those of the Rhinocerotidae as indicated by Van der Made (2010a), those of the Artiodactyla as indicated by Van der Made (1996) and Van der Made and Tong (2008), and those of the carnivores are taken in a comparable way. All measurements are given in mm, unless indicated otherwise. The measurements are indicated by same abbreviations as used by Van der Made (1996) and Van der Made and Tong (2008). DAP, DT, DMD, DLL mean respectively antero-posterior, transverse, medio-distal and labio-lingual diametre respectively. L and H mean length and height. Additions of letters as in DTa mean DT of the anterior lobe of a tooth or of the anterior side of a bone. Similarly: a = anterior, b = basal, dors = dorsal, f = of the facet, h = of the head (as in a calcaneum), l = lower, la = labial, li = lingual, max = maximum, mini = minimum, n = neck, o = occlusal, p = posterior, root = of the root, sf = at the height of the sustenacular facet, trigonid = of the trigonid, u = upper. Ta is the enamel thickness measured at the metaconid, h and l are alternative height and length of a bone. R1–5 are five dimensions of the distal condyle of the humerus, numbered from medial to lateral. Lint, Lm and Lext are the medial, middle and lateral lengths of the astragalus.
The terminology of the tooth morphology follows Van der Made (1996). If ungulate, phalanges, sesamoids and distal metapodials are indicated to be right or left, this means the position relative to the axis of the foot, not of the complete animal. Example a “right first phalanx” of an artiodactyl means thus a proximal phalanx III of the right foot or a phalanx IV of the left foot.
The fossils of the recent excavations at Azokh are housed in the Artsakh State Museum of History and Country Study in Stepanakert (ASMHCS). These fossils were compared with fossils from many other localities or bones of recent mammals. When such comparisons are made, either the relevant literature is cited, or the institute is indicated where the material was studied or where it is kept at present (which need not be the same institute). The institutes are indicated with the following acronyms: AUT = Aristotle University of Thessaloniki; BGR = Bundesanstalt für Geowisenschaften und Rohstoffe, Hannover; CENIEH = Centro Nacional de Investigación sobre la Evolución Humana, Burgos; CIAG = Centre d’Investigacions Arquelògics de Girona; EBD = Estación Biológica de la Doñana, Sevilla; FASMN = Römisch-Germanisisches Zentralmuseum, Forschungsinstitut für Vor- und Frühgeschichte, Forschungsbereich Altsteinzeit Schloss Monrepos, Neuwied; FBFSUJB = Forschungstelle Bilzingsleben, Friedrich Schiller-Universität Jena, Bilzingsleben; GIN = Geological Institute, Moscow; GSM = Georgian State Museum, Tbilisi; HGSB = Hungarian Geological Survey, Budapest; HMV = Historisches Museum, Verden; MNHUB – Museum für Naturkunde der Humboldt-Universität, Berlin; HUJ = Hebrew University, Jerusalem; IGF = Istituto di Geologia, Firenze; IPGAS = Institute of Palaeobiology, Georgian Academy of Sciences, Tbilisi; IPH = Institut de Paléontologie Humaine, Paris; IPS = Instituto de Paleontología, Sabadell; IQW = Institut für Quartärpaläontologie, Weimar; IVAU = Instituut Voor Aardwetenschappen, Utrecht; IVPP = Institute for Vertebrate Paleontology and Paleoanthopology, Academia Sinica, Beijing; LAUT = Laboratori de Arqeologia de la Universitat Rovira i Virgili, Tarragona; LPT = Laboratoire de Prehistoire de Tautavel, Université de Perpignan; LVH = Landesmuseum für Vorgeschichte, Halle; MCP = Musée Crozatier, Le Puy-en-Velay; MMB = Moravian Museum, Brno; MNCN = Museo Nacional de Ciencias Naturales, Madrid; MPRM = Musée de Préhistoire Régionale, Menton; MPT = Musée de la Préhistoire Tautavel; MRA = Museum Requien, Avignon; MUB = Medical University, Baku; NCUA = National and Capodistrian University of Athens; NHM = Natural History Museum, London; NHMB = Natural-Historical Museum, Baku; NMM = Naturhistorisches Museum, Mainz; NMMa = Natuurhistorisch Museum, Maastricht; NMP = National Museum, Prague; NNML = Nationaal Natuurhistorisch Museum, Leiden; PIN = Palaeontological Institute, Moscow; SIAP = Servei d’Investigacions Arqueològiques i Prehistòriques, Castellón; SMNK = Staatliches Museum für Naturkunde, Karlsruhe; SMNS = Staatliches Museum für Naturkunde, Stuttgart; SMS = Spengler Museum, Sangerhausen; TMH = Teylers Museum, Haarlem; TUC = Technische Universität Clausthal, Insitut für Geologie und Paläontologie; UCM = Universidad Complutense, Madrid; ZSM = Zhoukoudian Site Museum.
Systematic descriptions
New material
-
Unit II
-
E45-46B – third cuneiform.
Description of the new material and taxonomic classification
A third cuneiform bone shows size and articular facet morphology that clearly differ from Ursus spelaeus and Ursus deningeri, which indicates a small sized bear with narrow paws. If the measurements are plotted in the corresponding bivariate plot of the third cuneiform bones of Iberian U. deningeri, U. spelaeus and recent U. arctos (Torres 1989), it shows it to be smaller and more slender than U. spelaeus, clustering well with the other two species. Taking into account the general size of the Azokh bear skeletal elements, it seems very possible that there is a subtle presence of an ancient brown bear, but in some cave records of the Great Caucasus the presence of Ursus (Ursus) thibetanus G. Cuvier has been attested by Doronichev (2000). Therefore, we cannot ascertain to which of the two species this bone belongs.
New material
The specimens are listed and their measurements given in Tables 6.2, 6.3, 6.4, 6.5 and 6.6. Cave bear fossils studied here have been selected from the fossil collection recovered from Azokh, but some measurements could not be taken because most of these fossils are broken or damaged (see Marin-Monfort et al. 2016). The numbers of elements are indicated in brackets after each element type.
-
Unit Vm
-
Cuboid (1), first phalanx (2), second phalanx (1), I3 (1), P4 (1), M1 (1), M2 (3), I2 (1), M3 (1).
-
Unit Vu
-
Scapula fragment (1), radius (1), scapholunate (1), first metacarpal (1), fifth metacarpal (1), femur (1), fibula (1), cuboid (1), fourth metatarsal (2), sesamoid (2), first phalanx (4), third phalanx (3), M2 (1), I3 (2), lower canine (1), M1 (1), M2 (1), M3 (1).
-
Unit III
-
Humerus (1), radius (1), ulna (1), scapholunate bone (2), pisiform (1), magnum (1), second metacarpal (3), fibula (3), calcaneus (1), first metatarsal bone (1), fifth metatarsal (1), cervical vertebra (2), dorsal vertebra (1), lumbar vertebra (1), hyoid (cerato) bone (1), rib (1), sternum (xiphoid proc.) (1), pelvis (1), baculum (1), first phalanx (1), third phalanx (2), I3 (1), M2 (1), M3 (1), canine indet. (1).
-
Unit II
-
Skull fragment (1), maxilla fragment (10), mandible (1), scapula (6), humerus (9), radius (5), ulna (9), scapholunate (3), hamatum (2), magnum (3), pisiform (3), trapezoid (1), first metacarpal (2), second metacarpal (2), third metacarpal (3), fourth metacarpal (2), fifth metacarpal (5), femur (10), patella (3), tibia (3), fibula (9), calcaneus (5), astragalus (2), scaphoid (2), second cuneiform (1), third cuneiform (1), first metatarsal (1), second metatarsal (1), third metatarsal (2), fourth metatarsal (3), fifth metatarsal (1), hyoid-cerato bone (1), vertebra fragment (3), axis (1), dorsal vertebra (4), rib (1), pelvis (2), baculum (2), first phalanx (17), second phalanx (8), third phalanx (7), I1 (1), I2 (1), I3 (3), upper canine (4), P4 (1), M2 (2), I1 (1), I2 (1), I3 (2), lower canine (2), P4 (2), M1 (3), M2 (3), M3 (1) canine indet. (2).
-
Unit I
-
Femur fragment (1), I3 (1), M2 (1), I1 (2), I3 (2), CL (1), P4 (1)
Unit I is of Holocene age and mainly contains domestic animals. It has been heavily altered by recent animal burrowing. The result is the presence of fossils and stone tools reworked from Unit II and currently mixed with sediments from Unit I (Murray et al. 2016).
Most of the bear remains are from Unit II. The minimum number of individuals is: 1 in Unit I, 3 in Unit II, 1 in Unit III, 1 in Unit Vu, and 2 in Unit Vm, making a total number of eight individuals. This is not enough to ascertain any morphological change in skeleton and dentition in the recorded time-span.
Description of the new material and taxonomic classification
The most impressive specimen, at least according to its size (Table 6.3), is a complete and big right ulna (II C46 320 Z = 124). It is larger than those from the cave bear localities in the Iberian Peninsula that we used for comparison (cf. Torres 1989) (Fig. 6.2) and many other European localities (cf. Koby 1951). In order to discern whether this ulna falls into the spelaeus or the deningeri group, we used a bivariate analysis of the maximum anteroposterior diameter of the distal diaphysis against the total length of the bone (Fig. 6.2). In these diagrams the ulna from Azokh aligned with the U. spelaeus trend. The Azokh ulna, though bigger than all the ones comprised in the composite Iberian sample, matches well with the robustness of U. spelaeus individuals, diverging markedly from the small sample of U. deningeri and, in a more marked way, from the U. arctos group. Size differences (size trends) were explained by Kurten (1955).
Metrical comparison of the ulna of Ursus from Azokh 1 and those from the Iberian populations of U. spelaeus (El Reguerillo cave and Arrikrutz cave) composite sample), U. deningeri (La Lucia, Quintanilla Cantabria cave) and U. arctos (composite sample). Equiprobability (95%) ellipses were added. Anteroposterior diameter of the distal epiphysis is plotted against bone length. Data after Torres (1989) and Torres et al. (2006)
Though there is a low number of articular bones, their metrics are compared in bivariate plots (Fig. 6.3) with data of Ursus deningeri and Ursus spelaeus. In some cases, the plots of Torres (1989) were also used although they are not included in this chapter. Scapholunates II E 48 117B, II 132, II 256 and II D45 30 = 144–159 match with the U. spelaeus trend, but they are bigger. Vu D45 26 matches with the Ursus spelaeus trend (female sized). Magnums II D46 7 Z = 96, III D46 160 Z = 226 and II I49 22 match with the Ursus spelaeus trend and size, while II 25 matches with the Ursus spelaeus trend, although it is bigger. Hamates (or hook bones) II C46 303 and II C46 318 match with Ursus spelaeus in height. Pisiforms II C46 108 and II F51 1 match the Ursus spelaeus trend and are big sized. Trapezium II C46 204 matches the Ursus spelaeus trend. Calcanei II I50 9, II-69 and III D46 105 Z = 166 match the Ursus spelaeus trend. Astragali II 45 and II C46 150 match with the Ursus spelaeus trend. Cuboids Vu B, I, Vu B and Vm D42 12 Z = 90 match the Ursus spelaeus trend, while II F52 167 is slightly more robust. Scaphoid II C46 281 matches with the Ursus spelaeus trend and is big sized.
Metrical comparison of some carpals and tarsals of Ursus from Azokh 1 and those from the Iberian populations of U. spelaeus (El Reguerillo cave, Patones-Madrid and Arrikrutz cave, Oñati, Guipuzcoa) and U. deningeri (Sima de los Huesos, Atapuerca-Burgos). Equiprobability (95%) and regression lines have been added. For all cases (hamatum or hook bone excepted) antero-posterior diameter is plotted against transverse diameter. Data after Torres (1989)
The metrical relationships between the length and the transverse or anteroposterior diameter of epiphysis and diaphysis of the metapodials discriminate well between samples of U. deningeri and U. spelaeus (Torres 1989; Torres and Guerrero 1993; Torres et al. 2001). There are not enough complete metapodials from Azokh for a multivariate analysis, but Figs. 6.4 and 6.5 show bivariate diagrams of the transverse diameter of the distal epiphysis plotted against the total length of the bone. In all cases the size and robustness of the metapodials from Azokh match well the maximum values reached in the Iberian U. spelaeus samples and they are much larger and more robust than the metapodials of U. deningeri.
Metrical comparison of the metacarpals of Ursus from Azokh 1 with those from the Iberian populations of U. spelaeus (El Reguerillo cave, Patones-Madrid and Arrikrutz cave, Oñati-Guipuzcoa) and U. deningeri (Sima de los Huesos, Atapuerca-Burgos). Equiprobability (95%) and regression lines have been added. Transversal diameter of the distal epiphysis is plotted against bone length. Data after Torres (1989)
Metrical comparison of the metatarsals of Ursus from Azokh 1 and those from the Iberian populations of U. spelaeus (El Reguerillo cave, Patones-Madrid and Arrikrutz cave, Oñati-Guipuzcoa) and U. deningeri (Sima de los Huesos, Atapuerca-Burgos). Equiprobability (95%) and regression lines added. The transverse diameter of the distal epiphysis is plotted against bone length. Data after Torres (1989)
Dentition
The teeth form a mixed sample with elements from different Units and with different wear stages, which from a metrical point of view do not differ from Ursus spelaeus. In two second upper molars (III C46 7 Z = 173; I 54) the paracone is simply built, the protocone has a metaconule; the hypocone and metacone are duplicated and the talus is rounded.
The first (fourth) premolars (II C46 166 C = 193; II C46 294 Z = 104; IB) show a sharp protoconid with cutting anterior and posterior edges, an absent or poorly developed paraconid and a very small cusp (hypoconid) in the talonid region. In two first lower molars (II C46 294 Z = 104; II 86) the paraconid is simply built and with its oclusal face having a U. spelaeus-like arrangement: protoconid simple, metaconid duplicated, entoconid made of three cusplets of growing elevation towards the distal tip of the molar, hypoconid simple or more complex.
In the second lower molars (Vu D45 45 Z = 46, II C46 294 Z = 104, II 54), the protoconid consists of a single cusp, the metaconid duplicated (one case) or more complex, the entoconid highly variable, and the hypoconid simple. The two third lower molars (Vu D47 27 Z = 54; II C46 294 Z = 104) show a squared crown perimeter and lingual sinus well developed. Since the sample size is small, no conclusions can be drawn from these observations. The only remarkable aspect is the lower morphology of the fourth lower premolars that looks “archaic”. These morphologies were figured in the Spelaearctos deningeri subspecies of Baryshnikov (1998) but they also appear in low frequencies in large Ursus spelaeus samples.
Discussion
The bear remains from Azokh have been identified as U. spelaeus, matching well with those from Iberian localities that have been dated through amino acid racemization (AAR) to the upper part of the Middle Pleistocene (Torres et al. 2002). Similarly, Aliev (1969) identified the cave bear remains from Azokh as Ursus spelaeus, although other remains from the Caucasus have been identified as Spelaearctos deningeri kudarensis (Baryshnikov 1998, 2006; Doronichev 2000). However, today there is an almost general consensus to include the cave bear in the genus Ursus. Rohland et al. (2008) placed Spelaearctos deningeri kudarensis at the beginning of the MIS5 (120 ka) based on molecular chronology. This is confusing, since this date is much younger than the widely accepted last appearance of U. deningeri in west European localities, which are all of Early-Middle Pleistocene age): Petralona (Kurten and Poulianos 1977), Westbury (Andrews and Turner 1992), Sima de los Huesos (Torres and Cervera 1992; García et al. 1997), Santa Isabel cave (Torres et al. 2001), Cueto de la Lucia cave (Torres et al. 2006), L’Escale (Bonifay 1971, 1975a), Mosbach and Süssenborn (Soergel 1926), Hundsheim (Zapfe 1946), Cal Guardiola (Madurell-Malapeira et al. 2009).
Ursus deningeri has specific characters (Kurten and Poulianos 1977; Torres 1978; Rabeder et al. 2010), among others:
-
The ramus ascendensis of the mandible is tilted backwards in a characteristic way.
-
Bones and teeth are smaller than in Ursus spelaeus.
-
Limb and paw bones are more slender than in U. spelaeus.
-
Frequent, though erratic, presence of some of the first, second and third upper and lower premolars or their alveoli.
-
Frequently, but not in all the cases, the heel of the second upper molar shows an acute end.
-
The third lower molar is small and, in many cases, the crown perimeter is elliptical or almost circular.
-
In some cases the fourth lower premolar shows a simple architecture, the protoconid being the only cusp.
With the sole exception of the last one, these characters are absent in the Azokh Cave bear, but these more “carnivorous-like” premolars are present in 1% of the sample from the Iberian Peninsula (Torres 1989) compared with 14% of the Iberian sample of Ursus deningeri. We can conclude therefore that the Azokh bear can be placed in Ursus spelaeus.
Recent work based on fossil DNA (Rabeder et al. 2004) revealed a scenario that is more complex than expected, with three subspecies (U. spelaeus, U. s. ladinicus, U. s. eremus), while the new speleus-like species Ursus ingressus was also defined. Further DNA studies on Asian cave bears (Knapp et al. 2009) confirm differences between European cave bears (U. spelaeus and U. ingressus) and Asian cave bears (U. deningeri kudarensis) adding more confussion to the well known chronostratigraphical range of U. deningeri. Thus, the small morphological and metrical differences between the Azokh bears and typical U. spelaeus cannot be interpreted in the way of a “recent” U. deningeri representative, but we do not discard the possibility that they represent a local subspecies.
The presence of a Middle Pleistocene Ursus spelaeus matches very well with the interpretations of the first appearance of the species around 300 ka (Rabeder et al. 2004; Croitor and Brugal 2010), and with the numerical ages obtained through ESR and AAR dating of Azokh tooth samples (Murray et al. 2016). This also coincides with the ages obtained after systematic ESR and AAR dating of a large number of U. spelaeus localities that reveals that while most of them clustered in the Upper Pleistocene, two localities, El Reguerillo cave and Arrikrutz cave were much older: ca. 150–160 ka (upper part of the Middle Pleistocene; Torres et al. 2002). Cave bears remains from these two localities show a predominance of big sized bones and teeth.
New material
-
Unit Vu
-
Azokh 1, Unit Vu, D-45, 53 (z = 63, 7-8-08) – left mandible with P3-4 and M1, alveoles of P2 and Cx; P3: DAP = 5.8, DTa = 2.8, DTp = 3.4; P4: DAP = 7.0, DTa = 3.4, DTp = 4.0; M1: DAP = 17.1, DAPtrigonid = 9.1, DTa = 5.4, DTp = 7.9.
Description of the new material and taxonomic classification
The P3 and P4 (Fig. 6.6) are simple teeth with a main cusp from which anterior and posterior smooth crests descend. There are no cusps on the talonids. The crowns are short and high. Each tooth has two roots. The M1 is a carnassial with a trigonid with low cusps, the metaconid being well developed; the talonid is enlarged with four well developed cusps. From the protoconid backwards all cusps are heavily worn. The low trigonid on the carnassial and a very extended talonid points to Meles.
Discussion
Various species and subspecies of Meles have been named on the basis of fossils (e.g., Crégut-Bonnoure 1996). Wolsan (2001) noted that these species and subspecies fit within the ranges of variation of the living species, but refrained from formally synonymizing them until the problem is resolved about whether or not the living Asiatic badgers belong to a different species, called Meles anakuma. At present that species is not recognized as different from Meles meles (Wilson and Reeder 1993; Duff and Lawson 2004).
Material from Unit V was assigned to Meles meles, the living species of badger (Aliev 1969; Lioubine 2002; Rivals 2004). Likewise we assign the new material from Unit Vu to Meles meles. The badger appereared in Europe during the Late Pliocene with the species Meles thorali (Crégut-Bonnoure 1996), which is inseparable from the living species Meles meles (Wolsan 2001). A variety of species of the genus Meles are cited from the Early to the Late Pleistocene of north China, while Meles meles is cited from the Middle and Late Pleistocene (Xue and Zhang 1991). The species lives in wooded areas from western Europe to the Middle East and to Japan.
Material from Unit V was attributed to Martes cf. foina or Martes foina (Aliev 1969; Lioubine 2002; Rivals 2004), but the new collections do not include fossils that are attributable to this species. At present it lives in an area that extends from Europe to China. Excepting the larger species, the fossil record of the mustelids is not well known.
New material
-
Unit V
-
Azokh’03, uppermost platform, D-44, 10-8-03, 3 – left mandible with canine and P2-3: canine DAP = 4.9, DT ≥ 3.6; P2 DAP = 8.5, DTa = 2.5, DTp = 2.8; P3 DAP = 9.0, DTa = 2.6, DTp = 2.9.
-
Azokh’03, uppermost, D-45, rescue general finds – right mandible fragments with P4 and alveoles P3 and M1-3: P4 DAP = 9.5, DTa = 3.2, DTp = 3.7.
Description of the new material and taxonomic classification
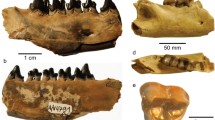
Both specimens seem to belong to the same individual. The mandible is gracile and shallow (Fig. 6.7). The canine is slender and relatively high, and the premolars are high and narrow. The P2 has a main cusp with anterior and posterior crests that are concave in side view. The P3 and P4 both have a cusp on the talonid. There are two alveoles for the P1 and one alveole for the M3. Size and morphology are similar to the recent and fossil Vulpes vulpes from l’Escale (Bonifay 1971).
Discussion
Material from Unit VI was assigned to Vulpes vulpes (Aliev 1969; Lioubine 2002; Rivals 2004). The new material shows this species to be present also in Unit II. Vulpes foxes were present already in the Pliocene. The red fox Vulpes vulpes is known in Europe from localities as old as Arago (Crégut-Bonnoure 1996). Vulpes vulpes is cited from the Late Pleistocene of northern China and Vulpes cf. vulgaris and Vulpes vulgaris from the Middle and Late Pleistocene, respectively (Xue and Zhang 1991). The latter species is considered to be synonymous with Vulpes vulpes (Wilson and Reeder 1993). At present the species occurs in an area extending from Europe to north Africa, northern Asia, the north of India and north America.
New material
-
Unit Vm
-
G-40, 6/9/02, G940 – right calacaneum: L = 39.6, Lu = 27.6, Ll = 14.5, DAPh = 12.4, DTh = 11.3, DAPn = 10.3, DTn = 7.2, DAPsf = 15.9, DTsf = 13.5.
Description of the new material
The calcaneum has the general morphology of a carnivore (Fig. 6.8). It is a little smaller and more gracile than that of Lynx spelaea, similar to that of Canis lupus, but much smaller. It is similar to those of Vulpes vulpes and Vulpes praeglacialis, but it is larger than several specimens attributed to these species (Bonifay 1971; Schmid and Garraux 1972; Dufour 1989).
Discussion
Material from Unit V was described as belonging to the jackal Canis aureus (Aliev 1969; Lioubine 2002; Rivals 2004). Canis aureus is of a size intermediate between C. lupus and Vulpes. It seems likely that the specimen described above belongs to this species. Morphological resemblances suggest a link between Canis aureus and the Late Pliocene Canis arnensis (Torre 1967; Crégut-Bonnoure 1996). The jackal is a living species in SE Europe, North Africa, the Middle East and south and central Asia.
New material
-
Unit II
-
AZUM’03, D46, 14 – left M1, talonid: DTp = 10.3. Figure 6.9.
-
Unit Vu
-
AZM Middle plat, cleaning, 26-07-05, right D4: DTp = 3.8. Figure 6.9.
-
Unit Vm
-
AZM’05/F38/1 – third phalanx: L = 15.8, DAPp = 10.1, DTp = 6.8. Figure 6.9.
Description of the new material and taxonomic classification
The talonid of the lower carnassial from Unit II has two major cusps (Fig. 6.9/1), as in Canis and unlike in Cuon and Lycaon. Size increase in European Canis is well illustrated by large numbers of measurements of the length of the lower carnassial (Van der Made 2010b, Fig. 4). Usually the maximum width of this tooth is given in the literature, but not the slightly smaller talonid width. As a consequence, the size trend in the talonid is illustrated here by fewer measurements (Fig. 6.9/2). The large size of the talonid of the M1 suggests that the material belongs to Canis lupus. A third phalanx (Fig. 6.9/4) and deciduous carnassial (Fig. 6.9/3) seem to belong to the same or a similar species.
1 Canis lupus from Azokh: AZUM’03, D46, 14 – talonid of left M1 (a–d occlusal, bucal, posterior, and lingual views). 2 Size increase of the width of the talonid (DTp) of the M1 in Canis. Localities in approximate stratigraphic order from old (bottom) to young (top): Mauer (SMNK), Mosbach (NMM), Atapuerca (LAUT, CENIEH), Neumark Nord (LVH), Azokh, Tutsvati (GSM), High Cave (GSM). 3 Canis cf. lupus from Azokh IV: AZM, middle plat, 26-7-05 -right D4 – lingual, occlusal and buccal views). 4 Canis cf. lupus from Azokh V: AZM’05, F38, 1 – third phalanx (a–d dorsal, side, plantar, and proximal views). Scale bars indicate 3 cm: left scale bar for M1 and right scale bar for the remaining photographs
Discussion
Material from Azokh V was assigned to Canis cf. lupus (Aliev 1969; Lioubine 2002; Rivals 2004). If the presence of that species in Unit V could be confirmed, this would be important for the biochronology of that unit, but the new material from Units IV and V is too poor; the new material that can be assigned to this species is from the younger unit Unit II.
At the end of the Pliocene, Canis dispersed from the New to the Old World. The first European species of the wolf lineage, Canis etruscus and Canis mosbachensis, were small, but they were replaced by Canis lupus, which may have evolved from the latter species or from a form close to it (Kahlke 1994). The wolf appeared initially with the somewhat larger subspecies Canis lupus lunellensis (in Lunel Viel, Heppenloch and TD10a; Bonifay 1971; Adam 1975; approximatly OIS 9-11) and later by the still larger Canis lupus lupus (Neumark Nord, Ehringsdorf, Chatillon St. Jean; OIS7). Canis lupus is cited from the middle and late Middle Pleistocene of China (Xue and Zhang 1991). The actual geographic distribution of the wolf extends from Europe and Asia to North America.
New material
-
Unit Vu
-
Middle plat., cleaning, 26-07-05 – left I3: DMD = 11.1, DLL = 11.1, Hli = 15.7, Hla = 17.0.
-
Unit Vm
-
Azokh, 28-7-05, plat middle, Unit V, z = 138-147, F-39, river sieving coarse – right mandible with canine and P2: Canine: DAP ≥ 15.4, DT ≥ 12.4; P2: DAP = 16.1, DTa = 9.5, DTp = 11.1, Hli = 9.8, Hla = 11.2.
Description of the new material and taxonomic classification
The mandible is massive (Fig. 6.10/1). The canine is stout and short; its tip is about level with the tip of the premolar and was probably not fully erupted. The diastema between canine and P2 is about 3.8 mm. The premolar is massive, as in the Hyaenidae, while in the Felidae it would be more elongate. It has a relatively low main cusp as in Crocuta and unlike in Hyaena, where the tip tends to be higher. No wear can be seen on this tooth, suggesting again that the individual was relatively young when it died. The I3 is very large and has a well developed lateral cusp (Fig. 6.10/2).
Discussion
Material from Unit V was assigned to Crocuta spelea (Aliev 1969; Lioubine 2002; Rivals 2004). Many authors consider this to be a subspecies of the living spotted hyena Crocuta crocuta (e.g., Crégut-Bonnoure 1996; García and Arsuaga 1999). During the earliest Pleistocene, the genus Crocuta was present in Africa and the Indian Subcontinent (De Vos et al. 1987; Turner 1990), The genus was present in Europe at about 1.4 Ma in Ubeidiyah and dispersed not later than at 0.8 Ma into western Europe (García and Arsuaga 1999), and the species Crocuta crocuta was present in the area long before the formation of Unit V at Azokh. There were different subspecies of C. spelaea, which may have stratigraphic significance (Crégut-Bonnoure 1996). The new material confirms the presence of Crocuta in Azokh, but it is insufficient for a subspecific assignment and a discussion of the biochoronological implications.
Felis chaus was cited from Unit V (Aliev 1969; Lioubine 2002; Rivals 2004). This species is not represented in the new collections. Felis chaus lives at present in an extensive area stretching from Egypt to the Middle East and to southern China and SE Asia. Though its vernacular name is jungle cat, it occurs in a variety of habitats, including dry environments.
Felis lynx was cited from Unit V (Aliev 1969; Lioubine 2002; Rivals 2004). The new collections do not include any lynx material. At present several species are recognised in the genus Lynx: the living Lynx pardina in the Iberian Peninsula and Lynx lynx in nothern Eurasia and the fossil Lynx pardina spelea of the late Middle and Late Pleistocene of large parts of Europe and a still older form called Lynx issiodorensis (Argant 1996). On the one hand, it has been suggested that material from Mauer and Soleilhac, that is usually assigned to the latter species, might in fact belong to L. pardina spelaea (Argant 1996), while on the other hand, it has been argued that the species Lynx issiodorensis should better be placed in the genus Caracal (Morales et al. 2003). The material from Azokh might be expected to belong to Lynx pardina spelaea, but we cannot confirm this.
New material
-
Unit III
-
Azokh uppermost, 21-8-03, D-46, in sample for palynology, z = 162 – left I3: DLL = 8.1, DT = 5.4.
-
Unit II
-
Azokh 1, Unit II, 3-8-08, C45, 21 (z = 123) – left humerus: DAPd = 37.2, DTd = 61.9, DTdf = 41.1, R1-4 = 28.4-18.3-23.9-21.7.
-
Azokh uppermost, 12-8-03, C-45, rescue, 19 (z = 134) – right calcaneum: L = 72.8, Lu = 51.7, Ll = 24.4, DAPh ≥ 23.7, DTh > 17.2, DAPn = 23.5, DTn ≥ 14.7, DAPsf = 29.1, DTsf = 29.3.
-
?Azokh uppermost, 14-8-03, D-46, 11 (z = 100) – first phalanx, distal part: DAPd = 9.6, DTd = 12.2, L ≫ 38.
Description of the new material and taxonomic classification
The distal humerus has a supracondylar foramen (Fig. 6.11), which is common in Felidae, but lacking in Hyaenidae, Canidae and Ursidae. The distal articulation is wide and with a relatively small radius of curvature. This is unlike in Hyaenidae and Canidae. The specimen is much smaller than its homologue of a recent or fossil Panthera leo (LAUT; Dufour 1989), smaller also than in Panthera onca gombaszoegensis (Hemmer 2001), but larger than those of recent and fossil Lynx (Hemmer 2001). The calcaneum is intermediate in size between those of a wolf and a lion.
Discussion
Panthera pardus was described or cited from Azokh Unit V (Aliev 1969; Lioubine 2002; Rivals 2004). The new finds show this species to be present in Units II and III as well. The leopard may have originated in Africa and it dispersed into Europe around 0.5–0.6 Ma ago (e.g., its presence in Mauer), where it survived until close to the end of the Pleistocene (Crégut-Bonnoure 1996). In China it is cited from the Early to Late Pleistocene (Xue and Zhang 1991).
New material
-
Unit V
-
?Azokh 1, Unit V, 21-8-09, H-41, 42 (z = 854) – fragment of a cheek tooth.
Description of the new material and taxonomic classification
The tooth fragment belonged to a tooth with a crown height of well over 45 mm. It is a small fragment consisting of enamel, dentine and cementum. The enamel is folded in a complex way as is common in the cheek teeth of Equus, but it is not possile to see which part it represents, and it is not possible to be sure of its species designation: for example it may belong to E. hydruntinus.
Discussion
Material from Unit VI was assigned to Equus caballus (Aliev 1969; Rivals 2004) but later the assignment seems to have been changed into Equus suessenbornensis (Lioubine 2002).
During the Middle and Late Pleistocene, there were two groups of equids in western Europe. One group included the relatively small and gracile “stenonid” species, with Equus altidens in the early Middle and Equus hydruntinus in the late Middle and Late Pleistocene. (Stenonid/caballoid refers to the shape of the lingua flexid, separating the genus in two groups, following Forsten 1992). The other group was made up of predominantly large forms with “caballoid” morphology. Some authors, like Eisenmann (1991) recognized many caballoid species, while others like Forsten (1988) recognized fewer species (E. mosbachensis, E. germanicus, E. caballus); still others, like Azzaroli (1990), recognized just the single species E. caballus. The very large stenonid Equus suessenbornensis may have given rise to the caballoid horses, which further declined in size. The transition must have occurred around 600 ka. At present the name Equus caballus is restricted to the domestic horse, while the wild form, including przewalski’s horse, is referred to as Equus ferus.
A second phalanx from Unit V in the collections in Baku is larger than a specimen from Unit 1 that is here assigned to Equus caballus (see below). It is also larger than the second phalanges from the Würmian of Villa Seckendorf, which were assigned to E. germanicus (Forsten and Ziegler 1995), larger than the phalanges from Taubach (Musil 1977) and Atapuerca TD10 (LAUT), but it is close in size and robusticity to two phalanges of E. suessenbornensis from Süssenborn (Musil 1969). The large size of this equid probably gave rise to the determination as E. suessenbornensis, but the material might well belong to a caballoid horse of the size of the Mosbach horse. In view of the likely age of Unit V, we favor Aliev’s (1969) earlier assignment, but with updated nomenclature: Equus cf. ferus.
New material
-
Unit I
-
Azokh 1, Unit I, subunit I, 14-7-2007, C-50, 4 (z = 116) – left second phalanx: L = 49.0, Ldors = 37.1, DTmini = 40.3, DTp = 47.5, DAPp = 30.7, DTd > 40.4, DAPd ≥ 25.2.
Description of the new material and taxonomic classification
The second phalanx from Unit I is of the common equid morphology (Fig. 6.12/1). It is relatively large and robust and it is larger than its homologue in E. hydruntinus, but similar in size to those of the wild E. ferus and its domestic descendant E. caballus. We are not able to distinguish between the wild and domestic species, but since Unit 1 is very recent, the phalanx probably represents Equus caballus.
Aliev (1969) assigned material from Units VI, V and III to Equus hydruntinus. This was a small and gracile species, probably closely related (or ancestral) to the living Equus hemionus, which was widespread during the late Middle and Late Pleistocene.
New material
-
Unit I
-
Azokh 1, Unit I, E-51, 49 (z = 46, 4-8-06) – right navicular: DT = 36.8.
Description of the new material and taxonomic classification
The navicular of equid morphology is very small (Fig. 6.12/2). The surface of the bone is smooth. The posterior part is broken, showing that the compact bone is very thin at this place. This might indicate that the individual was not fully adult, although the smooth surface of the bone suggests that the individual was nearly adult and may have attained more or less its adult size.
Discussion
The fossil bone may have belonged to an individual that was not fully adult, but neither was it very young, and its small size thus suggests a small species rather than a small individual of a large species. Equus hydruntinus is a small and gracile species, probably closely related (or ancestral) to the living Equus hemionus. Alternatively (and depending on its geological age), the bone may have belonged to the domestic donkey Equus asinus, which is a descendant of the african wild ass Equus africanus, and which was introduced in Eurasia during the Holocene. Since the material from Unit I is Holocene, it probably is a domestic donkey.
New material
-
Unit Vm
-
Azokh 1, Unit V, 27/7/09, D-15, 1 – left mandible fragment with M2-3; M2: DTp = 37.4; M3: DAP = 58.3, DAPb = 53.7, DTa > 28.9, DTp = 31.6, H = 29.6.
Description of the new material and taxonomic classification
In lingual view, the posterior valley of the third lower molar (Fig. 6.13/3) is U-shaped or slightly parabolus shaped. This is typical for Stephanorhinus kirchbergensis, while it is clearly V-shaped in Stephanorhinus hemitoechus (Van der Made 2000) and more variable and intermediate in Stephanorhinus hundsheimensis. The teeth have finely crenelated enamel, unlike in Coelodonta or S. hemitoechus, where the crenelation is much more coarse. The transverse diameter and crown height are in the range of S. kirchbergensis, while the latter variable is larger than in S. hundsheimensis (Fig. 6.13/1).
1 The third lower molar (M3) in Rhinocerotidae. Bivariate diagram of the width of the posterior lobe (DTp) versus the height (H) at the trigonid-talonid junction: Coelodonta from Steinheim (SMNS), Maastricht-Belvedère (NMMa), Backleben (IQW), Heldrungen (IQW), Eich (NMM); Stephanorhinus hundsheimensis from Voigtstedt (IQW), Süssenborn (IQW), Soleilhac (MCP), Mauer (SMNK); Stephanorhinus hemitoechus from Steiheim (SMNS), Taubach (IQW), Eich/Gimbsheim (NMM), Gimbsheim (NMM); Stephanorhinus kirchbergensis from Bilzingsleben (FBFSUJ), Mosbach (NMM, SMNS), Ehringsdorf (IQW), Taubach (IQW), Eich (NMM), Gimbsheim (NMM). 2 Material from Azokh 1, Unit V, 2-8-2009, I-4, 15 – left M3 of S. hemitoechus (occlusal, buccal and lingual views). 3 Azokh 1, Unit V, 27/7/09, D-15, 1 – left M2-3 of S. kirchbergensis from Unit V (buccal, occlusal and lingual views). The scale bar represents 5 cm. As can be seen the M3 of S. hemitoechus from Unit Vm is worn at the place where the height is measured; the value for H of this specimen is too low, which is indicated by an arrow in the bivariate diagram
Discussion
Aliev (1969) and Rivals (2004) assigned all rhinoceros material from Azokh to Dicerorhinus mercki, while Lioubine (2002), following Guérin and Barychnikov (1987), cited “Dicerorhinus etruscus brachycephalus (défenition C. Guérin)” from Unit VI. Most specialists now apply the names Stephanorhinus kirchbergensis and Stephanorhinus hundsheimensis, respectively, for these taxa (Fortelius et al. 1993).
A third molar (MUB 4/227) from Unit VI has a similar morphology, size and degree of hypsodonty as the specimen described above, but most other specimens in the old collections seem to belong to S. hemitoechus (see below).
Stephanorhinus kirchbergensis appears first in localities like Mosbach, with an age of 500–600 ka (Van der Made 2000, 2010a; Van der Made and Grube 2010). It is an “interglacial species”, dispersing during the interglacials from an unkown area into Europe. Though material from many localities in Spain was formerly assigned to S. mercki, in a revision by Cerdeño (1990) all this material was assigned to to S. hemitoechus. Dicerorhinus mercki (S. kirchbergensis) is cited from Zhoukoudian (Choukoutien) and other localities in China, suggesting a possible source area for the interglacial dispersals of that species to Europe (e.g., Xue and Zhang 1991). However, the material (IVPP; ZSM) is not completely identical and others assign it to Stephanorhinus choukoutienensis (or Dicerorhinus choukoutienensis). Stephanorhinus kirchbergensis was still abundant during the Eemian, but went extinct during a later part of the Late Pleistocene.
New material
-
Unit Vu
-
? – Azokh upper, 15/09/02, D-43, 10 (z = 72) – nasalia.
-
Unit Vm
-
Azokh 1, unit V, 2-8-2009, I-4, 15 (z = 251) – left M3: DAP = 52.9, DAPb = 51.0, DTa ≥ 31.3, DTp = 28.2, H > 27.4.
-
Akokh 1, Unit V, 4-8-2009, I-42, 41 (z = 847) – fragment of a left upper first or second molar, buccal wall: DAP = 46.5.
-
Azokh 1, Unit V, 26-7-2009, E-40, 7 (z = 861) – left Mc V: DAPp = 33.2, DTp = 24.0, L > 29.1.
Description of the new material and taxonomic classification
The M3 (Fig. 6.13/2) is moderately worn and has a well developed anterior contact facet, but no posterior facet. In lingual view, it has clear v-shaped lingual valleys, which is typical for Stephanorhinus hemitoechus. The enamel is crenelated as in that species, but not as strongly as in Coelodonta, and there is deposition of cementum in the valleys. The place where the crown height is measured is slightly worn out, so the value for H in Fig. 6.13/1 is a minimum value (indicated by the arrow in this figure). Despite dental wear, the crown is still high and must have been higher than in S. hundsheimensis. The tooth is smaller than in S. kirchbergensis and the same is the case for the foot bones (Fig. 6.14).
Stephanorhinus post cranial elements. 1 Bivariate diagram of the length (L) versus the distal width (at the articulation DTdf) of the third metacarpal (Mc III) of Stephanorhinus hundsheimensis from Untermassfeld (IQW), Soleilhac (MCP), Hundsheim (IPUW), Mauer (SMNK); S. hemitoechus from Bilzingsleben (FBFSUJ), Cova del Gegant (MNCN, cast), and Unit V (MUB); Stephanorhinus kirchbergensis from Bilzingsleben (FBFSUJ). 2 Bivariate diagram of the length (L) versus the distal width (DTd) of the second metacarpal (Mc II) of Stephanorhinus hundsheimensis from Soleilhac (MCP); S. kirchbergensis from Bilzingsleben (FBFSUJ) and S. hemitoechus from Unit V (MUB). 3 Material from Unit V: MUB 6/553 – right Mc II of S. hemitoechus (a–c proximal, axial and anterior views). 4 Azokh 1, Unit V, 26/7/09, E-40, 7 – left fifth metacarpal of Stephanorhinus hemitoechus (a–e lateral, posterior, medial, anterior, and proximal views). The scale bars represent 5 cm for the Mc II and 3 cm for the Mc V, respectively
A symetrical bone fragment with a more or less T-shaped transverse section from Unit Vu seems to represent the nasals of a rhino, the vertical bone being the ossified nasal septum, and the upper surface, which curves down at the sides, being the nasals. The preserved part is about 11 cm long and 6 cm wide. There is no suture visible between the nasals or between nasals and septum. All sides of the bone are broken before reaching a natural border. The upper surface is smooth. The minimum preserved thickness of the septum is 5.8 mm, while the minium thickness of the nasals near their presumed edge is about 1 mm.
Discussion
Nasals supported by an ossified septum occur in Stephanorhinus and Coelodonta. The living genera of Rhinocerotidae do not have ossified nasal septa. Stephanorhinus and Coelodonta tend to have thick nasals with a well developed “cauliflower structure” marking the spot where the horns originate. However, such a structure is not always well developed (e.g., Loose 1975, Pl. 4, Fig. 3). Azzaroli (1962) interpreted skulls with narrower nasals and a smoother surface as females, and it is also likely that the cauliflower structure is less developed in juveniles. The nasals described here are insufficient for a determination at the species level. The material of the old collections include a fragment of nasals, similar to the one described here, but with a moderate “cauliflower structure”.
Most specimens in the old collections have Stephanorhinus hemitoechus morphology and size. Most of the postcranial and dental specimens that can be measured are small, in particular the premolars which are too small for attribution to S. kirchbergensis. Stephanorhinus hundsheimensis is a species with large premolars, and the very small premolars from Azokh point to a species with reduced premolars like Stephanorhinus hemitoechus.
Stephanorhinus hemitoechus is assumed to have evolved from S. etruscus in an area outside western Europe and to have dispersed into the latter area around 450 ka ago, where it may have survived until the end of the Pleistocene (Guérin 1980; Fortelius et al. 1993; Van der Made 2000, 2010a; Van der Made and Grube 2010).
New material
-
Unit Vm
-
AZM’03, small finds (27-08-2003, Plat middle, Unit V, small finds) – left second phalanx: DAPp ≥ 16.2, DTp ≥ 16.6, L ~ 31.6, DAPd ~ 20.3, DTd–.
-
Unit II
-
Azokh uppermost, 11-8-03, D45, 19 (z = 133) – left Cm: Li = 23.8, La = 18.1, Po = 17.0.
-
Azokh 1, Unit II, 2-8-08, C-46, 269 (z = 99) – left Cf: DAP = 20.5, DT = 14.1.
-
Unit I
-
Azokh 1, Unit I, F-50, 3-8-06, 17 (z = 19) – juvenile right scapula: L = 41.6, DAPmax = 26.5, DAPn = 6.9, DTn = 3.9.
-
Azokh, 29-7-05, Unit II, square “passage into cave”, no surface find – fragment of the right side of the skull with occiput, and part of zygomatic arc.
Description of the new material and taxonomic classification
The Cm has a triangular section. Suid male lower canines are assigned to two types: the “scrofic section” with a posterior side that is wide, generally wider than the labial side, and the “verrucosic section” with a narrower posterior side. In the specimen from Azokh the posterior side is wide, but not wider than the labial side. The section is “scrofic” and such a section occurs in the genus Sus only in Sus scrofa and the rare and very small Sus salvanius, which is restricted to some area in Asia. The Cf (Fig. 6.15) is large.
Two fragments belong to a second phalanx. The morphology cannot be well seen because of the poor preservation of the specimen. If this is a lateral phalanx (digit II or V) it would be extremely large, but the size is acceptable for a central phalanx (III or IV) of a smaller representative of the species.
The skull fragment from Unit I has a very obtuse angle between the axis of the posterior side and dorsal side. This angle tends to be sharp in wild boars, resulting in an overhanging occiput, while in domestic pigs and juveniles, the angle tends to be obtuse and the occipital condyles are situated more posteriorly than the occiput. Between the brain and occiputal crest there is spongious bone instead of sinuses, another feature that is common in domestic pigs.
A small scapula of a very young individual has a triangular shape, as is common in Artiodactyla, and it has the spine in the middle of the blade, which is common in Suidae, but not near the anterior edge of the bone, as is common in ruminants.
Discussion
Suid material in the University of Baku comes from Units VI, V and III and that from Units V and III was assigned to Sus scrofa (Aliev 1969; Lioubine 2002; Rivals 2004). This material consists largely of postcrania and does not include well preserved elements that show clear Sus scrofa morphologies, such as the male upper and lower canines and upper fourth premolar. Nevertheless the material belongs most probably to this species, since no other species is known from the Middle Pleistocene of western Eurasia. The material from Unit I might belong to a domestic pig, but this cannot be confirmed.
Sus scrofa must have originated in eastern Eurasia and dispersed into Europe just before the Brunhes – Matuyama transition (it is present in the latest Early Pleistocene of Atapuerca TD6; Van der Made 1999). The early forms were larger than living Sus scrofa scrofa, but at later sites such as Mosbach and Mauer (about 0.5–0.6 Ma) and younger faunas, they are smaller. In Taubach (OIS5) they are large again, while in later faunas they are again smaller. At present there are slight geographic differences in size between Spain and Germany, while living wild boars of Israel and Georgia are larger. The female canine from Unit II must belong to such a large form. Since there are not many data on the size of this species in general, and from the Caucasus area in particular, these observations cannot be interpreted with reference to the age of the locality.
New material
-
Unit Vm
-
Middle plat., cleaning 26-7-05 – right axial sesamoid behind first phalanx: DAP = 6.3, L = 10.8, DT = 4.8.
-
Unit II
-
Azokh, 18-8-06, Unit II, G-48, 202 – right astragalus: Lext ≥ 34.6.
Description of the new material and comparison
The astraglus is damaged. Its length (Lext ≥ 34.6) is comparable to the length of the astragali of Capreolus priscus and Capreolus suessenbornensis (Fig. 6.17/5). The sesamoid has the typical artiodactyl shape. Its DAP or dorso-plantar diameter is small, so it is an axial and not an abaxial sesamoid. Apart from the shape of the dorsal facet, the transverse section is nearly symmetrical, with rounded corners at the latero-plantar and medio-plantar sides. In Bovidae like Capra, the plantar side is markedly a-symmetrical in such a way that the two sesamoids form a gulley at the plantar side. The size of the specimen is smaller than in Dama, but fits Capreolus.
Discussion
Material from Units V and III was assigned to Capreolus capreolus (Aliev 1969; Lioubine 2002; Rivals 2004). The new collections include some poor specimens from Units V and II that are compatible with Capreolus, but this taxon is well represented in the old collections from Units VI, V and III in Baku.
Roedeer of the genus Procapreolus were common in Europe, but disappeared after about 3.4 Ma (Heintz 1970; Kahlke 2001). Capreolus evolved from that genus and first appeared with the species Capreolus constantini in Udunga (Siberia) and other localities in Moldavia and Slovakia with ages as old as 3.5 Ma (Vislobokova et al. 1995). The earliest West European record attributed to Capreolus is C. cusanoides from Untermassfeld (Kahlke 2001), and with an age of about 1 Ma this species retains primitive characters present in Procapreolus but lost already in Capreolus constantini, so that this species seems to be an evolutionary side branch. The first morphologically clear European Capreolus is from the early Middle Miocene (Voigtstedt, Süssenborn, etc.). Pfeiffer (1998) recognized three species: Capreolus suessenbornensis, which is replaced by Capreolus priscus, of similar size but of different leg proportions, while the living species Capreolus capreolus is smaller. This size decrease must have occurred during the Late Pleistocene in Europe as well as in the Middle East (Fig. 6.16/1).
Left MUB 5/277 – left antler of Capreolus fom Unit III. The scale bar represents 5 cm. Right The variation in size of Capreolus as indicated by the width of the first lobe (DTa) of the M3. The localities are ordered in approximate stratigraphic order: Udunga (about 3.5 Ma; PIN, GIN), Untermassfeld (IQW), Stránska Skálá (MMB), Voigtstedt (IQW), West Runton (IQW), Süssenborn (IQW), Mosbach (NMM), Mauer (SMNS), Miesenheim (FASMN), Bilzingsleben (FBFSUJ), Orgnac 3 (MPT), Steinheim (SMNS), Unit III (MUB), Ehringsdorf (IQW), Taubach (IQW), Qafzeh (IPH), Congosto (MNCN), Valdegoba (UBU), Abric Romaní (LAUT), Zhoukoudian Upper Cave (IVPP), Recent material attributed to Capreolus capreolus and Capreolus pygargus in the GSM, Recent Capreolus capreolus from Germany (FASMN) and Spain (MNCN)
The living roe deer were formerly considered to belong to two or three subspecies (e.g., Whitehead 1993), but the current view is that they belong to two separate species C. capreolus (Europe and Middle East) and C. pygargus (Asia; Duff and Lawson 2004). The latter species is larger, has relatively larger antlers, and differs in the morphology of the antler base. Some authors included the populations from the Caucasus in the species or subspecies “capreolus”, while others included it in “pygargus”. The large recent material in the GSM in Tbilisi, attributed to this species, either represents C. pygargus, or a larger subspecies of C. capreolus. In either case, the material in the GSM seems to belong to a taxon that was different from the roe deer of most of Europe and Israel since the Late Pleistocene, at least.
The material from Unit VI is very poor, but the material from Unit III is larger than Capreolus capreolus (at least the west European form; Fig. 6.16/1), and the phalanges from Unit V are even larger than in C. priscus and C. suessenbornensis (Fig. 6.17/1, 2). This suggests, that the species from Azokh was very large and possibly was on a different lineage from the west European forms; possibly it was on a lineage leading to C. pygargus. Some antler remains are not as large as they may be in the living species C. pygargus (Fig. 6.16/2), and possibly the relatively large antlers in that species are relatively recent.
Capreolus and Saiga. 1, 2 Bivariate plots of the distal transverse diametre (DTd) versus the distal antero-posterior diametre (DAPd) and of the length (L) versus the DAPd of the first phalanx of Capreolus and Saiga: C. suessenbornensis from Süssenborn (IQW), Voigtstedt (IQW) and Koneprusy (NMP); C. priscus from Miesenheim (FASMN), Ehringsdorf (IQW) and Grotte des Cèdres (MRA), C. capreolus from Can Rubau (CIAG) and Cueva Morín (MNCN), Capreolus cf. pygargus from Unit V and III (MUB); the eight phalanges of of one individual of Gazella cuvieri (MNCN); an anterior and posterior phalanx of recent S. tatarica (NNML) and Saiga from Unit II. 3 Azokh, 18-8-06, Unit II, F-48, 94 – right first phalanx of Saiga from Unit II (a–f distal, dorsal, abaxial, plantar, axial, and proximal views). 4 MUB 471 – left astragalus of Capreolus from Unit V (a–f proximal, posterior, medial, anterior, laterla, and distal views). 5 Bivariate diagram of the lateral length (Lext) and distal width (DTd) of the astragalus of the deer and small bovids from Azokh (MUB), compared to Capreolus suessenbornensis and C. priscus (provenance of data as above) and Capreolus capreolus from Can Rubau and Spain (recent, MNCN). The scale bar represents 3 cm
New material
-
Unit VI
-
Found below the collumn of sediment, 13/09/02, VI – right D2: DAP = 14.4, DAPb = 12.8, DTa = 8.6, DTp = 9.9.
-
Unit Vm
-
Azokh middle, 6/09/02, G-41, general finds – tip of tine of an antler: length of the fragment about 5 cm, diameters at the base of the fragment 13.9 × 11.4.
-
C-43, 12-8-03, general find Unit III?, northern wall – fragment of branch of an antler (brow tine?): length of the fragment >93, width 29.0.
-
6-9-2002, plat middle, Unit V, z = 112, F41, 2 – fragment of tine or beam of an antler.
-
Azokh, 15-8-03, E-40, middle platform, Unit V, 3 (z = 122) – left humerus, distal part: DTd ≥ 41.1, DTdf = 37.3, R1 = 31.1, R2 = 23.3, R3 = 25.7, R4 = 17.7, R5 = 19.0.
-
AZUM’02, F40, 3 – fragment shaft of metatarsal: DTmini.18.4.
-
Azokh Cave, F42, split sample – left ulnar: DAP = 21.7, DT = 12.2, H = 24.2, Ha = 19.3.
-
14-09-02, plat upper, E-44, gen. finds – various finds, including a right I1: DT = 9.0, DMD = 7.0, DLL = 4.9, DTroot = 4.0, DLLroot = 4.9, Hli > 9.0.
-
Azokh 1, Unit V, 27-7-2009, I-42, 11 (z = 827) – right D4: DAPo = 17.1, DAPb = 15.2, DTa = 15.3, DTp = 14.9.
-
Azokh 1, Unit V, 1-8-2009, I-42, 26 (z = 844) – right D2: DAPo = 14.3, DAPb = 14.1, DTa = 7.8, DTp = 9.7.
-
Azokh 1, Unit V, 28-7-2009, I-42, 6 (z = 844) – left M2: DAPo = 21.8, DAPb = 19.9, DTa = 21.7, DTp = 21.4.
-
Azokh 1, Unit V, 25-7-2009, I-42, 5 (z = 825) – right M3: DAPo > 22.5, DAPb > 21.2, DTa = –, DTp = 22.4.
-
Azokh 1, Unit V, 4-8-2009, I-42, 40 (z = 848) – left M1: DAPo = 19.5, DAPb = 17.2, DTa = 18.2, DTp = 17.9.
-
Azokh 1, Unit V, 1-8-2009, I-42, 25 (z = 844) – left D4: DAPo = 16.9, DAPb = 14.0, DTa = 14.9, DTp = 14.8.
-
Azokh 1, Unit V, 25-7-2009, I-41, 2 (z = 857) – fragment of Mx (protocone of left M2?).
-
Azokh 1, Unit V, 28-7-2009, I-41, 4 (z = 839) – right I3: DT = 5.8, DMD = 5.0, DLL = 6.4; root: DT = 3.6, DLL = 5.2.
-
Unit V/VI
-
Azokh upper, 16/9/02, E-44, 6 (Z = 100) – left distal tibia: DAPd = 29.0, DTd = 34.9, DTfast = 24.0.
Description of the new material
Bones and teeth (Fig. 6.18/1–7) that are slightly smaller than those assigned to Cervus elaphus tend to have characters described by Lister (1996) as typical for Dama. For instance, a distal tibia has characters 3 and 4 developed as in Dama (Lister 1996). Though smaller than their homologues in Cervus elaphus, the Azokh bones and dental remains tend to be large for Dama and are on average larger than in any Dama dama and most Dama clactoniana.
Cheek tooth morphology in Dama aff. peloponesiaca from Unit V (figures 1–7) and Cervus elaphus from Unit Vm (8) and from Unit II (9). 1 Azokh 1, Unit V, 28-7-2009, I-42, 6 – left M3 (a, b buccal and occlusal views). 2 Azokh 1, Unit V, 27-7-2009, I-42, 11 – right D4 (a, b buccal and occlusal views). 3 Azokh 1, Unit V, 1-8-2009, I-42, 26 – right D2 (a, b buccal and occlusal views). 4 Azokh 1, Unit V, 1-8-2009, I-42, 25 – left D4 (a, b buccal and occlusal views). 5 Azokh 1, Unit V, 4-8-2009, I-42, 40 – left M1 (a, b buccal and occlusal views). 6 Azokh 1, Unit V, 25-7-2009, I-42, 5 – right M2 (a, b buccal and occlusal views). 7 MUB 6/234 (=6/253) – right P2-M1 (a–c lingual, occlusal, and buccal views). 8 Azokh 1, Unit V, E-44, 21 – right P4 (a–c occlusal, lingual, and buccal views). 9 Azokh 1, Unit II, N-49, 12 – right P4 (a–c occlusal, anterior and buccal views). The left scale bar applies to figures 1–7 and the right one to figures 8 and 9
Discussion
Material from Azokh was assigned to Cervus (Dama) cf. mesopotamica (Aliev 1969; Lioubine 2002; Rivals 2004). The new material broadly confirms the presence of Dama, but the old collections in Baku are much more abundant.
Basal parts of the antlers from Unit VI (Fig. 6.19/1) and Unit V (Fig. 6.20/2) have the first bifurcation (between brow tine-main beam) higher above the burr than in Dama dama, Dama mesopotamica and Dama clactoniana (Fig. 6.20). This bifurcation (as well as the second one) became progressively lower with time in the Dama-like deer, and in Dama mesopotamica it is particularly low and the brow tine is extremely short.
Antlers of Dama aff. peloponesiaca (1, 3, 6-7), Dama sp. (2) and Cervus elaphus (4-5) from Azokh. 1 MUB 1/206 – right antler from Unit Vu (a, b lateral and anterior views). 2 Azokh 1, Unit II, C-46, 327 – fragment of the palmation of an antler from Unit II (a–c distal and medial views, section). 3 MUB 7/839 – left (?) antler fragment from Unit III (a, b distal section and lateral view). 4 MUB 6/95 – crown of a left (?) antler from Unit V (a, b distal and medial views). 5 MUB 6/158 – close up of the surface of a fracture at the crown of an antler from Unit V; in the left upper corner the outer surface of the antler can be seen. 6 MUB 4/406 – fragment of the palmation; from Unit V. 7 MUB 6/623 – fragment of the palmation of a left (?) antler from Unit V (lateral view). The scale bar represents 4 cm for figures 2, 3 and 6, 7, and it represents 6 cm for figures 1 and 4; figure 5 is not to scale
The morphology of the basal part of the antler in the Dama-like deer. The variation in the height above the burr of the bifurcation of brow tine and main beam, expressed as the index 100× Hext/DAPb, in the Dama-like deer as shown in the picture: MUB 6/626 left shed antler of Dama aff. peloponesiaca from Azokh 1 Unit V (lateral view). The scale bar represents 5 cm. The localities in the graph are ordered in approximate stratigraphic order: Montopoli (IGF), Ponte a Elsa (IGF), Senèze (IQW), La Quercia (IGF), Tegelen (NNML, TMH, NMMa), Olivola (IGF), Dmanisi (GSM), Matasino (IGF), Valdarno sup. (IGF), Casa Frata (IGF), East Runton (NHM), Mundesley (NHM), Lachar (MNCN), Ubeidiyah (HUJ), Selvela (IGF), Venta Micena (IPS), Val di Chiana (IGF), Taman (PIN), Vallonnet (MPRM), Untermassfeld (IQW), Atapuerca TDinf (CENIEH), West Runton (NHM), Tiraspol (PIN), Soleilhac (MCP), Mosbach (NMM), Bacton (NHM), Megalopolis (NCUA, BGR), Arago (MPT), Bilzingsleben (FBFSUJ), Petralona (AUT), Atapuerca TD10+TG (CENIEH), Azokh (MUB), Clacton (NHM), Swanscombe (NHM), Neumark Nord (FBFSUJ, presenty kept in LVH), Lehringen (HMV), Pinilla del Valle (UCM), Kebara (HUJ), Recent Dama dama (EDB), Recent Dama mesopotamica (HUJ)
A specimen from Unit Vm (Fig. 6.19/7) consists of a large part of the palmation, which was wide and probably curved anteriorly as in Dama dama (the concave border of the left hand side of the photograph would then be the anterior border of the palmation). This is unlike Dama clactoniana and Dama mesopotamica. The oldest known palmate Dama is Dama clactoniana, appearing about 550 ka ago. Both Dama mesopotamica and Dama dama have more reduced brow tines, but this is especially so in the former. While Dama dama has a palmation that is better developed than in D. clactoniana, in D. mesopotamica it is like in the latter species, or, perhaps, even less developed. The material from Units VI and V does not seem to belong to any of these three species.
Previous to these three species, there were several “Dama-like deer”, which have broadly similar size and morphology, but which lack a palmation. Some authors place them in Dama (Azzaroli 1953; Van der Made 1996, 1999b, 2001; Pfeiffer 1999), but others assign them to different genera such as Pseudodama, Euraxis, Axis, Rusa, Metacervocerus and Cervus (s.l.) (Azzaroli 1992; Di Stefano and Petronio 1998, 2002; Kahlke 2001; Croitor 2006).
Teilhard de Chardin and Trassaert (1937) described Dama sericus from China. It has a palmation that is different from that of Dama dama, Dama mesopotamica and Dama clactoniana and has a first bifurcation that is much higher. Unfortunately it is not possible to compare these palmations to those of Dama peloponesiaca, which will be discussed below, because only fragments are known of the latter. Nor is it possible to compare bones or teeth, since these were not described by Teilhard de Chardin and Trassaert, who indicated the age as Plio-Pleistocene, probably Zone III or Villafranchian. Dama sericus (or better Dama serica?) was considered to be related to the Mio-Pliocene genus Cervocerus (Qiu 1979). If this is the case, this species is not related and is separated by time and distance from Dama or “Dama-like deer”.
A species which is not often discussed in the literature on Cervidae is Dama peloponesiaca. Sickenberg (1976) based the new name “Cervus (s. l.) peloponesiacus” on material from Megalopolis. There are older collections in the University of Athens. These collections include flattened tines, which suggest that they originated from a palmation. Because of the presence of a palmation and of other morphological similarities in antlers, teeth and bones, this species is here included in Dama, though the position of the first bifurcation is variable and in many cases is higher than in any Dama dama and Dama clactoniana (Fig. 6.20/1).
Dama peloponesiaca seems to be older than Dama mesopotamica, but its age is not exactly known. Sickenberg (1976) described material from various fossiliferous sites in the basin, but he treated it as if representing one fauna, including Praemegaceros verticornis and Bubalus marathousae. Bubalus is known from a number of localities in Germany, which are either OIS5 or OIS9 (Von Koenigswald 1986; Van der Made 2005b). The giant deer Preaemegaceros verticornis (or Megaceroides or Megaloceros solilhacus) is considered to be a “Cromerian” form, but still occurred in Atapuerca Galeria TG10, which might be as late as 300–400 ka (Berger et al. 2008). If the material from Megalopolis represents more or less one age, this age might be 300 ka (if the presence of Bubalus is believed to be coeval with the OIS9 dispersal of that genus), or about 400 ka (if a very young occurrence of the giant deer is not favored). In any case, it seems that Dama peloponesiaca is a side branch of the Dama lineage in the south eastern part of its area of distribution, similar to Dama mesopotamica in this respect, but earlier.
The material from Unit V and VI is similar in several characters to that of Megalopolis, but is clearly larger (Figs. 6.19 and 6.20). The deer from Azokh and Megalopolis share the combination of a primitive character in their high first bifurcation and a derived character of palmation, which is unique in Dama-like deer, but which differs in size. The size difference might be due to geographic or temporal separation, although the latter is perhaps more likely. These forms seem to belong to a branch or lineage that may have separated from the main west Eurasian Dama-lineage because of isolation in SE Europe or the Middle East. This may have happened before Dama mesopotamica separated from the main Dama lineage, which may have occured in OIS8, replacing the Dama peloponesiaca lineage (Fig. 6.21).
The third lower molar in Dama-like deer. 1 The variation in size of the Dama-like deer as indicated by the width of the first lobe (DTa) of the M3. The localities are ordered in approximate stratigraphic order: Montopoli (IGF), Tegelen (NNML, TMH, NMMa), Olivola (IGF), Almenara 1 (SIAP), Valdarno sup. (IGF), Il Tasso (IGF), Pyrgos (IVAU), Casa Frata (IGF), Ubeidiyah (HUJ), Selvella (IGF), Venta Micena (IPS; presently kept in the village of Orce), Vallonnet (MPRM), Untermassfeld (IQW), Atapuerca TDinf (CENIEH), West Runton (NHM), Megalopolis (NCUA, BGR), Arago (MPT), Bilzingsleben (FBFSUJ), Petralona (AUT), Azokh (MUB), Atapuerca TD10 & TG10-11(CENIEH), Orignac 3 (MPT), Swanscombe (NHM), Murr (SMNS), Neumark Nord (FBFSUJ, presently LVH), Qafzeh (IPH), Gimbsheim (NMM), Can Rubau (CIAG), Taglar (MUB), Recent Spain (MNCN). 2 MUB 6/350 – left M3 of Dama aff. peloponesiaca from Azokh 1 Unit V (a–c bucal, occlusal, and lingual views). The scale bar represents 3 cm
There is no new material of Dama from Unit III, but there is some material in the older collections. The largest antler fragment from this unit (Fig. 6.19/3) has a narrower palmation (right and left hand side in the photograph are natural borders, no fractures) than in the specimen from Unit V (Fig. 6.19/7). There is a small flat process, which protrudes less than 2 cm. Such processes occur in Dama dama at the back of the palmation. This narrow palmation does not seem to be a fragment from a different position in the antler of Dama aff. peloponesiaca, because there is no space for a section with this morphology between the lower part of the antler and the palmation as in Fig. 6.19/1 and 6.19/7. It does not seem to represent a different ontogenetic stage, because it is relatively large and straight for a juvenile antler (compared to Dama dama, where antlers of different ages are known). Alternatively it could belong to a different species, Dama mesopotamica, where the palmation is narrow. The oldest clear records of Dama mesopotamica are also of about OIS 7-8 (excluding Ubeidiyah and Gesher Benot Ya’akov; Di Stefano 1996).
Antler fragment MUB 7/839 (Fig. 6.19/3) has part of the surface of the antler with small pores. This suggests that the antler was not fully ossified at the moment of death of that individual. Shortly after full ossification the antler is cleaned of the velvet. In Dama dama this cleaning occurs at the end of August and the beginning of September (Ueckermann and Hansen 2002). Possibly the individual of MUB 7/839 died during August. This feature will be discussed more in detail under Cervus elaphus.
New material
-
Unit II
-
Azokh 1, Unit II, 03-8-08, C46, 327 (z = 119) – antler fragment (of a left antler?), including part of the palmation and the basis of a tine: diameters near the base of the tine 34.2 × 16.4.
-
Azokh uppermost, 11-9-03, D-45, rescue, 17 (Z = 130) – tip of the tine of an antler: length of the fragment about 10 cm, diametre at the base of the framgent 27.9 × 18.8.
-
Azokh uppermost, 16-8-03, D-45, 2 (Z = 132) – right calcaneum, juvenile: DAPn = 17.7, DTn = 11.0, DAPsf = 23.8, DTsf = 21.4.
-
Azokh 1, Unit II, J-48, 6 (z = 101, 8-8-2008) – right mandible with P3-4 (much worn) and alveoles of the P2; P3: DAP = 9.8, DTa = 6.3, DTp = 6.9; P4: DTa = 7.9.
-
Unit I
-
Azokh 1, 4-8-06, unit I, F51, 12 (z = 36) – condyle of a right mandible. Probably juvenile and might belong to other ruminants as well (e.g., Capra, Cervus?). Condyle DT = 20.1.
-
Azokh, Unit I, subunit c, 20-7-07, D-48, 16 (z = 201) – left magnum: DAP = 17.0, DT = 14.7, H = 10.9, h = 8.6.
Description of the new material
The antler fragment number 327 from Unit II (Fig. 6.19/2) contains part of the palmation and the beginning of a short tine. Another fragment of a tine of an antler (no. 17), also from Unit II, is flattened at the base, suggesting that it originated from a wide palmation, wider than in Dama mesopotamica.
Discussion
Within the Azokh sequence, the antler material from Unit II (Fig. 6.19/2) suggests again a wider palmation than in Unit 3, more like in the species from Unit V and Dama dama. Good antler bases are diagnostic between Dama peloponesiaca and Dama dama, but none have yet been recovered from Units II and I. Dama dama appeared not later than in OIS 7 (or about 220 ka) and at present extends its range into Anatolia. It is not impossible that the material from Units I and II belongs to Dama dama.
Aliev (1969) assigned a number of fossils from Azokh to Megaloceros giganteus, but no material from recent excavations can be assigned to this species or genus. Aliev’s material includes fragments of large antler, but we have found that all these antler fragments belong either to Cervus elaphus or to Dama. All the bones and teeth we have studied are smaller than in Megaloceros giganteus, but one tooth, a lower first or second molar from Unit V, belongs to a large deer (DAP = 30.9, DAPb = 25.3, DTa = 15.1, DTp = 15.0) (Fig. 6.22/2). Its size dimensions, however, are within the upper range of the M2 of Cervus elaphus from Azokh (Fig. 6.22/1). It is unworn, but the tip of the metaconid is broken off, so the standard measurement for crown height cannot be taken. At the entoconid, the height is 24.9 mm, which is relatively low for a Cervus elaphus M2. In the morphology of the styles at the lingual side, the tooth differs from Cervus, but recals Megaloceros. It is smaller than the M1 of Megaloceros giganteus, but it is in the ranges of the lower molars of deer of the type of Megaloceros solilhacus.
Deer of this type appeared in localities such as Pietrafitta and Ubeidiyah, with estimated ages around 1.4 Ma and are assigned to M. boldrini or M. obscurus. By the early Middle Pleistocene they had evolved into M. solilhacus. (Some authors recognize M. verticornis and M. dawkinsi as different species, and some authors place all of them in Megaceroides or Praemegaceros.) The last occurrence of that species in Western Europe is in Atapuerca TG10a (base of unit GIIb), which recently has been redated in the range 422–466 ka (Berger et al. 2008). Other late occurrences are in Petralona (probably OIS11 on the basis of biochronology) and Megalopolis (see discussion of the age of this locality under Dama aff. peloponesiaca). A Megaloceros sp. cited from Kudaro I-5b (Lioubine 2002) either belongs to this species or to Megaloceros giganteus. Megaloceros solilhacus is closely related to the highly modified species M. algericus, which appeared during the Late Pleistocene in North Africa. Thus a large part of the evolution of this branch of cervids is unkown and this new late record suggests that they may have lived in SW Asia immediately prior to their dispersal into northern Africa.
1 Bivariate plot of the first and second lower molar comparing Dama from Units III, V and VI (MUB), Cervus elaphus from Units III, V and VI (MUB), Megaloceros solilhacus from Pakefield (NHM), Voigtstedt (IQW, SMS), Süssenborn (IQW), West Runton (NHM), Mosbach (NMM), Megalopolis (NCUA, BGR), Atapuerca TG (CENIEH), Unit V (MUB), and Megaloceros giganteus from the Late Pleistocene Rhine sediments (NMM). 2 MUB 6/315 right M1 of Megaloceros solilhacus from Azokh 1 Unit V (a–c lingual, occlusal, and buccal views). The scale bar represents 3 cm
New material
-
Unit Vm
-
Azokh upper, D-43, Unit V, 12 (z = 105) – left metacarpal: L = 253.6, LIII = 245.5, LIV = 246.7, DAPp = 30.5, DTp = 42.0, DAPpf = 25.3, DTpf = 39.4, DAPm = 21.6, DTm = 27.1, DTd = 43.6, DAPIII = 28.9, DTIII = 19.9, DAPIV = 29.5, DTIV = 20.4.
-
Azokh middle, G-40, 7/9/02, Unit bag – fragment of shaft of metatarsal.
-
Azokh middle platform, Unit V, 17-8-03, E-41, 2 (z = 110) – right P3: DAP = 16.2, DAPb = 15.1.
-
Azokh’03, middle platform, D-42, 20-8-03, 11 (z = 92) – right P3: DAP = 17.5, DAPb = 15.8, DTa = 17.1, DTp = 17.8.
-
Azokh middle platform, Unit V, E-41, 22-8-03, 11 (z = 122) – right magnum: DAP = 21.8, DT = 22.1, H = 14.7, h = 10.0.
-
Azokh mid. platf. D41, 16-08-03, disturbed – left first phalanx: DAPd = 14.6, DTd = 17.2.
-
Azokh’03, Middle platform, Unit V, 19-8-03, F-42, 5 (z = 118) – left distal articulation of metacarpal: DT ≥ 20.2.
-
Azokh, plat. middle, 3-8-05, Unit V, F-40, 4 (Z = 137) – left scaphoid: DAP = 35.5, DT = 22.4, Ha = 25.1.
-
Unit Vu
-
Azokh upper, 17/9/02, E-44, 21 (Z = 92) – right P4: DAPo = 18.2, DAPb = 16.5, DTa = 11.9, DTp = 11.1.
-
Azokh Cave, 5/09/02, nivel IV, C-42, pared norte – fragment of branch of an antler: width of the fragment 27.2.
-
Azokh upper, 16/09/02, E-43, 2 (Z = 113) – right M2: DAP = 22,9, DAPb = 22.8, DTa = 14.8, DTp = 15.5, Ta = 0.9.
-
Azokh 1, Unit IV, D45, 10 (z = 24, 6-8-08) – right navicocuboid: DAP = 37.3, DT = 41.7, DTfast = 33.6.
-
Unit III
-
AZUM’03, D46, 151 – left I1: DLL = 8.4, DMDroot = 6.0, DLLroot = 6.2.
-
Unit 3/II
-
AZUM’03, D46, 72 – right distal humerus: DTd = 61.3, DTdf = 55.2, R1 = 44.8, R2 = 32.5, R3 = 36.1, R4 = 27.7, R5 = 29.8.
-
Unit II
-
Azokh 1, Unit II, 5-8-08, H-49, no. 12, z = 113 – right P4: DAP = 15.3, DAPb = 13.1, DT = 20.7.
-
Azokh 1, Unit II, 25-7-08, C45, 2 (z = 56) – left distal tibia: DAPd = 43.6.
-
Azokh uppermost, 15-08, D-46, 32 (Z = 108) – fragment of left distal tibia.
-
Azokh 1, Unit II, C-46, 232 (z = 97, 1-8-2007) – juvenile phalanx 1 without proximal articulation: DAPd = 14.1, DTd = 14.9.
-
Azokh, plat. uppermost, Unit II, 2-8-05, surface find, no. A – fragment of a right astragalus: DTd = 32.7.
-
Unit I
-
Azokh 1, Unit I, 7-8-06, D-51, 68 (z = 103) – right distal tibia: DAPd = 40.9, DTd = 54.4, DTdfast = 37.8.
-
Overburden
-
7-9-02, F-41, overburden – left third phalanx: L ≫ 40.
Description of the new material and taxonomic classification
A group of bones and teeth of cervid morphology larger than those of Dama and smaller than what is expected for Megaloceros (or Megaceroides, Praemegaceros), tend to have morphologies that are similar to those in Cervus elaphus. The metacarpal has a morphology that is typical of Cervus elaphus (characters 1 and 3–7 of Lister 1996; Fig. 6.23/2). It is small for fossil Cervus elaphus and approaches the size of large Dama (6.24/2). The navicocuboid has characters 1 and 2 of Lister (1996) as in Cervus and unlike in Dama. The profile of the lingual wall of the upper premolars, as seen in anterior or posterior view, has a convex upper profile (Fig. 6.18/9b), as in Cervus and unlike in Dama, where the lower part is convex and the upper part concave. This feature corresponds approximately to character 3 of Lister (1996) for the upper premolars.
1 Bivariate diagram of the metacarpal comparing distal width (DTd) and length (L) in Cervus elaphus and Dama: Cervus elaphus acoronatus from Voigtstedt (IQW); Cervus elaphus angulatus from Bilzingsleben (FBFSUJ) and Petralona (AUT); C. e. spelaeus from Neumark Nord (FBFSUJ, presently LVH), Cervus elaphus?maral from Roterberg, Heiligenstadt, Tingleff, Pinne, Dobschau, Wismar-Torfmoor and an unkown locality (all MNHUB); Cervus elaphus from Unit III (MUB) and Unit V (ASMHCS); Dama clactoniana from Petralona (AUT) and Riano, Clacton and Swanscombe (all Leonardi and Petronio 1976); D. dama geiselana from Neumark Nord (FBFSUJ, presently LVH); Dama dama from the Late Pleistocene of Lehringen (HMV), Gimbsheim (NMM), Danne (MNHUB) and Steinbeck (MNHUB) and recent D. d. dama (Leonardi and Petronio 1976); recent Dama mesopotamica (HUJ). 2 Azokh upper, D-43, Unit V, 12 – left metacarpal of Cervus elaphus from Unit V (a–e proximal, posterior, medial, anterior and distal views). The scale bar represents 5 cm
1 The variation in size of Cervus elaphus as indicated by the width of the first lobe (DTa) of the M3. The localities are ordered in approximate stratigraphic order: Dorn Dürkheim (FISF), Voigtstedt (IQW), West Runton (NHM), Koneprusy (NMP), Stránska Skálá (MMB), Süssenborn (IQW), Mosbach (NMM), Mauer (SMNK), Vérteszölös (HGSB), Miesenheim (FASMN), Arago (MPT), Bilzingsleben (FBFSUJ), Petralona (AUT), Azokh (MUB), Atapuerca TD10 & TG10-11 (CENIEH), Orignac 3 (MPT), Clacton (NHM), Swanscombe (NHM), Steinheim – Murr (SMNS), Neumark Nord (FBFSUJ, presently LVH), Ehringsdorf (IQW), Binagadi (NHMB), Schweinskopf (FASMN), Taubach (IQW), Lehringen (HMV), Can Rubau (CIAG), Abric Romaní (LAUT), Taglar (MUB), Sakazia (GSM), Cueto de la Mina (MNCN), Cueva Morin (MNCN), L’Arbreda (CIAG), Recent Germany (FASMN), Recent Spain (EBD, MNCN), Recent Georgia (GSM). 2 MUB 5/91 – right M2-3 from Azokh 1 Unit III (a–c lingual, occlusal, and buccal views). The scale bar represents 3 cm
Discussion
Aliev (1969) assigned material to Cervus elaphus from the collection in Baku, which includes basal antler fragments with a bez tine, and various fragments of a crown (Fig. 6.19/4). Both characters are very typical of Cervus elaphus. The new material confirms the presence of this species.
Cervus elaphus entered western Europe just before the Brunhes-Matuyama limit (Atapuerca TD4, Dorn Dürkheim; Van der Made 1996; Franzen et al. 2000). The earliest forms were large (Fig. 6.24/1) and lacked a crown, but they became smaller in Mosbach, where the subspecies Cervus elaphus acoronatus is defined (some authors consider this a separate species). This locality is about 600 or 500 ka old. In Mauer (with a range of dates around 500–600 ka for most of the section – Wagner et al. 2010), where the subspecies C. e. priscus is defined, there is still not a well developed crown. Possibly both subspecies are identical. Fully coronate antlers appeared about 400 ka ago (subspecies C. e. angulatus). The species became large again in OIS7 until OIS5 (C. e. spelaeus), and then late in OIS5 it became small again. In OIS 2, it became large and at present it is small again (C. e. elaphus). These size fluctuations seem to be independent of glacial-interglacial changes, since the species is large in Germany in OIS7 (warm), OIS6 (cold) and OIS5 (warm). These size fluctuations also are much larger than contemporary geographic size differences between representatives from Germany and Spain. Living Cervus elaphus are small in western Europe (with minor differences between Spain and Germany), while it is larger in the Caucasus area (subspecies C. e. maral).
Changes in body size of Cervus elaphus occured also in the Caucasus area: the species was small in Azokh VI, V and III, large in Binagadi (believed to be of Eemian age; e.g., Eisenmann and Mashkour 1999), small in Taglar and Ortvala and at present it is large (Fig. 6.24/1). Evidently, the Holocene size decrease did not occur in this area, although other size changes might have been synchronous with western Europe. If this is the case, the small size in Unit VI–III, in combination with well developed crowns, present in Unit V, indicates an age in the range OIS12 to 8 or late OIS5 to OIS3 for Units V–III, while Unit VI, from which no crown is known, might be as old as OIS13 or 14 (Fig. 6.24).
Some antler fragments from Azokh have porous outer bone, whereas antlers normally have compact bone at the outer surface. This compact bone is about 4–5 mm thick and below it the inner part of the antler is made up of spongeous bone with large pores. In deer living at middle and high latitudes, the antler cycle is determined by seasonal variation in the intensity of the light. Antlers are shed once a year, and when they grow again, they are made of cartilage initially, but within less than a month they are ossified. Antlers that are fossilized in the middle of the process of ossification give a relatively precise indication of the month in which the individual died. Ossification occurs from proximal to distal and the compact bone layer is initially spongeous with pores that are finer than those of the inner part of the antler. Figure 6.19/5 shows a detail of a crown (MUB 6/158), that is not fully mineralized: the spongeous inner bone is seen in the lower part of the photograph, then there is a layer of bone with finer pores reaching the outer surface in the upper part of the photograph. Some fragments of the lower part of the antler also show porous bone reaching the surface at different places. This is the case in MUB7/883 and in an un-numbered specimen kept with MUB 6/18 and 6/26. The latter is the tip of a tine, which is broken at its base, where at some places porous bone reaches the surface. When mineralisation of the antler is complete, the velvet dies off and the antler is cleaned. In Cervus elaphus this occurs in August (Lincoln et al. 1982), suggesting that the specimens from Unit V described above, belonged to individuals that died in August or the end of July.
New material
-
Unit III
-
Azokh 1, Rescue, Unit III, 1.046 (?), 12 (z = 173(?), 24-7-2008) – right maleolar bone: DAP = 54.5, DT = 28.3, H > 33.4.
-
Unit II
-
Azokh 1, Unit II, 2-8-05, E-48, section cleaning – left cuneiform II-III: DAP > 43.7, DT = 26.9.
-
Azokh 1, Unit II, C-46, 70 (Z = 70, 8-10-08) – right astragalus: Lext > 84.4, Lm = 68.6, Lint > 78.3, DTp = 50.4.
Description of the new material
Fossil bones from Unit II have the morphology and size of a large ruminant. The massiveness of the cuneiform and maleolar (Fig. 6.25/3) suggest they belong to a bovine. Heintz (1970) indicated that in the Bovidae, the large cuneiform (II + III) has a vertical facet on its lateral side for articulation with the cuboid part of the navicocuboid, where it is well developed unlike the conditionwhile in the Cervidae where it is reduced or absent. Though this side of the bone is partially eroded, a relatively large part of such a facet remains, indicating again that the fossils correspond to a bovid.
1 Bivariate diagram of the astragalus comparing axial length (Lm) and proximal width (DTp) in: Megaloceros boldrini and M. solilhacus from Ubeidiyah (HUJ), Bacton (NHM), Voigtstedt (IQW), Süssenborn (IQW), East Runton (NHM), West Runton (NHM) and Petralona (AUT); Megaloceros giganteus from Steinheim (SMNS) and Ireland (NHM); Bison schoetensacki from Vallonnet (MPRM), Akhalkalaki (GSM), Apollonia 1(AUT), Koneprusy (NMP), Pakefield (NHM), Vérteszölös (GSB), Süssenborn (IQW), Soleilhac (MCP), Mauer (SMNK), Jockgrim (SMNK), Bacton (NHM), Mundesley (NHM), Bilzingsleben (FBFSUJ), and Petralona (AUT); Bos primigenius from Miesenheim (FASMN), Megalopolis (AUT), Neumark Nord (FBFSUJ, presently LVH) and Lehringen (HMV) and “Azokh” including Bison schoetensacki from Unit V and cf. Bison schoetensacki from Unit II. 2 Azokh 1, Rescue, Bed II, 1.046 (?), 12 – right maleolar bone of cf. Bison schoetensacki from Azokh II (medial view). 3 Azokh 1, unit II, C-46, 70 – right astragalus of cf. Bison schoetensacki from Azokh II (anterior view). 4 AZM’05, E38, 3 – protocone of left upper molar of Bovidae indet. from Azokh 1 Unit V. The scale bars represent 3 cm (tooth) and 5 cm (bones)
There are no good morphological characters to separate cervid and bovid astragali (Heintz 1970). Bovini have very stout limb bones and the slenderness of the astragalus suggested that it might belong to a large cervid and not to a bovine. However, a metrical comparison of Bos, Bison and Megaloceros astragali (Fig. 6.25/1) does not show that these Bovini to have stouter astragali than a large cervid. The astragalus from the recent excavations (Fig. 6.25/4) is close in size to one recovered from the previous excavations at Azokh (Fig. 6.25/1), and both are larger than the astragali of large cervids of the type of Megaloceros soleilhacus (or Megaceroides, Praemegaceros, M. verticornis, M. dawkinsi) and M. boldrini (or M. obscurus).
Discussion
Material from Azokh Unit VI was assigned to Bison sp. or Bison schoetensacki (Aliev 1969; Lioubine 2002; Rivals 2004). The assignment of bones and teeth to Bos or Bison and in particular to different species of Bison is a dificult task. Some fragments of horn cores in the old collections from Azokh V have a surface with deep groves as occurs in the lower side and near the base of the horn cores of Bison. Likewise, distal articulations of metapodials from Unit III (and VI?) indicate the same genus.
The species of Bison differ in characters of the skull and horn cores, but also in the robusticity of the metapodials. The horn cores of Bison schoetensacki tend to be flattened (they have a relatively small transverse diameter in comparison to the anteroposterior diameter), while this tends to be less the case in Bison priscus. A relatively complete specimen from the old collections of Unit V has this “flattened” morphology. We follow the original assignment of the bovine material from Azokh to Bison schoetensacki. For the material from Unit II, which is much younger, other possibilities like Bison priscus or Bos primigenius are not to be excluded.
The origin of the genus Bison was probably in the plains of Asia. In western Europe there may have been three lineages: the Bison menneri-B. voigtstedtensis lineage (large, slender metapodials, narrow skulls) had an age range of about 1.2–0.5 Ma; the B. degiulii- B. schoetensacki lineage (initially small, increasing in size, and with robust metapodials and wide skulls) ranged about 1–0.1 Ma; and B. priscus (relatively small, with robust metapodials and wide skulls) might be related to the living B. bonasus (Van der Made 2005a). The moment of entry of B. priscus or related forms is interesting here, but the date is not well known beyond the notion that it was during the late Middle Pleistocene. The presence of B. schoetensacki in Azokh broadly confirms a Middle Pleistocene (or early Late Pleistocene) age for Unit II.
New material
-
Unit Vm
-
?Middle plat., cleaning 26-7-05 – left first phalanx distal part: DAPd = 6.3, DTd = 7.7.
-
Unit II
-
Azokh, 18-8-06, Unit II, F-48, 94 (z = 75) – right first phalanx: DAPp.15.3, L = 42.2, DAPd = 9.4, DTd = 9.9.
Description of the new material and taxonomic classification
The first phalanx from Unit II (Fig. 6.16/3) is damaged and its proximal morphology is unclear. It is smaller, however, and more slender and elongate than that of Capra, but proximally it is not as narrow or elongate as in Gazella (Fig. 6.16/2). It appears more gracile than the Capreolus phalanges (especially those of the manus) and this is confirmed to some extent by the measurements. It is much smaller than the Capreolus phalanges from Unit V and it is relatively elongate compared to the phalanges of C. priscus and C. capreolus. The dorsal surface of the proximal end is flatter than it tends to be in Capreolus. In size and proportions it is similar to phalanges of recent Saiga tatarica.
The phalanx from Unit Vm is fragmentary, but the remaining morphology is that of a ruminant. It is very small and even much smaller than the phalanx from Unit II (Fig. 6.16/1).
Discussion
Aliev (1969) assigned a horn core from Unit V to Gazella cf. subguturosa (see also list by Rivals 2004), but this taxon was absent from the list given by Lioubine (2002). Horn core MUB 209 (Fig. 6.26) originates directly above the orbit and curves backwards. The rugose part (the part that was in contact with the keratine sheath) has relatively deep grooves. The section is oval, with a slight bulge just posterior of the middle at the medial side. The horn core is wider than is the case in male gazellas, and it is larger than in female gazellas. Morphologically and metrically it is close to a saiga fossil from Pahren described by Kahlke (1990).
The phalanx from Unit II has more resemblance to Saiga than to Gazella or Capreolus. Though from a different Unit, the horn core again resembles Saiga. We assume the presence of saiga antelopes in Units II and V. Saiga is an antelope that at present lives in a restricted area of the steppes north of the Himalayas. During the last two cold periods it extended its range into western Europe (Kahlke 1990, 1994) and even reached the north of Spain (Altuna and Mariezkurrena 1996).
New material
-
Unit Vm
-
?AZM’05, E38, 3 – fragment of a left upper molar.
-
Unit I
-
Azokh 1, Unit Ib, 21-7-07, B51, 8 (z = 99) – right proximal metatarsal: DAPp = 26.9, DTp = 25.7, DAPpf = 25.8, DTpf = 24.9, DTmini = 17.5, L ≫ 132.
Description of the new material and taxonomic classification
On the proximal surface of the metatarsal from Unit I (Fig. 6.27/1), the posterior facet for the navico-cuboid is narrow and elevated at the medial end, as typical in Bovidae (and unlike the condition in Cervidae). The posterior area comprising this facet and the facet for the first cuneiform is narrow in comparison to the width at the major (anterior) facets. This is more evident in Alcelaphus (cited as far to the north as Ksâr’akil in Hooijer 1961), where the facet for the small cuneiform is situated on a pointed posterior extension. In most Caprinae, this area is wide, though it is not so wide in Ovis and Rupicapra.
The proximal articulation and a major part of the shaft of the metatarsal are preserved. The distal part lacks widening, so the metatarsal must have been a long one, much longer than in Capra and most other Caprinae, save for Ovis and Rupicapra, which among the Caprinae are the animals with the most elongate metapodials.
The anterior side of the bone lacks a clear furrow between the third and fourth metatarsals. Such a furrow is seen in Cervidae and some Bovidae, but it is lacking in Caprinae and some other Bovidae, where there is only a shallow depression. There is no clear longitudinal depression on the posterior side of the shaft.
In the morphology described above, the bone shows some similarities to the metatarsal of Saiga, but it is larger and the shaft is more robust. The closest resemblance is to the metatarsals of Ovis antiqua from Arago (MPT) and Bammenthal (SMNK), but it is a little smaller. It is larger than the metatarsal of Ovis vignei (NNML).
The molar fragment from Unit Vm consists of the protocone and part of the paracone. The preserved height of the protocone is 28 mm, but it must have been greater before it was worn. Wear was possibly not much advanced, and the paracone is higher than the protocone. The enamel is rugose, as is the case in the Bovini and some other Bovidae, like Alcelaphini and Hippotragini. In other bovids, like Caprinae, the enamel surface tends to be smooth.
The base of the protocone curved backwards, and the angle between the anterior side of the tooth, and what is preserved of the occlusal surface, both suggest that the tooth is an M3. However, the facet on the protocone may occasionally be inclined, so this observation may not be valid. The anteroposterior diameter of the protocone is about 10 mm, suggesting that the DAP of the complete tooth was about 20 mm. This is small for Bison schoetensacki, if the tooth is an M3. The estimated size of the specimen is not unlike in Ovis ammon.
The anterior and posterior crests of the protocone are straight, forming a smooth crescent, which limits a cresent shaped fossa. In living species of Alcelaphini and Hippotragini, there is a secondary crest on the inner sides of the anterior and of the posterior crest, resulting in a fossa with a more complex shape. These bovids also tend to have a well developed interlobular column, a minute additional fossa between the anterior and posterior lobes, and a flat occlusal surface. In all these characters they differ from the tooth fragment from Azokh.
Discussion
Ovis was not cited in early reports from Azokh (Aliev 1969; Lioubine 2002; Rivals 2004, p. 20). Rivals (2004, p. 31, Fig. 37, Table 6.9) assigned specimen MUB6/530 from Unit V (Fig. 6.27/2) to Ovis ammon antiqua. In size it is close to the specimen described above. A fragment of a large humerus from Unit III (MUB 5/48) has a distal articulation that is nearly cylindrical, not conical, and which has a small radius. It is large for Capra and might also represent Ovis ammon.
The metatarsal from Unit I is recent and could be from the wild species of Ovis that lives at present in the area. The recent species from this area is indicated as Ovis aries (Wilson and Reeder 1993), Ovis orientalis (Duff and Lawson 2004) or Ovis gmelini (Rivals 2004), and there does not seem to be any consensus on their names. The name Ovis aries is now applied to the domestic form. Ovis orientalis was cited at Mezmerskaya (Golanova et al. 1999) and Ovis ammon or Ovis cf. ammon was cited at Ortvala Klde, Tsona and Kudaro (Lioubine 2002; Rivals 2004, p. 20). The latter species is large, while Ovis orientalis and Ovis vignei are small (Rivals 2004). The bone from Unit I seems to belong to Ovis ammon.
Ovis ammon lives in an area extending from east Kazakhstan to south Siberia, Mongolia and northern China in the east and to northern Pakistan and northern India in the south. During the Early Pleistocene and again during the Middle Pleistocene, some 500 ka ago, it dispersed into western Europe, where the fossils are known as Ovis antiqua or Ovis ammon antiqua (Rivals 2004; Crégut-Bonnoure 2006).
New material
-
Unit Vm
-
Azokh’03, middle platform, Unit V, 17-8-03, 1 (Z = 117) – left M1/2: DAP = 20.1, DAPb = 14.9, H ≫ 30.
-
Azokh, 28-7-05, plat middle, Unit V, F-39, 3 (z = 139) – left M1/2: DAPo = 16.5, DAPb = 15.4, DTa = 14.9, DTp = 14.2.
-
Azokh, 8-9-02, plat. middle, F-43, 2 (z = 90) – right scapula: DAPn = 15.8, DTn = 11.8.
-
Azokh 1, Unit V, 4-8-2009, I-42, 39 (z = 860) – left M1: DAPo = 14.4, DAPb = 11.1, DTa = 10.7, DTp = 10.3.
-
Unit Vm-IV
-
17-9-02, plat north, E44, gen finds – very rolled antero-proximal fragment of a metatarsal (?)
-
Azokh upper, 14/9/02, F-43, general finds – right P4: DAP ≥ 10.2, DAPb ≥ 8.6, DT = 12.6.
-
Azokh, 13-9-02, F-44, dry sieve – sesamoid behind phalanx 1, right axial: L = 15.3, DAP = 8.8, DT = 9.5.
-
Azokh 1, Unit V, 27-7-2009, E-39, 8 (z = 871) – left ulnar: DAP = 16.7, DT ≥ 14.2, H > 22.2, Ha ≥ 16.5.
-
Unit Vu
-
Azokh 1, Unit IV, 7-8-08, O45, 31 (z = 60) – left lower molar (M1 or M2): DAP = 17.7, DAPb = 16.6, DTa = 9.7, DTp = 10.1.
-
Azokh upper, 17/9/02, D-45, 5 (Z = 73) – left distal articulation of metapodial, juvenile: DAP = 22.1, DT = 17.2.
-
Unit II
-
Azokh uppermost, 21-8-03, D-45, 16 (Z = 174) – right M3: DAPo = 24.3, DAP = 26.2, DAPb = 22.5, DTa = 14.7, DTp = 12.3.
Description of the new material and taxonomic classification
The molars have high crowns, smooth enamel and lack interlobular columns. The lower molars have a caprine fold and relatively flat lingual walls (Fig. 6.28/6). The upper molars have marked styles on the buccal walls, but the buccal walls are flat or concave buccally on the tips of the para- and meta-cones (Fig. 6.28/3). A third upper molar has a posterior expansion at the base of the postero-buccal corner, which is typical in Capra (Fig. 6.28/4).
1 Bivariate diagram of the distal width (DTd) and length (L) of the metacarpal of Capra: Capra from Unit V, C. causasica from Tsona (GSM), Akhalkalaki (GSM) and Tsona (GSM); recent C. cylindricornis (GSM); C. ibex recent (LPT) and from Petralona (AUT); recent C. falconeri (NMB), recent C. nubiana (NMB), recent C. sibirica (NMB), recent C. pyrenaica (MNCN), recent C. ?wali (NHM). Capra from Azokh 1 Unit V: 2 MUB 4/488 – right Mc (a–d proximal, anterior, medial, and distal views). 3 AZM’05, F39, 3 – left M1 (a–c occlusal, buccal and lingual views). 4 MUB 1/473 – left M3 (a–c buccal, lingual, and occlusal views). 5 MUB 6/354 – skull fagment (posterior view). 6 Azokh 1, Unit V, O45, 31 – left M2 (a–c buccal, occlusal and lingual views). The scale bars represent 5 cm (Mc and skull) and 3 cm (teeth)
A distal articulation of a metapodial has the typical caprine morphology with the abaxial part of the condyle small in diameter and a dorsal surface that is horizontal or slightly elevated at the abaxial side.
Discussion
Material from Units V and III was assigned to Capra aegagrus (Aliev 1969; Lioubine 2002; Rivals 2004). The collections in Baku also include Capra from Unit VI. These collections include fragments of very large horn cores (e.g., Fig. 6.28/5). We have not had the opportunity to study horn cores of adult males of most species of Capra and therefore cannot fully evaluate the information the specimens from Unit VI contain.
The number of living species of Capra recognized varies from author to author. Capra aegagrus is the wild ancestor of the domestic Capra hircus (Duff and Lawson 2004), and in some literature it was included in the latter species (e.g., Wilson and Reeder 1993). It occurs in a wide area including Crete, Turkey and the area from the Caucasus to Pakistan. Capra cylindricornis and Capra caucasica, which for some are a single species, occur also in the Caucasus. During the late Pleistocene, the latter gave rise to Capra pyrenaica (Crégut-Bonnoure 1992). Material from Tsona, Ortvala and Sakazia is believed to represent Capra caucasica (Lioubine 2002; Touchabramichvili 2003; Rivals 2004), but is much larger than the recent species (e.g., compare recent Capra caucasica in Fig. 6.28/1 with Tsona, which is the largest specimen in the group “Tsona-Akhalkalaki”). There must have been a considerable size decrease in the latter species, as was also the case in C. ibex. Capra ibex dispersed some 400 ka ago into Europe.
A metacarpal from Unit V (Fig. 6.28/2) is robust, much larger than recent Capra cylindricornis and close in size to recent Capra caucasica and a little smaller and more gracile than specimens from Akhalkalaki and Tsona (Fig. 6.28/1). It is in the lower range of Capra ibex from Petralona. The phalanges (Fig. 6.29/2–4) are more abundant than complete metacarpals. Some first phalanges from Unit V reach larger sizes than those of Capra ibex from Petralona (Fig. 6.29/1), suggesting that this might be the case also with the metacarpal, if that sample would be larger. The phalanges of Hemitragus show a wider range of variation in robusticity than those of Capra; possibly this is due to a greater difference between anterior and posterior phalanges. The phalanges from Azokh Unit V are similar in size and proportions to those from Tsona, Sakazia and Ortvala.
1 Bivariate diagram of the proximal width (DTp) and length (L) of the first phalanx of Capra and Hemitragus: H. bonali from Hundsheim (IPUW) and L’Escale (Bonifay 1975b), C. ibex from Petralona (AUT), Capra from Tsona (GSM), Sakazia (GSM), Ortvala (GSM), Capra from Azokh 1 Unit V (MUB) and Capra hircus from Unit I. 2 MUB 7/788 – left third phalanx of Capra from Unit III (a–e dorsal, abaxial, axial, plantar, and proximal views). 3 MUB 472 – left second phalanx of Capra from Unit V (a–f abaxial, dorsal, axial, plantar, proximal, and distal views). 4 MUB 1/315 – left first phalanx of Capra from Unit V (a–f proximal, distal, dorsal, axial, plantar, and abaxial views. The scale bar represents 5 cm
New material
-
Unit I
-
Azokh 1, Unit I, subunit c, 20-7-07, D48, 4 – right I1: DT = 5.3, DLL > 5.4.
-
Azokh 1, Unit I, passage, 22-7-07, C51, 57 (z = 124) – buccal side of left upper molar, probably M2: DAP = 17.7, DAPb = 16.6.
-
Azokh 1, Unit I, 4-8-06, F-51, 3 (z = 29) – fragment buccal cusp upper molar.
-
Azokh 1, Unit I, E-51 4-8-06, 25 (z = 39) – right M3: DAP = 32.4, DAPb = 30.5, DTa = 9.1, DTp = 9.8, DTpp = 6.5.
-
Azokh 1, Unit I, 4-8-06, E-51, 46 (z = 44) – left ulna, juvenile (?): DAPmax = 27.2, DTupperfacet = 8.7, DAPmini = 16.4, DTmax = 16.9.
-
?Azokh 1, Unit I, 4-8-06, E-51, 45 (z = 46) – left femur, juvenile.
-
Azokh 1, Unit J, 6-8-06, D-51, 31 (z = 64) – left first phalanx: DAPp = 17.4/16.6, DTp = 14.4, L = 43.5, DAPd = 11.5, DTd = 13.2.
-
Azokh 1, Unit I, subunit b, 21-7-07, B51, 10 (z = 102) right P4: DAP = 8.3, DAPb = 7.4, DT = 10.1; M1: DAP = 11.3, DAPb > 11.3, DTa = 12.3, DTp = 13.5; M2: DAP = 17.5, DAPb = 16.1, DTa ≥ 14.2, DTp = 13.1; M3: DAPo = 24.4, DAPmax = 27.4, DAPb = 26.1, DTa = 13.4, DTp = 11.4; Left P3: DAP = 8.1, DAPb = 7.7, DT = 9.5; P4: DAP = 7.9, DAPb = 7.7, DT ≥ 8.8; M1: DAP = 10.6, DAPb > 10.6.
Description of the new material and taxonomic classification
The teeth from Unit I have typical caprine morphology as described above. The ulna is much expanded laterally at the level of the facets with the radius, which is typical in the Caprini. It is not fused to the radius. In adult Capra ibex, the two bones tend to be fused, while they tend to remain separate in other genera of Caprini, such as the closely related Hemitragus. The ulna might be from a juvenile individual. A first phalanx of caprine morphology from Unit I is very small (Fig. 6.29/1). It might represent the domestic Capra hircus.
General Discussion and Conclusions
Aliev (1969) described the large mammals from Azokh recovered at that time. Rivals (2004) gave a composite faunal list based on Aliev (1969), while Lioubine (2002) gave faunal lists per unit and incorporated later work. Our updated faunal lists are based on these publications, with additions of new material and discussed modifications; if we did not consult the original material and do not have new material, we have not changed the original determination. The updated lists of large mammals from different Units from Azokh are diplayed in Table 6.7.
One of the most striking things about the Azokh large mammal fauna is that in the old collections, large mammals were only recovered from Units VI, V and III, while in the new collections they are also recovered from Unit II. In the most recent excavation, Unit V was separated into upper and middle levels (abbreviated here as Vm and Vu). In Table 6.7, Unit V of the earlier excavations is grouped with Unit Vm of the excavation and Unit Vu is given in a separate column. Observing the lists, it is striking that carnivore remains come mainly from Unit V, while the other units have mainly ungulates. This is probably a genuine result, because the most extensive collections were made from Unit V in the old seasons, and because fossils from the old seasons were dug from the entrance of the cave and the most recent excavations come from the rear of the cave.
Animals that tend to be typical of closed environments dominate the faunas of all units, while animals more typical of open environments are less common. They are present, however, and Caprinae species adapted to mountainous, rocky or arid environments also occur. All units contain taxa that are commonly associated with interglacial environments (Stephanorhinus kirchbergensis, Sus scrofa, Dama), and with the possible exception of Saiga, none contains taxa that are clearly associated to glacials. This suggests that the climate was temperate, either interglacial or of a glacial refugium. The area south of the Caucasus may have been a refugium for “interglacial” species during glacial times. However, during glacial times, the altitude of Azokh Cave (926 m above sea level) would result in a harsh environment in the immediate surroundings of the locality.
Figure 6.30 shows the faunas from the different levels of Azokh in a wider context, compared with other faunas of the region. A comparison is made with the stratigraphic distribution of the same taxa of Europe (solid lines). In the case of taxa not present in Europe, a comparison is made with the stratigraphic distribution in Africa and the Indian Subcontinent. The first observation that can be made is that most taxa present in the region are also present in Europe. Towards the south, European affinities decrease, but remain important. This pattern seems to be more or less constant in the time considered here. The faunas studied are biogeographically part of Western Eurasia, though African, Indian and central Asian elements are present.
The fauna from Azokh in the stratigraphic context of SW Asia (modified after Van der Made 2005a, b and based on the present study and the literature, principally: Lioubine 2002; Golanova et al. 1999; O’Regan et al. 2005; Tchernov et al. 1994; Ron et al. 2003; Hooijer 1961; Sharapov 1986; Janashvili 1978; Sotnikova and Vislobokova 1990; Vekua 1986, 1995). Solid squares indicate the presence of a taxon in the localities, open squares indicate “cf.”, “aff.”, “sp.” or “?”. In the cases of Cervus elaphus, Capreolus, Ursus and Canis, the information in the publications is insufficient to asses the grade of evolution of (sub)species and open squares are used. The fat vertical solid lines indicate the stratigraphic ranges of the taxa in Europe (left part of the figure), Africa and Asia (after Van der Made 2005b). Vertical fat dashed lines indicate uncertainty on the stratigraphic ranges. Fat oblique lines indicate phylogenetic relationships and dashed fat lines indicate assumed or possible relationships
Many of the localities and units in Fig. 6.30 are dated by some physical method (references in the figure caption), while some of the levels not yet dated form part of a sequence that includes dated levels. In a few cases, a site with a particular taxon in the study area has an age outside the temporal range for that taxon in Europe. These exceptions are: Ovis ammon, Megaloceros solilhacus, and Cervus elaphus maral, which all survived longer in the area, and Bos primigenius and Vulpes vulpes, which were present earlier than in Europe. The Holocene size decrease in Cervus elaphus that is so well known in Western Europe, did not occur here. The late occurrence of Megaloceros solilhacus is discussed under that species and there is no reason to believe that it is not a real result. The remains from Unit I show that Ovis ammon persisted in the area until the Present. Bos primigenius was present at Gesher Benot Ya’akov before it appeared in Europe. As discussed under Cervus elaphus, it seems that size changes south of the Caucasus follow those in western and central Europe, save for the Holocene. Leaving aside these exceptions, we can attempt to position the Units from Azokh in this scheme and thus estimate their ages on the basis of biochronology (Fig. 6.30).
The small sized Cervus elaphus identified in Units VI to IV marks a maximum age of 500–550 ka (OIS13 or 14), while the presence of well developed crowns of the antlers in Unit V indicates a maximum age of about 450–400 ka (OIS 11 or 12) for that and overlying units. The presence of Equus ferus and E. hydruntinus indicate maximum ages of about 500 ka (OIS13) for Units V and VI, but the material that we studied is too poor for certain identification. Stephanorhinus hemitoechus is identified in Units VI and V and indicates a maximum age of about 450 ka (or OIS12; see details on the temporal distribution in the discussion of the species). This species is assumed to have evolved outside western Europe and to have dispersed into Europe (Guérin 1980; Van der Made 2010a; Van der Made and Grube 2010a). Ursus spelaeus is present in Units VI, Vm, Vu, III, and II. It is assumed to have evolved from Ursus deningeri not later than 300 ka ago. In the period between about 450–240 kyr, the small Canis mosbachensis was replaced by the somewhat larger C. lupus lunellensis, which evolved into the large sized C. lupus. However, the material we studied from Units V and VI is to poor for assesing the grade of evolution. These data suggest a maximum age of around 300 ka for Unit VI and the overlying units. Dama peloponesiaca is an offshoot of the Dama lineage in the southeastern part of the geographical range of the genus and probably it was replaced there by Dama mesopotamica, when this species arose possibly during OIS8. This is in accordance with the small size of Cervus in Units VI–Vu (which in western Europe became large in OIS7). These data suggest that Units VI–Vu have ages between about 300 and 240 ka (corrsesponding to OIS10-8). In the case of Unit VI this is based on the bear material, which we did not study, and which was deposited with fluvial sediments (Murray et al. 2016). If the presence of “interglacial” taxa is taken as indicative, these units are to be correlated with OIS9. Radiometric dating indicates ages of about 300 ka for Unit Vm and 200 ka for Unit Vu (see Appendix, ESR), which is compatible with a correlation of Units VI and Vm to OIS9, while it suggests a younger age for Unit Vu.
Unit III has a fauna that is poorer but similar to that of the underlying units. The main difference is that there is a fragment of antler of Dama, which might belong to Dama mesopotamica. The material is not very abundant nor the character very clear, but if this attribution is correct, it suggests a younger age and correlation to OIS8 or more recent. Unit III also has a small Cervus elaphus, which in western Europe occurs until OIS9 or 8, and again from late OIS5 to OIS3. Radiometric dates of 200 ka from the underlying Unit Vu and of 185 ka from the bottom of the ovelying Unit II (see Appendix, ESR, and Murray et al. 2016), leaves a short time span for Units IV and III. If these dates are correct, size changes in Cervus in this area, do not follow the trend in western Europe. This would not be surprising, even though in several other localities sizes are in accordance with those in western Europe.
Unit II has rather poor faunal remains. Its main difference from Unit III is the indication of cervids with wide antler palmation, which suggests Dama dama rather than Dama mesopotamica. The material of Cervus is too poor to assess its evolutionary grade. The bottom of this unit has been dated around 185 ka and the top around 100 ka (Appendix, ESR), which is compatible with the biochronological data from this unit.
Unit 1 has remains of domestic animals. This suggests a Holocene age, which is compatible with a datation of 157 years BP (Appendix, radiocarbon).
References
Adam, K. D. (1975). Die mittelpleistozäne Säugetier-Fauna aus dem Heppenloch bei Gutenberg (Württemberg). Stuttgarter Beiträge zur Naturkunde, Serie B, 3, 1–245.
Aliev, S. D. (1969). Fauna Azikhskoy paleolitcheskoy stoyanki. Baku.
Aliev, S. D. (1989). Referred in Lioubine (2002).
Aliev, S. D. (1990). Referred in Lioubine (2002).
Altuna, J., & Mariezkurrena, K. (1996). Primer hallazgo de restos óseos de antílope Saiga (Saiga tatarica L) en la Península Ibérica. Munibe (Anthropologia-Arkeologia), 48, 3–6.
Andrews, P., & Turner, A. (1992). Life and death of the Westbury bears. Annales Zoologici Fennici, 28, 139–149.
Appendix: Fernández-Jalvo, Y., Ditchfield, P., Grün, R., Lees, W., Aubert, M., Torres, T., et al. (2016). Dating methods applied to Azokh Cave sites. In Y. Fernández-Jalvo, T. King, L. Yepiskoposyan & P. Andrews (Eds.), Azokh Cave and the Transcaucasian Corridor (pp. 321–339). Dordrecht: Springer.
Argant, A. (1996). Sous-famille des Felinae. In C. Guérin & M. Patou-Mathis (Eds.), Les grands mammifères Plio-Pléistocènes d’Europe (pp. 200–215). Paris, Milan & Barcelona: Masson.
Azzaroli, A. (1953). The deer of the Weybourn Crag and Forest Bed of Norfolk. Bulletin of the British Museum, Natural History, 2(1), 1–96.
Azzaroli, A. (1962). Validitá della species Rhinoceros hemitoechus Falconer. Palaeontographica Italica, 57, 21–33, pls. 16–20.
Azzaroli, A. (1990). The genus Equus in Europe. In E. H. Lindsay, V. Fahlbusch & P. Mein (Eds.), European Neogene Mammal Chronology (pp. 339–356). New York: Plenum Press.
Azzaroli, A. (1992). The cervid genus Pseudodama n.g. in the Villafranchan of Tuscany. Palaeontographia Italica, 79, 1–41.
Baryshnikov, G. F. (1991). Ursus mediterraneus v Pleistocene Caucasa i zamechania po istorii melkih medvedey Eurasii. Palaeoteriologicheskie issledovania fauny SSSR. Trudy Zoologischekoo Instituta AN SSSR, 238, 3–60.
Baryshnikov, G. F. (1998). Cave bears from the paleolithic of the Greater Caucasus. In J. J. Saunders, B. W. Styles & Baryshnikov (Eds.), Quaternary Paleozoology in the Northern Hemisphere. Illinois State Museum Scientific Papers, 27, 69–118.
Baryshnikov, G. F. (2006). Morphometrical variability of cheek teeth in cave bears. Scientific Annals, School of Geology Aristotle University of Thessaloniki, Special volume 98, 81–102.
Berger, G. W., Pérez-González, A., Carbonell, E., Arsuaga, J. L., Bermúdez de Castro, J.-M., & Ku, T.-L. (2008). Luminescence chronology of cave sediments at the Atapuerca paleoanthropological site, Spain. Journal of Human Evolution, 55, 300–311.
Bonifay, M. F. (1971). Carnivores Quaternaires du sud-est de la France. Mémoires du Museúm National d’Histoire Naturelle, novelle série, Série C, 21(2), 43–377, 27 pls.
Bonifay M. F. (1975a). Les Ursidés du gisement des Abimes de la Fage a Noailles (Corrèze) (Ursus deningeri von Reichenau). Nouvelles Archives Musée. Histoire Naturelle. Lyon, 13, 21–28.
Bonifay, M. F. (1975b). “Hemitragus bonali” Harlé et Stehlin, “Caprinae” de la Grotte de l’Escale (Saint-Estève-Janson, Bouches du Rhône). Quaternaria, 18, 215–302.
Burchak-Abramovitch, N. I., & Aliev, S. D. (1989). Iskopaemaya ornitofauna paleolitcheskoy stoyanki Azikhskoy peschery na Malom Caucase v Azerbaidjane (Chast I). Materialy po ekologii jivotnih v Azerbaidjane: Temat sbornik. Baku, 72–80.
Burchak-Abramovitch, N. I., & Aliev, S. D. (1990). Iskopaemaya ornitofauna paleolitcheskoy stoyanki Azikhskoy peschery na Malom Caucase v Azerbaidjane (Chast I). Materialy po ekologii jivotnih v Azerbaidjane: Temat sbornik. Baku, 44–57.
Cerdeño, E. (1990). Stephanorhinus hemitoechus (Falc.) (Rhinocerotidae, Mammalia) del Pleistoceno medio y superior de España. Estudios Geológicos, 6, 465–479.
Crégut-Bonnoure, E. (1992). Les Caprinae (Mammalia, Bovidae) du Pléistocène dÉurope: Intérêt biostratigraphique, peléoécologique et archéozoologique. Mémoires de la Société géologique de France, n.s., 160, 85–93.
Crégut-Bonnoure, E. (1996). Ordre des Carnivores. In C. Guérin & M. Patou-Mathis (Eds.), Les grands mammifères Plio-Pléistocènes d’Europe (pp. 155–230). Paris, Milano & Barcelona: Masson.
Crégut-Bonnoure, E. (2006). European Ovibovini, Ovini and Caprini (Caprinae, Mammalia) from the Plio-Pleistocene: New interpretations. Courier Forschungsinstitut Senckenberg, 256,139–158.
Croitor, R. (2006). Early Pleistocene small-sized deer of Europe. Hellenic Journal of Geosciences, 41, 89–117.
Croitor, R., & Brugal, J. F. (2010). Ecological and evolutionary dynamics of the carnivore community in Europe during the last 3 million years. Quaternary International, 212, 98–108.
de Vos, J., Leinders, J. J. M., & Hussain, S. T. (1987). A historical review of the Siwalik Hyaenidae (Mammalia, Carnivora) and description of two new finds from the upper Siwalik of Pakistan. Proceedings of the Koninklijke Nederlandse Akademie van Wetenschappen, Series B, 90(4), 333–369.
Di Stefano, G. (1996). The Mesopotamian fallow deer (Dama, Artiodactyla) in the Middle East Pleistocene. Neues Jahrbuch für Geologie und Paläontologie Abhandlungen, 199(3), 295–322.
Di Stefano, G., & Petronio, C. (1998). Origin of and relationships among Dama-like cervids in Europe. Neues Jahrbuch für Geologie und Paläontologie Abhandlungen, 207(1), 37–55.
Di Stefano, G., & Petronio, C. (2002). Systematics and evolution of the Eurasian Plio-Pleistocene tribe Cervini (Artiodactyla, Mammalia). Geologica Romana, 36, 311–334.
Doronichev V. B. (2000). Lower paleolithic occupation on the northern Caucasus. In D. Lordkipanidze, O. Bar-Josef & M. Otte (Eds.), Early Humans at the Gates of Europe. Études et Recherches Archéologiques de l’Université de Liège, 92, 67–77.
Duff, A., & Lawson, A. (2004). Mammals of the world: A checklist. London: A & C Black.
Dufour, R. (1989). Les carnivores Pléistocènes de la Caverne de Marlarnaud (Ariège). Bordeaux: Museum d’Histoire Naturelle de Bordeaux.
Eisenmann, V. (1991). Les chevaux Quaternaires européens (Mammalia, Perissodactyla). Taille, typologie, biostratigraphie et taxonomie. Geobios, 24(6), 747–759.
Eisenmann, V., Alberdi, M. T., de Giuli, C., & Staesche U. (1988). Volume 1: Methodology. In M. Woodburne & P. Sondaar (Eds.), Studying Fossil Horses (pp. 1–71). Leiden: E.J. Brill.
Eisenmann, V., & Mashkour, M. (1999). The small equids of Binagady (Azerbaidjan) and Qazvin (Iran): E. hemionus binagadensis nov. subsp. and E. hydruntinus. Geobios, 32(1), 105–122.
Fernández-Jalvo, Y., King, T., Andrews, P., & Yepiskoposyan, L. (2016). Introduction: Azokh Cave and the Transcaucasian Corridor. In Y. Fernández-Jalvo, T. King, L. Yepiskoposyan & P. Andrews (Eds.), Azokh Cave and the Transcaucasian Corridor (pp. 1–26). Dordrecht: Springer.
Forsten, A. (1988). Middle Pleistocene replacement of stenonid horses by caballoid horses – ecological implications. Palaeogeography, Palaeoclimatology, Palaeoecology, 65, 23–33.
Forsten, A. (1992). Early Equus dispersal and taxonomy: Conflicting opinions. Courier Forschungsintsitut Senckenberg, 153, 171–176.
Forstén, A., & Ziegler, R. (1995). The horses (Mammalia, Equidae) from the early Wuermian of Villa Seckendorff, Stuttgart-Bad Cannstatt, Germany. Stuttgarter Beiträge zur Naturkunde, Serie B (Geologie und Paläontologie), 224, 1–22.
Fortelius, M., Mazza, P., & Sala, B. (1993). Stephanorhinus (Mammalia: Rhinocerotidae) of the western European Pleistocene, with a revision of S. etruscus (Falconer, 1868). Palaeontographia Italica, 80, 63–155.
Franzen, J. L., Gliozzi, E., Jellinek, T., Scholger, R., & Weidenfeller, M. (2000). Die spätaltpleistozäne Fossillagerstätte Dorn-Dürkheim 3 und ihre Bedeutung für die Rekonstruktion der Entwicklung des rhenischen Flusssystems. Senckenbergiana Lethaea, 80(1), 305–353.
Gadziev, D. V., Guseinov, M. M., Mamedov, A. V., & Shirinov, N Sh. (1979). Kratkie rezul’taty complexnih issledovaniy Azikhskoy drevnepaleoitcheskoy stoyanki. Izvestia AN AzSSR, seriya nauk o zemle, 3, 10–16.
García, N., & Arsuaga, J. L. (1999). Carnivores from the Early Pleistocene hominid-bearing Trinchera Dolina 6 (Sierra de Atapuerca, Spain). Journal of Human Evolution, 37(3/4), 415–430.
García, N., Arsuaga, J. L., & Torres, T. (1997). The carnivore remains from the Sima de los Huesos Middle Pleistocene site (Sierra de Atapuerca, Spain). Journal of Human Evolution, 33(2/3), 130–154.
Golanova, L. V., Hoffecker, J. F., Kharitonov, V. M., & Romanova, G. P. (1999). Mezmerskaya Cave: A Neanderthal occupationin the Northern Caucasus. Current Anthropology, 40(1), 77–86.
Guérin, C. (1980). Les Rhinoceros (Mammalia, Perissodactyla) du Miocène terminal au Pléistocène Supérieur en Europe occidentale. Comparaison avec les espèces actuelles. Documents des Laboratoires de Géologie Lyon, 79(1–3), 1–1185.
Guérin, C., & Barychnikov, G. F. (1987). Le rhinocéros acheuléen de la gotte Koudaro I (Georgie, URSS) et le problème des espèces relictes du Pléistocène du Caucase. Geobios, 20(3), 289–396.
Heintz, E. (1970). Les cervidés Villafranchiens de France et d’Espagne. Mémoires du Muséum National d’Histoire Naturelle, nouv. sér., série C, Sciences de la Terre, 22, 1–303, 40 pls., 319 figs., 131 tables.
Hemmer, H. (2001). Die Feliden aus dem Epivillafranchium von Untermassfeld. In R. D. Kahlke (Ed.), Das Pleistozän von Untermassfeld bei Meinignen (Thüringen). Teil 2 (pp. 699–782, pls. 132–143). Bonn: Dr. Rudolf Habelt GMBH.
Hooijer, D. A. (1961). The fossil vertebrates of Ksâr’akil, a palaeolithic rock shleter in the Lebanon. Zoologische Verhandelingen, 49, 1–67, pls. 1–2.
Janashvili, R. (1978). The fauna of the caves. In Exploration of Caves in Colchis. Tbilisi 94–126.
Kahlke, H. D. (2001). Neufunde von Cerviden-Resten aus dem Unterplesitozän von Untermassfeld. In R. D. Kahlke (Ed.), Das Pleistozän von Untermassfeld bei Meinigen (Thüringen), Teil 2. Römisch-Germanisches Zentralmuseum Forschungsinstitut für Vor- und Frühgeschichte. Monographiën 40(2), 461–482, pls. 72–76.
Kahlke, R. D. (1990). Der Saiga-Fund von Pahren. Ein Beitrag zur Kenntnis der paläarktischen Verbreintungsgeschichte der Gattung Saiga Gray 1843 unter besonderer Berücksichtigung des Gebietes der DDR. Eiszeitalter und Gegenwart, 40, 20–37.
Kahlke, R. D. (1994). Die Entstehungs-, Entwicklungs- und Verbreitungsgeschichte des oberpleistozänen Mammuthus-Coelodonta-Faunenkomplexes in Eurasien (Grossäuger). Abhandlungen der Senckenbergischen Naturforschenden Gesellschaft, 546, 1–164.
Knapp, M., Rohland, N., Weinstock, J., Baryshnikov, G., Sher, A., Nagel, D., et al. (2009). First DNA sequences from Asian cave bear fossils reveal deep divergences and complex phylogeographic patterns. Molecular Ecology, 18, 1225–1238.
Koby, F. E. (1951). Les dimensions maxima et minima del os longs d’Ursus spelaeus. Eclogae Geologicae Helvetiae, 43(2), 287.
Kurten, B. (1955). Sex dimorphism and size trends in the cave bear. Acta Zoologica Fennica, 90, 1–47.
Kurten, B., & Poulianos, A. (1977). New stratigraphical and faunal material from Petralona cave with special reference to the carnivores. Anthropos, 4, 47–130.
Leonardi, G., & Petronio, C. (1976). The fallow deer of European Pleistocene. Geologica Romana, 15, 1–67.
Lincoln, G. A., Fraser, H. M., & Fletcher, T. J. (1982). Antler growth in male red deer (Cervus elaphus) after active immunization against LH-RH. Journals of Reproduction and Fertility, 66, 703–708.
Lioubine, V. P. (2002). L’Acheuléen du Caucase. Études et Reschrches Archéologiques de l’Université de Liège, 93, 1–140.
Lister, A. M. (1996). The morphological distinction between bones and teeth of fallow deer (Dama dama) and red deer (Cervus elaphus). International Journal of Osteoarchaeology, 6, 119–143.
Loose, H. (1975). Pleistocene Rhinocerotidae of W. Europe with reference to the recent two-horned species of Africa and S.E. Asia. Scripta Geologica, 33, 1–59.
Madurell-Malapeira, J., Alba, D. M., & Moyá-Solá, S. (2009). Carnivora from the Late Early Pleistocene of Cal Guardiola (Terrassa, Vallès-Penedès, Catalonia, Spain). Journal of Paleontology, 83(6), 969–974.
Marin-Monfort, M. D., Cáceres, I., Andrews, P., Pinto, A. C., & Fernández-Jalvo, Y. (2016). Taphonomy and site formation of Azokh 1. In Y. Fernández-Jalvo, T. King, L. Yepiskoposyan & P. Andrews (Eds.), Azokh Cave and the Transcaucasian Corridor (pp. 211–249). Dordrecht: Springer.
Markova, A. K. (1982). Microteriofauna iz paleoliticheskoy peschernoy stoyanki Azikh. Palaeontoligischeskoy sbornik, 19, 14–28.
Morales, J., Soria, D., Montoya, P., Pérez, B., & Salesa, M. J. (2003). Caracal depereti nov. sp. y Felis aff- silvestris (Felidae, Mammalia) del Plioceno Inferior de Layna (Soria, España). Estudios Geológicos, 59, 229–247.
Murray, J., Lynch, E. P., Domínguez-Alonso, P., & Barham, M. (2016). Stratigraphy and Sedimentology of Azokh Caves, South Caucasus. In Y. Fernández-Jalvo, T. King, L. Yepiskoposyan & P. Andrews (Eds.), Azokh Cave and the Transcaucasian Corridor (pp. 27–54). Dordrecht: Springer.
Musil, R. (1969). Die Equiden-Reste aus dem Pleistozän von Süssenborn bei Weimar. Paläontologische Abhandlungen, Abteilung A Paläozoologie, 3(3–4), 617–666, pls. 37–45.
Musil, R. (1977). Die Equidenreste aus den Travertinen von Taubach. Quartärpaläontologie, 2, 237–264.
O’Regan, H. J., Bishop, L. C., Lamb, A., Elton, S., & Turner, A. (2005). Large mammal turnover in Africa and the Levant between 1.0 and 0.5 Ma. In M. J. Head & P. L. Gibbard (Eds.), Early-Middle Pleistocene transitions: The land-ocean evidence. Geological Society, London, Special Publications 247, 231–249.
Pfeiffer, T. (1998). Capreolus suessenbornensis Kahlke 1956 (Cervidae, mammalia) aus den Mosbach-Sanden (Wiesbaden, Biebrich). Mainzer naturwissenschaftliches Archiv, 36, 47–76.
Pfeiffer, T. (1999). Die Stellung von Dama (Cervidae, Mammalia) im system pleisometacarpaler Hirsche des Pleistozäns. Phylogenetische Reconstruktion – Metrische Analyse. Courier Forschungsinstitut Senckenberg, 211, 1–218.
Qiu, Z. (1979). Some mammalian fossils from the Pliocene of Inner Mongolia and Gansu (Kansu). Vertebrata PalAsiatica, 17(3), 222–235.
Rabeder, G., Hofreiter, M., & Withalm, G. (2004). The systematic position of the cave bear from Potocka zijalka (Slovenia). Mitteilungen der Kommission für Quartärforschung der Österreichischen Akademie der Wissenchaften, 13, 197–200.
Rabeder G., Pacher M., & Withalm G. (2010). Early Pleistocene bear remains from Deutsch-Altenburg (Lower Austria) Mitteilungen der Kommission für Quartarforschung der Österreichischen Akademie der Wissenchaften, 17, 1–135.
Rivals, F. (2004). Les petits bovidés (Caprini et Rupicaprini) pléistocènes dans le bassin méditerranéen et le Caucase. Étude paléontologique, biostratigraphique, archéozoologizue et paléoécologique. British Archaeological Reports International Series, 1327, 1–252.
Rohland, N., Knapp, M., Weinstock, J., Baryshnikov, G., Sher, A., Nagel, D., et al. (2008). The complex biogeography of extinct Eurasian Cave bears. Society for Molecular Biology and Evolution Meeting 5–8 June, Spain.
Ron, H., Porat, N., Ronen, A., Tchernov, E., & Horwitz, L. K. (2003). Magnetostratigraphy of the Evron Member – Implications for the age of the Midle Acheulian site of Evron Quarry. Journal of Human Evolution, 44, 633–639.
Schmid, E., & O. Garraux (1972). Atlas of animal bones for prehistoirans, archaeologists and Quaternary geologists (pp. 1–159). Amsterdam, London & New York: Elsevier.
Sharapov, S. (1986). The Kurksay complex of Upper Pliocene Mammalian of Afghan-Tadjik Depression (pp. 1–269). Academy of Sciences of the Tadjik SST, Dushanbe.
Sickenberg, O. (1976). Eine Säugetierfauna des tieferen Bihariums aus dem becken von Megalopolis (Peloponnes, Griechenland). Annales Géologiques des Pays Helléniques, 1e série, 27, 25–73, pls. 6–10.
Soergel W. (1926). Der Bär von Suszenborn. Ein beitrag zur näheren Kenntinis der diluvialen Bären. Zeitschrift Deustchen Geologischen Gesellschaft, 77 Abteilung B (Geologie und Paläontologie), 115–156.
Sotnikova, M. V., & Vislobokova, I. A. (1990). Pleistocene mammals from Lakhuti, southern Tajikistan, U.S.S.R. Quartär-Paläontologie, 8, 144–237.
Tchernov, E., Kolska Horwitz, L., Ronen, A., & Lister, A. (1994). The faunal remains from Evron Quarry in Relation to other Lower Palaeolithic hominid sites in the southern Levant. Quatenary Research, 42, 328–339.
Teilhard de Chardin, P., & Trassaert, M. (1937). The Pliocene Camelidae, Giraffidae, and Cervidae of South Eastern Shansi. Palaeontologia Sinica, new series C, 1, 1–56, pls. 1–6.
Torre, D. (1967). I cani villafranchani della Toscana. Palaeontographia Italica, 63, 113–138, pls. 10–19.
Torres, T. (1978). Estudio comparativo de las mandíbulas de Ursus spelaeus Ros.-Hein. Ursus deningeri von Reich. y Ursus arctos Linn. Boletín Geológico y Minero, 89(3), 203–222.
Torres, T. (1989). Estudio de la filogenia, distribución estratigráfica y geográfica y análisis morfológico y métrico de esqueleto y dentición de los osos (Mammalia, Carnivora, Ursidae) del Pleistoceno de la Península Ibérica (U. deningeri Von Reichenau, Ursus spelaeus Rosenmüller-Heinroth, Ursus arctos Linneo). Madrid: Publicación Especial Instituto Geológico y Minero de España.
Torres T., & Cervera, J. (1992). Análisis Multivariante de la morfología dental de los Ursidos del Plio-Pleistoceno, con algunas consideraciones sobre la posición filogenética de Ursus deningeri von REICHENAU de Cueva Mayor (Sima de los Huesos), Atapuerca-Burgos. In. E. Aguirre, E. Carbonell & J. M. Bermúdez de Castro (Eds.), Evolución Humana en Europa y las Yacimientos de la Sierra de Atapuerca, 1 (pp. 123–135). Valladolid: Junta de Com. Castilla-León.
Torres T., & Guerrero P. (1993). Análisis multivariante de la morfología de los metápodos de osos espeloides del Pleistoceno Ibérico (Ursus deningeri von Reichenau y Ursus spelaeus Rosenmüller-Heinroth). Abstracts de las IX Jornadas de Paleontología, Málaga 49–54.
Torres, T., Nestares, T., Cobo, R., Ortiz, J. E., Cantero, M. A., Ortiz, J., et al. (2001). Análisis morfológico y métrico de la dentición y metapodios del oso de Deninger (Ursus deningeri Reichenau) de la cueva Santa Isabel de Ranero. Aminocronología (Valle de Carranza-Vizcaya-País Vasco), 51, 107–141.
Torres, T., Ortiz, J. E., Llamas, J. F., Canoira, L., Juliá, R., & García de la Morena, M. A. (2002). Cave bear dentine aspartic acid racemization analysis, proxy for Pleistocene Cave infills dating. Archaeometry, 44(3), 417–426.
Torres, T., Ortiz, J. E., Cobo, R., Julià, R., Camacho, A., Puch, C., & Llamas, J. F. (2006). Presence of two cave bear species in La Lucia cave (Lamasón, Cantabria, N Spain): Ursus deningeri von Reichenau and Ursus spelaeus Rosenmüller-Heinroth. Munibe, 57(1), 103–122.
Touchabramichvili, N. (2003). Les industries du Paléolithique inférieur dans le Caucase méridional. L’Anthropologie, 107, 565–576.
Turner, A. (1990). The evolution of the guild of larger terrestrial carivores during the Plio-Pleistocene in Africa. Geobios, 23(3), 349–368.
Ueckermann, E., & Hansen, P. (2002). Das Damwild. Biologie, Hege und Jagd. Kosmos, Stuttgart 1–327.
Van der Made, J. (1996). Listriodontinae (Suidae, Mammalia), their evolution, systematics and distribution in time and space. Contributions to Tertiary and Quaternary Geology 33(1–4), 3–254, microficha 54 pp.
Van der Made, J. (1999). Ungulates from Atapuerca-TD6. Journal of Human Evolution, 37(3–4), 389–413.
Van der Made, J. (2000). A preliminary note on the rhinos from Bilzingsleben. Praehistoria Thuringica, 4, 41–62.
Van der Made, J. (2001). Les ongulés d’Atapuerca. Stratigraphie et biogéographie. L’Anthropologie, 105, 95–113.
Van der Made, J. (2005a). La fauna, Capítulo 3 – Asia; Sección 3.4. In E. Carbonell (Ed.), Homínidos: Las primeras ocupaciones de los continentes (pp. 270–306). Barcelona: Ariel.
Van der Made, J. (2005b). La fauna del Pleistoceno europeo, Capítulo 4 – Europa; Sección 4.4. In E. Carbonell (Ed.), Homínidos: Las primeras ocupaciones de los continentes (pp. 394–432). Barcelona: Ariel.
Van der Made, J. (2010a). The rhinos from the Middle Pleistocene of Neumark Nord (Saxony-Anhalt). Veröffentlichungen des Landesamtes für Archeologie, 62, 432–527.
Van der Made, J. (2010b). Biostratigraphy – “Large Mammals”. In D. Höhne & W. Schwarz (Eds.), “Elefantentreich – Eine Fossilwelt in Europa”. Landesamt für Denkmalpflege und Archälogie Sachsen-Anhalt & Landesmuseum für Vorgeschichte, Halle 82–92.
Van der Made, J., & Grube, R. (2010). The rhinoceroses from Neumark-Nord and their nutrition. In D. Höhne & W. Schwarz (Eds.), Elefantentreich – Eine Fossilwelt in Europa (pp. 383–394). Halle: Landesamt für Denkmalpflege und Archälogie Sachsen-Anhalt & Landesmuseum für Vorgeschichte.
Van der Made, J., & Tong, H. W. (2008). Phylogeny of the giant deer with palmate brow tines Megaloceros from west and Sinomegaceros from east Eurasia. Quaternary International, 179, 135–162.
Vekua, A. (1986). The lower Pleistocene Mammalian Fauna of Akhalkalaki (southern Georgia, USSR). Palaeontographia Italica, 74, 63–96.
Vekua, A. (1995). Die Wirbeltierfauna des Villafranchium von Dmanisi und ihre biostratigraphische Bedeutung. Jahbuch des Römisch-Germanischen Zentralmuseums Mainz, 42, 77–180, pls. 7–54.
Velichko, A. A., Antonova, G. V., Zelikson, E. M., et al. (1980). Paleogeographia stoyanki Azikh – drevnejshego poselenia pervobytnogo cheloveka na territorii SSSR. Izvestia AN SSSR, seria geograph, 3, 20–35.
Vislobokova, I., Dmitrieva, E., & Kalmykov, N. (1995). Artiodactyls from the Late Pliocene of Udunga, western Trans-Baikal, Russia. Journal of Vertebrate Paleontology, 15(1), 146–159.
von Koenigswald, W. (1986). Beziehungen des pleistozänen Wasserbüffels (Bubalus murrensis) aus Europa zu den asiatischen Wasserbüffeln. Zeitschrift für Säugetierkunde, 51(5), 312–323.
Wagner, G. A., Krbetschek, M., Degering, D., Bahain, J. J., Shao, Q., Falguères, C., et al. (2010). Radiometric dating of the type-site for Homo heidelbergensis at Mauer, Germany. Proceedings of the National Academy of Sciences, 107(46), 19726–19730.
Whitehead, G. K. (1993). The Whitehead Encyclopedia of Deer. Shrewsbury: Swan Hill Press.
Wilson, D. E., & Reeder, D. A. M. (1993). Mammal species of the world – a taxonomic and geographic reference (2nd ed.). Washington & London: Smithsonian Institution Press.
Wolsan, M. (2001). Remains of Meles hollitzeri (Carnivora, Mustelidae) from the Lower Pleistocene site of Untermassfeld. In R. D. Kahlke (Ed.), Das Pleistozän von Untermassfeld bei Meinigen (Thüringen), Teil 2. Römisch-Germanisches Zentralmuseum Forschungsinstitut für Vor- und Frühgeschichte. Monographiën 40(2), 659–671.
Xue X., & Zhang Y. (1991). Chapter 10. Quaternary mammalian fossils and fossil human beings. In Z. Zhang, S. Shao, G. Tong & J. Cao (Eds.), The Quaternary of China (pp. 307–374). Beijing: China Ocean Press.
Zapfe, H. (1946). Die altplistozänen Bären von Hundsheim in Niederösterreich. Jahrbuch der Geologischen Bundesanstalt, 3–4, 95–164.
Acknowledgements
In the context of the project INTAS AO1051S, directed by H. de Lumley, JvdM had the opportunity to study collections in Baku and Tbilisi, in the care of S.D. Aliev and D. Lordkipanidze and benefitted from discussions with F. Rivals, P.E. Moullé, and A. Arellano. In addition JvdM received support by projects CGL2006-13532-C03-03 and CGL2008-03881 and YFJ by CGL 2007-66231 and CGL2010-19825 projects of the Spanish Ministerio de Ciencia y Inovación.
The following persons allowed access to material for comparison: F. Alférez Delgado, J. Barreiro, J.M. Bermúdez de Castro, C. Brauckmann, P. Cabot, E. Carbonell, E. Cioppi, E. Crégut-Bonnoure, G. Bossinski, A Currant, M. Dermitzakis, M. Esteban, O. Feifar, E. Frey, late J. Gibert, E. Gröning, J.H. Grünberg, F. Gusi, O. Hampe, W.-D. Heinrich, C. Heunisch, N. Ibañez, J. Jagt, R.D. Kahlke, F. Lacombat, L. Kordos, G. Koufos, D. Lordkipanidze, H. de Lumley, H. Lutz, G. Lyras, D. Mania, H. Meller, A.M. Moigne, W. Munk, R. Musil, M. Patou-Mathis, E. Pons, D. Reeder, J. Rodríguez, L. Rook, B. Sánchez Chillón, C. Smeenk, late P.Y. Sondaar, M. Sotnikova, U. Staesche, late E.Tchernov, Tong Haowen, E. Tsoukala, E. Turner, K. Valoch, I. Vislobokova, J. de Vos, R. van Zelst, R. Ziegler.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2016 Springer Science+Business Media Dordrecht
About this chapter
Cite this chapter
Van der Made, J., Torres, T., Ortiz, J.E., Moreno-Pérez, L., Fernández-Jalvo, Y. (2016). The New Material of Large Mammals from Azokh and Comments on the Older Collections. In: Fernández-Jalvo, Y., King, T., Yepiskoposyan, L., Andrews, P. (eds) Azokh Cave and the Transcaucasian Corridor. Vertebrate Paleobiology and Paleoanthropology. Springer, Cham. https://doi.org/10.1007/978-3-319-24924-7_6
Download citation
DOI: https://doi.org/10.1007/978-3-319-24924-7_6
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-24922-3
Online ISBN: 978-3-319-24924-7
eBook Packages: Earth and Environmental ScienceEarth and Environmental Science (R0)