Abstract
In Pectus Excavatum, the sternum is cast-down and depressed into a convex shape. The sternal malformation is caused by the extensive growth of the costal cartilages, inserting into the sternal body. The growth allows the cartilages to clump together and further push the sternum inwards. Having an appendicular derivative, the sternum develops from two sternal bar components. The following chapter aims to evaluate the embryological contributing factors to the development of Pectus Excavatum.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
Developmental Anatomy of Pectus Excavatum
In pectus excavatum, the sternum is cast-down and depressed into a convex shape. The sternal malformation is caused by the extensive growth of the costal cartilages, inserting into the sternal body. The growth allows the cartilages to clump together and further push the sternum inwards. Many pathophysiological hypotheses exist regarding the primary cause of pectus excavatum. These hypotheses refer to intrauterine mechanical factors, respiratory muscular imbalance in diseases like Spinal Muscular Atrophy type I and developmental delays being responsible. Present day theories centralise on the cause being a developmental disorder, allied with maturation disturbances of the sternocostal cartilage. To further support this hypothesis, histological changes in the sternocostal cartilage of those with pectus excavatum were observed [1]. Another instance of rib malformation is the cervical rib. This occurs when the thoracic wall of adults is arranged in an oblique manner, with the ribs angled forwards and downwards in an unorthodox fashion.
Embryological Development of the Thoracic Cage and Sternal Development
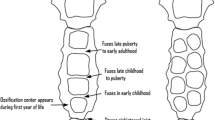
Having an appendicular derivative, the sternum develops from two sternal bar components. The sternum develops independently of both ribs and the pectoral girdle. It originates in the lateral somatopleuric mesoderm, from the ventromedial sclerotome at the body wall and forms as a pair of mesenchymal condensations. The pair of cranio-caudally orientated sternal bars assemble alongside the midline and fuse to form the cartilaginous model of the manubrium, sternabrae and xiphoid process (the three main components of the sternum). The primary ossification centres of the all parts of the sternum, excluding the xiphoid process, appear before birth. With respect to the xiphoid process, the centre of ossification is observed during childhood. In the case of incomplete fusion of the respective parts of the sternum, perforation becomes a major issue. Development of the sternal body begins in the 6th gestational week. The sternal primordia (tissue in early development) move towards each other and protract to congregate into sternal bars that make contact with the primordial ribs. The sternal bars commence fusing at the manubrium’s midline and proceed chondrify to a cartilaginous model by week 10 (Fig. 2.1) [2].
Embryologic and postnatal development of the human sternum [2]
The caudal extension of the sternal bars forms the xiphoid process. The segmentation of the mesosternum into the sternal body is influenced by the ribs and their respective attachment sites [3].
There are cases where the centre of ossification evolves before sternal bar component fusion. These centres of ossification can form in both bars. Conventionally, ossification of the sternum is much later than fusion; it begins during the 5th gestational month. The manubrium is the first to ossify, whereas the last to ossify is the xiphoid process. This stage of development only begins after 3 years of age – the age can be variable [4].
Rib Development
The ribs are also derived from the sclerotome portion of the paraxial mesoderm that forms the vertebrae’s costal processes. They form as elongations of the costal process of the thoracic vertebrae. Primary ossification centers appear in the body of the ribs at 14 weeks of development. The ribs are more horizontal in the infant, with less curvature, in comparison to the adult. The secondary ossification centres appear at the rib’s tubercle during puberty. Ribs 1–7 (referred to as true ribs) attach to the sternum through their own cartillages. The false ribs [5–8] attach to the sternum by the cartilage of another rib or adjacent ribs and the floating ribs don’t attach to the sternum [1, 9]. The costal cartilages’ embryological origin is through migrating sclerotome cells that move from the lateral somatic boundary into the lateral plate mesoderm. The costal cartilages join the sternal end of the ribs and then attach to the sternum at articular facets. Sternocostal joints formed here are synovial and are supported by ligaments that anchor the costal cartilages to the sternal sides. Articular cavities are commonly replaced by fibrocartilage, however, and are known as chondrosternal joints. The ribs attach when the fusion of sternum bars are complete; the sternal tissue band guides the formation of the ventral part of the rib. It is known that chondroblasts are involved in the development of the sternocostal articulation. Cells from the perichondrium proliferate between the sternum and ribs to form the sternocostal joints [5]. New hypotheses state that a faulty mechanism behind sternocostal joint development is a causative factor for congenital chest wall deformities and sternal depressions.
Similarities and Differences in Thoracic Development in Humans, Compared to Other Animals
Moreover, the vertebral column is derived from the somatic sclerotomes, whereas the ribs are formed by condensations of sclerotomal cells and the intercostal muscles are derived from the dermomyotomes [6]. Additionally, the distal parts of the ribs have been found to partially originate from the dermomyotomes [7]. Kato and Aoyama concluded that these findings were linked to the removal and the transplantation of the dermomyotomes in avian embryos. The results of R. Huang et al. disagree and show, with clarity, the proximal and the distal parts of the ribs being formed by the sclerotomal mesenchyme. Huang et al. go on to explain that Kato and Aoyama’s interpretation was due to a grafting technique in which three consecutive thoracic dermomyotomes with adjacent lateral plate mesoderm were grafted together. R. Huang’s method avoided the inclusion of sclerotomal cells into the graft by isolating a single dermomyotomes. The study also concludes that morphogenic Pax-1 protein is required for the formation of the proximal ribs. This is reinforced in a study by Braun et al., stating that the distal parts are missing in Myf5 and Pax-3 deficient mice. In a contrasting study by Wallin et al., the proximal ribs are not apparent in Pax-1 deleted mice [6]. Formation of the distal ribs may, therefore, depend on communication between the sclerotome and myotome. Both studies were conducted on mice, which have a similar embryological development to humans.
The formation of vertebrae and ribs in veterinary medicine is alike that in human medicine. Somite sclerotomes migrate to surround the neural tube as a mass. This mass proceeds to create a cartilaginous model. The diffuse region of one somite joins with the dense region of another neighbouring somite. Sclerotome mesenchyme forms annulus fibrous (outer coating of the intervertebral disc) and the notochord forms the nucleus pulposus (gel substance filling the spinal disc). The ribs then are developments from the thoracic vertebral processes.
Mammals have thoracic vertebral ribs only. Marsupials and placental mammals, on the other hand, have cervical and lumbar ribs that are found only as remnants fused to vertebral transverse processes. Birds have most of their ribs in the thoracic region with the exception of small fused cervical ribs. Fish have two sets of ribs attached to the vertebral column. The dorsal ribs are found in the septal area in between inferior and superior musculature and project sideways. In contrast, the ventral ribs begin caudal to the dorsal ribs. Sharks only have short ventral ribs and lack a dorsal set. Between amphibians and reptiles, there is variation in rib number. Turtles have eight pairs of ribs. These ribs form a plastron, which constitutes the flat part of a turtle’s shell and a cartilaginous carapace in the upper exoskeleton. Frogs have no ribs, except for a functional sacral pair forming the pelvis and allowing for stabilisation of movement. Surprisingly, dogs have 26 ribs. A Pekingese dog with hemivertebrae, rib malformations and spinal cord dysraphism (without spina bifida) was documented in a mid-90s study. Thoracic hemivertebrae was observed alongside anomalies of the dorsal median septum. To conclude, the dog showed malformations of the vertebral regions and ribs from mesodermal origin. Spinal dysraphism is of ectodermic origin, when considering embryological defaults. This suggests that mammalian thoracic defaults, between humans and dogs, can possibly be explained by embryological developmental problems [8].
Epidemiology
The prevalence of Pectus excavatum is estimated to be between 0.1 and 0.8 % of the cohort examined [5]. A large autopsy series conducted to estimate the incidence of pectus excavatum and other associated conditions has concluded a value lower than Brochhausen et al. of 0.12 %. Following survival analysis, it was found that pectus excavatum patients had a noticeable tendency to die earlier than the control group evaluated (P = .0001). In contrast, it was also found that the pectus excavatum patients who survived past the age of 56 years, survived longer than the same control group (P = .0001) [9]. A frequent birth prevalence estimate is 0.25 % (1/400 people) and pectus carinatum is two to four times less common than pectus excavatum [1]. A male to female predominance of pectus excavatum exists at a ratio of 5:1 or higher. In 15–40 % of cases there is a close relative on either side of the family with the same deformity. A higher preponderance among Caucasians was also found; out of all patients with idiopathic pectus excavatum studied in a teaching hospital in the USA, 89 % were Caucasians, 9 % were Hispanic and only 2 % were Asian [10]. The chest deformity is rare in Africa and authors have only observed 10 African-American patients out of more than 1000 patients studied. Furthermore, pectus excavatum comprises 87 % of chest wall deformities. Although this is the majority, pectus excavatum is linked with a number of associated ailments. For example, it is estimated that 19 % of pectus excavatum patients had clinical features suggestive of Marfan’s syndrome. Males have an increased risk of this deformity and females have an increased risk of associated scoliosis (scoliosis was identified in 29 % of patients.) In addition, Ehlers-Danlos syndrome was present in another 2.1 % of patients [11]. Eighty-six percent of PE cases are discerned in the first year of life and the majority of the remaining cases present in early adolescence. It is uncommon for resolution of the depression to occur spontaneously during this period and the depression is found to worsen during puberty [12]. There are limited family studies conducted to evaluate and determine the inheritance pattern of pectus excavatum. Creswick et al., however, surveyed 34 families and the majority (41 %) were found to display autosomal dominant inheritance. Autosomal recessive inheritance was apparent in 12 % and X-chromosomal inheritance in 18 % of families [13].
Probable Causes of Chest Wall Deformity
Causation of chest wall deformities is multifactorial. The condition is commonly associated with several genetic syndromes. Bauhinus first suggested an increase in diaphragmatic pressure, seen during embryonic development, to be a principal pathophysiologic attributing factor [5]. One opinion was that intrauterine pressure on the sternum through an abnormal position of the embryo could lead to repositioning and subsequent deformation [14]. Following viewpoints called upon permanent mechanical stress through extreme repositioning to be the main causative factor.
Brown published observations of sternal retraction due to a thickening of the ligamentum substernale [15–18]. Some suggested an imbalance between the anterior and posterior formation of muscle fibres of the anterior part of the diaphragm to cause sternal regression and further xiphoid movement. Other hypotheses discuss diseases such as syphilis or rickets as contributing factors to pectus excavatum (PE).
Studies assessing the histological changes in sternocostal cartilage in PE patients reveal premature ageing of the cartilage. Biochemical studies demonstrate decreased levels of zinc and increased levels of magnesium and calcium in the costal cartilages of PE patients. Reasons given for this observation are that zinc deficiency results in lower chondrocyte metabolic activity. Feng et al.’s study concluded a correlation between metabolic lesions and mechanical strength of the cartilage [18].
Most recent focus is on biomechanical weakness caused by an insufficient sternocostal cartilage metabolism. Nakaoka et al. showed that the costal cartilage on the side of deepest impression has no correlation with that of the contralateral side [16]. On the contrary, Fokin et al., found matrix disorganization in the cartilage of PE patients and suggested that to be the reason for sternal cartilage overgrowth [17].
In conclusion, both cartilaginous overgrowth and developmental disorders may contribute to the development of PE and other chest wall deformities.
My Hypothesis
I would like to propose the following hypothesis, stating that a growth factor-like signaling molecule is responsible for the rate and extent to which sternal cartilage and ribs unite. If there is a defect in the signaling pathway due to a mutation in one of the growth receptors or paracrine signals, the cartilage is either pushed forward or compressed downwards, leading to malformation and re-positioning (Fig. 2.2).
References
Cobben JM, Oostra RJ, van Dijk FS. Pectus excavatum and carinatum. Eur J Med Genet. 2014;57(8):414–7.
van der Merwe AE, Weston DA, Oostra RJ, Maat GJ. A review of the embryological development and associated developmental abnormalities of the sternum in the light of a rare palaeopathological case of sternal clefting. Homo. 2013;64(2):129–41.
Barnes E. Developmental defects of the axial skeleton in paleopathology. Niwat: University Press of Colorado; 1994.
Scheuer L, Black S. The juvenile skeleton. Amsterdam: Elsevier/Academic; 2004.
Brochhausen C, Turial S, Müller FK, et al. Pectus excavatum: history, hypotheses and treatment options. Interact Cardiovasc Thorac Surg. 2012;14(6):801–6.
Huang R, Zhi Q, Schmidt C, Wilting J, Brand-Saberi B, Christ B. Sclerotomal origin of the ribs. Development. 2000;127(3):527–32.
Kato N, Aoyama H. Dermomyotomal origin of the ribs as revealed by extirpation and transplantation experiments in chick and quail embryos. Development. 1998;125(17):3437–43.
Ruberte J, Añor S, Carretero A, et al. Malformations of the vertebral bodies and the ribs associated to spinal dysraphism without spina bifida in a Pekingese dog. Zentralbl Veterinarmed A. 1995;42(5):307–13.
Kelly Jr RE, Lawson ML, Paidas CN, Hruban RH. Pectus excavatum in a 112-year autopsy series: anatomic findings and the effect on survival. J Pediatr Surg. 2005;40(8):1275–8.
Koumbourlis AC. Pectus excavatum: pathophysiology and clinical characteristics. Paediatr Respir Rev. 2009;10(1):3–6.
Kelly Jr RE, Croitoru D, Nuss D. Chest wall anomalies: pectus excavatum and pectus carinatum. Adolesc Med Clin. 2004;15(3):455–71.
Obermeyer RJ, Goretsky MJ. Chest wall deformities in pediatric surgery. Surg Clinic North Am. 2012;92(3):669–84.
Kotzot D, Schwabegger AH. Etiology of chest wall deformities—a genetic review for the treating physician. J Pediatr Surg. 2009;44(10):2004–11.
Williams CT. Congenital malformation of the thorax great depression of the sternum. Trans Path Soc. 1872;24:50.
Brown AL. Pectus excavatum (funnel chest). J Thorac Surg. 1939;9:164–84.
Nakaoka T, Uemura S, Yano T, Nakagawa Y, Tanimoto T, Suehiro S. Does overgrowth of costal cartilage cause pectus excavatum? A study on the lengths of ribs and costal cartilages in asymmetric patients. J Pediatr Surg. 2009;44:1333–6.
Fokin AA, Robicsek F, Watts LT. Genetic analysis of connective tissue in patients with congenital thoracic abnormalities. Interact Cardiovasc Thorac Surg. 2008;7:56.
Feng J, Hu T, Liu W, Zhang S, Tang Y, Chen R, et al. The biomechanical, morphologic, and histochemical properties of the costal cartilages in children with pectus excavatum. J Pediatr Surg. 2001;36:1770–6.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2016 Springer International Publishing Switzerland
About this chapter
Cite this chapter
Kolvekar, S.K., Simon, N.L., Kolvekar, T. (2016). Developmental and Epidemiology. In: Kolvekar, S., Pilegaard, H. (eds) Chest Wall Deformities and Corrective Procedures. Springer, Cham. https://doi.org/10.1007/978-3-319-23968-2_2
Download citation
DOI: https://doi.org/10.1007/978-3-319-23968-2_2
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-23966-8
Online ISBN: 978-3-319-23968-2
eBook Packages: MedicineMedicine (R0)