Abstract
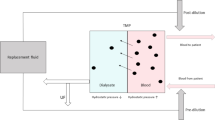
Mortality in patients under a conventional dialysis regimen of three 4-h sessions per week is four times higher than in the general population older than 65 years and new therapeutic regimens are required to improve patient survival. The thrice-weekly frequency of hemodialysis (HD), established the 1960s, has mainly been accepted and maintained since then for logistic, pragmatic and economic reasons. However, longer and more frequent dialysis sessions have produced excellent survival and clinical advantages. The results of the Tassin experience of long, slow-flow HD were first reported 30 years ago and showed excellent fluid and blood pressure control with the highest survival rates achieved at that time. Since then, multiple publications reported on the superiority of long-duration HD over conventional therapy. On-line postdilution hemodiafiltration (OL-HDF) offers an optimal form of extracorporeal treatment for patients with end-stage kidney disease. This technique, which combines diffusion with a considerable amount of convection, provides the highest clearances per unit of surface area for small, medium and large molecules. In this chapter we describe our experience with OL-HDF in two extended dialysis schemes: short daily OL-HDF and nocturnal, every-other-day, OL-HDF.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Daily dialysis
- Frequent dialysis
- Long dialysis
- Nocturnal dialysis
- Every-other-day dialysis
- Occupational rehabilitation
- Medication reduction
Introduction
Although the mortality rate for hemodialysis (HD) patients fell by 25 % from 2003 to 2012 in comparison with only 3 % from 1993 to 2002 [1], clinical outcome in this patient groups is still unacceptable poor and has recently been linked to the long inter-dialytic interval [2–4]. The limitations of thrice-weekly conventional HD in preventing the sequelae of chronic kidney disease (CKD), such as cardiovascular disease, CKD-mineral and bone disorder (CKD-MBD), hypertension, metabolic acidosis, hyperkalemia, inflammation, malnutrition and poor quality of life, are well known to the nephrology community [5]. Potential mechanisms for the adverse effects related to intermittent HD have recently been reviewed. [6] Most observational data agree that frequent dialysis schemes reduce fluctuations in metabolic and volume parameters, if compared with thrice-weekly schedules [7]. A recent review suggested that intensified dialysis schemes (daily, nocturnal or every-other-day) significantly improved blood pressure control, CKD-MBD and quality of life [8]. On-line postdilution hemodiafiltration (OL-HDF), which combines diffusion with high convective transport, provides the highest clearances per unit of surface area for small, medium, and large molecules. During the last 10 years, three randomized clinical trials have analyzed OL-HDF survival as a primary end point [9–11]. Only the ESHOL study [10] demonstrated improved survival in patients receiving OL-HDF and recent meta-analyses have confirmed the lower overall and cardiovascular mortality of this modality [12, 13]. This chapter aims to describe our personal experience with OL-HDF in two extended dialysis schemes: short daily OL-HDF and nocturnal, every-other-day, OL-HDF [14–16].
Daily Hemodiafiltration
Introduction
Daily HD has been shown to improve clinical outcomes and laboratory parameters, if compared to intermittent HD. The underlying mechanisms are the more physiological technique, which avoids volume overload and electrolyte and acid-base balance disturbances in the inter-dialytic periods, and better removal of uremic toxins [6]. The combination of daily dialysis and a high convective transport technique, such as OL-HDF, offers both higher removal of middle-sized and large molecules and better tolerance to dialysis, and is currently considered a good treatment option for patients requiring chronic renal replacement therapy [14].
Study Design and Practical Implementation
In 2003, we reported the first experience of combining a more physiological and effective dialysis schedule – daily dialysis – with the dialysis modality that offers the highest solute and uremic toxin removal (OL-HDF) [14]. This single-center, prospective and nonrandomized study, included eight stable patients treated for 4–5 h with 3×/week ‘standard’ OL-HDF (S-OL-HDF), who were switched to 2–21/2 h 6×/week ‘daily’ OL-HDF (D-OL-HDF). In both treatment options, bicarbonate based buffer was used, a 1.8 m2 high-flux polysulfone filter (HF80, Fresenius, Bad Homburg, Germany), a blood flow (Qb) of 445 ± 54 mL/min (350–560 mL/min), a dialysate flow (Qd) of 800 mL/min minus the infusion flow (Qi 80–150 mL/min) and a Fresenius 4008 monitor. Reinfusion was performed in the postdilution mode. All patients had native arteriovenous fistulas and 15-gauge needles were used in both OL-HDF schedules. Hence, the only changes were the frequency and duration of each session.
Impact of Daily Hemodiafiltration on Various Biomarkers
Although dialysis time was similar in both schedules, an increase in the dialysis dose was obtained with D-OL-HDF, confirming the beneficial effect of the higher frequency. Although weekly spKt/V and weekly eKt/V were similar between the two study periods, the EKR and standard Kt/V, proposed to measure the dialysis dose in dialysis regimens with different frequencies, were 26 % and 48 % higher on D-OL-HDF, respectively. Weekly urea reduction rate (URR) was 52 % higher in the daily schedule. This parameter is especially useful for showing differences between regimens with dissimilar frequencies and can be used for any solute.
Mean reduction ratios for urea, creatinin, osteocalcin, β2-microglobulin (B2M), myoglobin and prolactin were lower per session with D-OL-HDF, but the weekly reduction ratios were significantly increased on D-OL-HDF (see Fig. 21.1). The increased solute removal was more significant in solutes with greater molecular size or lower intercompartment mass-transfer coefficient (Kc) and could be explained by the creation of solute disequilibrium gradients by resistance to diffusion within tissues and organs. The resistance to diffusion can be quantified as Kc and is molecular size – sensitive. To obtain the same time average concentration in patients treated intermittently, a higher dialysis dose must be used than in daily or continuous treatment. This phenomenon is magnified in solutes with a lower Kc than urea.
Increase in the weekly percentage removal of a broad spectrum of solutes with short daily on-line hemodiafiltration (D-OL-HDF) in comparison with three times a week on-line hemodiafiltration (OLHDF). Abbreviations are: Crea creatinine, Osteo osteocalcin, β2-m microglobulin, Myo myoglobin, PRL prolactin (Reprinted from Maduell et al. [14]. With permission from Nature Publishing Group)
Although serum phosphate did not change, phosphate binders (calcium carbonate in seven patients and calcium acetate plus aluminium hydroxide in one patient) were reduced from 7.3 ± 3 tablets/day in S-OL-HDF to 2.9 ± 3 tablets/day after 3 months and 2.85 ± 4 after 6 months (P < 0.001) in D-OL-HDF. As reported by Daugirdas et al. [17] and Ayus et al. [18], for better control of predialysis phosphate levels, it is probably not enough to change the frequency of the schedule when the duration of weekly dialysis treatment is not increased as well.
The impact of frequent HD regimens on anemia control remains unclear. Some studies showed minor improvements in hemoglobin (Hb) and reductions in erythropoiesis-stimulating agent (ESA) requirements with more frequent weekly sessions, whereas others did not [8]. In our study, changes in Hb levels or ESA dose were not observed. Ferritin levels decreased over time and iron supplements were raised gradually to improve functional iron deficiency. The use of intravenous iron in daily dialysis experiences has not been clearly specified in the literature, but it is possible that iron needs are higher than in standard HD.
Clinical Impact of Daily Hemodiafiltration
After 6 months of treatment with D-OL-HDF, mean body weight increased by 1.5 kg, which was accompanied by an improvement in appetite and normalized protein catabolic rate (nPCR). These findings were confirmed in a 1-year extension of the study when the gain in body weight reached 3 kg [15]. There were no changes in nutritional parameters in the control group. A similar experience was published in children who were treated with daily predilution OL-HDF [19]. The authors demonstrated improved catch-up growth and significant weight gain with D-OL-HDF, which was related to better nutritional status and less uremic protein wasting, and possibly with a better response to growth hormone administration.
The most common cause of mortality in chronic HD patients is cardiovascular disease, amounting up to 50 % of cases. In our experience, the switch to D-OL-HDF improved several risk factors, such as hypertension, hyperuricemia and hyperhomocysteinemia. Although our patients were relatively well controlled at baseline (only three patients were hypertensive and two were receiving drugs), better blood pressure control was achieved without antihypertensive medications. In addition, we observed a marked regression of left ventricular hypertrophy (LVH) and left ventricular mass index (LVMI) 6 months after switching from S-OL-HDF to D-OL-HDF.
In all patients who were treated with D-OL-HDF, the treatment schedule was well accepted and tolerated. There were no local infections, thrombosis or bleeding of the vascular access. No changes were observed in the frequency of nausea, dizziness, cramps, or hypotensive episodes. In the first 4 weeks, a rapid improvement was reported in headache (n = 3), sleep (n = 3), sexual disorders (n = 2), thoracic pain (n = 1), appetite (n = 5) and thirst (n = 2). The most apparent benefit was the reduction in the mean post-dialysis fatigue intensity score, from 1.88 ± 1.2 in S-OL-HDF to 0.38 ± 0.7 in D-OL-HDF (P < 0.01), and the mean fatigue duration score from 1.75 ± 1.4 in S-OL-HDF to 0.25 ± 0.5 in D-OL-HDF (P < 0.01).
Summary of Daily Hemodiafiltration
D-OL-HDF is a well tolerated dialysis scheme, which improves clinical outcome and quality of life. For logistic and economic reasons, however, this modality should be restricted to certain patient groups, such as those with severe cardiovascular disease not allowing long inter-dialytic periods and patients with poorly controlled hypertension, as recommended by the European Best Practice Clinical Guidelines [20]. D-OL-HDF schedule is also suitable for patients with hyperphosphatemia, but only if accompanied by an increased duration of the dialysis treatment. See Table 21.1.
Teaching Points (I)
-
Daily hemodiafiltration recommended in
-
Severe cardiovascular disease
-
Poorly controlled hypertension
-
Hyperphosphatemia
-
-
Clinical advantages of daily hemodiafiltration
-
Higher dialysis dose (Kt/V)
-
Improved nutritional state
-
Regression of LVH
-
Improved phosphate control
-
Reduction in phosphate binders and antihypertensive medication
-
Nocturnal, Every-Other-Day Hemodiafiltration
Introduction
More than 30 years have passed since the Tassin group reported their experience of long, slow-flow HD sessions, showing excellent blood pressure and fluid control with the highest survival rates achieved at that time [21]. Since then, multiple publications have demonstrated the superiority of long-duration HD (8 h) over conventional therapy (3–4 h) in terms of blood pressure control, reduction of LVH and reduced serum phosphate levels, often allowing phosphate binders to be discontinued [22–24]. Apart from these clinical advantages, longer (nocturnal) HD also improves quality of life and patient survival [25]. Interest in this thrice weekly prolonged HD modality has increased in the last 10 years, resulting in publications from Canada [23], Germany [24], USA [25], the United Kingdom [26], and Turkey [27]. An every-other-day HD scheme has been used by the Lecce group in Italy since 1972. Survival at 10 years was 60 %, with a lower incidence of ischemic heart disease, stable high depurative efficiency and improvements in anemia, acid-base and nutritional status [28].
Study Design and Practical Implementation
Our experience of combining a more physiological and effective dialysis schedule – long (nocturnal) and more frequent (every-other-day) dialysis – with the dialysis modality that offers the highest solute and uremic toxin removal (OL-HDF) was the first reported in the literature. The study began in September 2007, and the first data published were the results of 26 patients receiving this dialysis schedule for at least 12 months. The study was initially designed as a cross-sectional study, which compared the effect of a switch from 4–5 h 3×/week OL-HDF to 7–8 h nocturnal, every-other-day OL-HDF with the same (20–30 L) or higher (35–50 L) convective volume to evaluate the impact of this schedule on solute removal and analytical and clinical outcomes [16]. Since the publication of these data, we have increased the number of patients to 52, and 26 patients have completed 24 months of follow up (unpublished data). As all these patients were stable and had good prospects for improved occupational, psychological and social rehabilitation, they were younger than the general dialysis population. In nocturnal dialysis schemes, patients are free to carry out their routine activities during the day, which enhances their quality of life.
At baseline, OL-HDF parameters consisted of conventional OL-HDF, 4–5 h 3×/week with bicarbonate buffered dialysate, 1.4–1.8 m2 high-flux helixone filters (FX60 or FX80, Fresenius), Qb of 440 ± 33 mL/min (400–500 mL/min), Qd 800 mL/min, Qi 90–110 mL/min and a Fresenius 4008 or 5008 dialysis monitor. Reinfusion was always performed in the postdilution mode. All patients had native arteriovenous fistulae and only 15-G needles were used. The duration of the sessions increased from 273 ± 19 min (240–300 min) at baseline to 471 ± 22 min (420–480 min) in nocturnal every-other-day OL-HDF. Qb was 439 ± 33 mL/min at baseline and 421 ± 32 mL/min at 12 months. The convection volume was 26.7 ± 2 L at baseline, 27.5 ± 2 with the unchanged volume and 42.9 ± 4 L with the higher volume. Once these patients finished the 12-month follow-up, all received the maximum convective volume, the average being 45.8 ± 4 L. This study was performed with a new generation of dialysis machines with auto-substitution systems as described in Chaps. 5, 6, 7, 8, 9, and 10. We have used these 5008 monitors since 2013 and observed a 13 % increase in the convective volume [29].
During the study, the surface area of the dialyzers was reduced to 1.0–1.4 m2, while Qd fall to 500 mL/min. At present, it is unknown whether a high Qd improves convective transport. Recently, we reported our experience of varying the Qd (300, 400, 500, 600 and 700 ml/min) on convection volume and removal efficacy in 59 patients treated with OL-HDF. As expected, dialysate volume increased from 86.5 ± 4 L (Qd 300 ml/min) to 201 ± 10 L (Qd 700 ml/min) per session, while changes in the amount of substitution volume were not observed. Kt increased from 67.98 ± 6.9 L (Qd 300 ml/min) to 75.53 ± 7.3 L (Qd 700 ml/min). No changes were observed in other medium and large molecules studied. As the variation of Qd in OL-HDF did not change the convection volume we recommend reducing it as far as possible to ensure an adequate dialysis dose at the lowest consumption of water and dialysis concentrate [30].
Impact of Nocturnal Every-Other- Day Hemodiafiltration on Various Biomarkers
To match the dialysis dose in frequent dialysis regimens, stdKt/V has been proposed, and in comparison with thrice-weekly dialysis schemes, our study employed a higher dose per session. Lecce [28] reported a weekly Kt/V of 4.6, which is comparable to a stdKt/V of 2.36, while in our study stdKt/V increased from 1.75 at baseline to 3.77 at the end of the study [16].
All studies of long-duration dialysis have reported excellent anemia control. Initially, we observed a reduction in ESA dosing, while at the end of the study, ESA was discontinued in 29 % of the patients [16]. In a subsequent follow-up in a larger number of patients, ESA dosing fall by 40 % and was discontinued in 35 % of the patients, while the erythropoietin resistance index (ERI) decreased by 50 %.
During follow-up, bicarbonate levels increased significantly. Comparable results are reported by Ok et al. [27] with nocturnal dialysis and by the Lecce experience with every-other-day dialysis [28]. As expected, blood-urea-nitrogen (BUN) and serum creatinin levels were also significantly reduced.
We also found better phosphate control (pre-treatment phosphate decreased from 4.93 to 3.74 mg/dL) and a decreased need for phosphate binders, from 77 to 4 %. Actually, addition of phosphorus supplements in the dialysate was required in 55 % of the patients (see Fig. 21.2). This improved phosphate control could be explained by the sum of several factors. First, the dialysis dose was higher than in other studies; second, some studies have demonstrated that OL-HDF increases phosphate depuration with a reduction in predialysis levels [31, 32]. Finally, the increased frequency (every-other-day) is another advantage, as studies of daily nocturnal dialysis have observed excellent phosphate control without phosphate binders and with phosphate supplementation in the dialysate [33, 34]. In children, Thumfart et al. also reported a significant reduction in serum phosphate and PTH levels in both dialysis modalities (NHD and NHDF) in comparison with standard HD, despite discontinuation of phosphate binders [5].
Evolution of serum phosphate, phosphorus binders and phosphate dialysis supplement when switching from thrice-weekly OL-HDF to nocturnal every-other-day OL-HDF. Patients were randomized to 6 months with the same convective volume as previously (20–30 L) followed by 6 months with a higher (35–50 L) convective volume (Group A) or to the same two schedules but in reverse order (Group B) (Reprinted from Maduell et al. [16]. With permission from Oxford University Press)
Surprisingly, plasma β2M levels did not decrease during follow-up, despite a significant increase in convection volume, treatment time and frequency. Of note, Ok et al. [27] did not observe reduced pre-dialysis β2M levels just by changing the dialysis duration. The result of β2M removal, as a marker of middle molecule solutes, largely depends on convection processes. In our study, there were no differences between values at baseline and at 6 months with the same convective volume, indicating that removal of β2M mainly depends on the total convective volume, independent of the dialysis time.
Different patterns of solute removal were observed, which were related to dialysis time, convection volume, and/or Qi. To confirm this clinical observation, we performed a new study to evaluate the influence of dialysis duration and infusion flow on the removal of different molecular weight solutes and to verify the usefulness of two-compartment mathematical models in quantifying the changes in removal kinetics when the type of dialysis is changed. In this study, the removal of β2M was significantly increased after changes in both Qi and treatment duration, resulting in an 11 % higher reduction ratio, on average, by doubling the treatment time, and a 6 % improved reduction ratio by doubling convection. These results were confirmed in the mathematical two-compartment model [35]. The removal of larger molecules, such as myoglobin and prolactin, was significantly lower when the same convection volume was applied (see Fig. 21.3). For these high molecular weight molecules the impact of Qi is clearly independent of dialysis time [35].
Comparison of percentages of the reduction ratio in myoglobin (17,184 Da) and prolactin (23,000 Da) for each study situation. Group A (n = 12): convective volume of 20–30 L for the first 6 months followed by 6 months of 35–50 L. Group B (n = 12): convective volume of 35–50 L for the first 6 months followed by 6 months of 20–30 L (Reprinted from Maduell et al. [16]. With permission from Oxford University Press)
Clinical Impact of Nocturnal Every-Other-Day Hemodiafiltration
Mean body weight (measured as dry weight after dialysis) increased from 70.1 ± 19 to 72.2 ± 19 Kg (P < 0.01) and was accompanied by a greater interdialytic weight gain and protein intake. The improvement in nutrition was not accompanied by changes in inflammation markers, probably because the patients were not previously inflamed and received treatment with biocompatible dialyzers, ultrapure dialysis fluid and convective techniques. These nutritional advantages could also be explained by the reduced fluid overload and uremic milieu after the switch to 7–8 h nocturnal, every-other-day OL-HDF. These results have been corroborated by Thumfart et al. in children [5].
Regarding cardiovascular risk factors, Chan et al. found that the switch from conventional HD, three 4 h sessions per week, to nocturnal HD, 8–10 h six nights per week, resulted in an improvement in the heart rate response to pulsatile blood pressure changes (baroreceptor response and arterial compliance) [36]. In our study, blood pressure control improved and only 8 % of patients required antihypertensive medications at the end of the observation period. In the study by Thumfart et al. the switch from conventional HD to NHD and NHDF resulted not only in discontinuation of antihypertensive therapy in five out of seven children but also in fewer intradialytic hypotensive episodes [5]. Another independent cardiovascular risk factor strongly associated with mortality in dialysis patients is LVH, which is present in 70–80 % of this population. Echocardiographic assessment revealed a 12 % decrease in LVMi after 1 year.
In our experience of 52 patients, 60 % were working and continued working throughout the study with practically no absenteeism, in many different occupations, varying from restaurant workers to a university professor. All patients completed a fatigue index questionnaire on the intensity, duration and frequency of postdialysis fatigue [37], which showed no significant changes over time.
Summary of Nocturnal Every-Other-Day Hemodiafiltration
Conversion from 4–5 h thrice weekly OL-HDF to 7–8 h every-other-day OL-HDF shows excellent clinical tolerance and patient acceptance, adequate social and occupational rehabilitation, better dialysis adequacy, marked improvement in nutritional status, regression of LVH, good phosphate and hypertension control, and a marked reduction of phosphate binders and antihypertensive medication. Different patterns of solute removal were observed, which were related to dialysis time, convective volume and/or to the infusion flow rate. Therefore, long-term, nocturnal, in-center, every-other-day OL-HDF with high convective volumes appears to be a good therapeutic dialysis scheme with improvements in clinical and social-occupational rehabilitation.
Intensified Hemodiafiltration Schemes: Conclusion
D-OL-HDF is a well tolerated dialysis scheme with adequate clinical outcomes and improved quality of life. Due to logistic and economic reasons, however, this modality should be restricted to patients with severe cardiovascular disease and/or poorly controlled hypertension. In case of hyperphosphatemia D-OL-HDF needs to be accompanied by an increase in dialysis time. Nocturnal every-other-day OL-HDF shows excellent clinical tolerance and patient acceptance, adequate social and occupational rehabilitation, better dialysis adequacy, marked improvement in nutritional status, regression of LVH, good phosphate and hypertension control and a marked reduction of phosphate binders and antihypertensive medication. This modality could be prescribed to patients who need to improve clinical and biochemical parameters (hypertension, hyperphosphatemia and other cardiovascular risk factors) and social-occupational rehabilitation. Our recommendations for intensified HDF schemes are summarized in Table 21.1.
Teaching Points (II)
-
Nocturnal every-other-day hemodiafiltration recommended for/in
-
Optimal work-life balance
-
Poorly controlled hypertension
-
Hyperphosphatemia
-
-
Clinical advantages of Nocturnal every-other-day hemodiafiltration
-
Higher dialysis dose (Kt/V)
-
Improved nutritional state
-
Regression of LVH
-
Improved phosphate control
-
Reduction in phosphate binders and antihypertensive medication
-
References
United States Renal Data System. 2014 annual data report: epidemiology of kidney disease in the United States. Bethesda: National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases; 2014.
Flythe JE, Lacson Jr E. Outcomes after the long interdialytic break: implications for the dialytic prescription. Semin Dial. 2012;25:1–8.
Foley RN, Gilbertson DT, Murray T, Collins AJ. Long interdialytic interval and mortality among patients receiving hemodialysis. N Engl J Med. 2011;365:1099–107.
Krishnasamy R, Badve SV, Hawley CM, et al. Daily variation in death in patients treated by long-term dialysis: comparison of in-center hemodialysis to peritoneal and home hemodialysis. Am J Kidney Dis. 2013;61:96–103.
Thumfart J, Puttkamer CV, Wagner S, Querfeld U, Muller D. Hemodiafiltration in a pediatric nocturnal dialysis program. Pediatr Nephrol. 2014;29:1411–6.
Georgianos PI, Sarafidis PA. Pro: should we move to more frequent haemodialysis schedules? Nephrol Dial Transplant. 2015;30:18–22.
Zhang H, Schaubel DE, Kalbfleisch JD, et al. Dialysis outcomes and analysis of practice patterns suggests the dialysis schedule affects day-of-week mortality. Kidney Int. 2012;81:1108–15.
Diaz-Buxo JA, White SA, Himmele R. Frequent hemodialysis: a critical review. Semin Dial. 2013;26:578–89.
Grooteman MP, van den Dorpel MA, Bots ML, et al. Effect of online hemodiafiltration on all-cause mortality and cardiovascular outcomes. J Am Soc Nephrol. 2012;23:1087–96.
Maduell F, Moreso F, Pons M, et al. High-efficiency postdilution online hemodiafiltration reduces all-cause mortality in hemodialysis patients. J Am Soc Nephrol. 2013;24:487–97.
Ok E, Asci G, Toz H, et al. Mortality and cardiovascular events in online haemodiafiltration (OL-HDF) compared with high-flux dialysis: results from the Turkish OL-HDF Study. Nephrol Dial Transplant. 2013;28:192–202.
Mostovaya IM, Blankestijn PJ, Bots ML, et al. Clinical evidence on hemodiafiltration: a systematic review and a meta-analysis. Semin Dial. 2014;27:119–27.
Nistor I, Palmer SC, Craig JC, et al. Convective versus diffusive dialysis therapies for chronic kidney failure: an updated systematic review of randomized controlled trials. Am J Kidney Dis. 2014;63:954–67.
Maduell F, Navarro V, Torregrosa E, et al. Change from three times a week on-line hemodiafiltration to short daily on-line hemodiafiltration. Kidney Int. 2003;64:305–13.
Maduell F, Navarro V, Rius A, et al. Improvement of nutritional status in patients with short daily on-line hemodiafiltration. Nefrologia. 2004;24:60–6.
Maduell F, Arias M, Duran CE, et al. Nocturnal, every-other-day, online haemodiafiltration: an effective therapeutic alternative. Nephrol Dial Transplant. 2012;27:1619–31.
Daugirdas JT, Chertow GM, Larive B, et al. Effects of frequent hemodialysis on measures of CKD mineral and bone disorder. J Am Soc Nephrol. 2012;23:727–38.
Ayus JC, Achinger SG, Mizani MR, et al. Phosphorus balance and mineral metabolism with 3 h daily hemodialysis. Kidney Int. 2007;71:336–42.
Fischbach M, Terzic J, Menouer S, Dheu C, Seuge L, Zalosczic A. Daily on line haemodiafiltration promotes catch-up growth in children on chronic dialysis. Nephrol Dial Transplant. 2010;25:867–73.
Tattersall J, Martin-Malo A, Pedrini L, et al. EBPG guideline on dialysis strategies. Nephrol Dial Transplant. 2007;22 Suppl 2:ii5–21.
Charra B, Calemard E, Cuche M, Laurent G. Control of hypertension and prolonged survival on maintenance hemodialysis. Nephron. 1983;33:96–9.
Bayliss G, Danziger J. Nocturnal versus conventional haemodialysis: some current issues. Nephrol Dial Transplant. 2009;24:3612–7.
Bugeja A, Dacouris N, Thomas A, et al. In-center nocturnal hemodialysis: another option in the management of chronic kidney disease. Clin J Am Soc Nephrol. 2009;4:778–83.
David S, Kumpers P, Eisenbach GM, Haller H, Kielstein JT. Prospective evaluation of an in-centre conversion from conventional haemodialysis to an intensified nocturnal strategy. Nephrol Dial Transplant. 2009;24:2232–40.
Lacson Jr E, Xu J, Suri RS, et al. Survival with three-times weekly in-center nocturnal versus conventional hemodialysis. J Am Soc Nephrol. 2012;23:687–95.
Powell JR, Oluwaseun O, Woo YM, et al. Ten years experience of in-center thrice weekly long overnight hemodialysis. Clin J Am Soc Nephrol. 2009;4:1097–101.
Ok E, Duman S, Asci G, et al. Comparison of 4- and 8-h dialysis sessions in thrice-weekly in-centre haemodialysis: a prospective, case-controlled study. Nephrol Dial Transplant. 2011;26:1287–96.
Mastrangelo F, Alfonso L, Napoli M, DeBlasi V, Russo F, Patruno P. Dialysis with increased frequency of sessions (Lecce dialysis). Nephrol Dial Transplant. 1998;13 Suppl 6:139–47.
Maduell F, Rodriguez N, Sahdala L, et al. Impact of the 5008 monitor software update on total convective volume. Nefrologia. 2014;34:599–604.
Maduell F, Ojeda R, Arias M, et al. Optimizing dialysate flow in online hemodiafiltration. Nefrologia. 2015. doi: 10.1016/j.nefro.2015.06.019 [in press].
Davenport A, Gardner C, Delaney M. The effect of dialysis modality on phosphate control: haemodialysis compared to haemodiafiltration. The Pan Thames Renal Audit. Nephrol Dial Transpl. 2012;25:897–901.
Penne EL, van der Weerd NC, van den Dorpel MA, et al. Short-term effects of online hemodiafiltration on phosphate control: a result from the randomized controlled Convective Transport Study (CONTRAST). Am J Kidney Dis. 2010;55:77–87.
Lockridge Jr RS, Anderson H, Coffey L, et al. Nightly home hemodialysis in Lynchburg, Virginia: economic and logistic considerations. Semin Dial. 1999;12:440–7.
Mucsi I, Hercz G, Uldall R, Ouwendyk M, Francoeur R, Pierratos A. Control of serum phosphate without any phosphate binders in patients treated with nocturnal hemodialysis. Kidney Int. 1998;53:1399–404.
Maduell F, Sanchez J, Net M, et al. Mathematical modeling of different molecule removal on on-line haemodiafiltration: influence of dialysis duration and infusion flow. Blood Purif. 2015; 39:288–96.
Chan CT, Shen XS, Picton P, Floras J. Nocturnal home hemodialysis improves baroreflex effectiveness index of end-stage renal disease patients. J Hypertens. 2008;26:1795–800.
Sklar A, Newman N, Scott R, Semenyuk L, Schultz J, Fiacco V. Identification of factors responsible for postdialysis fatigue. Am J Kidney Dis. 1999;34:464–70.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2016 Springer International Publishing Switzerland
About this chapter
Cite this chapter
Maduell, F., Ojeda, R., Arias-Guillén, M. (2016). Intensified Hemodiafiltration. In: Nubé, M., Grooteman, M., Blankestijn, P. (eds) Hemodiafiltration. Springer, Cham. https://doi.org/10.1007/978-3-319-23332-1_21
Download citation
DOI: https://doi.org/10.1007/978-3-319-23332-1_21
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-23331-4
Online ISBN: 978-3-319-23332-1
eBook Packages: MedicineMedicine (R0)