Abstract
A great deal of evidence has now accumulated on the ability of extracorporeal convective therapies to enhance removal of compounds of different molecular weight which are markers or causative agents of severe uremic pathology, such as cardiovascular disease, chronic inflammation, anemia and bone metabolism derangement. A general reduction of the uremic toxicity might be the link with the clinical benefits reported in patients undergoing convective therapies. These benefits may eventually contribute to improving patient survival provided that high convective volume and, thus, high removal of middle-sized compounds is achieved, as suggested by the results of the recently published large trials. Post-dilution hemodiafiltration (HDF), combining diffusion and convection as mechanisms of solute removal, is the most widespread infusion mode in HDF and commonly held as the most efficient in removing middle molecules. Alternative convective and mixed convective-diffusive therapies, exploiting the more common mechanisms of solute transport in different ways, have been developed and proposed in the past years and more recently with the common aim to enhance removal of toxic solutes of different size. An overview of their principles, technical aspects and transport mechanisms on which they are based is provided in this chapter.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Convective therapies
- Convective volume
- Hemodiafiltration
- Hemodialysis
- Hemofiltration
- Middle molecules
- Mid-dilution hemodiafiltration
- Mixed hemodiafiltration
- Solute transport
- Ultrafiltration
Introduction
A new blood-purification modality based on convection as a mechanism of middle molecular toxins removal was first applied in patients with end stage-renal failure by Henderson and colleagues in 1967 at the University of Pennsylvania [1]. The new technique, coupling diffusion and convection as a transport mechanism, was called ‘diafiltration’. The fundamental principles and mathematical relationships of their technique were published some years later [2, 3], but its clinical application was started in Europe by Leber [4], who first proposed the original term ‘hemodiafiltration’ (HDF) for the new technique, and by Quellhorst, who reported in 1983 promising results of a series of studies in patients over a long time period [5].
Continuous evolution of HDF took place from its birth until the more recent modalities of its application. Introduction of bicarbonate buffer in dialysis fluid and replacement solutions minimized the relevant side-effects caused by the acetate or lactate contained in the original fluids. The development of new synthetic highly biocompatible and permeable membranes with selective cut-off extended the range of removed compounds to small molecular weight proteins and beyond, while minimizing albumin loss. On-line production of indefinite amount of ultrapure dialysate/substitution fluid at low cost replaced the cumbersome and expensive use of fluids in sterile bags. The ultrafiltration (UF) control systems, introduced to control body weight (BW) loss with fluximeters measuring the differential flow between outlet and inlet dialysate compartment, were adapted to optimize and safely modulate the infusion rate (Qinf) through a feedback mechanism controlled by the trans-membrane pressure (TMP). Nowadays, technological progress of dialysis systems grants a high level of efficiency and safety to the convective techniques with the application of advanced feedback devices, operating automatically and easily controlled through a friendly user interface. In the last years, different infusion modalities in HDF have been proposed as alternatives to the traditional post-dilution and pre-dilution modes, combining convection, diffusion and adsorption to a different extent, but with the common aim to improve the operational and clinical feasibility of convective therapies and to achieve maximal solute removal in a wide spectrum of molecular weights.
Water and Solute Transport in Convective Therapies
Ultrafiltration
Ultrafiltration (UF) of plasma water occurs as a consequence of a pressure gradient across the dialyzer membrane modulated by applying a negative pressure in the dialysate compartment of the filter. The driving force for water filtration at every point of the capillary length of the dialyzer is the resultant of the hydraulic pressure inside the fiber (PB) and in the dialysate compartment (PD) and the oncotic pressure exerted by plasma proteins (π), which opposes to filtration. The average pressure gradient across a dialyzer membrane (TMP) may be calculated as:
where the suffix in and out indicate the inlet and outlet ports of the two dialyzer compartments.
The volumetric water flux (Jf) is a function of TMP according to the equation [6, 7]:
where Lp is the hydraulic permeability of the membrane for water, i.e. the water flow rate per unit area of membrane (A) per unit TMP gradient (ml/min/cm2/mmHg).
Water permeability of a dialyzer membrane is defined in clinical practice with its UF coefficient (KUF, ml/h/mmHg/m2) according to the equation:
where QUF is the UF rate.
When referred to the overall membrane surface of a dialyzer KUF becomes KUFD, ml/h/mmHg:
KUFD corresponds to the slope of the regression equation [6] relating QUF with TMP and characterizes numerically the hydraulic permeability of that dialyzer, which largely depends on the surface and characteristics of the membrane (mainly the pore radius), and on the dialyzer geometry. A nominal KUFD >40 ml/h/mmHg is a requisite for high-flux dialyzers.
Lower than nominal KUFD values are found in vivo as a consequence of the protein layer formation on the inner face of the membrane (secondary membrane). Loss in hydraulic permeability is negligible and quite constant along low-flux HD sessions conducted at moderate QUF [8]. Progressive and even substantial reduction in KUFD may be observed during HDF and hemofiltration (HF) sessions when higher QUF and filtration fraction (FF) are applied, as an effect of the solute and protein polarization on the inner membrane surface and thickening of the secondary membrane [9, 10]. In addition, the colloid osmotic pressure exerted by the concentrated plasma proteins counteracts the filtration pressure [11]. As a consequence, the modern feedback systems are very effective in preventing this risk by adapting QUF to the actual operating conditions and reducing it automatically whenever TMP rises to dangerous values. Besides the hydraulic permeability properties of the membrane, maximal QUF level is mainly a function of the blood flow rate (QB) permeating the capillaries of the dialyzer. Therefore, high QB are preferential for the production of high UF and convective removal and, thus, to achieve high efficiency in the application of convective therapies.
Convection
Convection is the main transport mechanism of middle molecular size solutes in mixed convective-diffusive therapies. Convective solute removal is the result of the bulk movement of the solvent (plasma water) across the membrane driven by the hydrostatic pressure gradient between blood and dialysate compartments. Convective transport is constant over a wide range of molecular weight solutes but decreases as the hydrated molecular size approaches that of the pores of the membrane. In general, the degree to which convection increases total solute removal is proportional to QUF and to the molecular weight of the solute [12]. The membrane characteristics (electro-chemical properties and structure, pore radius and conformation) also play an important role [13–16]. The ability of a membrane to remove a specific solute from plasma by convection is determined by its sieving properties and expressed mathematically with an index, the sieving coefficient (SC, dimensionless), unique for that solute and that membrane. Sc, measured in vitro in a defined experimental setting and in the absence of diffusion, is the ratio between the solute concentration detected in the UF (Cuf) and its average plasma concentration within the dialyzer [6]:
According to Eq. 2.5, Sc value is inversely related to the solute molecular weight and varies between 1 for a freely permeable molecule and 0 for a molecule to which the membrane is completely impermeable. However, in a clinical setting, the same events that limit the hydraulic permeability of the membrane and reduce QUF may also affect removal of middle-molecular solutes by convection. As a consequence of the progressive thickening of the secondary membrane layer the in vivo SC value (apparent sieving) for molecules such as beta2-microglobulin (β2-m) may results in lower values than those measured in vitro and may even approach zero in post-dilution HF at very high QUF [17].
Convective transport of a solute (JC) may be expressed with the mathematical equation which defines its relation with the solute plasma concentration (C) and the rate of fluid transfer across the membrane QUF, limited by the solute and membrane Sc:
The equation also defines the clearance of the solute when pure convection is applied in HF.
Diffusion
Diffusion is the main transport mechanism of small molecular size solutes also in mixed convective-diffusive therapies. Solute diffusion follows a trans-membrane concentration gradient between blood and dialysis fluid according to a first-order kinetics and the process is represented mathematically by the Fick’s law:
where JD is the rate of solute diffusive flux per unit area of the membrane (A), proportional to the solute concentration gradient (dc/dx), and Ko is the overall mass transfer coefficient (or solute diffusion coefficient), which is a property of the membrane and the solute and characterizes the overall resistance to a definite solute flux across a unit area of that membrane. When referred to the overall surface of a dialyzer, its diffusion coefficient for a specific solute is defined with the expression KoA (i.e. overall mass transfer coefficient *area). According to the Eq. 2.7, diffusive transport is proportional to the surface area of the membrane: progressive increase in dialyzer surface at constant QUF results in moderate enhancement of the diffusive transport according to a curve that achieves its plateau faster for small molecular solutes. The level of the plateau is a function of the diffusive permeability of the membrane.
Diffusion property of a dialyzer is also influenced by blood and dialysate flow rates (QB, QD), and the relative role of each factor depends on KoA, according to the equation by Michaels [18]
where KD is the diffusive dialyzer clearance. The effect of increasing QB up to 500–600 ml/min progressively increases KD of small solutes to a greater extent than that of middle-high molecular weight solutes, which is scarcely affected by QB values beyond 200–250 ml/min [19]. An increase in QD from 500 to 800 ml/min results in a small-moderate enhancement of small solute removal by diffusion but not of the larger solutes [20–22].
Interactions Between Diffusion and Convection
Convection and diffusion act simultaneously as solute transport mechanisms in HDF, even if to a different extent according to the molecular weight of the removed solute. However, the overall mass transport is not the sum of the two separate components because of an interaction between them, which is more prominent at the high QUF of HDF. Their effects cannot be distinguished from each other, but some mathematical models have attempted to quantify their combined effect in term of solute removal. The simplest model is described by the equation [23]:
where KHDF is the overall (convective + diffusive) clearance, and T is the transmittance coefficient, a parameter which is a function of the flow conditions and membrane properties. An expression for T that is universal for all solutes is:
Absorption
Absorption assumes relevance as a removal mechanism particularly in the case of some high-flux membranes carrying electrical charges, such as polyacrilonitrile and polymethyl-metacrilate [24, 25], and may significantly enhance the dialyzer clearance of β2-m and of several cytokines. Polysulfone membranes show minor absorptive capacity and remove middle molecule compounds mainly by convection [26]. Electrochemical interaction between these membranes and certain hydrophobic compounds like peptides and proteins may cause them to adhere on the inner surface of the membrane within the pore structure [27]. Therefore, the open pore structure of high-flux membranes affords more absorptive potential than do low-flux membranes, and synthetic hydrophobic membranes are generally much more absorptive than hydrophilic cellulosic membranes [28]. Albumin coats the membrane immediately after exposure to blood, with the effect to reduce its in vivo permeability. Absorption characteristics of high-flux membranes are more extensively defined in another chapter.
Teaching Points I
-
Convection is the main transport mechanism of middle molecular size solutes in mixed convective-diffusive therapies
-
Convective solute removal is the result of the movement of plasma water across the membrane, driven by the hydrostatic pressure gradient between blood and dialysate compartments.
-
The driving force for water filtration is the resultant of this pressure gradient and the oncotic pressure exerted by plasma proteins, which opposes filtration.
-
Diffusion is the main transport mechanism of small molecular size solutes also in mixed convective-diffusive therapies. Solutes follow a trans-membrane concentration gradient between blood and dialysis fluid according to a first-order kinetics
-
Convection and diffusion act simultaneously as solute transport mechanisms in HDF
Modalities of Convective Therapies
Internal Hemodiafiltration (iHDF)
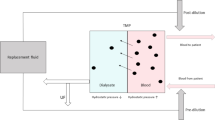
A certain amount of solute removal by convection may also be obtained during prevalent diffusive treatments when high-flux dialyzers are used. In this case, the TMP gradient established in the proximal part of the dialyzer promotes large water transfer from blood to the dialysate. Water acts as solvent drag and favors removal of middle molecular compounds by convection. Hydraulic pressure on the blood side drops progressively along the fibers, while oncotic pressure increases with plasma protein concentration until, at a certain point of the dialyzer length, the pressure gradient across the membrane reverses its direction and, accordingly, UF ceases and water moves from the dialysate compartment to blood (Fig. 2.1). This mechanism, called ‘back-filtration’ or ‘internal filtration’ is the underlying principle of high-flux HD and its effect is an enhancement of small- and middle-molecular solute removal by convection [29]. iHDF works just as a high-flux HD, but it requires the convective dose to be clinically relevant, quantifiable and possibly adjustable by the operator. This technique entails the use of a dedicated dialyzer with geometric characteristic suitable for increasing internal filtration. iHDF improves convective transport by direct filtration and backfiltration without the need of substitution fluid infusion [30]. A user-friendly mathematical model has been designed to quantify the internal filtration/backfiltration flux taking place during the treatment. Flux is predicted on the basis of the machine settings and hematocrit/plasma protein concentration [31, 32].
Hemofiltration (HF)
This technique realizes pure convective solute transport without solute exchange by diffusion in the absence of dialysate flow and, thus, more closely mimics the glomerular filtration of the human kidney than any other dialysis technique. As a consequence, HF promotes a higher rate of medium- and large molecules removal than low- and high-flux HD but lesser removal of small solutes which are mainly removed by diffusion. Achievement of high convective volume is often difficult in the post-dilution mode of HF, during which rapidly progressive hemoconcentration in the dialyzer and significant loss of hydraulic membrane permeability may occur at very high QUF. Only the pre-dilution mode may partially obviate these drawbacks of HF by improving flux rheology, membrane permeability and convective removal of all solutes thanks to the increased flow along the dialyzer capillaries. Some clinical benefit of this technique in terms of hemodynamic stability was reported in the past as a consequence of a better vascular reactivity in the absence of vasodilator acetate in dialysis fluid [33, 34], and was variably attributed to the removal of vasoactive destabilizing factors with convection [35, 36], blood cooling after mixing with the substitution fluid [37], or sodium retention and positive sodium balance due to the Donnan effect [38]. These advantages of HF faded when bicarbonate buffer was introduced and temperature and sodium balance were matched with HD and HDF with the modern dialysis systems. Thus, the positive effect of HF on hemodynamic instability remains unexplained. Moreover, more recent observations have reported lower incidence of intradialytic hypotension during on-line HDF than on HF and high-flux HD, see also Chap. 17 [39].
Hemodiafiltration
High QUF may be obtained in HDF, in the absence of significant back-filtration due to a constantly positive pressure gradient between blood and dialysate along the dialyzer capillary. Solutes with diameter up to that of the membrane pores are dragged across the membrane with the UF flow independently of their molecular size, while transfer of small-molecular toxic compounds from blood to dialysate occurs by diffusion according to a concentration gradient. Combining both removal mechanisms into a single treatment (HDF) is undoubtedly the strategy enabling the high potential of hydraulic and solute permeability of synthetic membranes to be most properly exploited.
Post-dilution HDF
This technique is the most widespread infusion mode in HDF and commonly held as the most efficient in removing middle molecules [12, 19, 40, 41]. Sterile substitution fluid is produced on-line from the dialysate by the more recent HDF systems and is infused after the filter to replace the excess fluid lost by the patient with the high UF (Fig. 2.2a). Up to 5–6 l of UF per hour may be obtained by applying appropriate flux-pressure regimen. Proportional increase in β2-m removal is achievable in post-dilution HDF with increasing QUF [12, 19] and lower β2-m basal level have been associated with a reduced death risk in dialysis patients [42]. Indeed, observational [43] and prospective randomized trials [44–46] have shown that post-dilution on-line HDF may obtain a substantial reduction of the death risk in dialysis patients, with improved survival of around 30 % compared to low- and high-flux HD, provided that high convective volume is achieved per session (21–23 l).
Thus, clinical application of on-line HDF requires operating conditions to be set in order to achieve this goal and maximally exploit the convective potential of high-flux membranes. At any given blood flow the maximal efficiency in convective removal is obtained at the highest FF [40], but the highest achievable FF value is often unpredictable. When very high QUF are applied in post-dilution HDF, hemoconcentration increases blood viscosity and resistance to flow inside the fibers, especially when high rates of weight loss are necessary to achieve the dry body weight and when the individual capacity to recruit fluid from the extra-vascular space during dehydration (refilling) is scarce. In these conditions, a critical reduction of the membrane permeability is likely to occur as a consequence of the events described above [9–11] and the relationship between QUF and TMP, linear up to a certain TMP value (200–300 mmHg for high-flux membranes), becomes curvilinear and progressively increasing TMP is necessary to maintain the programmed filtration, until a plateau is reached [6], beyond which the system becomes unstable [47], increasingly higher TMP gradients fail in the attempt to maintain the planned QUF and sudden dangerous pressure peaks are likely to result from small changes in blood flow or viscosity, venous pressure, or for clinical reasons, particularly in patients with cardiac failure, diabetes or hemodynamic instability. In such circumstances circuit clotting and residual irreversible reduction in the performance of the dialyzer may be observed. Historically, the limit beyond which the adverse events of high TMP levels and hemoconcentration may occur was set empirically at a plasma water FF of 0.5 [6]. Setting QUF purely on the basis of the in vitro KUFD may be misleading for several reasons.
The present technology of HDF machines helps to automatically plan a session of post-dilution HDF in order to safely accomplish this task with the use of feedback devices which sets and maintains the infusion rate under TMP control and reduces it whenever TMP increases beyond its maximum limit as a consequence of the progressive decline of the membrane permeability through the session (Fig. 2.3c).
(a) Schematic representation of the hardware for mixed HDF implemented on the 5008 Fresenius Therapy system. Instantaneous mean TMP values are calculated from the measures of four pressure probes (P) placed at the inlet and outlet blood and dialysate compartments. Infusion lines are connected at the inlet and outlet lines of the extracorporeal circuit. (b) In Mixed dilution HDF, the TMP/UF feedback system maintains TMP values within the maximum safe range by modulating the total infusion and the ratio between post- and pre-dilution infusion. (c) In post-dilution HDF, TMP is controlled by modulating the total infusion. The diagrams represent the mechanism by which the TMP/UF feedback works in the two modalities. More details are in the text
However, high convective volume may only be achieved by applying high QB in order to maximally increase the capillary flow of plasma water available for UF and better preserve the membrane permeability by enhancing the stirring and thinning actions exerted by the blood flow on the protein layer on the blood side of the membrane.
Pre-dilution HDF
This technique may ensure more favorable rheological and hydraulic conditions than the post-dilution mode by better preserving the permeability of the membrane, as the replacement fluid added to blood at the dialyzer inlet prevents excessive hemoconcentration and increases the rate of flow within the capillaries with enhanced shear-rate effect on the secondary protein layer (Fig. 2.2b). This advantage may be offset by the dilution effect of the plasma solute concentrations available for diffusion and convection, with consequent reduction of the cumulative solute transfer [19, 40, 41, 48]. Accelerated extraction of diffusible small solutes from the intracellular space has been described as an effect of a more favorable transcellular gradient [2, 49], but this mechanism is unable to fully compensate for loss in efficiency. Only a substantial increase of the infusion rate up to a value approximately double with respect to post-dilution HDF may result in similar removal of middle molecular solutes between the two infusion modalities [19]. Clinical application of pre-dilution HDF is limited by the above drawbacks and by the cost related to the increased amount of replacement solution to be prepared from the dialysate. It may be indicated in patients with high hematocrit or hemorrhagic to help in the anticoagulation of the extracorporeal circuit.
Mixed HDF
This technique, in which the replacement fluid is simultaneously infused to a variable ratio at the inlet and outlet port of the dialyzer, was developed in the last decade (Figs. 2.2c and 2.3a). The aim is to overcome limits and risks implicit in the traditional infusion modes in HDF while coupling their advantages [40, 50, 51]. The basic concept is that more favourable rheological and hydraulic conditions than in post-dilution HDF are ensured within the dialyzer by splitting the infusion between pre- and post-filter. An increase in blood flow rate obtained with partial and controlled pre-dilution may better preserve the characteristics of water and solute permeability of the membrane, while avoiding the excessive dilution of the inlet solute concentrations characteristics of the pre-dilution mode. In mixed HDF, a convective volume of up to 40–45 l/session may be attained under the control of an original feedback system device which ensures maximal filtration fraction by favoring the infusion at the post-dilution port (60–70 % of the total infusion). The feedback system maintains TMP within the highest range of safety during the session by splitting small amounts of substitution fluid from the post- to the pre-dilution site whenever TMP rises to its highest safety limit (300–350 mmHg) without reducing the total infusion rate (Fig. 2.3b) [50, 52].
Validation studies have shown that greater β2-m and phosphate removal may be safely obtained in on-line mixed HDF than in post-dilution HDF by ensuring optimal operating conditions of the technique and forcing QUF to achieve the most efficient convective transport [48, 52–55].
Mixed HDF may be of special advantage in patients with high pre-dialysis hematocrit and an increased risk of filter clotting with post-dilution HDF due to hemoconcentration [56], and more in general in all those patients who cannot achieve the desired convective volume in post-dilution HDF, due to different clinical and technical situations.
Mid-dilution HDF (MD-HDF)
This technique was proposed by Krieter as a step ahead in terms of improved convective solute transport (Fig. 2.4) [57]. It is based on the use of dedicated hemodiafilters which include a unique U-shaped blood capillary bundle and a special two-port header cap (Olpur™ MD 190 and MD 220, Nephros, New York, USA). Blood flows through the annular region of the fiber bundle, mixes with substitution fluid infused through a middle infusion port placed at the point where blood flow reverses its direction and flows in the reverse direction through the core region of the fiber bundle. Blood and dialysate flow counter-current in the annular region of the capillaries where post-dilution is performed and co-currently in the core, pre-dilution region.
This infusion technique has been claimed to achieve greater efficiency when compared to traditional post-dilution HDF [57]. However, a prospective comparative analysis between on-line mixed HDF and MD-HDF showed that MD-HDF was carrying with it serious membrane permeability impairment when applied as proposed in the original study because considerably high TMP in the post-dilution section of the hemofilter were necessary to achieve the planned UF of about 10 l/h [58]. This problem was overcome by devising a new configuration, called reverse MD-HDF, in which blood inlet and outlet were inverted. In the new setting blood flows through the core region of the fiber bundle, mixes with substitution fluid at the other end, and flows in the reverse direction through the annular region of the fiber bundle [59]. Anyway, safe rheologic and hydraulic conditions in MD-HDF may only be maintained by carrying out treatments with the larger MD 220 hemofilter (2.2 m2) in reverse MD-HDF configuration [60]. The total solute removal of reverse MD-HDF with the larger MD 220 hemofilter and post-dilution HDF appears to be not different from post-dilution HDF for both small water-soluble and protein-bound compounds [61]. An efficient pressure control system with modulation of the infusion rate according to the operational conditions of the treatments, would be useful to improve safety and performance in the clinical application of this technique.
HDF with Endogenous Reinfusion (HFR) (Fig. 2.5)
This technique was designed to separate the two main transport processes, convection and diffusion, with the use of a two-chamber filter and a sorbent cartridge [62–66]. Isolated plasma UF and solute convection take place through the polyethersulfone high-flux membrane of the first chamber of the dialyzer. The UF produced in the first chamber is ‘regenerated’ while flowing through a sorbent cartridge and then infused in the second dialyzer chamber as endogenous replacement solution. The diffusion stage occurs in the second chamber through a low-flux polyethersulfone membrane. The sorbent cartridge contains a hydrophobic styrenic resin which has high affinity and adsorbs several uremic toxins and MM, such as β2-m, homocysteine, parathyroid hormone and several cytokines. Electrolytes and small solutes such as urea, creatinin and uric acid are not adsorbed and are managed in the second, diffusive section of the dialyzer [63, 67]. Lower impact on oxidative stress [68] and sparing effect on amino acids loss [69] have been reported in HFR compared to HD and acetate-free biofiltration (AFB), respectively. The recent development of HFR equilibrium, based on the combination of HFR with dialysate sodium and UF profile, has been shown to improve intradialytic hemodynamics [39].
Push/Pull Hemodiafiltration (PP-HDF)
This technique is one of the most widespread modalities used in Japan and South Korea (Fig. 2.6). It’s based on a double-cylinder piston pump (push/pull pump) implemented on the effluent dialysate line of the dialysis machine. Based on this alternate pump device, alternate fast cycles of UF (pull) and backfiltration (push) are performed through a high-flux dialyzer [70, 71]. During the UF phase, uremic substances are eliminated both by diffusive and convective transport. During the backfiltration phase, dialysate is forced to the blood side in order to balance the excessive reduction in body fluid developed during the previous UF phase. Body fluid replacement volume is over 120 l during a 4-h treatment. Since the UF and backfiltration times are much shorter in PP-HDF than the time for blood to pass through the dialyzer, blood is concentrated and diluted many times before it leaves the dialyzer. High removal rate of middle molecules and reduction of symptoms of dialysis-related amyloidosis have been reported with this technique [72, 73].
Double High-Flux Hemodiafiltration (DHF-HDF)
This technique was designed in the beginning of 1980s in order to achieve a drastic reduction of treatment time over conventional HD and increase convective transport without the need for ultrapure substitution fluid and consequently dedicated machines. DHF-HDF (Fig. 2.7) consists of two high-flux dialyzers connected in series by blood and dialysate lines [74]. Fluid and solutes are removed in the first dialyzer with a mixed diffusion-convection process, while backfiltration of sterile dialysate takes place in the second dialyzer. An adjustable flow-restrictor is placed on the dialysis fluid pathway between the two dialyzers to induce TMP variations and modulate UF in the first dialyzer and backfiltration in the second one [75]. Studies have shown that DHF-HDF with very high QB (450–650 ml/min) may provide higher removal of small molecules than standard HD and HF over shorter treatment time [76], and β2-m clearance similar to that in on-line HDF [77, 78]. Increased treatment cost and scarce data about long term effects [76] have limited the diffusion of DHF-HDF, which might provide the benefits of convective therapy to patients in situations where on-line techniques cannot be implemented [78].
Acetate Free Biofiltration (AFB)
This modality was proposed in 1984 as the first HDF technique employing buffer-free dialysis solutions [79, 80]. Correction of acidosis was obtained with infusion in postdilution mode of a solution of sodium bicarbonate supplied in bags at fixed concentration of 120, 145 or 167 mmol/l at a rate of 8–10 l/session (Fig. 2.8). An automatic control system was implemented on dedicated dialysis machines to balance infusion to UF rate. The use of polyacrylonitrile hollow-fiber dialyzers with consistent absorptive power [81] and the absence of acetate resulted in reduced stimulation of inflammatory mediators [82]. Other encouraging traits have been added over the years, such as the possibility to modulate the concentration of potassium in the dialysate, thus reducing the risk of arrhythmias [83–85], and the possibility to monitor blood volume changes during treatment, thereby reducing intradialytic hypotension episodes and predialysis systolic blood pressure values [86]. Nowadays AFB retains an historical value as one of the first alternative convective therapies but it can hardly be included in modern convective therapies because of the low convective volume it can provide [86], which is comparable to the amount of internal filtration in high-flux HD [30].
Conclusion
On-line post-dilution HDF is at present the most widespread infusion mode in HDF and commonly held as the most efficient in removing middle molecules. The exciting results of this technique in terms of prolonged patient survival may depend on several factors, such as the high biocompatibility of the systems and the dialysate/infusate produced on-line which reduce the chronic inflammatory status of dialysis patients, and the better hemodynamic stability which prevents episodes of severe ischemic cardiac damage. Among those factors, high convective volume and, thus, enhanced middle and small molecular weight solute removal, appears to play an important role and it may only be achieved with the use of high-flux, highly permeable membranes and high blood flow rates. For further reading see Chaps. 16 and 23. Alternative convective and mixed convective-diffusive therapies exploiting the more common mechanisms of solute transport in different ways have been reviewed here. Alternative convective therapies may play a role in enhancing convective removal in definite settings when post dilution HDF fails. Clinical validation with larger numbers and longer follow-up is necessary for an extended application.
Teaching Points II
-
Different treatments are described in which convection plays a role:
-
Internal hemodiafiltration: solute removal by convection occurring during treatment with high-flux dialyzers, which is compensated by backfiltration of (ultrapure) dialysis fluid.
-
Hemofiltration: pure convective solute transport (ultrafiltration) without solute exchange by diffusion in the absence of dialysate flow. The ultrafiltrate is replaced online or offline by sterile substitution fluid
-
Hemodiafiltration: solute removal through both convection (hemofiltration) and diffusion (hemodialysis). The ultrafiltrate is replaced online or offline by sterile substitution fluid
-
-
Different modes of hemodiafiltration:
-
Post-dilution (online) HDF: Sterile substitution fluid is produced on-line from the ultrapure dialysate and infused after the filter to replace the amount of ultrafiltrate, minus weight gain during the interdialytic interval. Most widespread infusion mode in HDF and currently considered as the most efficient modality for removing middle molecules.
-
Pre-dilution (online) HDF: Sterile substitution fluid is produced on-line and infused before the filter to replace the excess fluid that is extracted from the blood of the patients. Pre-dilution substitution prevents hemoconcentration, but results in the dilution of plasma solute concentrations, thereby reducing the cumulative solute transfer.
-
Mixed HDF: sterile replacement fluid is simultaneously infused to a variable ratio at the inlet and outlet port of the dialyzer.
-
Mid-dilution HDF: dedicated hemodiafilters are used, with infusion of sterile substitution fluid through a middle- and a post-dilution infusion port.
-
HDF with endogenous reinfusion: using a two-chamber filter and a sorbent cartridge, isolated UF takes place through the high-flux membrane of the first chamber. The ultrafiltrate is ‘regenerated’ while flowing through a sorbent cartridge and infused in the second chamber as endogenous replacement solution. Here, diffusion occurs through a low-flux membrane.
-
Push/pull hemodiafiltration: alternate fast cycles of UF (pull) and backfiltration (push) of dialysate are performed through a high-flux dialyzer, using a double-cylinder piston pump (push/pull pump) implemented on the effluent dialysate line.
-
Double high-flux hemodiafiltration: two high-flux dialyzers are placed in series; fluid and solutes are removed in the first dialyzer, while backfiltration of dialysate takes place in the second dialyzer.
-
Acetate Free Biofiltration (AFB): a mode of offline post-dilution HDF, in which the bicarbonate substitution fluid is supplied in bags.
-
References
Henderson LW, Besarab A, Michaels AS, Bluemle LW. Blood purification by ultrafiltration and fluid replacement (diafiltration). Trans Am Soc Artif Intern Organs. 1967;13:216–26.
Colton CK, Henderson LW, Ford CA, Lysaght MJ. Kinetics of hemodiafiltration. I. In vitro transport characteristics of a hollow-fiber blood ultrafilter. J Lab Clin Med. 1975;85:355–71.
Henderson LW, Colton CK, Ford CA. Kinetics of hemodiafiltration. II. Clinical characterization of a new blood cleansing modality. J Lab Clin Med. 1975;85:372–91.
Leber HW, Wizemann V, Goubeaud G, Rawer P, Schutterle G. Hemodiafiltration: a new alternative to hemofiltration and conventional hemodialysis. Artif Organs. 1978;2:150–3.
Quellhorst E. Long-term follow up in chronic hemofiltration. Int J Artif Organs. 1983;6:115–20.
Henderson LW. Biophysics of ultrafiltration and hemofiltration. In: Maher JF, editor. Replacement of renal function by dialysis. Dordrecht: Kluwer; 1989. p. 300–26.
Hoenich NA. Membranes and filters for haemodiafiltration. Contrib Nephrol. 2007;158:57–67.
Bosch T, Schmidt B, Samtleben W, Gurland HJ. Effect of protein adsorption on diffusive and convective transport through polysulfone membranes. Contrib Nephrol. 1985;46:14–22.
Rockel A, Hertel J, Fiegel P, Abdelhamid S, Panitz N, Walb D. Permeability and secondary membrane formation of a high flux polysulfone hemofilter. Kidney Int. 1986;30:429–32.
Vilker VL, Colton CK, Smith KA. Concentration polarization in protein ultrafiltration. Am Inst Chem Eng. 1981;27:632–6.
Vilker VL, Colton CK, Smith KA, Green DL. The osmotic pressure of concentrated protein and lipoprotein solutions and its significance to ultrafiltration. J Membr Sci. 1984;20:63–77.
Lornoy W, Becaus I, Billiouw JM, Sierens L, Van Malderen P. Remarkable removal of beta-2-microglobulin by on-line hemodiafiltration. Am J Nephrol. 1998;18:105–8.
Floege J, Granolleras C, Deschodt G, et al. High-flux synthetic versus cellulosic membranes for beta 2-microglobulin removal during hemodialysis, hemodiafiltration and hemofiltration. Nephrol Dial Transplant. 1989;4:653–7.
Kim ST. Characteristics of protein removal in hemodiafiltration. Contrib Nephrol. 1994;108:23–37.
Morti SM, Zydney AL. Protein-membrane interactions during hemodialysis: effects on solute transport. ASAIO J. 1998;44:319–26.
Ronco C, Heifetz A, Fox K, et al. Beta 2-microglobulin removal by synthetic dialysis membranes. Mechanisms and kinetics of the molecule. Int J Artif Organs. 1997;20:136–43.
David S, Cambi V. Hemofiltration: predilution versus postdilution. Contrib Nephrol. 1992;96:77–85.
Michaels AS. Operating parameters and performance criteria for hemodialyzers and other membrane-separation devices. Trans Am Soc Artif Intern Organs. 1966;12:387–92.
Wizemann V, Kulz M, Techert F, Nederlof B. Efficacy of haemodiafiltration. Nephrol Dial Transplant. 2001;16 Suppl 4:27–30.
Ward RA, Schmidt B, Hullin J, Hillebrand GF, Samtleben W. A comparison of on-line hemodiafiltration and high-flux hemodialysis: a prospective clinical study. J Am Soc Nephrol. 2000;11:2344–50.
Hauk M, Kuhlmann MK, Riegel W, Kohler H. In vivo effects of dialysate flow rate on Kt/V in maintenance hemodialysis patients. Am J Kidney Dis. 2000;35:105–11.
Leypoldt JK, Cheung AK, Agodoa LY, Daugirdas JT, Greene T, Keshaviah PR. Hemodialyzer mass transfer-area coefficients for urea increase at high dialysate flow rates. The Hemodialysis (HEMO) study. Kidney Int. 1997;51:2013–7.
Jaffrin MY, Ding LH, Laurent JM. Simultaneous convective and diffusive mass transfers in a hemodialyser. J Biomech Eng. 1990;112:212–9.
Bouman CS, van Olden RW, Stoutenbeek CP. Cytokine filtration and adsorption during pre- and postdilution hemofiltration in four different membranes. Blood Purif. 1998;16:261–8.
Lonnemann G, Koch KM, Shaldon S, Dinarello CA. Studies on the ability of hemodialysis membranes to induce, bind, and clear human interleukin-1. J Lab Clin Med. 1988;112:76–86.
Clark WR, Hamburger RJ, Lysaght MJ. Effect of membrane composition and structure on solute removal and biocompatibility in hemodialysis. Kidney Int. 1999;56:2005–15.
Clark WR, Macias WL, Molitoris BA, Wang NH. Membrane adsorption of beta 2-microglobulin: equilibrium and kinetic characterization. Kidney Int. 1994;46:1140–6.
Clark WR, Macias WL, Molitoris BA, Wang NH. Plasma protein adsorption to highly permeable hemodialysis membranes. Kidney Int. 1995;48:481–8.
Ronco C. Backfiltration in clinical dialysis: nature of the phenomenon, mechanisms and possible solutions. Int J Artif Organs. 1990;13:11–21.
Ronco C, Orlandini G, Brendolan A, Lupi A, La GG. Enhancement of convective transport by internal filtration in a modified experimental hemodialyzer: technical note. Kidney Int. 1998;54:979–85.
Lucchi L, Fiore GB, Guadagni G, et al. Clinical evaluation of internal hemodiafiltration (iHDF): a diffusive-convective technique performed with internal filtration enhanced high-flux dialyzers. Int J Artif Organs. 2004;27:414–9.
Righetti M, Filiberti O, Ranghino A, et al. Internal hemodiafiltration versus low-flux bicarbonate dialysis: results from a long-term prospective study. Int J Artif Organs. 2010;33:796–802.
Altieri P, Sorba G, Bolasco P, et al. Predilution haemofiltration – the Second Sardinian Multicentre Study: comparisons between haemofiltration and haemodialysis during identical Kt/V and session times in a long-term cross-over study. Nephrol Dial Transplant. 2001;16:1207–13.
Altieri P, Sorba GB, Bolasco PG, et al. On-line predilution hemofiltration versus ultrapure high-flux hemodialysis: a multicenter prospective study in 23 patients. Sardinian Collaborative Study Group of On-Line Hemofiltration. Blood Purif. 1997;15:169–81.
Fox SD, Henderson LW. Cardiovascular response during hemodialysis and hemofiltration: thermal, membrane, and catecholamine influences. Blood Purif. 1993;11:224–36.
Henderson LW. Hemodynamic instability during different forms of dialysis therapy: do we really know why? Blood Purif. 1996;14:395–404.
van Kuijk WH, Hillion D, Savoiu C, Leunissen KM. Critical role of the extracorporeal blood temperature in the hemodynamic response during hemofiltration. J Am Soc Nephrol. 1997;8:949–55.
De Vries PM, Olthof CG, Solf A, et al. Fluid balance during haemodialysis and haemofiltration: the effect of dialysate sodium and a variable ultrafiltration rate. Nephrol Dial Transplant. 1991;6:257–63.
Locatelli F, Altieri P, Andrulli S, et al. Hemofiltration and hemodiafiltration reduce intradialytic hypotension in ESRD. J Am Soc Nephrol. 2010;21:1798–807.
Pedrini LA, De Cristofaro V, Pagliari B, Sama F. Mixed predilution and postdilution online hemodiafiltration compared with the traditional infusion modes. Kidney Int. 2000;58:2155–65.
Ahrenholz P, Winkler RE, Ramlow W, Tiess M, Muller W. On-line hemodiafiltration with pre- and postdilution: a comparison of efficacy. Int J Artif Organs. 1997;20:81–90.
Cheung AK, Rocco MV, Yan G, et al. Serum beta-2 microglobulin levels predict mortality in dialysis patients: results of the HEMO study. J Am Soc Nephrol. 2006;17:546–55.
Canaud B, Bragg-Gresham JL, Marshall MR, et al. Mortality risk for patients receiving hemodiafiltration versus hemodialysis: European results from the DOPPS. Kidney Int. 2006;69:2087–93.
Grooteman MP, van den Dorpel MA, Bots ML, et al. Effect of online hemodiafiltration on all-cause mortality and cardiovascular outcomes. J Am Soc Nephrol. 2012;23:1087–96.
Ok E, Asci G, Toz H, et al. Mortality and cardiovascular events in online haemodiafiltration (OL-HDF) compared with high-flux dialysis: results from the Turkish OL-HDF study. Nephrol Dial Transplant. 2013;28:192–202.
Maduell F, Moreso F, Pons M, et al. High-efficiency postdilution online hemodiafiltration reduces all-cause mortality in hemodialysis patients. J Am Soc Nephrol. 2013;24:487–97.
Jenkins RD, Funk JE, Chen B, Golper TA. Operational instability in extracorporeal filtration of blood. Blood Purif. 1992;10:292–308.
Pedrini LA, Zerbi S. Mixed-dilution hemodiafiltration. Contrib Nephrol. 2007;158:123–30.
Cheung AK, Alford MF, Wilson MM, Leypoldt JK, Henderson LW. Urea movement across erythrocyte membrane during artificial kidney treatment. Kidney Int. 1983;23:866–9.
Pedrini LA, De Cristofaro V. On-line mixed hemodiafiltration with a feedback for ultrafiltration control: effect on middle-molecule removal. Kidney Int. 2003;64:1505–13.
Pedrini LA, De Cristofaro V, Pagliari B, Filippini M, Ruggiero P. Optimization of convection on hemodiafiltration by transmembrane pressure monitoring and biofeedback. Contrib Nephrol. 2002;137:254–59
Pedrini LA, Cozzi G, Faranna P, et al. Transmembrane pressure modulation in high-volume mixed hemodiafiltration to optimize efficiency and minimize protein loss. Kidney Int. 2006;69:573–9.
Creput C, Toledano D, Petitclerc T. Ionic dialysance and determination of Kt/V in on-line hemodiafiltration with simultaneouspre- and post-dilution. Int J Artif Organs. 2013;36:327–34.
de Sequera P, Albalate M, Perez-Garcia R, et al. A comparison of the effectiveness of two online haemodiafiltration modalities: mixed versus post-dilution. Nefrologia. 2013;33:779–87.
Potier J, Le RF, Faucon JP, et al. Elevated removal of middle molecules without significant albumin loss with mixed-dilution hemodiafiltration for patients unable to provide sufficient blood flow rates. Blood Purif. 2013;36:78–83.
Kuhlmann MK. Phosphate elimination in modalities of hemodialysis and peritoneal dialysis. Blood Purif. 2010;29:137–44.
Krieter DH, Falkenhain S, Chalabi L, Collins G, Lemke HD, Canaud B. Clinical cross-over comparison of mid-dilution hemodiafiltration using a novel dialyzer concept and post-dilution hemodiafiltration. Kidney Int. 2005;67:349–56.
Feliciani A, Riva MA, Zerbi S, et al. New strategies in haemodiafiltration (HDF): prospective comparative analysis between on-line mixed HDF and mid-dilution HDF. Nephrol Dial Transplant. 2007;22:1672–9.
Santoro A, Ferramosca E, Mancini E, et al. Reverse mid-dilution: new way to remove small and middle molecules as well as phosphate with high intrafilter convective clearance. Nephrol Dial Transplant. 2007;22:2000–5.
Pedrini LA, Feliciani A, Zerbi S, Cozzi G, Ruggiero P. Optimization of mid-dilution haemodiafiltration: technique and performance. Nephrol Dial Transplant. 2009;24:2816–24.
Eloot S, Dhondt A, Van LM, Waterloos MA, Vanholder R. Removal of water-soluble and protein-bound solutes with reversed mid-dilution versus post-dilution haemodiafiltration. Nephrol Dial Transplant. 2012;27:3278–83.
de Francisco AL, Botella J, Escallada R, et al. Haemodiafiltration with sorbent-regenerated ultrafiltrate as replacement fluid: a multicenter study. Nephrol Dial Transplant. 1997;12:528–34.
de Francisco AL, Pinera C, Heras M, et al. Hemodiafiltration with on-line endogenous reinfusion. Blood Purif. 2000;18:231–6.
Ghezzi PM, Botella J, Sartoris AM, Gervasio R, Diez C. Use of the ultrafiltrate obtained in two-chamber (PFD) hemodiafiltration as replacement fluid. Experimental ex vivo and in vitro study. Int J Artif Organs. 1991;14:327–34.
Ghezzi PM, Gervasio R, Tessore V, Sartoris AM, Botella J. Hemodiafiltration without replacement fluid. An experimental study. ASAIO J. 1992;38:61–5.
Sanz-Moreno C, Botella J. Hemodiafiltration in two chambers without replacement fluid: a clinical study. Artif Organs. 1995;19:407–10.
Wratten ML, Ghezzi PM. Hemodiafiltration with endogenous reinfusion. Contrib Nephrol. 2007;158:94–102.
Calo LA, Naso A, Carraro G, et al. Effect of haemodiafiltration with online regeneration of ultrafiltrate on oxidative stress in dialysis patients. Nephrol Dial Transplant. 2007;22:1413–9.
Borrelli S, Minutolo R, De NL, et al. Intradialytic changes of plasma amino acid levels: effect of hemodiafiltration with endogenous reinfusion versus acetate-free biofiltration. Blood Purif. 2010;30:166–71.
Maeda K, Shinzato T. Push/pull hemodiafiltration: technical aspects and clinical effectiveness. Nephron. 1995;71:1–9.
Miwa M, Shinzato T. Push/pull hemodiafiltration: technical aspects and clinical effectiveness. Artif Organs. 1999;23:1123–6.
Shinzato T, Miwa M, Kobayakawa H, et al. Effectiveness of new push/pull hemodiafiltration for arthralgia in long-term hemodialysis patients. Contrib Nephrol. 1995;112:111–8.
Shinzato T, Maeda K. Push/pull hemodiafiltration. Contrib Nephrol. 2007;158:169–76.
von Albertini B, Miller JH, Gardner PW, Shinaberger JH. Performance characteristics of high flux haemodiafiltration. Proc Eur Dial Transplant Assoc Eur Ren Assoc. 1985;21:447–53.
Ronco C, Brendolan A, Lupi A, Metry G, Levin NW. Effects of a reduced inner diameter of hollow fibers in hemodialyzers. Kidney Int. 2000;58:809–17.
Bosch JP, Lew SQ, Barlee V, Mishkin GJ, von Albertini B. Clinical use of high-efficiency hemodialysis treatments: long-term assessment. Hemodial Int. 2006;10:73–81.
Susantitaphong P, Tiranathanagul K, Katavetin P, et al. Efficacy of convective-controlled double high-flux hemodiafiltration versus on-line hemodiafiltration: 1-year prospective study. Blood Purif. 2010;29:35–43.
Tiranathanagul K, Yossundharakul C, Techawathanawanna N, et al. Comparison of middle-molecule clearance between convective control double high-flux hemodiafiltration and on-line hemodiafiltration. Int J Artif Organs. 2007;30:1090–7.
Santoro A, Ferrari G, Spongano M, Badiali F, Zucchelli P. Acetate-free biofiltration: a viable alternative to bicarbonate dialysis. Artif Organs. 1989;13:476–9.
Zucchelli P, Santoro A, Raggiotto G, Degli EE, Sturani A, Capecchi V. Biofiltration in uremia: preliminary observations. Blood Purif. 1984;2:187–95.
Galli G, Panzetta G. Acetate free biofiltration (AFB): from theory to clinical results. Clin Nephrol. 1998;50:28–37.
Amore A, Cirina P, Mitola S, et al. Acetate intolerance is mediated by enhanced synthesis of nitric oxide by endothelial cells. J Am Soc Nephrol. 1997;8:1431–6.
Munoz RI, Montenegro J, Salcedo A, et al. Effect of acetate-free biofiltration with a potassium-profiled dialysate on the control of cardiac arrhythmias in patients at risk: a pilot study. Hemodial Int. 2008;12:108–13.
Santoro A, Mancini E, Fontanazzi F, Paolini F. Potassium profiling in acetate-free biofiltration. Contrib Nephrol. 2002;137:260–67
Severi S, Vecchietti S, Cavalcanti S, Mancini E, Santoro A. Electrocardiographic changes during hemodiafiltration with different potassium removal rates. Blood Purif. 2003;21:381–8.
Tessitore N, Santoro A, Panzetta GO, et al. Acetate-free biofiltration reduces intradialytic hypotension: a European multicenter randomized controlled trial. Blood Purif. 2012;34:354–63.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2016 Springer International Publishing Switzerland
About this chapter
Cite this chapter
Pedrini, L.A., Zerbi, S. (2016). Convective Techniques. In: Nubé, M., Grooteman, M., Blankestijn, P. (eds) Hemodiafiltration. Springer, Cham. https://doi.org/10.1007/978-3-319-23332-1_2
Download citation
DOI: https://doi.org/10.1007/978-3-319-23332-1_2
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-23331-4
Online ISBN: 978-3-319-23332-1
eBook Packages: MedicineMedicine (R0)