Abstract
This chapter addresses the acute and chronic cardiovascular effects of convective therapies. The most important acute cardiovascular complication in intermittent dialysis therapies is intra-dialytic hypotension (IDH) which causes patient discomfort, but is also related to end organ ischemia and mortality. The pathogenesis of IDH is multifactorial, in which both patient- and treatment-related factors are involved. The effect of the dialysis treatment on IDH is mediated by three factors: a decline in blood volume, an impaired reactivity of the resistance and capacitance vessels and myocardial contractility.
Various studies have shown that the incidence of IDH is reduced by the use of convective techniques. Available evidence suggests that the most important responsible factor for the positive hemodynamic effects of convective techniques is an improved reactivity of the resistance and capacitance vessels as compared to hemodialysis (HD). This phenomenon also appears to be at least partly mediated by thermal factors. Post-dilution hemodiafiltration (HDF) has an increased cooling effect as compared to HD due to additional heat loss from the infusion line. Smaller studies showed an equivalent incidence of IDH and hemodynamic response between HD and convective techniques after control for thermal factors. As for the chronic cardiovascular effects of convective therapies, available evidence does not suggest a major role of convective therapies on inter-dialytic blood pressure, arterial stiffness or left ventricular mass. Evidence on cardiovascular events and outcomes are as yet conflicting, one randomized study showing a positive effect of post-dilution on-line HDF on cardiovascular mortality and incidence of stroke, whereas other studies did not show a significant effect on cardiovascular outcomes. Future randomized studies, carefully controlled for thermal factors, are needed to fully establish the potential of convective techniques in preventing both short-and long-term cardiovascular complications in dialysis patients.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Hemodiafiltration
- Intra-dialytic hypotension
- Vascular reactivity
- Thermal balance
- Arterial stiffness
- Hypertension
- Left ventricular hypertrophy
- Cardiovascular mortality
Intradialytic Hypotension
Introduction
The most important acute complication of dialysis therapies is intra-dialytic hypotension (IDH). IDH is a frequently occurring phenomenon which can cause significant patient discomfort but can, in some cases, even lead to severe complications. IDH has been defined in different ways. By K/DOQI and the European Best Practice Guidelines (EBPG), IDH is defined as a decline in systolic blood pressure (BP) ≥20 mmHg or a decline in mean arterial pressure (MAP) by 10 mmHg versus baseline, associated with clinical events and need for nursing interventions [1, 2]. However, in the literature, also other definitions, e.g. based on the nadir BP have been proposed [3].
The incidence of IDH during hemodialysis is significant. Historically, IDH is assumed to occur in 20–30 % of dialysis sessions [4]. More recent surveys have addressed this issue in more detail. In a survey from Great Britain in 2,193 patients including 6,579 dialysis sessions, symptomatic IDH (defined as a sudden decline in BP, which required intravenous fluid replacement) occurred in 14.9 % of non-diabetic and 20.3 % of diabetic dialysis patients [5]. In a study from the US in 1,137 patients including 44,801 treatments [6], IDH (defined as an intradialytic decline in systolic BP by more than 30 mmHg to a level of less than 90 mmHg) occurred in 17.2 % of patients with a large intra-individual variability: whereas 25.1 % of patients did not experience IDH at all, in 16.2 % IDH occurred in more than 35 % of treatments. The incidence of IDH also varies between centers [7]. In a report based on audits in the Greater London area in the UK including 11 centers, the incidence of IDH varied between 7 % and 28 % of treatments.
In the largest survey available so far, Stefansson et al. studied records of 39,497 patients in the USRDS database during a 90 days assessment period. IDH, defined in line with the K/DOQI guidelines (≥20 mmHg fall in systolic BP plus ≥2 responsive measures) was observed in 31 % of patients at least once [8]. In a study in 1,409 patients of the HEMO cohort, the incidence of IDH according to the K/DOQI definition was 9.6 % [3]. Summarizing, even in contemporary dialysis treatment, IDH remains a common problem. However, it also becomes clear that the definition of IDH used in the literature varies widely.
The consequences of IDH are substantial. On the short term, IDH leads to clinical symptoms such as nausea, vomiting, cramps and cardiovascular collapse. It has also been involved in the pathogenesis of vascular access thrombosis [9]. At a subclinical level, indirect evidence suggests that IDH as such may contribute to reversible regional myocardial dysfunction (“stunning”) as well as circulating endotoxemia due to splanchnic hypoperfusion [10–12].
In addition, various [3, 8, 13] reports found a relation between IDH and outcome. In a study in 1,244 dialysis patients, Shoji et al. observed that a fall in intra-dialytic systolic BP of more than 40 mmHg, was associated with an increase in 2-years mortality as compared to patients with a lower intra-dialytic fall in systolic BP after adjustment for age, gender, diabetic status, serum creatinine, ultrafiltration per body weight, and body weight after HD [13]. In a study by Stefansson et al. the occurrence of one or more episodes of IDH during a 90 day period was associated with an increased risk for all-cause and cardiovascular mortality, as well as for major adverse cardiac events during a mean follow-up time of 398 days [8]. Despite correction for comorbid factors, these observations do not necessarily imply causation, although for instance, repetitive cardiac stunning might result in persistent left ventricular dysfunction and is also in itself an important risk factor for mortality [14, 15].
The relation between IDH and outcome also appears to depend on its definition. In a recent analysis in 11,801 patients, the strongest association with mortality was observed with a nadir in systolic BP of 90 mmHg or less in patients with pre-dialytic systolic BP below 160 mmHg. In patients with pre-dialytic systolic BP levels of 160 mmHg or higher, the strongest association was observed with nadir systolic BP levels below 100 mmHg. Unlike the results of Shoji et al. [13], in this study, symptoms, interventions or the magnitude of the decline in BP per se were not associated with outcome [3].
Nevertheless, regardless of the differences in the literature and the uncertainties with regard to causation, it is well established that IDH is an important risk factor for mortality in dialysis patients and that both for this reason, as well as to prevent patient discomfort, its prevention is of great clinical importance.
Pathophysiology of IDH
The pathophysiology of IDH is multifactorial, but three major components can be distinguished [2, 16]. In analogy to hypovolemic shock, the first driver is the decline in circulating blood volume leading to a decline in venous return to the heart [17, 18]. However, in contrast to previously healthy persons, in whom a decline in plasma volume up to 15 % (and in some cases up to 25 %) is not associated with significant clinical features, IDH can occur at a much lower decline in blood volume. In a survey in 60 IDH-prone patients, intra-morbid events (two out of three related to IDH) occurred at a decline in relative blood volume varying between 2 % and 29 % [19].
The fact that IDH may occur at a much lower decline in blood volume as compared to healthy subjects indicates that the normal compensatory response to hypovolemia can be disturbed in dialysis patients. The acute compensatory response to hypovolemia, subsequently activated by low and high pressure receptors in the cardiovascular system, results in an increase in myocardial contractility and heart rate, as well as an increase in peripheral arterial and venous tone through sympathetic activation [17, 20].
In dialysis patients, both patient as well as treatment related factors may interfere with the hemodynamic response during dialysis. Patient related-factors contributing to IDH, which will not be discussed in detail further in this chapter, include factors such as age and dialysis vintage, as well as structural cardiovascular abnormalities, such as a reduction in left ventricular systolic or diastolic function, a reduction in compliance of the venous system, and autonomous neuropathy [2, 6, 14, 21, 22]. Treatment related factors contributing to the occurrence or prevention of IDH can be conceptually summarized as factors influencing respectively blood volume, vascular reactivity and myocardial contractility [2].
Ultrafiltration volume, the major determinant of the decline in blood volume during dialysis [23], is mainly influenced by ultrafiltration rate, a resultant of the inter-dialytic weight gain and treatment time. Various studies [6, 8] showed inter-dialytic weight gain to be important predictors of IDH. Next to ultrafiltration, an important treatment-related determinant of the fall in blood volume is the sodium concentration of the dialysate [24].
When blood volume declines, an adequate vascular reactivity is of pivotal importance to maintain BP. This reactivity concerns both a constriction of the resistance vessels, leading to an increase in systemic vascular resistance, as well as a constriction of the capacitance vessels. The latter contain 80 % of circulating blood volume, and mobilization of so-called “unstressed” (i.e. hemodynamically inactive blood volume [20]) allows for maintenance of venous return and preservation of cardiac output despite a fall in blood volume [25]. During dialysis, this process may be impaired. In search for the pathogenesis of this phenomenon, it has become clear that thermal factors play a major role.
The dialysis membrane is an efficient heat exchanger due to the close and continuous contact between the blood and dialysis fluid. An important determinant of body temperature changes during dialysis is therefore the ratio between the pre-dialytic body temperature of the patient and the dialysate temperature [26, 27]. It has been shown that core temperature generally increases in patients with a dialysate temperature of 37–37.5 °C [26, 28, 29], which may interfere with the normal reactivity of the vascular system by inducing vasodilation of the cutaneous blood vessels in order to remove the excess heat. One of the most potent methods to prevent IDH is cooling of the patient by reducing the dialysate temperature [30, 31], which is mainly explained by its beneficial effect on vascular reactivity [29]. In a systematic review, the incidence of IDH with the use of cool dialysis was reduced by 7.1 times [32].
Interestingly, core temperature increases during dialysis even without addition of heat from the extracorporeal circuit [33], which suggests that, apart from the effects of dialysate temperature, the dialysis treatment itself contributes to the increase in core temperature. Available literature suggests that both an initial reduction in heat loss from the skin due to peripheral vasoconstriction in response to a decline in blood volume (later followed by vasodilation), but also as yet unidentified factors related to the hemodialysis procedure per se play a role in the increase in core temperature during dialysis [33–35]. Without additional removal of thermal energy from the extracorporeal circuit, a mean increase in arterial temperature of 0.47 °C was observed during dialysis [36].
The amount of thermal energy which needs to be removed in order to keep body temperature stable (isothermic) during dialysis is substantial, and has been assessed by monitoring extracorporeal heat flow (Jex) during dialysis by a specific device (Blood Temperature Monitor®). Jex is calculated by the formula: \( {\mathrm{J}}_{\mathrm{ex}}=-\mathrm{c}\rho \left({\mathrm{T}}_{\mathrm{art}}-{\mathrm{T}}_{\mathrm{ven}}\right)*\left({\mathrm{Q}}_{\mathrm{b}}-\mathrm{U}\mathrm{F}\mathrm{R}\right) \) Footnote 1 [35]. The product c ρ (3.81 J/°C/m3) refers to the heat capacity and density of blood, Tart and Tven to respectively the temperature in the arterial and venous blood line, Qb to extracorporeal blood flow rate and UFR to ultrafiltration rate. One study found a Jex of −0.25 W/kg during isothermic treatments, corresponding to 24 % of the resting energy expenditure, whereas in another study a mean Jex of −17.9 W was observed [33, 36]. Whether it suffices to maintain body temperature or whether further cooling is needed to maintain optimal hemodynamic stability during dialysis remains to be determined, although only small, albeit significant differences in the blood pressure decline during dialysis were observed between isothermic treatments (in which core temperature was kept stable) and dialysis during which the core temperature was decreased by 0.5 °C [37].
Regarding cardiac contractility, in important treatment-related factor is dialysate calcium [38], which may have relevance for the intra-dialytic blood pressure course [38, 39]. In addition, the dialysis procedure itself, but especially ultrafiltration may induce myocardial stunning [14, 15]. Whether the latter phenomenon also plays a role in the pathogenesis of IDH remains to be determined.
IDH During HDF
The first study showing a difference in the hemodynamic response between convective therapies (conventional hemofiltration [HF] with infusion of bags) and HD was already published in 1980 by Quellhorst et al. [40]. (These results were confirmed in later studies with conventional HDF [41]. However, different studies also showed a reduction in IDH with on line convective therapies as compared to hemodialysis, both for on-line HF as well as HDF [42–44]. In the largest study so far (ESHOL study), in which 906 patients were randomized to post-dilution o-HDF or HD with a mean follow up of 1.9 years, the incidence rate ratio of IDH with on-line HDF (oHDF) was 0.72 [CI 0.68–0.77] as compared to HD [43]. In this study, IDH was not clearly defined, but the results are of significant relevance given the reduction in CV mortality and stroke observed in this study with the use of HDF. In a multicenter study in 146 patients randomly allocated to either pre-dilution oHDF (n = 40), on-line HF (n = 36) or HD (n = 70), a reduction [44] of IDH was observed with both o-HF (OR 0.69; 95 % confidence interval 0.51–0.92) as well as o-HDF (OR 0.46, 95 % confidence interval 0.33–0.63). In this study, IDH was defined as a rapid symptomatic fall of systolic BP by at least 30 mmHg or that required nursing and/or medical intervention. In a meta-analysis of RCT published in 2013 in which 1,006 patients divided over 12 study arms were pooled, the relative risk of IDH with convective therapies (which also included the use of high flux treatments) was 0.55, 95 % CI 0.35, 0.87, P = 0.01) as compared to low-flux HD [45]. Comparable results (RR, 0.49; 95 % CI, 0.30–0.81) in which HF or HDF therapies were compared to HD were observed in a later meta-analysis [46] in five trials with in total 1,259 participants, as well as in another meta-analysis (RR 0.72 [CI 0.66–0.88]) [47]. Summarizing, there is extensive evidence that IDH is reduced by the use of convective treatments.
Effects of Convective Therapies on the Pathophysiologic Determinants of IDH
Whereas the benefits of HDF on hemodynamic instability have been independently shown in various trials, the mechanism behind this effect has not been completely elucidated. Previous reports with conventional HF suggested that, possibly due to an increase in the Donnan effect due to protein coating of the dialyzer, sodium removal was lower during convective therapies [48, 49], which could result in improved blood volume preservation [50, 51]. However, other studies with on-line HF or HDF [52] did not observe differences in sodium removal, blood volume preservation, or body water compartments [50, 53, 54] between convective therapies and on-line convective therapies. In contrast, one study even observed a larger decline in blood volume during post-dilution on on-line HDF as compared to HD [55]. With regard to myocardial contractility, no study as yet addressed potential differences between HD and convective therapies.
The main mechanism affected by convective therapies appears to be an improved vascular reactivity [56]. Studies from the early 1980s showed an increase in systemic vascular resistance as well as plasma noradrenaline levels during conventional HF as compared to HD [40, 57, 58]. These results were later confirmed by others [59, 60], showing both an increase in peripheral vascular resistance as well as venous tone. The mechanisms behind the differences in vascular response between convective therapies and HD have not been definitely elucidated. Various mechanisms, such as differences in removal of larger molecular weight vasoactive substances such as calcitonin-related gene peptide, or ouabain-like factors, or a reduction in inflammatory mediators have been suggested [56, 61–63]. However, most available evidence suggests an important role of extracorporeal cooling as an important contributory factor to the improved hemodynamic response during convective strategies [55, 59].
Effects of Convective Therapies on Thermal Balance
As discussed previously, the temperature in the venous blood line (Tven) is an important contributor to the extracorporeal heat flow rate Jex. Tven is dependent on the temperature of the dialysate, and the heat loss from the venous line to the environment (which is approximately 7–15 W) [35, 64]. From this, it becomes clear that, irrespective of dialysate temperature, post-dilution HDF leads to additional cooling of the patient because of heat loss from the infusion line, next to the heat loss from the venous blood lines. This has been quantified in the study of Donauer et al. in which mean Jex was −5.4 W during HD and −16.6 W during post-dilution on-line HDF with a mean dialysate/infusate temperature of 36.8 °C and an infusion rate of 50 ml/min [55]. In this study, the rise in mean blood temperature in the arterial line was significantly higher during HD (0.39 °C) as compared to on-line HDF (0.26 °C). In order to achieve the same Jex during HD as compared to on-line HDF, the dialysate temperature had to be lowered to a mean of 35.6 °C in order to achieve the same Jex as post-dilution on-line HDF. It should be noted that the infusion rate in this study was substantially less as compared to recent recommendations [65]. However, in a more recent study, mean Jex was 16.2 W during post-dilution on-line HDF with a mean infusion rate of 59 ml/min and a dialysate temperature between 35.5 and 36.5 °C [53]. The thermal effects are different for pre-dilution on-line HDF, where this additional heat loss does not play a role because the infusion fluid enters the blood stream before the dialyzer. This was confirmed by a study comparing pre-dilution HDF with HD, during which the body temperature of the patient was kept stable (isothermic) by the feedback module of the Blood temperature monitor®. During a 4.5 h treatment, the mean energy which needed to be removed to allow an isothermic treatment was 155 kJ during HD and 135 kJ during pre-dilution on-line HDF, corresponding to an approximate Jex of 9.6 and 8.3 W respectively [66].
With regard to the other, less commonly used convective strategies, no detailed in vivo data on thermal balance are available. For post-dilution on-line HF, the cooling effect will likely be larger as compared to HD with an equivalent dialysate temperature, because the additional heat exchange due to contact between blood and dialysate does not take place and because of the heat loss through the infusion line, as discussed previously for oHDF [46]. For pre-dilution HF, the cooling effect will likely be less pronounced because the infusion volumes are generally high and because the additional cooling due to the venous line does not take place [46]. In an in vitro study, the estimated thermal balance (expressed as kJ/h) was −35 kJ/h with pre-dilution HF (−9.7 W) at an infusate temperature of 37 °C, as compared to 72 kJ/h with post-dilution HF (−20.0 W, −10 kJ/h (−2.8 W) with conventional HD and −170 kJ/h (−47.2 W) with cool dialysis (35.5 °C). However, translation from in vitro to in vivo data is hazardous because regulation of “arterial” temperature, which occurs constantly in vivo, is not possible in the in vitro setting.
The heat loss may be larger with conventional convective techniques given the fact that the temperature of the infusion fluid is generally lower as compared to on-line convective therapies, with fluids mostly infused at room temperature [35]. This explains the finding that during conventional HDF, the cooling effect was dependent upon the infusion volume. In a crossover study in 12 patients, mean Jex was comparable between HD 35.5 °C (−26.6 W) and post-dilution HDF with an infusion rate of 2.5 L/h (mean −25.3 W) and was significantly more negative compared with HD 37.5 °C (−3.5 W) and HDF at an infusion rate of 1 L/h (−15.9 W) [41].
The Relation between Extracorporeal Cooling and the Hemodynamic Response to HDF
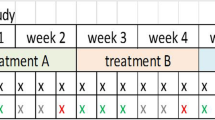
These thermal effects appear to have a major impact on the hemodynamic stability during treatments. In a crossover study in 17 patient with frequent IDH, in which 25 treatments were compared between 3 different treatment settings the incidence of IDH (defined as a decline in systolic BP below 100 mmHg in the presence of symptoms) was 40 % higher during HD as compared to on-line HDF without correction for this additional energy loss, whereas no difference in hypotensive episodes between HD and on-line HDF was observed when the dialysate was additionally cooled during HD (4 % during both modalities), in order to achieve a comparable energy balance [55], see Fig. 17.1. In addition, in a single treatment study, no differences in the hemodynamic response to HD and on-line HDF were observed with comparable negative Jex [53], see Fig. 17.2. Another study in 12 dialysis patients found a significantly larger decline in BP during HD with a dialysate temperature of 37.5 °C as compared to conventional HDF, but no difference in the BP fall between HDF and cool dialysis (temperature 35.5 °C) [41]. In earlier study, by van Kuijk et al. vascular reactivity was clearly different between HD and conventional HF, when the latter was associated with a significant cooling effect whereas no difference was observed when the temperature of the infusion fluid was heated in order to obtain comparable thermal effects [59]. In a non-controlled prospective study in which 44 patients on cooled HD (median dialysate temperature 35 °C) were compared to 34 patients on post-dilution oHDF (median dialysate/infusate temperature 36 °C, infusion volume 65–85 ml/min), the incidence of IDH was even higher in the oHDF group (25.9 % versus 16.5 %; p = 0.01) [62]. In a crossover study in 12 patients, no difference in change in cardiac output, BP or total peripheral resistance was observed between pre-dilution on-line HDF and HD under thermal controlled conditions [66]. In contrast to these findings, in the study of Locatelli, IDH was significantly reduced despite the fact that pre-dilution HDF was used [44]. As discussed previously, theoretically, this should have resulted in the comparable thermal balance between the convective techniques and HD, although data on this aspect were not available. Also in the ESHOL study, in which a significant reduction in IDH was observed with post-dilution HDF, which has likely resulted in significant cooling effects, unfortunately no data on thermal effects of the different modalities were available [43].
This figure shows that the incidence of IDH is significantly reduced by on line HDF as compared to HD without correction for thermal energy balance (a), but a comparable reduction in IDH during HD when both treatments were matched for thermal balance (Temp-HD) (b) (Reprinted from Donauer et al. [55]. With permission from Oxford University Press)
Figure showing that the change in systolic blood pressure (BP) during treatment was more dependent on the duration of the treatment than on the modality choice of HD (mean dialysate temperature 35.9 °C) or on-line HDF. The number behind the modalities reflect the treatment duration in hours (Reprinted from Cornelis et al. [53]. With permission from Elsevier)
Thus, there is substantial clinical evidence for an important effect of thermal balance as an important contributing factor to the improved hemodynamic stability during convective therapies. Whether additional factors are also involved in the reduction of IDH during convective therapies should be investigated in future randomized trials with strict control of thermal balance between HD and HDF.
Long Term Effects on Cardiovascular Parameters
Cardiovascular events are the most important contributor to the greatly increased risk of mortality in dialysis patients [67]. Uncontrolled hypertension and structural cardiovascular abnormalities such as increased arterial stiffness and left ventricular hypertrophy are important risk factors for mortality in dialysis patients [68–70]. It has been suggested that convective techniques are associated with improved cardiovascular outcomes, but also with an improved BP regulation and cardiovascular structure due to increased removal of larger molecular weight uremic toxins and vasoactive substances such as asymmetric dimethylarginine (ADMA) [71]. In the following paragraphs, the available evidence for an effect of convective techniques on BP regulation, cardiovascular structure and outcomes will be summarized.
Hypertension
Earlier reports suggested an improved regulation of hypertension with the use of conventional HF [72]. However, these results were not confirmed in later randomized studies with longer follow-up durations. Beerenhout et al. did not observe a difference in BP regulation, assessed by 48-h ambulatory BP measurements with the use of pre-dilution on-line HF [71]. Notably, in this study, also no effect of oHF on serum levels of ADMA was observed. Neither in the ESHOL nor in the CONTRAST an effect of oHDF on BP were observed [43, 73], whereas in the Turkish on-line HDF study significantly higher time averaged systolic BP levels were observed with oHDF (129 ± 13 versus 126 ± 13 mmHg, P = 0.001) as compared to HD [74]. Also a cross-sectional study did not show differences in pre-dialytic BP between patients treated with HD or oHDF [62]. Therefore, there is at present no evidence for a direct positive additional effect on inter-dialytic BP regulation and hypertension control. It cannot be excluded that the earlier positive results of conventional HF on BP resulted from a better volume regulation due to an improved hemodynamic tolerance during HF.
Structural Cardiovascular Parameters
Few studies have assessed the effect of convective therapies on structural cardiovascular parameters. In two observational studies, no differences in pulse wave velocity, as a marker of arterial stiffness, were observed between patients on oHDF and matched HD patients [75, 76].
Two randomized studies have studied the effect of convective techniques on structural cardiovascular parameters. In a study in patients comparing on-line HF with low-flux HD during a follow-up time of 1 year, arterial stiffness or left ventricular mass did not differ between the groups [71]. Also in a subgroup of the CONTRAST study, no differences in arterial stiffness or left ventricular mass were observed between the groups randomized either to low-flux HD or post-dilution HDF [73].
Cardiovascular Outcomes
Three large randomized controlled trials were recently published which, in addition to all-cause mortality, also assessed the risk of cardiovascular mortality and/or events.
The CONTRAST study did not find a difference in the composite cardiovascular outcomes between low-flux HD and post-dilution on-line HDF (hazard ratio, 1.07; 95 % confidence interval, 0.83–1.39) [77]. Also in the Turkish OL-HDF study, comparing on-line HDF with high flux HD, no difference in cardiovascular mortality or events was observed in the primary analysis, although an improved cardiovascular outcome was observed in the subgroup which achieved higher substitution volumes [74]. In the ESHOL study, a near significant difference in cardiovascular mortality (HR, 0.67; 95 % CI, 0.44–1.02; P = 0.06) between post-dilution on-line HDF and high-flux dialysis was observed in the primary analysis. A reduction in stroke risk was a significant contributor to the reduced cardiovascular mortality in this study [43]. The reason for the improved cardiovascular outcome in this study was not clear, although the authors hypothesized that a reduction in systemic inflammation might be involved. However, it should be noted that in this study also a significant reduction in IDH was observed, which might have contributed to lesser variations in cerebral perfusion.
Also in systematic reviews, the effect of convective techniques on cardiovascular outcomes has yielded conflicting results. In one analysis, no effect of convective techniques (defined as filtration techniques and high-flux dialysis) on cardiovascular outcomes was observed as compared to low-flux dialysis [46]. Another systematic review observed a reduction in cardiovascular mortality, but not in non-fatal cardiovascular events between convective techniques (including HDF, HD and acetate-free biofiltration) as compared to HD techniques [47].
Summarizing, there is no solid evidence for a beneficial effect of convective techniques on either inter-dialytic BP regulation or structural cardiovascular parameters. The effect on convective techniques on cardiovascular outcome is conflicting. One randomized study observed a near significant reduction in cardiovascular outcome and a reduction in stroke incidence. More studies are needed to definitely address the effect of convective techniques on cardiovascular outcome in dialysis patients.
Teaching Points
-
The most important acute cardiovascular complication in intermittent dialysis therapies is intra-dialytic hypotension (IDH), which is related to end organ ischemia and mortality.
-
The effect of the dialysis treatment on IDH is mediated by three factors: a decline in blood volume, impaired reactivity of the resistance and capacitance vessels and myocardial contractility.
-
The incidence of IDH is reduced by the use of convective techniques.
-
The most important responsible factor for the positive hemodynamic effects of convective techniques is an improved reactivity of the resistance and capacitance vessels as compared to hemodialysis (HD). This phenomenon appears to be at least partly mediated by thermal factors.
-
Post-dilution hemodiafiltration (HDF) has an increased cooling effect as compared to HD due to additional heat loss from the infusion line.
-
Available evidence does not suggest a major role of convective therapies on inter-dialytic blood pressure, arterial stiffness or left ventricular mass.
-
Evidence on the effect of HDF on cardiovascular outcome is yet conflicting.
-
Future randomized studies, carefully controlled for thermal factors, are needed to fully establish the potential of high-volume post-dilution HDF in preventing both short-and long-term cardiovascular complications in dialysis patients.
Notes
- 1.
A negative Jex reflects heat flow from the patient to the extracorporeal system (“cooling”)
References
K/DOQI Workgroup. K/DOQI clinical practice guidelines for cardiovascular disease in dialysis patients. Am J Kidney Dis. 2005;45(4 Suppl 3):S1–153.
Kooman J, Basci A, Pizzarelli F, Canaud B, Haage P, Fouque D, Konner K, Martin-Malo A, Pedrini L, Tattersall J, Tordoir J, Vennegoor M, Wanner C, ter Wee P, Vanholder R. EBPG guideline on haemodynamic instability. Nephrol Dial Transplant. 2007;22 Suppl 2:ii22–44.
Flythe JE, Xue H, Lynch KE, Curhan GC, Brunelli SM. Association of Mortality Risk with Various Definitions of Intradialytic Hypotension. J Am Soc Nephrol. 2015;26:724–34.
Palmer BF, Henrich WL. Recent advances in the prevention and management of intradialytic hypotension. Nephrol Dial Transplant. 2005;20:1155–63.
Davenport A, Cox C, Thuraisingham R. Blood pressure control and symptomatic intradialytic hypotension in diabetic haemodialysis patients: a cross-sectional survey. Nephron Clin Pract. 2008;109:c65–71.
Sands JJ, Usvyat LA, Sullivan T, Segal JH, Zabetakis P, Kotanko P, Maddux FW, Diaz-Buxo JA. Intradialytic hypotension: frequency, sources of variation and correlation with clinical outcome. Hemodial Int. 2014;18:415–22.
Davenport A, Cox C, Thuraisingham R. Achieving blood pressure targets during dialysis improves control but increases intradialytic hypotension. Kidney Int. 2008;73:759–64.
Stefánsson BV, Brunelli SM, Cabrera C, Rosenbaum D, Anum E, Ramakrishnan K, Jensen DE, Stålhammar NO. Intradialytic hypotension and risk of cardiovascular disease. Clin J Am Soc Nephrol. 2014;9:2124–32.
Chang TI, Paik J, Greene T, et al. Intradialytic hypotension and vascular access thrombosis. J Am Soc Nephrol. 2011;22:1526–33.
Jefferies HJ, Crowley LE, Harrison LE, Szeto CC, Li PK, Schiller B, Moran J, McIntyre CW. Circulating endotoxaemia and frequent haemodialysis schedules. Nephron Clin Pract. 2014;128:141–6.
Jefferies HJ, Virk B, Schiller B, Moran J, McIntyre CW. Frequent hemodialysis schedules are associated with reduced levels of dialysis-induced cardiac injury (myocardial stunning). Clin J Am Soc Nephrol. 2011;6:1326–32.
Burton JO, Jefferies HJ, Selby NM, McIntyre CW. Hemodialysis-induced cardiac injury: determinants and associated outcomes. Clin J Am Soc Nephrol. 2009;4:914–20.
Shoji T, Tsubakihara Y, Fujii M, Imai E. Hemodialysis associated hypotension as an independent risk factor for two-year mortality in hemodialysis patients. Kidney Int. 2004;66:1212–20.
Assa S, Hummel YM, Voors AA, Kuipers J, Westerhuis R, de Jong PE, Franssen CF. Hemodialysis-induced regional left ventricular systolic dysfunction: prevalence, patient and dialysis treatment-related factors, and prognostic significance. Clin J Am Soc Nephrol. 2012;7:1615–23.
Burton JO, Jefferies HJ, Selby NM, McIntyre CW. Hemodialysis-induced repetitive myocardial injury results in global and segmental reduction in systolic cardiac function. Clin J Am Soc Nephrol. 2009;4:1925–31.
van der Sande FM, Kooman JP, Leunissen KM. Intradialytic hypotension – new concepts on an old problem. Nephrol Dial Transplant. 2000;15:1746–8.
Worthley LI. Shock: a review of pathophysiology and management. Part I. Crit Care Resusc. 2000;2:55–65.
Hardaway RM. Monitoring of the patient in a state of shock. Surg Gynecol Obstet. 1979;148:339–52.
Barth C, Boer W, Garzoni D, Kuenzi T, Ries W, Schaefer R, Schneditz D, Tsobanelis T, van der Sande F, Wojke R, Schilling H, Passlick-Deetjen J. Characteristics of hypotension-prone haemodialysis patients: is there a critical relative blood volume? Nephrol Dial Transplant. 2003;18:1353–60.
Funk DJ, Jacobsohn E, Kumar A. The role of venous return in critical illness and shock-part I: physiology. Crit Care Med. 2013;41:255–62.
Ritz E, Rambausek M, Mall G, Ruffmann K, Mandelbaum A. Cardiac changes in uraemia and their possible relationship to cardiovascular instability on dialysis. Nephrol Dial Transplant. 1990;5 Suppl 1:93–7.
Kooman JP, Wijnen JA, Draaijer P, van Bortel LM, Gladziwa U, Peltenburg HG, Struyker-Boudier HA, van Hooff JP, Leunissen KM. Compliance and reactivity of the peripheral venous system in chronic intermittent hemodialysis. Kidney Int. 1992;41:1041–8.
Mann H, Ernst E, Gladziwa U, Schallenberg U, Stiller S. Changes in blood volume during dialysis are dependent upon the rate and amount of ultrafiltrate. ASAIO Trans. 1989;35:250–2.
Brummelhuis WJ, van Geest RJ, van Schelven LJ, Boer WH. Sodium profiling, but not cool dialysate, increases the absolute plasma refill rate during hemodialysis. ASAIO J. 2009;55:575–80.
Kooman JP, Gladziwa U, Böcker G, van Bortel LM, van Hooff JP, Leunissen KM. Role of the venous system in hemodynamics during ultrafiltration and bicarbonate dialysis. Kidney Int. 1992;42:718–26.
van der Sande FM, Kooman JP, Burema JH, Hameleers P, Kerkhofs AM, Barendregt JM, Leunissen KM. Effect of dialysate temperature on energy balance during hemodialysis: quantification of extracorporeal energy transfer. Am J Kidney Dis. 1999;33:1115–21.
Fine A, Penner B. The protective effect of cool dialysate is dependent on patients’ predialysis temperature. Am J Kidney Dis. 1996;28:262–5.
Provenzano R, Sawaya B, Frinak S, Polaschegg HD, Roy T, Zasuwa G, Dumler F, Levin NW. The effect of cooled dialysate on thermal energy balance in hemodialysis patients. ASAIO Trans. 1988;34:515–8.
van der Sande FM, Gladziwa U, Kooman JP, Böcker G, Leunissen KM. Energy transfer is the single most important factor for the difference in vascular response between isolated ultrafiltration and hemodialysis. J Am Soc Nephrol. 2000;11:1512–7.
Schneditz D, Ronco C, Levin N. Temperature control by the blood temperature monitor. Semin Dial. 2003;16:477–82.
Maggiore Q, Dattolo P, Piacenti M, Morales MA, Pelosi G, Pizzarelli F, Cerrai T. Thermal balance and dialysis hypotension. Int J Artif Organs. 1995;18:518–25.
Selby NM, McIntyre CW. A systematic review of the clinical effects of reducing dialysate fluid temperature. Nephrol Dial Transplant. 2006;21:1883–98.
van der Sande FM, Rosales LM, Brener Z, Kooman JP, Kuhlmann M, Handelman G, Greenwood RN, Carter M, Schneditz D, Leunissen KM, Levin NW. Effect of ultrafiltration on thermal variables, skin temperature, skin blood flow, and energy expenditure during ultrapure hemodialysis. J Am Soc Nephrol. 2005;16:1824–31.
Schneditz D, Rosales L, Kaufman AM, Kaysen G, Levin NW. Heat accumulation with relative blood volume decrease. Am J Kidney Dis. 2002;40:777–82.
Schneditz D. Temperature and thermal balance in hemodialysis. Semin Dial. 2001;14:357–64.
Maggiore Q, Pizzarelli F, Santoro A, Panzetta G, Bonforte G, Hannedouche T, Alvarez de Lara MA, Tsouras I, Loureiro A, Ponce P, Sulkovà S, Van Roost G, Brink H, Kwan JT, Study Group of Thermal Balance and Vascular Stability. The effects of control of thermal balance on vascular stability in hemodialysis patients: results of the European randomized clinical trial. Am J Kidney Dis. 2002;40:280–90.
van der Sande FM, Wystrychowski G, Kooman JP, Rosales L, Raimann J, Kotanko P, Carter M, Chan CT, Leunissen KM, Levin NW. Control of core temperature and blood pressure stability during hemodialysis. Clin J Am Soc Nephrol. 2009;4(1):93–8.
van der Sande FM, Cheriex EC, van Kuijk WH, Leunissen KM. Effect of dialysate calcium concentrations on intradialytic blood pressure course in cardiac-compromised patients. Am J Kidney Dis. 1998;32:125–31.
Gabutti L, Bianchi G, Soldini D, Marone C, Burnier M. Haemodynamic consequences of changing bicarbonate and calcium concentrations in haemodialysis fluids. Nephrol Dial Transplant. 2009;24:973–81.
Quellhorst E, Schuenemann B, Hildebrand U, Falda Z. Response of the vascular system to different modifications of haemofiltration and haemodialysis. Proc Eur Dial Transplant Assoc. 1980;17:197–204.
van der Sande FM, Kooman JP, Konings CJ, Leunissen KM. Thermal effects and blood pressure response during postdilution hemodiafiltration and hemodialysis: the effect of amount of replacement fluid and dialysate temperature. J Am Soc Nephrol. 2001;12:1916–20.
Altieri P, Sorba G, Bolasco P, Asproni E, Ledebo I, Cossu M, Ferrara R, Ganadu M, Cadinu F, Serra G, Cabiddu G, Sau G, Casu D, Passaghe M, Bolasco F, Pistis R, Ghisu T, Second Sardinian Multicentre Study. Predilution haemofiltration – the Second Sardinian Multicentre Study: comparisons between haemofiltration and haemodialysis during identical Kt/V and session times in a long-term cross-over study. Nephrol Dial Transplant. 2001;16:1207–13.
Maduell F, Moreso F, Pons M, Ramos R, Mora-Macià J, Carreras J, Soler J, Torres F, Campistol JM, Martinez-Castelao A, ESHOL Study Group. High-efficiency postdilution online hemodiafiltration reduces all-cause mortality in hemodialysis patients. J Am Soc Nephrol. 2013;24:487–97.
Locatelli F, Altieri P, Andrulli S, Bolasco P, Sau G, Pedrini LA, Basile C, David S, Feriani M, Montagna G, Di Iorio BR, Memoli B, Cravero R, Battaglia G, Zoccali C. Hemofiltration and hemodiafiltration reduce intradialytic hypotension in ESRD. J Am Soc Nephrol. 2010;21:1798–807.
Susantitaphong P, Siribamrungwong M, Jaber BL. Convective therapies versus low-flux hemodialysis for chronic kidney failure: a meta-analysis of randomized controlled trials. Nephrol Dial Transplant. 2013;28:2859–74.
Wang AY, Ninomiya T, Al-Kahwa A, Perkovic V, Gallagher MP, Hawley C, Jardine MJ. Effect of hemodiafiltration or hemofiltration compared with hemodialysis on mortality and cardiovascular disease in chronic kidney failure: a systematic review and meta-analysis of randomized trials. Am J Kidney Dis. 2014;63:968–78.
Nistor I, Palmer SC, Craig JC, Saglimbene V, Vecchio M, Covic A, Strippoli GF. Convective versus diffusive dialysis therapies for chronic kidney failure: an updated systematic review of randomized controlled trials. Am J Kidney Dis. 2014;63:954–67.
Pedrini LA, Ponti R, Faranna P, Cozzi G, Locatelli F. Sodium modeling in hemodiafiltration. Kidney Int. 1991;40:525–32.
de Vries PM, Olthof CG, Solf A, Schuenemann B, Oe PL, Quellhorst E, Schneider H, Donker AJ. Fluid balance during haemodialysis and haemofiltration: the effect of dialysate sodium and a variable ultrafiltration rate. Nephrol Dial Transplant. 1991;6:257–63.
Kumar S, Khosravi M, Massart A, Potluri M, Davenport A. Haemodiafiltration results in similar changes in intracellular water and extracellular water compared to cooled haemodialysis. Am J Nephrol. 2013;37:320–4.
Quellhorst E, Schuenemann B, Hildebrand U. How to prevent vascular instability: haemofiltration. Proc Eur Dial Transplant Assoc. 1981;18:243–9.
Beerenhout C, Dejagere T, van der Sande FM, Bekers O, Leunissen KM, Kooman JP. Haemodynamics and electrolyte balance: a comparison between on-line pre-dilution haemofiltration and haemodialysis. Nephrol Dial Transplant. 2004;19:2354–9.
Cornelis T, van der Sande FM, Eloot S, Cardinaels E, Bekers O, Damoiseaux J, Leunissen KM, Kooman JP. Acute hemodynamic response and uremic toxin removal in conventional and extended hemodialysis and hemodiafiltration: a randomized crossover study. Am J Kidney Dis. 2014;64:247–56.
Caplin B, Alston H, Davenport A. Does online haemodiafiltration reduce intra-dialytic patient symptoms? Nephron Clin Pract. 2013;124:184–90.
Donauer J, Schweiger C, Rumberger B, Krumme B, Böhler J. Reduction of hypotensive side effects during online-haemodiafiltration and low temperature haemodialysis. Nephrol Dial Transplant. 2003;18:1616–22.
Santoro A, Mancini E, Zucchelli P. The impact of haemofiltration on the systemic cardiovascular response. Nephrol Dial Transplant. 2000;15 Suppl 2:49–54.
Baldamus CA, Ernst W, Lysaght MJ, Shaldon S, Koch KM. Hemodynamics in hemofiltration. Int J Artif Organs. 1983;6(1):27–31.
Baldamus CA, Ernst W, Frei U, Koch KM. Sympathetic and hemodynamic response to volume removal during different forms of renal replacement therapy. Nephron. 1982;31(4):324–32.
van Kuijk WH, Hillion D, Savoiu C, Leunissen KM. Critical role of the extracorporeal blood temperature in the hemodynamic response during hemofiltration. J Am Soc Nephrol. 1997;8:949–55.
Fox SD, Henderson LW. Cardiovascular response during hemodialysis and hemofiltration: thermal, membrane, and catecholamine influences. Blood Purif. 1993;11:224–36.
Henderson LW. Hemodynamic instability during different forms of dialysis therapy: do we really know why? Blood Purif. 1996;14:395–404.
Pinney JH, Oates T, Davenport A. Haemodiafiltration does not reduce the frequency of intradialytic hypotensive episodes when compared to cooled high-flux haemodialysis. Nephron Clin Pract. 2011;119:c138–44.
Mora-Bravo FG, De-La-Cruz G, Rivera S, Ramírez AM, Raimann JG, Pérez-Grovas H. Association of intradialytic hypotension and convective volume in hemodiafiltration: results from a retrospective cohort study. BMC Nephrol. 2012;13:106.
Santoro A, Mancini E, Canova C, Mambelli E. Thermal balance in convective therapies. Nephrol Dial Transplant. 2003;18 Suppl 7:vii41–5.
Canaud B, Bowry SK. Revisiting frontiers of tolerability and efficacy in renal replacement therapy. Am J Kidney Dis. 2014;64:171–3.
Karamperis N, Sloth E, Jensen JD. Predilution hemodiafiltration displays no hemodynamic advantage over low-flux hemodialysis under matched conditions. Kidney Int. 2005;67:1601–8.
Foley RN, Parfrey PS, Sarnak MJ. Epidemiology of cardiovascular disease in chronic renal disease. J Am Soc Nephrol. 1998;9(12 Suppl):S16–23.
Agarwal R, Flynn J, Pogue V, Rahman M, Reisin E, Weir MR. Assessment and management of hypertension in patients on dialysis. J Am Soc Nephrol. 2014;25:1630–46.
Briet M, Boutouyrie P, Laurent S, London GM. Arterial stiffness and pulse pressure in CKD and ESRD. Kidney Int. 2012;82:388–400.
Middleton RJ, Parfrey PS, Foley RN. Left ventricular hypertrophy in the renal patient. J Am Soc Nephrol. 2001;12:1079–84.
Beerenhout CH, Luik AJ, Jeuken-Mertens SG, Bekers O, Menheere P, Hover L, Klaassen L, van der Sande FM, Cheriex EC, Meert N, Leunissen KM, Kooman JP. Pre-dilution on-line haemofiltration vs low-flux haemodialysis: a randomized prospective study. Nephrol Dial Transplant. 2005;20:1155–63.
Quellhorst E, Schuenemann B, Doht B. Treatment of severe hypertension in chronic renal failure by haemofiltration. Proc Eur Dial Transplant Assoc. 1977;14:129–35.
Mostovaya IM, Bots ML, van den Dorpel MA, Grooteman MP, Kamp O, Levesque R, Ter Wee PM, Nubé MJ, Blankestijn PJ. A randomized trial of hemodiafiltration and change in cardiovascular parameters. Clin J Am Soc Nephrol. 2014;9:520–6.
Ok E, Asci G, Toz H, Ok ES, Kircelli F, Yilmaz M, Hur E, Demirci MS, Demirci C, Duman S, Basci A, Adam SM, Isik IO, Zengin M, Suleymanlar G, Yilmaz ME, Ozkahya M, Turkish Online Haemodiafiltration Study. Mortality and cardiovascular events in online haemodiafiltration (OL-HDF) compared with high-flux dialysis: results from the Turkish OL-HDF Study. Nephrol Dial Transplant. 2013;28:192–202.
Charitaki E, Davenport A. Does hemodiafiltration reduce vascular stiffness measured by aortic pulse wave velocity compared with high-flux hemodialysis? Hemodial Int. 2014;18:391–5.
Georgianos PI, Sarafidis PA, Karpetas A, Kosmidis D, Sioulis A, Liakopoulos V, Stamatiadis DN, Papagianni A, Zebekakis PE, Nikolaidis P, Lasaridis AN. Hemodiafiltration does not have additional benefits over hemodialysis on arterial stiffness, wave reflections and central aortic pressures. Blood Purif. 2014;37:18–26.
Grooteman MP, van den Dorpel MA, Bots ML, Penne EL, van der Weerd NC, Mazairac AH, den Hoedt CH, van der Tweel I, Lévesque R, Nubé MJ, ter Wee PM, Blankestijn PJ, CONTRAST Investigators. Effect of online hemodiafiltration on all-cause mortality and cardiovascular outcomes. J Am Soc Nephrol. 2012;23:1087–96.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2016 Springer International Publishing Switzerland
About this chapter
Cite this chapter
Kooman, J.P., van der Sande, F.M., Leunissen, K.M.L. (2016). Hemodynamic Stability and Cardiovascular Effects of Convective Therapies. In: Nubé, M., Grooteman, M., Blankestijn, P. (eds) Hemodiafiltration. Springer, Cham. https://doi.org/10.1007/978-3-319-23332-1_17
Download citation
DOI: https://doi.org/10.1007/978-3-319-23332-1_17
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-23331-4
Online ISBN: 978-3-319-23332-1
eBook Packages: MedicineMedicine (R0)