Abstract
High incidence of phytotoxicity and inconsistent results in nematode suppression of soil amended with phytonematicides had been limiting the adoption of phytonematicides in various countries. The efficacy of phytonematicides depended on allelochemicals as active ingredients, which are naturally phytotoxic. Plant species respond to increasing concentrations of allelochemicals through density-dependent growth patterns, which have three phases, namely, stimulation, neutral and inhibition, with each phase having a range of concentrations. The curve-fitting allelochemical response dosage model was used for two triterpenoid phytonematicides, nemarioc-AL and nemafric-BL phytonematicides, to develop the non-phytotoxic concentrations of the products within the stimulation phase of tomato (Solanum lycopersicum) plants. The concept called for the development of the application interval, culminating in the formulation of the application frequency and the dosage, which ameliorated the incidence of phytotoxicity. The application intervals of the derived non-phytotoxic concentrations for the products were such that the life cycle of the root-knot (Meloidogyne species) nematodes was continuously disrupted, thereby ensuring consistent results in nematode suppression in soils amended with phytonematicides.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
The global withdrawal of environment-unfriendly synthetic nematicides from agrochemical markets resulted in the emergence of various alternatives for managing plant-parasitic nematodes (Chedekal 2013; Stirling 2014). However, the introduced alternatives had inherent drawbacks. For instance, most crude extracts from plants with acceptable efficacies on suppression of nematodes were highly phytotoxic and could therefore not be sanctioned for use in crop husbandry. The European and Mediterranean Plant Protection Organization (EPPO 2010) and other such legal entities in various countries have zero tolerance on products that induce phytotoxicity on crops which are being protected against pests. Invariably, some non-phytotoxic products have had inconsistent results on target pests, which raised credibility issues for their registration. Incidentally, most products to be used in agricultural pests such as plant-parasitic nematodes have to undergo registration after intensive efficacy and non-phytotoxic trials.
Plant-parasitic nematodes are among the most injurious soilborne pests in cropping systems , with yield losses ranging from 5 % to 15 % (Stirling 2014) and translating to billions of US dollars (Chitwood 2002; Khan et al. 2008). Following the withdrawal of highly effective nematicides, the use of nematode-resistant genotypes had been in the forefront as a management strategy of choice in reducing nematode densities to below injurious levels. However, in plant genotypes without nematode resistance such as watermelon (Citrullus lanatus), peppers (Capsicum annuum) and potatoes (Solanum tuberosum), the yield losses escalate to as high as 50 % and at times to complete crop failure due to infection by the root-knot (Meloidogyne species) nematodes (Pofu et al. 2012). Reliance on nematode resistance was not sustainable due to the existence of nematode races and sensitivity of nematode-resistant genotypes to environmental factors such as high soil temperature (Dropkin 1969), salinity (Mashela et al. 1992) and honeydew-inducing foliar insects (Pofu et al. 2012). Lack of nematode-resistant genotypes in certain economically important crops and incompatibility of intergeneric nematode-resistant rootstocks and scions also negated the widespread adoption of nematode resistance technology (Pofu et al. 2012). Notwithstanding the listed drawbacks and the degree of nematode resistance in a given cultivar, the extent of crop losses is also depended upon the aggressiveness of the target nematode. For example, Meloidogyne species, lesion nematode (Pratylenchus species), sting nematode (Belonolaimus longicaudatus) and burrowing nematode (Radopholus similis) are highly aggressive, and, therefore, each may induce excessive damage to the host plants. In contrast, the citrus nematode (Tylenchulus semipenetrans) is not aggressive, but could be highly damaging in soils with salinity problems (Mashela and Nthangeni 2002; Duncan 2009).
Management of plant-parasitic nematodes in cropping systems is indispensable if crop enterprises are to be profitable and thereby improve food security on a global scale. Due to various setbacks on nematode resistance , organic amendments and/or other biological agents were tested on a grand scale for the suppression of population nematode densities. Notably, higher plants, biocontrol agents and fungi have since provided a broad spectrum of active compounds for use in nematode managemen t (Chitwood 2002; Okwute 2012; Chedekal 2013). Phytonematicides as an alternative management strategy in nematode suppression comprise a class of plant-based nematicides, which are available as aqueous plant extracts (Egunjobi and Afolami 1976; Rossner and Zebitz 1987; Chedekal 2013), methanol plant extract s (Usman 2013), ethanol plant extract s (Khan et al. 2008), oilcake s (Muller and Gooch 1982), essential oils (Meyer et al. 2008), fermented crude plant extract s (Kyan et al. 1999; Ncube 2008; Pelinganga and Mashela 2012; Pelinganga et al. 2013a), powder s (Ahmad et al. 2013) or granule s (Mashela et al. 2008, 2011, 2012).
Phytonematicides differ from conventional organic amendments, which may include crop residues , manures, compost, organic manures, agro-industrial wastes and sewage sludg e (Castagnone-Sereno and Kermarrec 1991; D’Addabbo 1995; Thoden et al. 2011; Stirling 2014). Generally, phytonematicides were introduced to mitigate the drawbacks of conventional organic amendments in suppression of plant-parasitic nematodes (Mashela 2002), which include (1) inconsistent results in nematode suppression ; (2) large quantities (10–500 t/ha) which were required to achieve nematode suppression; (3) unavailability of the materials; (4) high transport costs to haul the materials from the production site to that of use; (5) negative period, with the subsequent time-lag to allow for microbial decomposition in order to avoid negative periods; and/or (6) decrease in soil pH, which inherently imbalances the availability of essential nutrient elements in the soil (Jafee et al. 1994; Belair and Tremblay 1995; McSorley and Gallaher 1995; Mashela 2002; Kimpinski et al. 2003; Thoden et al. 2011; Stirling 2014). Inputs for most phytonematicides are locally collected from indigenous plants (Muller and Gooch 1982; Akhtar and Malik 2000; Oka 2010; Mashela et al. 2011; Ahmad et al. 2013), which possess complex allelochemical compounds (Chitwood 2002; Okwute 2012). In purified formulation, most phytonematicides lose their nematode suppression capabilities (Wuyts et al. 2006; Oka 2010; Ntuli and Caboni 2012; Okwute 2012) and are accompanied by unacceptable high phytotoxicity levels on crops being protected against nematodes (Mian and Rodriguez-Kabana 1982a, b; Meyer et al. 2008). Generally, phytonematicides rely on allelochemicals as their active ingredients and are used in vivo for defence against invading pathogens (Rice 1984; Inderjit et al. 1999). Roots of certain allelochemical-producing plants exude copious quantities of allelochemicals to provide competitive edge against competitors during interference (Inderjit et al. 1999; Rice 1984). The objective of this overview was to provide the dosage model as an alternative strategy in managing plant-parasitic nematodes with specific reference to addressing efficacy, phytotoxicity and inconsistent result issues of phytonematicides.
2 Distinction Between Phytonematicides and Organic Amendments
In the original overview on organic amendments, Muller and Gooch (1982) noted that between 1971 and 1981, out of 33 organic amendment trials, those with at least 91 % success frequency on nematode suppression were in the form of powders and oilcakes from neem (Azadirachta indica), peanuts (Arachis hypogaea) and castor (Ricinus communis). Later, other reviews (Alam 1993; Ferraz and de Freitas 2004; Oka 2010) confirmed that neem extracts, particularly those from seed kernels, had high bioactivities on nematode populations. Mashela et al. (2011) introduced a classical model on the ground leaching technology (GLT) system, with the research focus being on powdered plant products from selected plant organs with the view of ameliorating the numerous drawbacks of conventional organic amendments in smallholder tomato (Solanum lycopersicum) farming systems in South Africa. In the GLT system, powdered materials were derived from unshelled dried castor bean, fever tea (Lippia javanica) leaves, wild cucumber ( Cucumis myriocarpus ) fruit and wild watermelon ( Cucumis africanus ) fruit. In all cases, the four products each consistently reduced population densities of Meloidogyne species and T. semipenetrans. Overall, the GLT system uses 0.2–0.7 powdered materials/ha for 4000 tomato plants when compared with 10–500 t organic amendments/ha required to effect consistent results in nematode suppression (Mashela 2002). In order to distinguish the powdered materials with their small quantities required in suppression of nematodes relative to large quantities required in conventional organic amendments, the former were referred to as phytonematicides (Mashela et al. 2011). Phytonematicides at the concentration used are intended to consistently suppress population densities of the target nematodes, while stimulating growth of the protected crops instead of inhibiting plant growth and productivity (Pelinganga 2013). Certain phytonematicides can be highly effective in nematode suppression. Incidentally, the efficacy of powdered materials from C. myriocarpus fruit in nematode suppression was similar to those of aldicarb and fenamiphos nematicides (Mashela et al. 2008).
The major distinctions between phytonematicides and conventional organic amendments could be:
-
1.
The empirically based small quantities applied to achieve consistent nematode suppression under diverse conditions as opposed to large quantities.
-
2.
In GLT system there is gradual release of active ingredients from crude extracts into the rhizosphere which is achieved through irrigation water or rainfall as opposed to microbial degradation in conventional organic amendments.
-
3.
Phytonematicide products mimic synthetic chemical nematicides since they could be commercially packaged in relatively small containers with label information which includes active ingredients, along with efficacy features.
-
4.
Phytonematicides like most non-fumigant nematicides do not have negative periods and could therefore be applied as post-planting products.
-
5.
These products are required to comply with relevant legislation in terms of avoiding health risks to end users, nontarget organisms and the environment.
The drawback of the GLT system was its high labour costs since products were manually applied, which rendered the system less appealing to large commercial tomato producers (Mashela et al. 2011). An alternative technology, referred to as botinemagation (Mashela et al. 2011), was developed for use in large-scale tomato farming systems, where crude extracts from fermented plant organs were used through drip irrigation systems. Using dried fruits of C. myriocarpus and C. africanus fruits, fermented crude extracts as liquid formulations consistently reduced population densities of Meloidogyne species in tomato production (Pelinganga et al. 2013a, b).
Not all plant organs contain allelochemicals with nematicidal properties. In South Africa, Van Wyk et al. (2002) listed 372 plant species on the basis of their toxicity to humans and animals, which were for the purpose of this discussion classified into six using their degree of toxicity (Table 7.1). Approximately 22.6, 18.3 and 6.7 % of the listed plants were described as being poisonous, very poisonous and deadly, respectively, to humans and livestock. The degree of toxicity to humans and animals does not confer a plant a better status to be a candidate for serving as source of phytonematicides. Cucumis myriocarpus and R. communis, from which two phytonematicides were developed for the GLT system (Mashela 2002; Mashela and Nthangeni 2002), were regarded as being poisonous and very poisonous, respectively (Van Wyk et al. 2002). However, the deadly oleander (Nerium oleander) and tamboti (Spirostachys africana) did not have phytonematicidal properties against Meloidogyne species (Mashela et al. 2011). In contrast, certain plants listed as ‘not really poisonous’, namely, fever tea (Lippia javanica) and Brassica species, produced potent phytonematicides (Mashela et al. 2010). Among the listed plant species, only 0.8 % plant species were tested against nematodes in Sou th Africa, with only 0.55 % having some nematicidal properties. At a global level, among the 45 papers of biological control agents of nematodes discussed at the 2014 International Congress of Nematology in Cape Town, South Africa, 42, 36, 18 and 4 % were on botanicals, fungi, bacteria and enzymes, respectively. The highest percentage of phytonematicide papers clearly illustrated the potential importance and interest in this group of biological control agents in plant nematology.
3 Efficacy of Phytonematicides
The majority of in vitro trials have had in excess of 90 % suppression of nematode numbers from phytonematicides (Okwute 2012). However, due to their high phytotoxicities and restricted measures (EPPO 2010), a large number of botanicals with potent nematicidal properties do not make it beyond in vitro tests. Notwithstanding the high rejection of most products, detailed assessments on mode of action for certain phytonematicides had been undertaken.
4 Mode of Action of Phytonematicides
The distinguishing feature of synthetic pesticides is their single active ingredients, with clearly defined bioactivities. In synthetic insecticides, such single active ingredients had high incidents of insect resistance, particularly in insects with high reproductive capabilities (Nzanza and Mashela 2012). However, although certain nematode species have high reproductive capabilities, resistance to synthetic nematicides in plant-parasitic nematodes had not been observed (Van Gundy and McKenry 1975). In contrast to synthetic pesticides, phytonematicides have multiple active ingredients, with complementary modes of action. For instance, in wild garlic (Tulbaghia violacea), the plant bulb contains sacrid volatile oils and sulpho-oxides—each being a derivative of allicin, which has insecticidal and nematicidal properties (Vijayalakshmi et al. 1996; Nzanza and Mashela 2012; Mashela et al. 2012). In insects the mode of action for the allicin derivatives had been identified as antifeedant, repellent and insecticidal (Vijayalakshmi et al. 1996; Dhanalakshmi 2006). Similarly, in insects, azadirachtin in neem had been shown to have antifeedant, repellent and anti-ovipositor properties, with capabilities for delaying or preventing moulting in insects. Apparently, using phytopesticides confers a broad spectrum of active ingredients, with multiple modes of action. In phytonematicides, observations on mode of action had been limited to chemotaxis, juvenile motility, egg hatch, juvenile mortality or juvenile paralysis, with limited information on behavioural responses of adult nematodes.
4.1 Chemotaxis
Chemotaxis is a phenomenon where nematodes direct their movement according to the gradient of selected chemical cues in the environments (Bargmann and Mori 1997). Positive chemotaxis occurs when movement is towards the increasing gradient of chemical cues. Conversely, movement towards the opposite direction of the increasing gradient is described as negative chemotaxis (Bargmann and Mori 1997). The nematode is literally exposed to both liquid- and airborne volatilised chemicals in the air-water interface of the soil, which could either be water-soluble and/or volatile chemoattractants or chemorepellents. According to Bargmann and Mori (1997), water-soluble chemoattractants are detected by chemoreceptors in nematodes at micromolar concentrations, while the volatile chemoattractants are detected at picomolar concentrations. Water-soluble and volatile chemoattractants are used for short- and long-distance chemotaxes, respectively (Prot 1980). In contrast, water-soluble and volatile chemorepellents are toxic and could cause either paralysis or death of the nematode. In phytonematicides, both chemoattractants and chemorepellents are important. Chemoattractant phytonematicides may disorientate the nematode from being guided by chemoattractant cues produced by potential host plants, thereby deferring penetration and attack of host by nematodes (Wuyts et al. 2006). In contrast, chemorepellents may induce various behavioural changes in the nematode, including paralysis and death (Bargmann and Mori 1997).
The body of a nematode is ‘wired’ with chemoreceptors, particularly on the frontal and cervical regions (Ferraz and Brown 2002), suggesting that chemoattractants and chemorepellents play important roles in behavioural activities of nematodes. Plants release numerous chemicals through exudation, leaching, volatilisation and microbial degradation for different reasons (Stirling 2014). Similarly, phytonematicides release potent chemicals either through leaching, volatilisation or microbial degradation (Mashela et al. 2011, 2012). Generally, increasing concentrations of phytochemicals could interfere with chemotaxis in one of three ways: no effect (neutral chemotaxis), attract (positive chemotaxis) and repel (negative chemotaxis). Responses characterised by these three phases in the environment subscribe to density-dependent growth (DDG) curves (Salisbury and Ross 1992; Liu et al. 2003), which constitute an important part of this review.
Using purified phytochemical compounds, Wuyts et al. (2006) demonstrated that certain chemical compounds from Philenoptera violacea in the Fabaceae family had similar and/or different effects on chemotaxis—which is dependent much on the nematode species. In their work (Wuyts et al. 2006), among the tested chemical compounds produced through the shikimic acid pathway, 26 % repelled R. similis, 2.6 % attracted this nematode, while 45 % were neutral. In contrast, of the 37 % tested chemical compounds on P. penetrans, even those that were chemorepellent to R. similis had no effect on this nematode. In contrast, some chemorepellents to R. similis were also repellent to M. incognita. Although the approach used by Wuyts et al. (2006) did not provide information on physiological activities of the target chemicals in nematode bodies, it provided broad clues in terms of what we want to convey using DDG patterns later on in this overview. The three nematode species used depicted neutrality to the largest number of chemical compounds produced through the mevalonate pathway, followed by inhibition of motility and then repellence as depicted in chemotaxis (Wuyts et al. 2006). A remarkable feature in the work of Wuyts et al. (2006) was, therefore, the agreement of their observations with the concept of DDG patterns , particularly with the observed repeated neutral responses.
4.2 Motilit y
Juveniles from unhatched eggmasses which were previously exposed to crude extracts from leaves of Borelin remained motile, while those exposed to crude extracts of garlic bulb or neem seed kernels had impact on juvenile motility (Agbenin et al. 2005). According to DDG principles, different concentrations of phytonematicides might have no effect (neutral) on, stimulate and/or inhibit motility of nematodes (Salisbury and Ross 1992; Liu et al. 2003). Wuyts et al. (2006) observed that a chemical compound which was neutral in one nematode species could inhibit juvenile motility in another nematode species, vice versa. Similarly, those that were chemoattractants in chemotaxis for one nematode species might be neutral and/or inhibitive in juvenile motility for another nematode species. Oka et al. (2000) showed that essential oils from 12 of 27 plants immobilised more than 80 % M. javanica J2s after a 2-day exposure, with immobilisation being amenable to DDG patterns.
4.3 Egress
Egress in M. incognita was inversely proportional to concentrations of crude extracts from garlic and neem (Agbenin et al. 2005; Chedekal 2013). Although egress is a physical process, in most plant-parasitic nematodes, it is stimulated by external chemical cues from roots (Prot 1980). According to Wuyts et al. (2006), some phytochemical compounds were neutral towards egg hatch, while others were inhibitive. In contrast, one flavanone, which is a hesperetin chemical compound, was both stimulatory and inhibitive to egress in R. similis (Wuyts et al. 2006). Most active ingredients from phytonematicides have the capability to penetrate eggmasses, where J1s become exposed to aqueous solutions (Hirschmann 1985; Parmar 1987; Agbenin et al. 2005). Incidentally, the materials interfered with stylet development, rendering it incapable of piercing through the eggshell and, therefore, resulting in complete failure of egress (Hirschmann 1985; Parmar 1987).
Using in vitro trials, essential oils from 27 different plant species, at 1000 μL/L only 30 % of plants inhibited egress, while at 600 μL/L only 15 % of plants had oils with inhibitive properties (Oka et al. 2000). Ojo and Umar (2013) demonstrated that crude extracts from testa of cocoa bean (Theobroma cacao) plants had significantly higher effects on egress of M. javanica than oil palm fibre, with differences attributed to different chemical constituents. Cocoa bean testa contains alkaloids and flavonoids, with egress inhibition being directly proportional to the concentration of the listed chemical compounds (Ojo and Umar 2013). However, in the same study, Ojo and Umar (2013) observed that oil palm fibre, which was devoid of alkaloids and flavonoids, had negligent effects on egress. Okeniyi et al. (2013) demonstrated increasing concentrations (0, 10, 25, 50 and 100 %) of leaf crude extracts from the coastal golden leaf (Bridelia micrantha), euphorbia (Mallotus oppositifolius), abeere (Hunteria umbellate) and citron (Citrus medica)—each increased inhibition of egress in M. incognita. Removal of eggs from the chemical compounds resulted in reversal of the extract effects.
4.4 Mortality
In vitro exposure of Meloidogyne J2s to crude extracts from hen’s nettle (Fleurya interrupta), panicled peristrophe (Peristrophe bicalyculata) and king of bitters (Andrographis paniculata) resulted in 100 % mortalities (Mukherjee and Sukul 1978). Similarly, high Meloidogyne J2 mortalities were observed in crude extracts from leaves of marigold (Tagetes species), Indian gooseberry (Emblica officinalis) and Christ’s thorn (Carissa carandas) during in vitro exposure (Toida and Moriyama 1978; Haseeb et al. 1980). Also, in vitro exposure of M. incognita J2s to crude extracts or aqueous extracts from fresh leaves of various plants resulted in high mortalities (Agbenin et al. 2005; Chedekal 2013). Similarly, crude extracts of either cocoa bean testa or oil palm fibre resulted in high mortalities of M. javanica juveniles (Ojo and Umar 2013). Juvenile mortalities were directly proportional to increasing concentrations of phytonematicides and exposure time (Agbenin et al. 2005). In some instances, ‘mortalities’ were reversible when J2s were removed from the chemicals (Wuyts et al. 2006).
4.5 Paralysis
Paralysis involves irreversible interference of nematicides with the nervous systems of J2s. Generally, affected J2s can still wiggle, but have complete loss of coordinated mobility. Phytonematicide-induced paralysis reports on plant-parasitic nematodes are uncommon. An exceptional case is that in Ntalli et al. (2011), where paralysis of Meloidogyne J2s was regularly observed when exposed to aliphatic ketones from rue (Ruta chalepensis).
5 Variation in Efficacy of Phytonematicides
Incidentally, biological entities respond to various abiotic and/or biotic factors through a myriad of complex processes and mechanisms. For instance, when various plant-parasitic nematodes infect plants at population densities below the tolerance limit, plant growth is invariably stimulated (Wallace 1973), while at high population densities, growth is reduced (Seinhorst 1967). Similarly, infection by different nematode species on various legumes either stimulated, had no effect on or inhibited nodulation and/or nitrogen fixation (Huang 1987). Vesicular-arbuscular mycorrhizal (VAM) fungi on various host plants also resulted in positive, neutral or negative growth responses (Smith 1987). Different fertilisers and/or salinity levels can also induce such growth responses in plants. In soil allelochemical residue (SAR) trials, it was shown that while SAR effects from one phytonematicide stimulated growth of the successor crop, SAR effects consistently reduced population densities of Meloidogyne species (Mashela and Dube 2014), with reduced population densities subscribing to similar inconsistent growth patterns (Zasada and Ferris 2003). Mashela (2014) showed that SAR effects had inhibitive effects on nodulation by Bradyrhizobium japonicum in cowpea (Vigna unguiculata). Others (Mashela and Dube 2014) argued that for phytonematicides to be successful, their inhibition concentration range to nematodes should overlap the stimulation range to the crop being protected against nematodes.
Sites of action in organisms by allelochemicals are not yet established. However, cucurbitacins from fruits of wild Cucumis species were shown to have the potential to inhibit cell division in cancer at high concentrations, while the materials were highly cytotoxic to healthy cells (Lee et al. 2010). In contrast, when used at low concentrations, cytotoxicity was avoided, but division of healthy cells was stimulated, thereby rendering the materials cancerous (Lee et al. 2010). These observations in cancer trials provided clues on the site of action of cucurbitacins—the cellular level.
Reports which demonstrated that conventional organic amendments increased population densities of nematodes in Europe (Belair and Tremblay 1995; Kimpinski et al. 2003), had no effect on nematode numbers in Florida, USA (Jafee et al. 1994; McSorley and Gallaher 1995) and reduced nematode numbers (Stirling 2014) raised credibility issues on organic amendments due to the ‘perceived’ inconsistent results (McSorley 2011). The efficacy of phytonematicides is dependent upon the concentration of allelochemicals in the organ used for processing the intended products. Generally, the accumulation of secondary metabolites in organs varies from season to season (Mudau et al. 2008), with high inconsistent results in nematode suppression and high phytotoxicities during certain seasons. However, the variability that leads to inconsistent results should not be confounded with DDG patterns in allelochemical-containing products. Although the variability of concentrations of allelochemicals in a particular organ could be associated with DDG patterns in certain cases, DDG principles are primarily related to responses of living entities in response to increasing concentrations of allelochemicals ex vitro. In organs such as fruits or bulbs where the accumulation of secondary metabolites appears to level off with maturity, variability in efficacy of phytonematicides on nematode suppression had mostly been due to different concentrations in the processed product (Meyer et al. 2008). Generally, sources that result in the final product being of high variability are undesirable, particularly when commercial products are envisaged. On the basis of the three phases (stimulation, neutral and inhibition ) being characterised by different concentration ranges, one could argue that the various materials of plant origin did not have ‘inconsistent’ results on nematode suppression, but what was being observed in a particular time was a reflection of differences in concentrations with respect to the allelochemicals involved.
5.1 Density-Dependent Response Patterns in Phytonematicides
At low concentrations, crude extracts of neem leaf were shown to stimulate growth of maize (Zea mays) and tomato seedlings, while at high concentrations, the opposite occurred (Egunjobi and Afolami 1976; Rossner and Zebitz 1987). Similarly, Inderjit et al. (1999) noted that at low concentrations root leachate from golden crownbeard (Verbesina encelioides) consistently stimulated plant growth of various plant species. Also, at low concentrations, nemarioc-B phytonematicide stimulated growth of tomato seedlings, where the product was viewed as having a ‘fertiliser effect’ (Mashela 2002). However, detailed analysis of essential nutrient elements in leaves did not support the ‘fertiliser effect’ view since the product had negligible effect on accumulation of essential nutrient elements. In subsequent studies (Mafeo et al. 2011a, b; Pelinganga et al. 2012, 2013a, b), it was shown that various plant variables (y-axis) when subjected to lines of the best fit on increasing concentrations of nemarioc-A (x-axis) invariably resulted in quadratic relationships, which is a strong indicator for the existence of DDG patterns (Salisbury and Ross 1992; Liu et al. 2003). A myriad of complex models regarding DDG patterns exist in biological entities, including plant-parasitic nematodes (Ferris and Wilson 1987; Duncan and McSorley 1987). The DDG tenets are closely related to the original conceptual framework of the carrying capacity (Nicholson 1933), which had since been used in a wide range of disciplines. DDG patterns have three distinct growth responses: stimulated, saturated (neutral) and inhibited growth (Salisbury and Ross 1992; Liu et al. 2003), with biological indices which had been used to unravel diverse biological responses to increasing pressures from their environments. DDG principles have the ultimate aim of improving decision-making systems in sustainable management of natural resources. Generally, plants, nematodes and microbes respond to increasing concentrations of allelochemicals through DDG patterns (Rice 1984; Ferris and Wilson 1987; Zasada and Ferris 2003; Liu et al. 2003), with attempts to investigate the mechanisms involved still being at conceptual stages, except that the site of action is at the cellular level (Lee et al. 2010).
Biological entities respond to increasing concentrations of allelochemicals in phytonematicides through DDG patterns, which comprise three phases, namely, stimulation, neutral and inhibition phases (Salisbury and Ross 1992; Liu et al. 2003; Pelinganga et al. 2012, 2013a, b). DDG patterns are an advanced modification of the 1933 Nicholson’s carrying capacity model, which had been adapted and used in various disciplines. Liu et al. (2003) quantified concentrations of allelochemicals which lead to three stages that characterise DDG patterns for various organisms using the curve-fitting allelochemical response dosage (CARD) computer-based model. The CARD model quantifies the three phases through seven biological indices : (1) threshold stimulation (D m ) = the allelochemical concentration that initiates the stimulation phase, (2) saturation point (R h ) = the concentration that terminates stimulation or starts the neutral phase, (3) 0 % inhibition (D0) = the concentration that terminates the neutral phase, (4) 50 % inhibition (D50) = the concentration at half the distance of the inhibition phase, (5) 100 % inhibition (D100) = the concentration at the end of the inhibition phase), (6) the sensitivity index (k) = provides the degree of sensitivity of an organism to the test product and (7) the coefficient of determination (R2) = provides the degree of the strength of the CARD model. Generally, stimulated (D m −R h ) and inhibited (D0−D100) growth concentrations are ideal representatives for phytonematicides and herbicides, respectively. The CARD model had since been empirically adapted to generate phytonematicide concentrations which stimulate plant growth while reducing population densities of nematodes using fruits as organs of preference in order to avoid confounding variability of allelochemical concentrations in the source and the actual concentration of allelochemicals in the processed product (Mafeo and Mashela 2010; Pelinganga and Mashela 2012). Using the three phases of the CARD model , we are currently in a position to argue that observations that nematode populations were not consistently suppressed by application of conventional organic amendments, which were dubbed ‘inconsistent’ since the materials sometimes stimulated (Belair and Tremblay 1995; Kimpinski et al. 2003), had no effect on (Jafee et al. 1994; McSorley and Gallaher 1995; Thoden et al. 2011) or inhibited population densities of nematodes (Mashela et al. 2011), were biologically incorrect. Incidentally, it should also be noted that not all plant organs or species have allelochemicals which have potent nematicidal properties (Mashela et al. 2011). In most plants with nematicidal allelochemicals , due to their auto-allelopathy, the chemical compounds in vivo are compartmentalised in organs not always preferred for use in conventional organic amendments. For instance, in C. myriocarpus fruit, cucurbitacin A is compartmentalised in seeds (Jeffrey 1978), which are hardly used in conventional organic amendments for fear of spreading the ‘weed’ through seed dispersal . Similarly, in neem the active ingredient, azadirachtin, is primarily concentrated in seed kernels (Parmar 1996).
Another important feature of DDG patterns in the CARD model is that the variable (y-axis) and the concentration of allelochemicals (x-axis) invariably have quadratic relationships (Salisbury and Ross 1992; Liu et al. 2003; Pelinganga et al. 2013a, b; Pelinganga and Mashela 2012). On this basis, should there be a positive linear instead of quadratic relationship between dependent and independent variables, results could be suggesting that the concentrations of the phytonematicide used were within the stimulation range—as observed in various trials. Incidentally, no effective response of dependent variables over increasing independent variable levels could suggest that phytonematicide concentrations were either within the neutral range (R h −D0) or below D m . In contrast, negative linear relationship invariably suggested that the concentration of allelochemicals tested was within the inhibition range (D0−D100). In biology, literature is replete with responses to abiotic and/or biotic factors that can be described as relationships that have positive (D m −R h ), neutral (R h −D0) or negative linear responses (D0−D100) (Salisbury and Ross 1992). Interestingly, such responses had not attracted attention as those in conventional organic amendments , where the responses were broadly viewed as evidence that phytonematicides were unsuitable for use in management of plant-parasitic nematodes since they were unpredictable.
5.2 Fluctuations in Concentrations of Allelochemicals In Vivo
Allelochemicals in plants are produced for ‘unknown’ physiological roles through various pathways, with the major ones being the (a) shikimic acid pathway, (b) malonic acid pathway and (c) mevalonic acid pathway (Lai 2008). Concentrations of any allelochemical within the pathways are in continuous state of fluctuation as depicted by a large number of precursors and reversible chemical reactions which are linked to the end of glycolysis just prior to the Krebs cycle of respiration (Lai 2008). Primarily, the formation of secondary metabolites helps to remove excess end products along the respiration pathway, particularly the acetyl co-A at the end of glycolysis (Campbell 1990). Responses to a phytonematicide in the plant being protected against nematodes are primarily a reflection of the phytonematicide concentration level at the time that particular organ was harvested for the development of the phytonematicide in question. For instance, phytonematicides derived from leaves such as those of fermented crude extracts from L. camara plants have the tendency of being highly inconsistent in nematode suppression due to seasonal variation of active ingredients in leaves. Also, the drying conditions of the organ after harvest might have deleterious effects on concentrations of the allelochemicals (Makkar 1991). For instance, shade-, sun- and oven-dried plant materials from the same plant organ may eventually contain different chemical concentrations due to differential chemical losses through volatilisation. Also, exposure of the harvested materials to rainfall as is common in maturation of conventional organic amendments may result in leaching out of allelochemicals since they are primarily nonstructural.
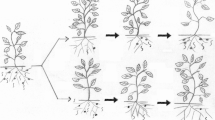
Timing of nematode sampling with reference to the initial application time of the phytonematicide could also be an important factor to consider in the perceived ‘inconsistent’ effect of organic materials in nematode management. In GLT systems, nematode sampling for Meloidogyne species was empirically established at 56 days after inoculation of plants (Mashela et al. 2011). When Maila and Mashela (2013) increased the sampling time from 56 to 150 days in citrus seedlings treated with nemarioc-AG and nemafric-BG, the highest population densities of T. semipenetrans were in phytonematicide-treated plants than in untreated controls. The unexpected observation was explained on the basis of cyclic growth patterns of population nematode densities, which subscribe to DDG patterns due to inherent competition for infection sites (Fig. 7.1). Generally, soon after the application of a phytonematicide, the product reduced population nematode densities, while those in untreated control increased, resulting in a situation where growth in the two populations was unsynchronised in a way that when the treated reached the trough the control was reaching the peak (Maila and Mashela 2013). By 150 days, nematode numbers under the untreated control were approaching the trough, while those from the treated seedlings were approaching the peak after reaching the trough within approximately 56-day application interval. Pofu and Mashela (2014) quantified the cyclic growth of population nematode densities of Meloidogyne species in four hemp (Cannabis sativa) cultivars and concluded that from inoculation to the peak of the nematode densities approximately 56 days were required, which was in agreement with the 56-day application interval for phytonematicides in GLT systems.
5.3 Confounding Survival Adaptations with Phytonematicidal Effects
Nematodes have evolved unique survival strategies , which rendered them the status of ‘undefeatable enemies’ after attempts to annihilate them failed. The survival strategies had been classified into (1) intrinsic adaptations in the life cycle of the nematode and (2) extrinsic rapid responses to environmental stresses (McSorley 2003). Intrinsic adaptations occur at three levels: (a) diapause in the egg (J1), (b) developmental dormancy prior to egress in various nematodes and (c) sex reversal, mainly in Meloidogyne species (Triantaphyllou 1973; Papadopoulou and Triantaphyllou 1982). Extrinsic rapid responses to environmental stresses (cryptobiosis = anabiosis) involve modifications in nematode cuticles which eventually decrease their permeability to water and related gases during J2, J3 and J4 stages, depending on the nematode species (Bird and Bird 1991; McSorley 2003). Cryptobiotic responses to drought, low temperature, osmotic stress, low oxygen and high concentrations of toxic chemical compounds had been referred to as anhydrobiosis, cryobiosis, osmobiosis, anoxybiosis and chemiobiosis, respectively (McSorley 2003). Both intrinsic and extrinsic adaptations might in many respects be confounded to nemastatic responses observed in non-fumigant synthetic nematicides (Van Gundy and McKenry 1975). For example, when eggs used in hatching in vitro trials are allowed gradual permeation of chemicals to J1s, juveniles may enter the diapause stage, with the resultant failure of egress. Similarly, when cryptobiosis coincides with the application of any nematicide, the product might be rendered unfit for the intended purpose. Notwithstanding, conditions should be improved and specified during in vitro trials to establish efficacy of phytonematicides on nematodes in order to avoid confounding survival strategies induced by gradual adverse effects on various stages of nematodes with the effects of phytonematicides.
6 Magnitude of Phytotoxicity in Phytonematicides
Allelochemicals as active ingredients in phytonematicides are naturally phytotoxic to other plant species during interference (Wuyts et al. 2006; Okwute 2012; Ntuli and Caboni 2012). In banana (Musa acuminata) trial, application of 200–400 g powdered neem seed kernels per mat at 6-month application interval induced phytotoxicity—characterised by complete wilting prior to fruiting (Musabyimana et al. 2000). Additionally, in survivor plants, the inflorescence failed to emerge (Musabyimana et al. 2000), resulting in a condition called choking, where the inflorescence could not emerge through the whorl of the pseudostem. Wild garlic (Tulbaghia violacea) bulbs contain sacrid volatile oils and sulpho-oxides—both being derivatives of allicin (Vijayalakshmi et al. 1996). Crude extracts of garlic bulb at 50 % concentration reduced population densities of plant-parasitic nematodes, but was highly phytotoxic to tomato seedlings (Sukul et al. 1974; Egunjobi and Afolami 1976). However, at 20 % concentration, there were no noticeable effects on tomato plant growth, while the product suppressed population densities of M. incognita (Agbenin et al. 2005). Oil from clove (Eugenia caryophyllata), when drenched using 0.1, 0.2 and 0.3 % concentrations at 0, 2, 5 and 7 days prior to transplanting cucumber (Cucumis sativus), muskmelon (C. melo), pepper and tomato seedlings, all concentrations were highly phytotoxic to all crops while reducing nematode populations (Meyer et al. 2008). Sensitivities of seedlings to clove oil from E. caryophyllata varied with plant species, with tomato seedlings being the most sensitive among all the test plants (Meyer et al. 2008). Generally, at transplanting, seedlings from various crops were all affected by oil at 0.2 and 0.3 % concentrations. The product contains eugenol as an active ingredient, which is naturally herbicidal at low concentrations (Walter et al. 2001; Tworkoski 2002; Waliwitiya et al. 2005; Bainard et al. 2006; Boyd and Brennan 2006; Boyd et al. 2006). Incide ntally, oilcakes from different plant species have high levels of phytotoxicity to various crops at various concentrations (Haseeb et al. 1980; Mian and Rodriguez-Kabana 1982a, b; Muller and Gooch 1982; Parmar 1996). Ahmad et al. (2013) demonstrated that ground leaves of adulsa (Justicia adhatoda) at 3 % (w/w) concentration were highly phytotoxic to tomato seedlings. Similar phytotoxic effects were observed from high concentrations of L. camara root extracts on various plant species (Shaukat et al. 2003).
Two phytonematicides from fruits of indigenous Cucumis species in South Africa are available in granular formulation, nemarioc-AG and nemafric-BG (Mashela et al. 2011), and liquid formulation, nemarioc-AL and nemafric-BL (Pelinganga et al. 2013a). Nemarioc-AG phytonematicide was shown to be highly phytotoxic to eight monocotyledonous and ten dicotyledonous crops, with most crops failing to emerge when 5 g crude extracts were applied as pre-emergent drenches (Mafeo and Mashela 2009a, b, 2010). Similarly, both nemafric-BL and nemarioc-AL were highly phytotoxic to tomato seedlings when applied at above 10 % concentration after transplanting (Pelinganga and Mashela 2012; Pelinganga et al. 2013a, b). Nemafric-BL has cucurbitacin B (C32H48O8), while nemarioc-AL contains two active ingredients, namely, cucumin (C27H40O9) and leptodermin (C27H38O8) (Rimington 1938; Jeffrey 1978). Except in rare cases such as pyrethrins that account for 80 % global uses of botanical pesticides, in purified form most active ingredients of phytonematicides, including azadirachtin-containing products, are not effective on nematode suppression, while they are highly phytotoxic to crops (Wuyts et al. 2006; Okwute 2012). Subsequently, most active ingredients in phytonematicides are applied as crude extracts.
7 Management of Phytotoxicity in Phytonematicides
Due to phytotoxicity and its zero tolerance in m ost legislative frameworks on products used in agriculture, literature is replete with efficacy trials which do not go beyond in vitro status. Using the concept of DDG patterns, there are basically three concentration ranges, namely, stimulation, neutral and inhibition concentration ranges (Fig. 7.2). Using the latter, we developed the concept of mean concentration stimulation range in an attempt to answer the farmers’ question ‘How much concentration of nemarioc-AL or nemafric-BL to apply?’ which was followed by ‘What is the application interval for the recommended concentration?’. The two questions were empirically answered, with avoidance of phytotoxicity and the efficacy of the products on nematode suppression in mind.
7.1 Establishing the Mean Stimulation Concentration Range
The potential uses of the CARD model rely on th e availability of empirically generated data (Mafeo et al. 2011a, b, c; Pelinganga et al. 2012, 2013a, b). As an illustration, an experiment was conducted on tomato plants inoculated with 5000 eggs and second-stage juveniles (J2s) of M. incognita/plant and subjected to 0, 2, 4, 8, 16, 32 and 64 % concentrations of nemafric-BL (Fig. 7.3). At 56 days after initiating the treatments, plant variables were subjected to analysis of variance, with significant (P ≤ 0.05) treatment means (Table 7.2) being further subjected to the CARD model to generate the quadratic relationships.
From the CARD-generated biological indices (Table 7.3), the actual values of R h for the variables measured were computed (Table 7.4). The mean actual D m and actual R h values were used to establish the concentration stimulation range (CSR), which is representative of the stimulated growth in the test plant (Table 7.5). Half the distance of the integrated CSR is referred to as the mean concentration stimulation range (MCSR). By definition, MCSR is the concentration of a phytonematicide which stimulates plant growth, while suppressing population densities of the target nematode (Pelinganga et al. 2013a; Mashela et al. 2014) and is quantified as:
Using actual mean D m and Rh, values in the MCSR formula provided the values of 2.63 and 2.99 % for nemafric-BL and nemarioc-AL, respectively, in tomato plants (Pelinganga 2013). The MCSR value, which is empirically based on a series of phytonematicide concentrations, should be interpreted alongside the overall k-value of the plant to the test phytonematicide. The usefulness of a given product for use as a phytonematicide is entirely dependent on the overall sensitivity (∑k) of the plant being protected to the product used (Liu et al. 2003). The k-values, which are plant and product specific, are generated using the CARD model and are defined as the number of In (D + 1) transformations that serves as a biological indicator of the degree of sensitivity of an organism to increasing concentrations of an allelochemical (Liu et al. 2003). The lower is the mean k-value, the higher is the sensitivity of the plant to the test allelochemicals and vice versa (Liu et al. 2003; Mafeo and Mashela 2010; Pelinganga and Mashela 2012). In CARD model, as the mean sensitivity (∑k/n) values increase, coefficients of determination (R2) also increase to a peak, where k = i, followed by decreases from i + 1 transformations until the model stops running (Liu et al. 2003). The three DDG patterns and the selected biological indices for nemarioc-AL phytonematicide on tomato plants were illustrated for various potential purposes (Fig. 7.4).
In both nemafric-BL and nemarioc-AL phytonematicides, MCSR values were established as being equivalent to 3 % concentration (Pelinganga et al. 2013b). In other words, for every 3 L stock solution of nemafric-BL or nemarioc-AL phytonematicides, 100 L chlorine-free water is used for application through drip irrigation. After empirically determining the amount to be applied per irrigation, the next step is to determine the application interval, which allows the computation of the application frequency—a factor required in the computation of dosage (D) = MCSR × application frequency.
7.2 Determining Phytonematicide Application Interval
The application interval (T) in days for the derived MCSR cannot be established using the CARD model since the latter is exclusively used when the x-axis represents increasing concentration of allelochemicals (Liu et al. 2003). The concept ‘weeks-of-30-day-month’ for the x-axis was developed for Meloidogyne species, where the x-axis was equivalent to 0, 1, 2, 3 and 4 ‘weeks-of-30-day-month’ (Pelinganga and Mashela 2012; Pelinganga et al. 2013b). The unit ‘weeks-of-30-day-month’ was developed to enhance the capability of a phytonematicide to break the life cycle of Meloidogyne species since their life cycles under optimum conditions in tropical and subtropical areas is approximately 30 days. In T. semipenetrans with the life cycle of approximately 42 days, the unit would be ‘weeks-of-42-day-month’. Since empirical information is required to establish the application interval, experiments are usually established with each tomato seedling inoculated with 5000 eggs and J2s of M. incognita under greenhouse conditions (Pelinganga and Mashela 2012). Nematode population densities were managed using the empirically established MCSR value of 3 % for nemafric-BL at 0-day (untreated control), 7.5-day (1 week × 30 days/4 weeks), 15-day (2 weeks × 30 days/4 weeks), 22.5-day (3 weeks × 30 days/4 weeks) and 30-day (4 weeks × 30 days/4 weeks) application interval. At 56 days after the treatment, plant variables (y-axis) are then subjected to ANOVA, with significantly (P ≤ 0.05) different treatment means being subjected to lines of the best fit to generate the quadratic relationships (Y = b 2 x 2 + b 1 x + a), where the optimum application interval was determined using x = −b 2/2b 1 in weeks (Table 7.5). In nemafric-BL 3 % and nemarioc-AL 3 %, the application intervals were 18 and 16 days, respectively (Pelinganga et al. 2012, 2013a). Doubling the concentration from 3 % to 6 % concentration had negligent effect on application interval of nemarioc-AL phytonematicide, but increased that of nemafric-BL from 18 days (2.40 weeks × 30 days/4 weeks) to 20 days (Pelinganga et al. 2013a).
In the use of nematicides applied into the soil, the concept of dosage is important and should be distinguished from dose and concentration (Van Gundy and McKenry 1975). Dose is an amount of chemical taken up by the target pest to effect detrimental behavioural changes, which may include disruption of juvenile development in eggs, egress, disoriented motility and/or mortality in nematodes (Van Gundy and McKenry 1975). In contrast, dosage (D) is the product of concentration (C) and the application frequency (T ca ), which could be summarised as:
The T ca is the proportion of the crop cycle (days) to the application interval (days), with the factor being unit-less. For instance, at 56 days under greenhouse or microplot conditions, T ca values for nemafric-BL 3 % and nemafric-AL 3 % were 3.11 and 3.50, respectively. The model is primarily for seasonal crops, but can also be adapted for perennial crops since nematode population dynamics for various crops, particularly in citriculture, are well established (Duncan 2009).
8 Soil Allelochemical Residual Effects
The soil allelochemical residue (SAR) effects investigate post-application effects of phytonematicides on various successor crops and nematode population densities. Increasing the concentration and shortening the application intervals inherently increase the dosage in the soil and, therefore, might defeat the purpose of establishing the MCSR and T ca values which are intended to ameliorate phytotoxicity. Doubling the concentration of phytonematicides may have negligent effects on the application interval, but serious consequences on dosage (Pelinganga et al. 2013a) and, thereby, SAR effects. The SAR effects of phytonematicides from Cucumis species were shown to have inhibitive effects of nodulation in B. japonicum (Mashela and Dube 2014) while having stimulation effects on growth of sweet-stem sorghum (Mashela 2014). In both cases, SAR effects reduced population nematode densities of Meloidogyne species. Additional work is still being under way to understand the chemistry of the SAR effects from phytonematicides.
9 Conclusion
Higher plants provide a broad spectrum of active ingredients for use in the management of plant-parasitic nematodes, with their principal drawback being phytotoxicity since their active ingredients comprise allelochemicals. The development of a phytonematicide where phytotoxicity is to be avoided consists of a series of steps. Firstly, there is need to establish whether the plant organ intended for use as a phytonematicide has the potential to reduce population nematode densities under in vitro and/or ex vitro conditions. Secondly, a series of concentrations with known bioactivity effects on the target nematode under greenhouse conditions are used to establish the MCSR value, which is a non-phytotoxic concentration to the crop which is to be protected against nematodes. Thirdly, the MCSR is used to establish the application interval (days), which should be based on the unit that would allow the product to interrupt the life cycle of the target nematode. Using the proposed procedures, commercial phytonematicides could be a reality in the management of plant-parasitic nematodes.
References
Agbenin NO, Emechebe AM, Marley PS, Akpa AD (2005) Evaluation of nematicidal action of some botanicals on Meloidogyne incognita in vivo and in vitro. J Agric Rural Dev Trop Subtrop 106:29–39
Alam MM (1993) Bioactivity against phytonematodes. In: Parmar BS, Randhawa NS (eds) Neem research and development. Society of Pesticide Science, New Delhi
Ahmad F, Siddiqui MA, Babalola OO (2013) Characterization of nematicidal activity of plant residues and their application with moisture approach against Meloidogyne incognita in tomato. Afr J Agric Res 8:93–101
Akhtar M, Malik A (2000) Role of organic soil amendments and soil organisms in the biological control of plant-parasitic nematodes: a review. Bioresour Technol 74:35–47
Bainard LD, Isman MB, Upadhyaya MK (2006) Phytotoxicity of clove oil and its primary constituent eugenol and the role of leaf epicuticular wax in the susceptibility to these essential oils. Weed Sci 54:833–837
Bargmann CI, Mori I (1997) Chemotaxis and thermotaxis. In: Blumenthal T, Meyer BJ, Priess JR, Riddle DL (eds) C. elegans II. Cold Spring Harbour Laboratory, New York, pp 717–737
Bird AF, Bird J (1991) The structure of nematodes, 1st edn. Academic, San Diego
Boyd NS, Brennan EB (2006) Burning nettle, common purslane, and rye response to a clove oil herbicide. Weed Technol 20:646–650
Boyd NS, Brennan EB, Fennimore SA (2006) Stale seedbed techniques for organic vegetable production. Weed Technol 20:1052–1057
Belair M, Tremblay N (1995) The influence of chitin-urea amendments applied to an organic soil on a Meloidogyne hapla population and on the growth of greenhouse tomato. Phytoprotection 76:75–80
Campbell NA (1990) Biology, 1st edn. Cummings, Redwood City
Castagnone-Sereno P, Kermarrec A (1991) Invasion of tomato roots and reproduction of Meloidogyne incognita as affected by raw sewage sludge. Suppl J Nematol 23:724–728
Chedekal AN (2013) Effect of four leaf extracts on egg hatching and juvenile mortality of root-knot nematode Meloidogyne incognita. Int J Adv Life Sci 6:68–74
Chitwood DJ (2002) Phytochemical based strategies for nematode control. Annu Rev Phytopathol 40:221–249
D’Addabbo T (1995) The nematicidal effect of organic amendments: review of the literature 1982–1994. Nematol Mediterr 23:299–305
Dhanalakshmi DN (2006) Studies on storability of indigenous materials and their utilization on okra sucking pests. M.Sc. (Agri.) dissertation, University of Agricultural Sciences, Dharwad
Dropkin VH (1969) The necrotic reaction of tomato and other hosts resistant to Meloidogyne: reversal by temperature. Phytopathology 59:1632–1637
Duncan LW (2009) Managing nematodes in citrus orchards. In: Ciancio A, Mukerji KG (eds) Integrated management of fruit crops and forest nematodes. Springer, Milton Keynes, pp 135–173
Duncan LW, McSorley R (1987) Modeling nematode population. In: Veetch JA, Dickson DW (eds) Vistas on nematology: a commemoration of the twenty- fifth anniversary of the society of nematologist. Society of Nematologists, Hyattsville, pp 377–389
Egunjobi OA, Afolami SO (1976) Effects of neem (Azadirachta indica) leaf extracts on population of Pratylenchus brachyurus and on the growth and yield of maize. Nematologica 22:125–132
EPPO (2010) Efficacy of evaluation of plant protection products: phytotoxicity assessment. European and mediterranean plant protection organisation. EPPO PP1/135(3)
Ferraz ICCB, Brown DJF (2002) An introduction to nematodes: plant nematology, 1st edn. Pensoft, Sofia-Moscow
Ferraz S, de Freitas LG (2004) Use of antagonistic plants and natural products. In: Chen ZX, Chen SY, Dickson DW (eds) Nematology advances and perspectives. Tsinghua University Press, Beijing
Ferris H, Wilson LT (1987) Concepts and principles of population dynamics. In: Veetch JA, Dickson WD (eds) Vistas on nematology: a commemoration of the twenty-fifth anniversary of the society of nematologist. Society of Nematologists, Hyattsville, pp 372–376
Haseeb A, Singh B, Khan AM, Saxena SK (1980) Effect of watering and mode of application of oil cakes and nematodes on their efficiency in controlling root- knot nematode on tomato. Acta Bot Indica 8:193–195
Huang JS (1987) Interactions of nematodes with Rhizobia. In: Veetch JA, Dickson WD, Soc N (eds) Vistas on nematology: a commemoration of the twenty-fifth anniversary of the society of nematologist. Society of Nematologist, Hyattsville, pp 301–306
Hirschmann H (1985) The genus Meloidogyne and morphological characters differentiating its species. In: Sasser JN, Carter CC (eds) An advanced treatise on Meloidogyne, vol I, Biology and control. North Carolina State University Graphics, Raleigh
Inderjit K, Asakawa C, Dakshini KMM (1999) Allelopathic potential of Verbesina encelioides root leachate in soil. Can J Bot 77:1419–1424
Jafee BA, Ferris H, Stapleton JJ, Norton MVK, Muldoon AE (1994) Parasitism of nematodes by the fungus Hirsutella rhossiliensis as affected by certain organic amendments. J Nematol 26:152–161
Jeffrey C (1978) Cucurbitaceae. In: Launert E (ed), Flora Zambezi, 1st edn. London
Kimpinski J, Gallant CF, Henry R, Macleod JA, Sanderson JB, Sturz AV (2003) Effect of compost and manure soil amendments on nematodes and yields of potato and barley: a 7-year study. J Nematol 35:289–293
Khan IA, Sayed M, Shaukat SS, Handoo ZA (2008) Efficacy of four plant extracts on nematodes associated with papaya in Sindh, Pakistan. Nematol Mediterr 36:93–98
Kyan T, Shintani M, Kanda S, Sakurai M, Ohashi H, Fujisawa A, Pongdit S (1999) Kyusei nature farming and the technology of effective microorganisms. Asia Pacific Natural Agriculture Network, Bangkok
Lai E (2008) Secondary metabolites and plant defense. In: Taiz L, Zeiger E (eds) A companion to plant physiology, 1st edn. I.K. International, New Delhi
Lee DH, Lwanski GB, Thoennissen NH (2010) Cucurbitacin: ancient compound shedding new light on cancer treatment. Sci World J 10:413–418
Liu DL, An M, Johnson IR, Lovett JV (2003) Mathematical modeling of allelopathy. III. A model for curve-fitting allelochemical dose responses. Nonlinear Biol Toxicol Medic 1:37–50
Mafeo TP, Mashela PW, Mphosi MS (2011a) Allelopathic responses of various seeds to crude extracts of Cucumis myriocarpus fruits when used as a pre- emergent bio-nematicide. Acta Hortic (ISHS) 938:409–414
Mafeo TP, Mashela PW, Mphosi MS (2011b) Sensitivity of selected Alliaceae seedlings to crude extracts of Cucumis myriocarpus fruits. Afr J Agric Res 6:158–164
Mafeo TP, Mashela PW, Mphosi MS, Pofu KM (2011c) Modelling responses of maize, millet and sorghum seedlings to crude extracts of Cucumis myriocarpus fruit as pre-emergent bio-nematicide. Afr J Agric Res 6:3678–3684
Mafeo TP, Mashela PW (2010) Allelopathic inhibition of seedling emergence in dicotyledonous crops by Cucumis bio-nematicide. Afr J Biotechnol 9:8349–8354
Mafeo TP, Mashela PW (2009a) Responses of germination in tomato, watermelon and butternut squash to a Cucumis bio-nematicide. J Agric Environ Sci 6:215–219
Mafeo TP, Mashela PW (2009b) Responses of four monocotyledonous crops to crude extracts of Cucumis myriocarpus fruit as a pre-emergent bio- nematicide. Afr Crop Sci Proc 9:631–634
Maila KD, Mashela PW (2013) Responses of the citrus nematode (Tylenchulus semipenetrans) to nemarioc-A phytonematicide with and without effective micro-organisms in citrus production. Afr Crop Sci Soc Conf 11:177 (Abst.)
Makkar HPS (1991) Quantification of tannins in tree foliage. IAEA working document. IAEA, Viena
Mashela PW, Duncan LW, McSorley R (1992) Salinity reduces resistance to Tylenchulus semipenetrans in citrus rootstocks. Nematropica 22:7–12
Mashela PW (2002) Ground wild cucumber fruits suppress numbers of Meloidogyne incognita on tomato in micro-plots. Nematropica 32:13–19
Mashela PW, Nthangeni ME (2002) Efficacy of Ricinus communis fruit meal with and without Bacillus species on suppression of Meloidogyne incognita and growth of tomato. J Phytopathol 150:399–402
Mashela PW, Shimelis HA, Mudau FN (2008) Comparison of the efficacy of ground wild cucumber fruits, aldicarb and fenamiphos on suppression of the root-knot nematode in tomato. J Phytopathol 156:264–267
Mashela PW, Shimelis HA, De Waele D, Mokgalong MN, Mudau FN, Ngobeni LG (2010) Fevertea (Lippia javanica) as a root-knot nematode suppressant in tomato production. Afr Plant Prot 6:1–6
Mashela PW, Pofu KM, Nzanza B (2012) Suitability of Brassica oleracea leaves in managing Meloidogyne incognita through the ground leaching technology system under microplot conditions. Acta Agric Scand 63:19–24
Mashela PW, De Waele D, Pofu KM (2011) Use of indigenous Cucumis technologies as alternative to synthetic nematicides in management of root- knot nematodes in low-input agricultural farming systems: a review. Sci Res Essay 6:6762–6768
Mashela PW (2014) Soil allelochemical residue effects in a tomato cowpea rotation – nodulation and productivity of cowpea and nematode suppression. Acta Agric Scand 64:372–375
Mashela PW, Dube ZP (2014) Soil allelochemical residue effects of nemafric-BL and nemarioc-AL phytonematicides on soil health, growth of sweet sorghum and Meloidogyne species. Acta Agric Scand 64:79–84
Mashela PW, Pofu KM, Dube ZP (2014) Managing phytotoxicities in phytonematicides: the dosage model. In: 6th international conference of nematology, pp 69–70
McSorley R (2003) Adaptations of nematodes to environmental extremes. Florida Entomol 86:138–142
McSorley R (2011) Overview of organic amendments for management of plant- parasitic nematodes, with case studies from Florida. J Nematol 43:69–81
McSorley R, Gallaher RN (1995) Cultural practices improve crop tolerance to nematodes. Nematropica 25:53–60
Meyer SLF, Lakshman DK, Zasada IA, Vinyard BT, Chitwood DJ (2008) Phytotoxicity of clove oil to vegetable crop seedings and nematotoxicity to root-knot nematodes. Horti Technol 18:631–638
Mian IH, Rodriguez-Kabana R (1982a) Soil amendments with oilcakes and chicken litter for control of Meloidogyne arenaria. Nematropica 12:205–220
Mian IH, Rodriguez-Kabana R (1982b) Survey of the nematicidal properties of some organic materials available in Alabama as amendments to soil for control of Meloidogyne arenaria. Nematropica 12:235–246
Mudau FN, Mogotlane ID, Mashela PW, Soundy P (2008) Seasonal variation of total antioxidant contents of wild bush tea. Acta Hortic (ISHS) 802:273–275
Mukherjee SN, Sukul NC (1978) Nematicidal action of three species of wild herb. Indian J Res 2:12
Muller R, Gooch PS (1982) Organic amendments in nematode control: an examination of the literature. Nematropica 12:319–326
Musabyimana T, Saxena RC, Kairu EW, Ogol KZR (2000) Powdered neem seed and cake for management of banana weevil and parasitic nematodes. Phytoparasitica 28:321–330
Ncube L (2008) Evaluation of effective micro-organisms (EM) on soil chemical properties and yield of selected vegetables in the Eastern Cape, South Africa. MSc dissertation, University of Fort Hare, Alice, South Africa
Nicholson AJ (1933) The balance of animal populations. J Anim Ecol 2:132–178
Ntalli NG, Manconi F, Leonti M, Maxia A, Caboni P (2011) Aliphatic ketones from Ruta chalepensis (Rutaceae) induce paralysis on root-knot nematodes. J Agric Food Chem 59:7098–7103
Ntuli NG, Caboni P (2012) Botanical nematicides: a review. Agric Food Chem 60:9929–9940
Nzanza B, Mashela PW (2012) Control of whiteflies and aphids in tomato by fermented plant extracts of neem leaf and wild garlic. Afr J Biotechnol 11:16077–16082
Ojo GT, Umar I (2013) Evaluation of some botanicals on root-knot nematode (Meloidogyne javanica) in tomato (Lycopersicon esculentum Mill) in Yola Adamawa State, Nigeria. Biol Forum 5:31–36
Oka Y (2010) Mechanisms of nematode suppression by organic soil amendments: a review. Appl Soil Ecol 44:101–115
Oka Y, Nacar S, Putievsky E, Ravid U, Yaniv Z, Spiegel Y (2000) Nematicidal activity of essential oils and their components against the root-knot nematode. Phytopathology 90:710–715
Okeniyi MO, Afolami SO, Fademi OA, Oduwaye OF (2013) Effect of botanical extracts on root-knot nematode (Meloidogyne incognita) infection and growth of cashew (Anacardium occidentale) seedlings. Acad J Biotechnol 1:81–86
Okwute SK (2012) Plants as potential sources of pesticidal agents: a review. In: Soundararajan RP (ed) Pesticides: advances in chemical and botanical pesticides. In Tech, pp 208–232
Papadopoulou J, Triantaphyllou AC (1982) Sex differentiation in Meloidogyne incognita and anatomical evidence of sex reversal. J Nematol 14:549–566
Parmar BS (1987) An overview of neem research and use in India during the years 1983–1986. In: Proceedings of the 3rd international neem conference abstract, Nigeria
Parmar BS (1996) An overview of neem research and use in India during the years 1983–1986. In: Schmutter H, Ascher KRS (eds) Natural pesticides from the neem tree and other tropical plants, vol 3, Proceedings of the international neem conference. GTZ, Eschborn, pp 50–80
Pelinganga OM (2013) Developing bio-pesticides using indigenous Cucumis africanus and Cucumis myriocarpus fruits for tomato production systems. PhD thesis, University of Limpopo, Sovenga
Pelinganga OM, Mashela PW (2012) Mean dosage stimulation range of allelochemicals from crude extracts of Cucumis africanus fruit for improving growth of tomato plant and suppressing Meloidogyne incognita numbers. J Agric Sci 4:8–12
Pelinganga OM, Mashela PW, Mphosi MS, Mafeo TP (2013a) Using computer- based model to determine phytotoxicity concentration of nemarioc-A phytonematicide in tomato production. Afr Crop Sci Soc Conf (Abst) 11:168
Pelinganga OM, Mashela PW, Mphosi MS, Nzanza B (2013b) Optimizing application frequency of diluted (3%) fermented Cucumis africanus fruit in tomato production and nematode management. Acta Agric Scand 63(3):278–282
Pelinganga OM, Mashela PW, Nzanza B, Mphosi MS (2012) Baseline information on using fermented crude extracts from Cucumis africanus fruit for suppression of Meloidogyne incognita and improving growth of tomato plant. Afr J Biotechnol 11:11407–11413
Pofu KM, Mashela PW, Shimelis H (2012) Intergeneric grafting in watermelon for managing Meloidogyne species: a review. Sci Res Essay 7:107–113
Pofu KM, Mashela PW (2014) Density-dependent growth patterns of Meloidogyne javanica on hemp cultivars: establishing nematode-sampling timeframes in host-status trials. Am J Exp Agric 4:639–650
Prot JC (1980) Migration of plant-parasitic nematodes towards plant roots. Rev Nematol 3:305–318
Rossner J, Zebitz CPW (1987) Effect of neem products on nematodes and growth of tomato (Lycopersicon esculentum) plants. In: Schmutterer H, Ascher KRS (eds) Naturalpesticides from the neem tree (Azadirachta indica A. Juss) andothertropical plants, vol 3, Proceedings of the international neem conference. GTZ, Eschborn, pp 611–621
Rice EL (1984) Allelopathy, 1st edn. Academic, New York
Rimington P (1938) Medicinal and poisonous plants of South and East Africa. University of Natal Press, Pietermaritzburg
Salisbury FB, Ross CW (1992) Plant physiology, 2nd edn. Wadsworth, Belmont
Seinhorst JW (1967) The relationship between population increase and population density in plant-parasitic nematodes. 3. Definition of the terms host, host- status and resistance. 4. The influence of external conditions on the regulation of population density. Nematologica 13:429–442
Shaukat SS, Siddiqui IA, Ali NI (2003) Nematicidal and allelopathic responses of Lantana camara root extract. Phytopathol Mediterr 42:71–78
Smith GS (1987) Interactions of nematodes with mycorrhizal fungi. In: Veetch JA, Dickson DW (eds) Vistas on nematology: a commemoration of the twenty- fifth anniversary of the society of nematologist. Society of Nematologist, Hyattsville, pp 292–300
Stirling GR (2014) Biological control of plant parasitic nematodes: soil ecosystem management in sustainable agriculture, 2nd edn. CAB International, Wallingford
Sukul NC, Das PK, De GC (1974) Nematicidal action of edible crops. Nematologica 20:187–191
Thoden TC, Korthals GW, Termorshuizen AJ (2011) Organic amendments and their influences on plant-parasitic and free-living nematodes: a promising method for nematode management? Nematology 13:133–153
Toida Y, Moriyama H (1978) Effects of the Marigold on the control of nematodes in Mulberry. 2. Nematicidal effects of the Mexican marigold by application. Helminthol B 49:696, Abstr
Triantaphyllou AC (1973) Environmental sex differentiation of nematodes in relation to pest management. Annu Rev Phytopathol 11:441–462
Tworkoski T (2002) Herbicide effects of essential oils. Weed Sci 50:42–431
Usman A (2013) Studies on the integrated management of phytonematodes attacking some vegetable crops. PhD thesis, Aligarh Muslim University, Aligarh
Van Gundy SD, McKenry MV (1975) Action of nematicides. In: Horsefall JG, Cowling EB (eds) Plant disease: an advanced treatise, Ith edn, How diseaseis managed. Academic, New York
Van Wyk B, Heerden F, Oudshoorn B (2002) Poisonous plants of South Africa. Briza, Pretoria
Vijayalakshmi K, Subhashini B, Shivani VK (1996) Plant in pest control using garlic and onion. Centre for Indian Knowledge System, Chennai
Wallace HR (1973) Nematode ecology and plant disease, 1st edn. Edward Arnold, London
Waliwitiya R, Isman MB, Vernon RS, Riseman A (2005) Insecticidal activity of selected monoterpenoids and rosemary oil to Agriotes obscurus (Coleoptera: Elateridae). J Econ Entomol 98:1560–1565
Walter M, Jaspers MV, Eade K, Frampton CM, Stewart A (2001) Control of Botrytis cinerea in grape using thyme oil. Austr Plant Pathol 30:21–25
Wuyts N, Swennen R, De Waele D (2006) Effects of plant phenylpropanoid pathway products and selected terpenoids and alkaloids on the behaviour of the plant parasitic nematodes Radopholus similis, Pratylenchus penetrans and Meloidogyne incognita. Nematology 8:89–101
Zasada IA, Ferris H (2003) Sensitivity of Meloidogyne javanica and Tylenchulus semipenetrans to isothiocyanates in laboratory assays. Phytopathology 93:747–750
Acknowledgements
The authors are grateful to the Land Bank Chair of Agriculture—University of Limpopo, the Flemish Interuniversity Council (VLIR) and the Agriculture Research Council of South Africa for funding the Green Technologies Research Programme at the University of Limpopo.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2015 Springer International Publishing Switzerland
About this chapter
Cite this chapter
Mashela, P.W., Dube, Z.P., Pofu, K.M. (2015). Managing the Phytotoxicity and Inconsistent Nematode Suppression in Soil Amended with Phytonematicides. In: Meghvansi, M., Varma, A. (eds) Organic Amendments and Soil Suppressiveness in Plant Disease Management. Soil Biology, vol 46. Springer, Cham. https://doi.org/10.1007/978-3-319-23075-7_7
Download citation
DOI: https://doi.org/10.1007/978-3-319-23075-7_7
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-23074-0
Online ISBN: 978-3-319-23075-7
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)