Abstract
In chronic liver disease management, the emergence of noninvasive tests, mainly transient elastography, to estimate liver fibrosis has resulted in a major impact in daily clinical practice, in the epidemiology of chronic liver disease, and it is challenging some of the current recommendations for liver cirrhosis. In this chapter, we will explain the importance of the use of TE in the management of severe chronic liver disease, we will also propose a new term to adequately describe these patients detected in the early phases of advanced chronic liver disease, and we will provide simple rules to avoid unnecessary procedures in such patients.
A new term of compensated advanced chronic liver disease (cACLD) defining patients in the early phases of severe chronic liver disease will be described, including both patients with severe fibrosis or pre-cirrhotic patients and patients with compensated cirrhosis. The term might be helpful for both clinical practice and research purposes. Simple clinical rules to avoid unnecessary endoscopies and hepatic venous pressure gradients in these cACLD patients will be also provided. With the combination of simple rules using liver stiffness measurement and platelet count, 40–45 % of screening endoscopies could be avoided in these patients, with an acceptable risk of missing varices. Similarly, using only liver stiffness a subgroup of cACLD patients can be safely considered as having clinically significant portal hypertension. These recommendations will definitely decrease the number of unneeded procedures in these patients.
Access provided by Autonomous University of Puebla. Download conference paper PDF
Similar content being viewed by others
Keywords
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
From Cirrhosis to Compensated Advanced Chronic Liver Disease
Chronic liver disease progresses through different stages as a consequence of increased liver fibrosis. As a result of continued liver injury, there is progressive accumulation of fibrous tissue in the liver. When accumulation exceeds degradation and remodeling, the process results in cirrhosis, the end stage of chronic liver disease. Cirrhosis is a histological diagnosis defined by the presence of regenerative nodules surrounded by fibrous tissue that leads to angioarchitectural distortion. Liver biopsy has been the “gold standard” in the assessment of the severity of fibrosis and in the diagnosis of cirrhosis. However, the limitations of the procedure are widely known (invasiveness, complications, sampling error, etc.), and in part due to these limitations, liver biopsy is not adequate for continuous monitoring of liver disease progression and does not provide a dynamic information of the process [1, 2].
In addition to liver biopsy, cirrhosis is also usually defined from a clinical point of view by the presence of a combination of clinical signs and biochemical (low platelets, liver dysfunction tests), imaging (nodular liver or signs of portal hypertension: splenomegaly and collateral circulation), and endoscopic parameters (varices). This practical definition has become popular among liver specialists, but the sensitivity and specificity of these clinical criteria are very variable and precisely defined criteria and consensus are lacking.
The development of portal hypertension is a crucial event in the evolution of cirrhosis. When the hepatic venous pressure gradient (HVPG) increases to 10 mmHg (clinically significant portal hypertension, CSPH), cirrhotic patients become at risk of developing varices and clinical decompensation [3–5]. HVPG is an accurate and reproducible method, although again invasive and, most importantly, only available in specialized centers. The reality is that HVPG has not entered universal routine clinical practice. In addition, detecting varices by endoscopy in cirrhotic patients with CSPH is an important hallmark in the natural history of cirrhosis, since it carries prognostic significance and sets the indication for primary prophylaxis of variceal bleeding [6, 7]. Therefore, international guidelines indicate that if possible, HVPG measurement should be used for diagnosis and therapeutic indications in cirrhotic patients and that all cirrhotic patients should be screened (by endoscopy) for varices at diagnosis [7, 8]. In addition to screening for portal hypertension, cirrhotic patients also should initiate surveillance for hepatocellular carcinoma [9].
For the reasons outlined before, in the last years, methods aimed at determining noninvasively the presence of liver fibrosis, cirrhosis, CSPH, and varices have been developed and extensively investigated. The appearance of such methods and their widespread use have somehow changed the clinical scenario of chronic liver disease, notably increasing the number of patients with significant chronic liver disease detected in the very early phases of the process. These previously undetected patients are now being labeled as cirrhotic patients, although we know that at least 10–15 % of them have no cirrhosis by histology [10]. In these patients also the decision to screen for varices and CSPH – which obviously has to be considered – may entail an important increase in the use of unnecessary procedures [11]. To adequately frame and describe this new clinical situation in chronic liver disease and provide recommendations, it might be helpful to use the term of compensated advanced chronic liver disease (cACLD), instead of liver cirrhosis that could be still used for patients with biopsy-proven cirrhosis, patients with evident signs of portal hypertension (varices), or decompensated patients. The incorporation of information from some noninvasive tests into the definition of cACLD might also be helpful and concur with current clinical practice in many centers.
Role of Elastography in Changing the Epidemiology of cACLD
Over the last years, several different approaches have explored the possibility of identifying by different noninvasive methods the degree of liver fibrosis and consequently recognizing cirrhosis, varices, or CSPH. Among the different modalities, including direct and indirect serum biomarkers of fibrosis and physical approaches that measure liver stiffness, transient elastography (TE) using FibroScan® (Echosens, Paris, France) has achieved wide acceptance, has been shown to possess excellent performance, and is currently incorporated as a valuable tool in the assessment of chronic liver disease in many centers, especially in Europe. Liver biopsy for staging purposes has substantially been reduced in many hospitals. TE has very good performance in detecting cirrhosis and excluding significant fibrosis [10, 12].
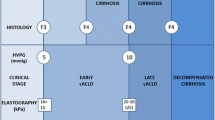
The impact of TE in changing the epidemiology of chronic liver diseases can be illustrated by different approaches, but the most remarkable is the fact that TE is able to uncover cACLD in patients with chronic liver disease in whom the caring physician did not suspect it. This effect represents a substantial increase in the number of patients needing close follow-up and surveillance (Fig. 5.1). Prospective studies specifically aimed at identifying this occult cACLD among chronic liver disease patients are not available, but some information could be extracted from other studies.
Screening studies performed with TE in unselected healthy populations may help to understand what the prevalence of cACLD in general population is. In a French study carried out in more than 1000 normal subjects (general population), a 7.5 % prevalence of liver stiffness measurement (LSM) ≥8 kPa was found, among them 10 % with LSM >13 kPa (0.8 of the total population) [13]. In the Rotterdam cohort, with 1324 participants older than 65 years, the prevalence of LSM >9.5 kPa and >13 kPa was 4.2 % and 1.1 %, respectively [14]. Similar studies carried out in Asian populations, including more than 3000 individuals, have detected LSM values indicative of F3 fibrosis in 1–2 % of the individuals [15, 16, 17]. Nonalcoholic fatty liver disease (NAFLD) was the predominant etiology of liver disease in all studies.
A different way to analyze the importance of TE to uncover occult cACLD is by systematically studying series of patients with chronic liver disease without any clinical sign of cirrhosis (normal platelets and abdominal sonography). Results from Barcelona [18], Montreal [19], and Seoul [20] indicate a prevalence of 8–14 % of patients with LSM ≥13–13.6 kPa in these cohorts (Fig. 5.2. Panel a). These patients with occult cACLD represented 24–37 % of the total number of patients with LSM ≥13–13.6 kPa included in the first two prior cohorts, plus patients from the ANTICIPATE study [21] (Fig. 5.2. Panel b). The ANTICIPATE study is a cooperative study (Edmonton, Barcelona, Toulouse, Cluj-Napoca) aimed at assessing noninvasive tools to identify the risk of CSPH and varices in patients with presumed or confirmed compensated liver cirrhosis. Therefore, patients with occult cACLD account for a substantial portion of patients in the different studies.
Prevalence of patients with LSM ≥13–13.6 kPa in three series of patients with chronic liver disease without any clinical sign of cirrhosis (normal platelets and abdominal sonography) (Panel a). Prevalence of occult ACLD (normal platelets and abdominal sonography) in three series of patients with LSM ≥13–13.6 kPa (Panel b)
Finally, it is also worth to mention that in the study by Chen et al. [19], patients with occult cACLD received significant less surveillance than patients with clinically evident liver cirrhosis, and this resulted in a higher rate of late diagnosis (advanced hepatocellular carcinoma, variceal bleeding). The results of this observational study suggest that patients with occult cACLD are frequently underdiagnosed and under-monitored compared to patients with evident liver cirrhosis.
Why Compensated Advanced Chronic Liver Disease?
When a patient with chronic liver disease develops clinical decompensation, there is no doubt that liver cirrhosis is present, and the same could be applied to a patient in whom varices are detected by endoscopy or CSPH by HVPG. However, the presence or not of cirrhosis in its early stages might be challenging, and since the spectrum of severe fibrosis and cirrhosis is actually a continuum that is difficult to be distinguished without liver histology, the new term of what we have called cACLD might be helpful in this setting. The new definition would be useful for several reasons: (1) to select patients for clinical and therapeutic studies; (2) to adequately frame a clinical situation; and (3) to provide recommendations for screening of hepatocellular carcinoma, varices, and CSPH.
The grounds for this new term of cACLD that would include both patients with severe fibrosis and patients with compensated cirrhosis, especially in the earliest stages, could be the following:
-
1.
Liver cirrhosis is a histological diagnosis.
-
2.
Cirrhosis is not histologically present in every patient classified as F4 by noninvasive methods.
-
3.
There is no consensus in a clinical definition of liver cirrhosis.
-
4.
Patients in pre-cirrhotic stages may have portal hypertension [22, 23].
-
5.
Hepatocellular carcinoma surveillance might be indicated in pre-cirrhotic stages [9, 24].
-
6.
Noninvasive tests have changed the clinical scenario of chronic liver disease.
A patient with cACLD would be a patient with chronic liver disease with signs of severe liver fibrosis or compensated liver cirrhosis with or without signs of portal hypertension. The identification of a patient with cACLD would imply referral by primary care physicians to a liver disease specialist for follow-up and treatment. Considerations for closer follow-up and hepatocellular carcinoma surveillance, and CSPH and varices evaluation should be made at this point by the liver disease specialist.
Different parameters that liver specialists with experience in cACLD and cirrhosis use to classify patients as having or suspecting cACLD are shown in the results of the questionnaire answered by the panelists of the present consensus workshop (Table 5.1). Two aspects are worth to mention from this survey: (1) Many of the parameters are the ones we have been using for years with different performance for the clinical diagnosis of liver cirrhosis, and (2) experts consider now noninvasive tests useful for classifying patients as cACLD patients, and among them only TE possesses wide acceptance. In consequence, recommendations for ruling out and ruling in cACLD based on TE results are now included in the final statements of the consensus workshop. In essence, an LSM below 10 kPa (high negative predictive value) will exclude cACLD, and values above 15 kPa (high positive predictive value) will be highly indicative of cACLD; for the rest of TE results between these two points, additional work-up would be needed.
What Patients with cACLD Could Avoid Screening Endoscopy?
One of the main challenges of detecting cACLD by noninvasive methods is the large number of unnecessary endoscopies that would potentially be performed in patients with a very low risk of varices [11]. TE has been evaluated as a predictor of varices in several studies. In general, studies show that TE performs better in ruling out (high sensitivity and negative predictive value) than in ruling in (high specificity and positive predictive value) the presence of varices [12, 18, 25, 26]. However, heterogeneity in the results and cutoffs, and overall low predictability has impeded translation into clinical practice. Since TE seems to work better to rule out varices, it is important to decide what would be an acceptable risk when using this technique for prescreening purposes. For all varices a 20 % risk of missing might be acceptable, but for varices needing treatment (VNT: medium-large varices or small with red signs), it should be near 0 or 5 % at the most.
Diagnostic performance for varices seems to improve when LSM is combined with simple clinical parameters, mainly including platelets and spleen size. The LSPS (LSM-spleen diameter to platelet ratio) [27, 28] and the VRS (variceal risk score) [29] are very good examples of this strategy, and both perform better than LSM alone for varices prediction. Also in the ANTICIPATE study cohort, LSPS was the best predictor for varices and VNT in a risk prediction modeling analysis [21].
However, these combined noninvasive tests require more or less complex calculation and threshold memorization to be applied to daily clinical practice. Simple, visual, practical clinical rules using these parameters could be equally useful and easily implementable. Three studies using just a combination of LSM and platelets are summarized in Table 5.2 [18, 30, 31]. In addition, the validation of the classification rules of these studies in the ANTICIPATE cohort is also shown. Results indicate that using a combination of LSM with a cutoff of 25 kPa and platelet count with cutoffs between 100 and 150 ×103 mm3, 20–40 % of screening endoscopies could be avoided in these patients, with an acceptable risk of missing VNT (5 % in the worst case). The simplicity and readiness-to-use of the classification rule could allow doctors to easily defer endoscopy while visiting the patient and consequently contribute to the incorporation of the classification rule into clinical practice.
What Patients with cACLD Could Be Classified as Having CSPH?
Similarly to varices detection, TE has also been utilized to predict CSPH. Detecting patients at very high risk of having CSPH could be useful to select patients for clinical studies and indicate empiric prophylactic therapy for decompensation (provided future studies show its usefulness). However, it is quite clear that TE will never be capable of predicting the numerical value of HVPG and it is probably not suitable for monitoring HVPG changes. TE seems to be a good predictor of CSPH and in general, tends to perform better in ruling in (high specificity and positive predictive value) than in ruling out (high sensitivity and negative predictive value) the presence of CSPH [12, 18, 25, 26]. In terms of selecting patients with CSPH, positive predictive values (ruling in) higher than 90 % can be achieved with an LSM cutoff of 25 kPa; these numbers decrease slightly to 85–90 % if LSM cutoff is lowered to 20–21 kPa. Again data from the ANTICIPATE study [21] shown in Fig. 5.3 indicates that with an LSM ≥25 kPa (37 % of the cohort), 96 % of these patients can be assumed as having CSPH.
The ability of TE to rule in CSPH is not substantially improved by adding simple clinical information (platelets and spleen size), as in the LSPS or the PH (portal hypertension) risk scores [28, 29]. Also in the ANTICIPATE study cohort, LSPS was only slightly better than LSM alone to predict CSPH in a risk prediction modeling analysis [21]. By contrast and although TE is not very accurate in ruling out CSPH, a subgroup of patients with less than 20 % risk of having CSPH can be detected combining LSM and platelet count. As shown in Fig. 5.3, patients with LSM <25 kPa and normal platelets have a risk of CSPH of 17 %. These patients, representing 25 % of the total cohort, can probably be monitored and safely avoid immediate CSPH evaluation. Other studies [18, 32] with lower number of patients have revealed very similar results. The study by Kitson et al. [32] found a 90 % negative predictive value (10 % risk) of CSPH with the same classification rule, LSM <25 kPa and platelet count >150 ×103 mm3. As for the rest of patients positioned in the gray zone, patients with LSM <25 kPa and low platelets, representing 35–40 % of the population (Fig. 5.3), the prevalence of CSPH ranges from 40 to 60 %, and if CSPH is to be diagnosed, an HVPG should be performed.
Summary
A new term of cACLD defining patients in the early phases of severe chronic liver disease has been proposed, including both patients with severe fibrosis or pre-cirrhotic patients and patients with compensated cirrhosis. The term will be helpful for both clinical practice and research purposes. Simple clinical rules to avoid unnecessary endoscopies and HVPG in these cACLD patients are also provided. With the combination of LSM <25 kPa and platelet count ≥100 × 103 mm3, 40–45 % of screening endoscopies could be avoided in these patients, with an acceptable risk of missing 5 % VNT. Similarly, patients with LSM ≥25 kPa can be safely considered as having CSPH, and patients with LSM <25 kPa and normal platelets can be classified as not having CSPH; 60 % of unnecessary procedures might be avoided. These recommendations will definitely decrease the number of unneeded procedures in these patients.
References
Bedossa P, Dargère D, Paradis V (2003) Sampling variability of liver fibrosis in chronic hepatitis C. Hepatology 38:1449–1457
Bedossa P, Carrat F (2009) Liver biopsy: the best, not the gold standard. J Hepatol 50:1–3
Groszmann RJ, Garcia-Tsao G, Bosch J, Grace ND, Burroughs AK, Planas R, Escorsell A, Garcia-Pagan JC, Patch D, Matloff DS, Gao H, Makuch R, Portal Hypertension Collaborative Group (2005) Beta-blockers to prevent gastroesophageal varices in patients with cirrhosis. N Engl J Med 353:2254–2261
Ripoll C, Groszmann R, Garcia-Tsao G, Grace N, Burroughs A, Planas R, Escorsell A, Garcia-Pagan JC, Makuch R, Patch D, Matloff DS, Bosch J, Portal Hypertension Collaborative Group (2007) Hepatic venous pressure gradient predicts clinical decompensation in patients with compensated cirrhosis. Gastroenterology 133:481–8
de Franchis R (2000) Updating consensus in portal hypertension: report of the Baveno III Consensus Workshop on definitions, methodology and therapeutic strategies in portal hypertension. J Hepatol 33:846–852
D’Amico G, Garcia-Tsao G, Pagliaro L (2006) Natural history and prognostic indicators of survival in cirrhosis: a systematic review of 118 studies. J Hepatol 44:217–231
de Franchis R, Baveno V Faculty (2010) Revising consensus in portal hypertension: report of the Baveno V consensus workshop on methodology of diagnosis and therapy in portal hypertension. J Hepatol 53:762–768
Garcia-Tsao G, Sanyal AJ, Grace ND, Carey W, Practice Guidelines Committee of the American Association for the Study of Liver Diseases; Practice Parameters Committee of the American College of Gastroenterology (2007) Prevention and management of gastroesophageal varices and variceal hemorrhage in cirrhosis. Hepatology 46:922–938
European Association For The Study Of The Liver; European Organisation For Research And Treatment Of Cancer (2012) EASL-EORTC clinical practice guidelines: management of hepatocellular carcinoma. J Hepatol 56:908–943
Castera L (2012) Noninvasive methods to assess liver disease in patients with hepatitis B or C. Gastroenterology 142:1293–1302
Rudler M, Benosman H, Lebray P, Nghiem D, Ngo Y, Munteanu M, Poynard T, Thabut D (2011) Screening for esophageal varices in patients newly diagnosed in 2011: 84% of upper gastrointestinal endoscopies are futile. Hepatology 54(Suppl):935A (abstract)
Castera L (2011) Elastography in the non-invasive evaluation of the extent of fibrosis and in the diagnosis of portal hypertension. In: de Franchis R (ed) Proceedings of the fifth Baveno international consensus workshop. Wiley-Blackwell, Oxford, UK, pp 18–27
Roulot D, Costes JL, Buyck JF, Warzocha U, Gambier N, Czernichow S, Le Clesiau H, Beaugrand M (2011) Transient elastography as a screening tool for liver fibrosis and cirrhosis in a community-based population aged over 45 years. Gut 60:977–984
Koehler EM, Schouten JNL, Hansen BE, Stricker BH, Castera L, Janssen HLA (2012) Prevalence and risk factors of severe fibrosis in the elderly: transient elastography in a population-based study. Abstract book, Autumn meeting of the Dutch Society of Hepatology, Zeist, 4–5 Oct 2012. p 13
Wong VW, Chu WC, Wong GL, Chan RS, Chim AM, Ong A, Yeung DK, Yiu KK, Chu SH, Woo J, Chan FK, Chan HL (2012) Prevalence of non-alcoholic fatty liver disease and advanced fibrosis in Hong Kong Chinese: a population study using proton-magnetic resonance spectroscopy and transient elastography. Gut 61:409–415
Fung J, Lee CK, Chan M, Seto WK, Lai CL, Yuen MF, Hong Kong Liver Health Census Study Group (2015) High prevalence of non-alcoholic fatty liver disease in the Chinese – results from the Hong Kong liver health census. Liver Int 35:542–549
You SC, Kim KJ, Kim SU, Kim BK, Park JY, Kim do Y, Ahn SH, Lee WJ, Han KH (2015) Factors associated with significant liver fibrosis assessed using transient elastography in general population. World J Gastroenterol 21:1158–1166
Augustin S, Millán L, González A, Martell M, Gelabert A, Segarra A, Serres X, Esteban R, Genescà J (2014) Detection of early portal hypertension with routine data and liver stiffness in patients with asymptomatic liver disease: a prospective study. J Hepatol 60:561–569
Chen T, Wong R, Wong P, Rollet-Kurhajec KC, Alshaalan R, Deschenes M, Ghali P, Sebastiani G (2015) Occult cirrhosis diagnosed by transient elastography is a frequent and under-monitored clinical entity. Liver Int. doi:10.1111/liv.12802 [Epub ahead of print]
Kim MN, Kim SU, Kim BK, Park JY, Kim DY, Ahn SH, Song KJ, Park YN, Han KH (2015) Increased risk of hepatocellular carcinoma in chronic hepatitis B patients with transient elastography-defined subclinical cirrhosis. Hepatology. doi:10.1002/hep.27735 [Epub ahead of print]
Abraldes J, Bureau C, Stefanescu H, Augustin S, Ney M, Procopet B, Bosch J, Genesca J, Berzigotti A (2015) Non-invasive tools and risk of varices and clinically significant portal hypertension in compensated cirrhosis: the “ANTICIPATE” Study. J Hepatol 62(Suppl 2):S198–S199 (abstract)
Krogsgaard K, Gluud C, Henriksen JH, Christoffersen P (1984) Correlation between liver morphology and portal pressure in alcoholic liver disease. Hepatology 4:699–703
van Leeuwen DJ, Howe SC, Scheuer PJ, Sherlock S (1990) Portal hypertension in chronic hepatitis: relationship to morphological changes. Gut 31:339–343
Rinella ME, Sanyal AJ (2015) NAFLD in 2014: genetics, diagnostics and therapeutic advances in NAFLD. Nat Rev Gastroenterol Hepatol 12:65–66
Castera L, Pinzani M, Bosch J (2012) Non invasive evaluation of portal hypertension using transient elastography. J Hepatol 56:696–703
Shi KQ, Fan YC, Pan ZZ, Lin XF, Liu WY, Chen YP, Zheng MH (2013) Transient elastography: a meta-analysis of diagnostic accuracy in evaluation of portal hypertension in chronic liver disease. Liver Int 33:62–71
Kim BK, Han KH, Park JY, Ahn SH, Kim JK, Paik YH, Lee KS, Chon CY, Kim do Y (2010) A liver stiffness measurement based, noninvasive prediction model for high-risk esophageal varices in B-viral liver cirrhosis. Am J Gastroenterol 105:1382–1390
Colecchia A, Montrone L, Scaioli E, Bacchi-Reggiani ML, Colli A, Casazza G, Schiumerini R, Turco L, Di Biase AR, Mazzella G, Marzi L, Arena U, Pinzani M, Festi D (2012) Measurement of spleen stiffness to evaluate portal hypertension and the presence of esophageal varices in patients with HCV-related cirrhosis. Gastroenterology 143:646–654
Berzigotti A, Seijo S, Arena U, Abraldes JG, Vizzutti F, García-Pagán JC, Pinzani M, Bosch J (2013) Elastography, spleen size, and platelet count identify portal hypertension in patients with compensated cirrhosis. Gastroenterology 144:102–111
Montes Ramirez ML, Pascual-Pareja JF, Sánchez-Conde M, Bernardino De la Serna JI, Zamora Vargas FX, Miralles P, Castro JM, Ramírez M, Gutierrez I, Gonzalez-García J, Berenguer J, Arribas López JR (2012) Transient elastography to rule out esophageal varices and portal hypertensive gastropathy in HIV-infected individuals with liver cirrhosis. AIDS 26:1807–1812
Ding NS, Nguyen T, Iser DM, Hong T, Flanagan E, Wong A, Luiz L, Tan JY, Fulforth J, Holmes J, Ryan M, Bell SJ, Desmond PV, Roberts SK, Lubel J, Kemp W, Thompson AJ (2015) Liver stiffness plus platelet count can be used to exclude high risk oesophageal varices. Liver Int. doi:10.1111/liv.12916 [Epub ahead of print]
Kitson MT, Roberts SK, Colman JC, Paul E, Button P, Kemp W (2015) Liver stiffness and the prediction of clinically significant portal hypertension and portal hypertensive complications. Scand J Gastroenterol 50:462–469
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2016 Springer International Publishing Switzerland
About this paper
Cite this paper
Augustin, S., Pons, M., Santos, B., Ventura, M., Genescà, J. (2016). Identifying Compensated Advanced Chronic Liver Disease: When (Not) to Start Screening for Varices and Clinically Significant Portal Hypertension. In: de Franchis, R. (eds) Portal Hypertension VI. Springer, Cham. https://doi.org/10.1007/978-3-319-23018-4_5
Download citation
DOI: https://doi.org/10.1007/978-3-319-23018-4_5
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-23017-7
Online ISBN: 978-3-319-23018-4
eBook Packages: MedicineMedicine (R0)