Abstract
Early diagnosis of patients with compensated advanced chronic liver disease (cACLD) and portal hypertension is challenging in clinical practice. A growing amount of evidence regarding noninvasive diagnostic methods, and in particular liver stiffness measurement (LSM), suggests that these tools could be used in clinical practice and might potentially limit the use of invasive, reference diagnostic tools (HVPG measurement and endoscopy).
Our panel aimed at better understanding the opinion of the Baveno faculty regarding the current practice and use of invasive and noninvasive methods in the field of screening and surveillance of varices; a specific questionnaire was electronically sent to all the faculty members. The results suggested that the experts agreed on the use of noninvasive methods to rule out/identify patients with cACLD. They also indicated that the persistence or removal of the causal agent which led to cirrhosis should guide the choice of using the shortest or the longest interval among those recommended for surveillance endoscopies. Finally, the use of noninvasive methods in these clinical scenarios was pointed out as a relevant field for future research.
Access provided by Autonomous University of Puebla. Download conference paper PDF
Similar content being viewed by others
Keywords
- Portal Hypertension
- Sustained Virological Response
- Noninvasive Method
- Transient Elastography
- Surveillance Endoscopy
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
Introduction
In the previous Baveno consensus workshop, it was underlined that in any patient with compensated chronic liver disease, the identification of cirrhosis is crucial, since it marks the beginning of an increased risk of complications and death. On diagnosis, endoscopic screening of esophageal varices and ultrasound screening of hepatocellular carcinoma should be initiated, and patients should undergo an appropriate surveillance thereafter.
As will be explained in other chapters in this book, the term “cirrhosis” has been recently challenged [1]; it has been suggested that it should be replaced by the term “advanced chronic liver disease” or “compensated advanced chronic liver disease” (cACLD) that better responds to new concepts, among others those related to the difficulty of distinguishing between severe fibrosis and early cirrhosis in patients without previous decompensation of cirrhosis, and the potential reversibility of liver disease due to advances in treatment [2].
Independent of the terminology used, it is undoubtedly important to provide criteria to allow identification of this stage in asymptomatic, compensated patients, who should be referred to centers with expertise in liver diseases for confirmation and appropriate monitoring. In this group of patients, portal hypertension can be present and should be assessed as the next mandatory step due to its prognostic relevance.
Liver biopsy, despite several drawbacks (sampling errors, intra- and interobserver variability), is still considered as the standard reference method for staging fibrosis and diagnosing cirrhosis [3], while the gold standard method to assess portal hypertension is hepatic venous pressure gradient (HVPG) measurement obtained during hepatic vein catheterization [4]; upper gastrointestinal tract endoscopy is the reference method to assess the presence and severity of esophageal and gastric varices.
These invasive methods, however, are not available in all centers, require specific expertise, and hold a high cost and some potential risks. In the last Baveno consensus conference (Baveno V), it was underlined that there was a need to develop noninvasive methods to better select patients who should be referred to endoscopy [5, 6]. This implies that noninvasive tests should be able to identify or rule out (a) cACLD, (b) clinically significant portal hypertension, and (c) varices (or at least varices requiring treatment).
During the last years several noninvasive methods (Fig. 4.1), and in particular liver stiffness measurement (LSM) by transient elastography (TE) and serum biomarkers, emerged as reliable surrogates of fibrosis [7]; in addition, LSM has been evaluated for diagnosing portal hypertension [4] and has been shown to hold prognostic significance for hard clinical endpoints such as clinical decompensation.
Diagnostic methods currently used (or proposed) in patients with compensated liver disease. Liver stiffness by TE is a well-validated method that has changed clinical practice by allowing an early identification of patients in a pre-cirrhotic or early cirrhosis stage who are now grouped under the term “cACLD” that were previously often not detected due to the absence of other specific signs. These patients require further evaluation by invasive and noninvasive techniques to rule out or identify portal hypertension and varices
In this changing scenario, our panel is aimed at better understanding the opinion of the experts in the field of portal hypertension on the current practice and use of invasive and noninvasive methods in the following aspects:
-
(a)
When screening of varices should be initiated or, in other words, how and when ACLD/cirrhosis is diagnosed
-
(b)
Screening of varices
-
(c)
Surveillance of varices
A questionnaire was sent to all Baveno experts faculty (n = 52). The questionnaire obtained 48 answers (92 %) and was completely filled in by 47 respondents. The main results are presented in the following paragraphs.
Diagnosis of Compensated Advanced Chronic Liver Disease (cACLD)
Question 1
Which parameters do you use to classify a patient as having compensated advanced chronic liver disease that requires initiation of HCC surveillance and evaluation of CSPH and varices?
Respondents were allowed to choose more than one answer to summarize all the diagnostic methods they use. Eighty-three percent of experts use liver biopsy to diagnose cACLD; in addition, 81.3 % answered that they use the finding of varices on endoscopy, suggesting that, according to what is recommended by previous Baveno statements, once a clinical diagnosis/suspicion of cirrhosis was made, endoscopy was performed, and the observation of signs of portal hypertension was considered sufficient as a confirmatory sign.
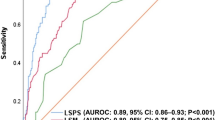
Among noninvasive parameters, LSM by TE is the most widely accepted (83.3 % of experts), indicating that this is currently the most commonly used technique to rule out cACLD and to identify it even when no other sign is present. Cutoffs used varied among respondents (≥13.6 kPa, 60.4 % of respondents; ≥10 kPa, 16.6 %; other cutoffs, 6.3 %).
Imaging techniques (ultrasound, CT scan, or MRI) are the next most trusted; with these methods, respondents look for signs of definite cirrhosis (nodular liver surface, 75.9 % of respondents) and signs of portal hypertension (portosystemic collaterals, a pathognomonic sign of portal hypertension, 58.3 % of answers; splenomegaly, a sensitive but not a specific sign of cirrhosis and portal hypertension, 29.2 % of answers). Forty-eight percent of experts indicated an HVPG > 5 mmHg as confirmatory of cACLD.
Only 4 experts (8.3 %) considered liver stiffness by ARFI (with a cutoff of ≥1.75 m/s) as a diagnostic parameter, and no other newer elastographic techniques were specifically discussed. Laboratory tests and their combination with other techniques obtained only a minority of answers (platelet count <150,000 mm3, 18.7 %; platelet count <150,000 mm3 + splenomegaly ≥13 cm, 37.5 %; platelet count/spleen diameter >909, 2 %; FibroTest ≥0.60, 4.1 %; FibroTest ≥0.75, 6.25 %).
The respondents had the possibility to insert comments to this question. Among them, some suggested that a stepwise approach is preferable; this approach would be based on identification of cACLD by LSM or multiple noninvasive tests as a first step, followed by invasive reference standard methods to be used in case of discordance of noninvasive methods, preferably in referral hospitals.
Screening of Varices
Question 2
Do you perform screening endoscopy in patients with cACLD at the time of diagnosis to detect the presence of gastroesophageal varices?
Question 3
Do you use noninvasive methods to restrict the performance of endoscopy to the patients at higher risk of having varices?
Question 4
If you do, which method do you use?
There is a clear consensus regarding the first two points: 95.8 % of the respondents confirmed that they use screening endoscopy once cirrhosis is diagnosed (Q2); according to the previous Baveno statements, this was done without any selection of higher-risk patients based on noninvasive methods (89.1 %) (Q3). Few respondents (10 %) use noninvasive methods to stratify patients before endoscopy; several methods were pointed out, but most answers indicated LSM by TE and ultrasound signs of portal hypertension.
Surveillance of Varices
According to the Baveno V consensus conference, patients should undergo surveillance by endoscopy to detect formation of varices when they were not present on first endoscopy and to detect growth of varices in patients with small varices at diagnosis. The intervals varied from 1–2 year to 2–3 years according to the first endoscopy findings and to the presence of clinical decompensation [8]. However, data suggest that the risk of developing varices is decreased in patients with alcoholic cirrhosis who stop drinking, in those with HBV-related cirrhosis who achieve a sustained suppression of HBV-DNA, and in those with HCV-related cirrhosis achieving a sustained virological response (SVR).
Question 5
After performing screening endoscopy do you use any invasive or noninvasive method to follow up the portal hypertensive status in your patients?
Question 6
If you answered YES to the previous question, which method and what interval (e.g., once a year, every 6 months, only when clinical changes appear) do you use to follow up portal hypertension in your patients?
Question 7
Do you always use the intervals for surveillance endoscopy suggested at the last Baveno consensus conference independent of any clinical/laboratory/imaging data?
Question 8
If you answered NO to the previous question, which are the data you consider to reduce the interval for surveillance endoscopy? Please tick all that apply.
Question 9
Similarly which are the data you consider to increase the interval for surveillance endoscopy? Tick all that apply.
74.4 % of the responders reported that they are following the last Baveno consensus conference recommendations regarding surveillance endoscopy (Q7). However, about a half of respondents (53.2 %) also use noninvasive methods and/or HVPG during the follow-up to periodically reevaluate the portal hypertensive status of their patients; more than one answer was allowed. LSM by TE is the most frequently used method (60 %) [4, 9], followed by the HVPG measurement (44 %) and by the follow-up of ultrasound signs of portal hypertension/check of patency of the portal venous system (44 %). As for the frequency of controls, we received 15 answers; there was a large variability in methods and intervals used; most indicated US every 6 months (in the context of HCC screening) and LSM at an interval of 12 months.
The 12 experts (25 % of the respondents) who do not always follow Baveno recommendations mostly consider ongoing alcohol intake (66.6 %), lack of SVR in case of HCV cirrhosis (50 %), and appearance/worsening of ultrasound signs of portal hypertension (50 %) to reduce the interval of surveillance endoscopy. Conversely, most of these experts consider a longer interval for surveillance in case of alcohol abstinence (60 %) and achievement of SVR for HCV cirrhosis (50 %).
Endoscopic Surveillance Interval in Specific Conditions
Question 10 and 11
What interval for surveillance endoscopy do you use for a patient with compensated alcoholic cirrhosis and ongoing drinking with no varices (Q10)/small varices (Q11) at screening endoscopy?
Question 12 and 13
What interval for surveillance endoscopy do you use for a patient with compensated HBV-related cirrhosis with no varices (Q12)/small varices (Q13) at screening endoscopy who has not achieved HBV-DNA suppression under antiviral treatment?
Question 14 and 15
What interval for surveillance endoscopy do you use for a patient with compensated HBV-related cirrhosis with no varices (Q14)/ small varices (Q15) at screening endoscopy who has achieved HBV-DNA suppression under antiviral treatment?
Question 16 and 17
What interval for surveillance endoscopy do you use for a patient with compensated HCV-related cirrhosis with no varices (Q16)/small varices (Q17) at screening endoscopy who has no t achieved SVR under antiviral treatment?
Question 18 and 19
What interval for surveillance endoscopy do you use for a patient with compensated HCV-related cirrhosis with no varices (Q18)/small varices (Q19) at screening endoscopy who has achieved SVR under antiviral treatment?
Correction of the underlying etiologic factor has been shown to favorably impact the natural history of cirrhosis. The questions posed to the audience were aimed at assessing whether published data on this topic changed the clinical practice of experts in the field regarding the intervals of surveillance endoscopy. The results are summarized in Table 4.1.
As shown, accordingly to the data of our survey, there is consensus among expert regarding the use of a shorter interval for surveillance endoscopies in patients with ongoing liver injury due to the persistence of the etiologic factor. Namely, respondents stated that they repeat endoscopy at 2-year intervals when no varices were seen on index endoscopy and at 1-year intervals when small varices were present at index endoscopy in patients who have ongoing drinking (alcoholic cirrhosis) or did not achieve HBV-DNA suppression (HBV-related cirrhosis) or did not achieve SVR (HCV-related cirrhosis). In patients in whom the causal factor was removed or under control, there is no clear consensus on the interval to be used, but the overall experts’ opinion is that the upper limit of the recommended interval can be used (3-year intervals when no varices were seen on index endoscopy and at 2-year intervals when small varices were present at index endoscopy). Comments underlined that cofactors (e.g., obesity) should be always taken into consideration when assessing whether liver injury has been removed or not.
Conclusions
The results of this survey suggest that the experts agree on the use of noninvasive methods and in particular LSM to rule out/identify patients with cACLD (compensated patients with severe fibrosis/pre-cirrhosis or early cirrhosis). This is relevant, since patients without cACLD according to the most sensitive noninvasive method so far (LSM) do not require further work-up for CSPH and varices, while those who belong to this stage of CLD require further evaluation (preferably in referral centers which have invasive methods available). The questionnaire confirmed that screening endoscopy and surveillance are used by the experts according to the recommendations stated in previous Baveno workshops. However, the results also indicated that the persistence or removal of the causal agent which led to cirrhosis seems to guide the choice of using the shortest or the longest interval among those recommended for surveillance endoscopies.
Finally, the answers and comments of the experts indicated that fields for future research regard: (a) the use of LSM and other noninvasive methods in the follow-up to tailor the interval of surveillance endoscopy or even to stop it in case of long-term stability after removal of the causal agent of CLD and (b) the relevance of cofactors of liver disease in the natural history of gastroesophageal varices, in particular in patients in whom the main cause of cirrhosis has been removed (e.g., SVR in HCV).
References
Rosselli M, Macnaughtan J, Jalan R, Pinzani M (2013) Beyond scoring : a modern interpretation of disease progression in chronic liver disease. Gut 62:1234–1241
Hytiroglou P, Snover DC, Alves V, Balabaud C, Bhathal PS, Bioulac-Sage P et al (2012) Beyond “cirrhosis” a proposal from the International Liver Pathology Study Group. Am J Clin Pathol 137:5–9
Castera L, Pinzani M (2010) Biopsy and non-invasive methods for the diagnosis of liver fibrosis: does it take two to tango? Gut 59:861–866
Berzigotti A, Seijo S, Reverter E, Bosch J (2013) Assessing portal hypertension in liver diseases. Expert Rev Gastroenterol Hepatol 7:141–155
de Franchis R (2005) Evolving consensus in portal hypertension. Report of the Baveno IV consensus workshop on methodology of diagnosis and therapy in portal hypertension. J Hepatol 43:167–176
de Franchis R (2010) Revising consensus in portal hypertension: report of the Baveno V consensus workshop on methodology of diagnosis and therapy in portal hypertension. J Hepatol 53:762–768
EASL (2014) Clinical practice guidelines: management of hepatitis C virus infection. J Hepatol 60:392–420
de Franchis R (2000) Updating consensus in portal hypertension: report of the Baveno III Consensus Workshop on definitions, methodology and therapeutic strategies in portal hypertension. J Hepatol 33:846–852
Castera L, Pinzani M, Bosch J (2012) Non invasive evaluation of portal hypertension using transient elastography. J Hepatol 56:696–703
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2016 Springer International Publishing Switzerland
About this paper
Cite this paper
Procopet, B., Berzigotti, A. (2016). Results of the Questionnaire. In: de Franchis, R. (eds) Portal Hypertension VI. Springer, Cham. https://doi.org/10.1007/978-3-319-23018-4_4
Download citation
DOI: https://doi.org/10.1007/978-3-319-23018-4_4
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-23017-7
Online ISBN: 978-3-319-23018-4
eBook Packages: MedicineMedicine (R0)