Abstract
Competing risks hamper the occurrence of other clinically relevant events or modify the probability that they occur, therefore altering the correspondence between rate and risk for the outcome of interest. When the correspondence between rate and risk is lost, the Kaplan-Meier estimator provides systematically upward biased estimates of the risk. In this situation, the appropriate analysis is the cumulative incidence function (CIF). The use of competing risks analysis allows to build multistate models to describe the course of diseases. Appropriate multistate models in cirrhosis may allow to assess the risk of major events such as hepatocellular carcinoma, decompensation, refractory ascites, liver-related mortality, and other clinically relevant outcomes. A five-state model has been proposed to fit the clinical course of cirrhosis including two states in compensated and three states in decompensated cirrhosis.
Prognosis research by competing risks analysis may allow to identify prognostic indicators and to develop prognostic models. Specific statistical tools have been developed to assess discrimination and calibration of prognostic models developed by competing risks analysis, in validation studies.
Competing risks situations are frequent in the clinical course of cirrhosis and competing risks analysis should be appropriately used to achieve unbiased estimates.
Access provided by Autonomous University of Puebla. Download conference paper PDF
Similar content being viewed by others
Keywords
- Competing Risk Analysis
- Cumulative Incidence Function (CIF)
- Competing Risks Situation
- Decompensated Cirrhosis
- Five-state Model
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
Introduction
Growing evidence has emerged in the last years suggesting that the clinical course of cirrhosis may be described by a multistate model. This evidence has been developed on the long-lasting knowledge that compensated cirrhosis has a much longer survival than decompensated cirrhosis [1]. Moreover, patients with compensated cirrhosis have an acceptable or at all good quality of life, do not usually experience symptoms, and may remain in this disease state for many years, if not indefinitely. By contrast, patients with decompensated cirrhosis not only have a significantly shorter survival but also a worse quality of life: they present clear evidence of clinically advanced disease, with bleeding and/or ascites, encephalopathy, or jaundice. These marked clinical differences have recently brought about the concept that compensated and decompensated cirrhosis are two different clinical disease states [2, 3].
Therefore, the basic model for cirrhosis is a three-state model: compensated disease, decompensated disease, and death. On this basis a more complex model has been proposed by introducing two disease states in compensated cirrhosis, defined by the presence or absence of esophageal varices, and three states in decompensated cirrhosis defined by variceal bleeding alone, first non-bleeding decompensation, and any second decompensation [4]. Sepsis and renal failure are events characteristic of the more advanced disease states and both are associated with a significant increase of death risk [5, 6]. Hepatocellular carcinoma may arise in any disease state and, whenever it develops, significantly worsens outcome.
To build up a multistate disease model, the risks of transition across the disease states have to be assessed. Since the transition toward a different state will compete with the transition toward another state, a competing risks analysis has to be used to properly set the multistate model.
The concept of competing risks will be illustrated in this chapter by examples from liver cirrhosis.
Definition of Competing Risks
A competing risk is the risk of an event whose occurrence hampers the occurrence of another event and hence modifies the probability that it occurs.
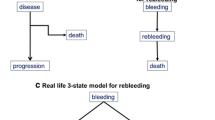
To illustrate this definition, suppose that a group of patients with compensated cirrhosis is followed to observe the occurrence of decompensation. If, by the end of the observation period, each patient was decompensated or still alive and compensated, then one could conclude that all compensated patients will develop, earlier or later, decompensation without any competing event. This would imply that death would only occur after decompensation. However, this does not occur in real life because several patients do die before decompensation. Therefore, in some patients death precludes the occurrence of decompensation and hence modifies the risk of decompensation of the whole group (Fig. 3.1).
Recognizing competing risks is important because when assessing the risk of the event of interest in the presence of competing risks, specific analysis models are required. This is essentially because the survival analysis by the Kaplan-Meier model [7], usually extended to the analysis of the incidence of specific events, is only suitable for a two-state model, typically alive → dead. In the presence of competing events, the competing risks analysis should be used instead. This analysis is based on the cumulative incidence function (CIF) [8], which partitions the probability of any event in the probabilities of each event, in such a way that the probability of any event (or the sum of the probabilities of each event) ranges from 0 to 1.
Rate and Risk
The reason why the Kaplan-Meier model may not be used in the presence of competing risks lies in the relationship between rate and risk, which in different ways measure the occurrence of the same event.
In epidemiology, risk is typically defined as the ratio D/N, where D is the number of subjects who develop the disease over a given time and N is the number of subjects disease-free at the beginning of that time [9]. The rate (or incidence rate) is the ratio D/Y where D is the number of subjects developing the disease and Y is the total amount of person-time at risk: it is essentially a measure of the speed of the occurrence of the event of interest. It is to note that while the risk necessarily increases along time (because D increases with time), the incidence may increase, remain unchanged, or decrease, according to the length of the follow-up.
Hazard (h) is the instantaneous risk of failing at each observation time t and is expressed by the ratio of the number of events to the number of patients exposed to the risk during the instantaneous time t. The hazard function, therefore, provides a dynamic description of how the instantaneous risk of failing varies along time; when the instantaneous risk is roughly constant, the cumulative hazard, ĥ, is equal to the rate, D/Y, and also estimates the instantaneous risk h. There is therefore a unique “one-to-one” correspondence between risk and rate. As a consequence of this correspondence, for a given hazard function, it is possible to compute the cumulative incidence function. The correspondence between the hazard function and the incidence rate allows to calculate the survival function in survival analysis models, like the Kaplan-Meier [8] and the Cox [10] models.
However, in the presence of competing risks, the correspondence between risk and rate is lost because the subjects experiencing the competing event are not any more at risk for the event of interest; therefore, the hazard function varies in a different way than the incidence rate function. The consequence of this lost relationship is that the Kaplan-Meier risk estimates are systematically upward biased in the presence of competing risks.
Censoring
Typically, in survival analysis or in analysis of time to an event of interest, the exact time to the event is known only for a part of the included subjects. For all the others, it is only known that at the time the analysis is performed, the event of interest had not yet occurred: the observation of these subjects has been truncated before the occurrence of the event. This condition is known as censoring (in this case, right censoring). Although the time to the event is not known for these patients, they provide an important information about the probability of remaining free of the event for at least the time period they were observed.
Clearly, if censoring is caused by some event related to the outcome of interest, as, for example, significant clinical deterioration or improvement, the analysis will be biased toward falsely pessimistic or optimistic conclusions. Therefore, censoring must be independent of the outcome of interest. A second important requirement for censoring is that it must be uninformative, meaning that the events causing censoring must be unimportant for the clinical course of the disease [11, 12]. The end of the study period is typically such an event because the truncation of the observation is not at all informative with regard to the disease course.
In a competing risks situation, the competing event, which usually causes censoring in the Kaplan-Meier model, hardly fulfills the censoring requirements of being independent and uninformative. In fact, the competing event is frequently death, which in no way may be uninformative. Yet, when the competing event is not death, it is usually another clinically relevant event, likely informative for the course of the disease.
Because of these characteristics of censoring, in the presence of competing risks, the Kaplan-Meier model may not be used to assess the cumulative incidence. In this situation also the Cox proportional hazards model may lead to misleading results when the correspondence between rate and risk is lost. Both the Kaplan-Meier and the Cox models may however be used when the interest lies in the cumulative incidence of an outcome of interest or on the pure association between a covariate and the outcome, ignoring the competing risks. This is usually the case when looking for causal factors potentially involved in biological mechanisms of the outcome. In this situation the interest of the analysis lies in the incidence rate, i.e., in the total number of patients who will develop the outcome according to some given characteristics and not the in the real risk of the outcome occurrence observed in clinical practice, which may be affected by some competing risk.
Competing Risks Analysis
The Kaplan-Meier model computes the risk of only one event, the event of interest, and does not account for competing events, which are instead considered as censoring events. Since censored patients are treated as if they could experience the event of interest in the future [11], the Kaplan-Meier model, systematically overestimates the absolute risk. As a consequence of this overestimate, the sum of the probabilities of two competing events, each computed by the Kaplan-Meier model, may reach values greater than 1 [11], while by definition it should span between 0 and 1. The appropriate analysis for competing risks is based on the cumulative incidence function (CIF) [8] and the Nelson-Aalen estimator [12]. In this analysis the competing events are not censored but correctly counted as occurred events. Moreover, calculation of the risk is based on an additive approach, as compared to the multiplicative approach of the Kaplan-Meier model; this results in an overall probability of events ranging from 0 to 1, as expected. Details of the differences in calculation of risks between the Kaplan-Meier and the Nelson-Aalen estimators have been illustrated elsewhere [12, 13-14]. A visual example of how the Kaplan-Meier model overestimates the cumulative risks compared to the Nelson-Aalen estimator is provided in Fig. 3.2. The cumulative risk of death and of bleeding is computed by the two methods in a series of 402 patients with cirrhosis and newly diagnosed varices. Death is, of course, a competing risk for bleeding. By the Kaplan-Meier method (Fig. 3.2, left panel), the 10-year risk of bleeding is 0.54. The corresponding figure by the Nelson-Aalen estimator is 0.30 (Fig. 3.2, right panel). The explanation for the difference is that there is a 0.25 probability of death before bleeding which is not accounted for by Kaplan-Meier model, in which death before bleeding is a censoring event. On the other hand, the survival curve plotted by the Kaplan-Meier model does not account for bleeding and informs on overall survival.
Multistate Models and Competing Risks in Cirrhosis
There are no pre-definite multistate models in cirrhosis. Competing risk analysis should be applied whenever a competing risk may hamper correct risk assessment for an outcome of interest and the relevant multistate model should be appropriately built. Examples of this kind of situations are reported in Fig. 3.3. A typical situation where a multistate model is required is the assessment of liver specific mortality, where mortality for other causes is a competing outcome. Several applications of this kind of analysis may be appropriate when defining risks along the course of the disease. As outlined above, the assessment of the risk of decompensation for patients with compensated cirrhosis should account for the competing risk of death before decompensation. Likewise, a multistate model should be set to assess the risk of developing hepatocellular carcinoma, or the risk of resistant ascites or other major clinical events, to account for death before the event of interest as a competing outcome. The example of rebleeding after a first episode of variceal bleeding is reported in Fig. 3.2. Many other similar situations may be recognized, along the clinical course of cirrhosis.
More complex multistate models may be built to fit the clinical course of cirrhosis [2]. For alcoholic cirrhosis a model has been proposed including compensated cirrhosis, variceal bleeding, ascites, ascites plus bleeding, and encephalopathy as disease states [15]. More recently a five-state model has been set for mostly viral and alcoholic cirrhosis [4]: compensated cirrhosis without esophageal varices, compensated cirrhosis with esophageal varices, variceal bleeding without other decompensating events, first non-bleeding decompensating event, and any second decompensating event. In this model, the probability of 5-year mortality increased from 0.015 in state 1 to 0.88 in state 5 (Fig. 3.4).
Schematic representation of a five-state disease model for cirrhosis. Five-year transitioning rate across disease states and to death is shown for a series of 494 patients. Arrows represent transitions and the numbers close to each arrow are the relevant transition rates. A fairly steady increase in death rate was found across stages
Competing risks analysis may be used also when assessing which is the next relevant clinical event to occur in a definite clinical condition. In this situation, several events may compete with each other to occur first. This kind of information is usually clinically relevant to plan the appropriate follow-up schedules and preventive interventions when available. As an example a competing risks analysis to assess the probability of the next clinical event in patients with compensated cirrhosis and without varices is reported in Fig. 3.5. The analysis shows that in this series of 202 consecutive patients, the probability of occurring as the first new event was 0.07 for death, 0.43 varices, 0.20 ascites, and 0.07 jaundice or encephalopathy, while the probability of remaining free of any event in the observation period was 0.23.
Competing Risks and Prognosis Research
Prognosis research is aimed at assessing outcome probability and relevant predictors of outcome in a given time. By combining several predictors, prognosis research may also result in clinical prediction rules which, if appropriately validated, may assist physicians in clinical decision-making and in providing correct information to the patient [16, 17].
Predictors may be patient characteristics or biological or physiological disease characteristics and may be associated to the outcome either through a causal mechanism (causal factors) or without any causal relationship, simply as indicators of the risk (predictive factors). When the interest is on causal factors, the analysis should identify any significant association between the candidate factors and the event rate or incidence and not the risk. In this case, it is important to assess whether the outcome of interest did occur more frequently in patients presenting the candidate causal factor compared to those without. Here, the measure of the risk observed in real practice or whether some competing event may alter the probability of occurrence of the event of interest is not important. In this situation a competing risks analysis is not required, and hence the Kaplan-Meier model may be used for incidence analysis and the proportional hazards Cox model may be safely used. By contrast, when the interest is in predictive factors, the analysis is centered on the cause specific risk: here, it is therefore essential to account also for competing risks, which may modify the risk of the event of interest. In this condition, neither the Kaplan-Meier nor the Cox models are appropriate, and an analysis properly accounting for competing risks should be used instead.
Prognostic Models with Competing Risks
Subgroup competing risks analysis of cumulative incidence allows to assess the association of candidate predictors with the outcome of interest in the presence of competing risks [18]. A multiple regression model has also been developed for competing risks [19]. The model allows to compute sub-hazard functions for prognostic indicators and provides regression coefficients which allow to calculate a prognostic score for individual patients to predict the probability of the outcome of interest at a given time. Comparing the predicted risk with the observed risk may inform on the calibration of the prediction. The standard Cox model, as expected, usually overestimates the risk, while the Fine and Gray model provides reliable predictions [19].
Validity of prognostic models with competing risks may be assessed in groups of patients independent from the derivation sample, and statistical tools to assess discrimination, reclassification index, and calibration of the model are available [20].
Conclusions
Competing risks modify the probability that an event of interest occurs. In this situation the correspondence between risk and rate is lost, and the Kaplan-Meier model systematically results in upward biased risk estimation. Therefore, in the presence of competing risks, the specifically developed Nelson-Aalen estimator should be used to compute the cumulative incidence function (CIF) .
Competing risks analysis allows to build multistate models, which may satisfactorily represent typical clinical conditions in which it may be important to investigate the risk of a specific event. In cirrhosis, such models may provide reliable information on the probability of occurrence of major clinical events like hepatocellular carcinoma, bleeding, ascites, refractory ascites, and any other event of interest in the presence of competing risks.
Multistate models for cirrhosis have been proposed to fit the clinical course of the disease. These models are essentially based on compensated and decompensated disease and on the presence of esophageal varices and decompensating events.
Multiple regression analysis with competing risks may also be performed and allows to compute prognostic scores which may be validated by assessing discrimination, reclassification, and calibration by specific statistical approaches. A competing risk approach to prognostic research in cirrhosis may help to improve the performance of prediction rules.
References
Saunders JB, Walters JRF, Davies P, Paton A (1981) A 20-year prospective study of cirrhosis. Brit Med J (Clin Res Ed) 282:263–266
D’Amico G, Garcia-Tsao G, Pagliaro L (2006) Natural history and prognostic indicators of survival in cirrhosis: a systematic review of 118 studies. J Hepatol 44:217–231
Garcia-Tsao G, Friedman S, Iredale J et al (2010) Now there are many stages where before there was one: in search of pathophysiological classification of cirrhosis. Hepatology 51:1445–1449
D’Amico G, Pasta L, Morabito A, D’Amico M, Caltagirone M, Malizia G et al (2014) Competing risks and prognostic stages in cirrhosis: a 25-year inception cohort study of 494 patients. Aliment Pharmacol Ther 39:1180–1193
Arvaniti V, D’Amico G, Fede G, Manousou P, Tsochatzis E, Pleguezuelo M, Burroughs AK (2010) Infections in cirrhosis increase mortality four-fold and should be used in determining prognosis. Gastroenterology 139:1246–1256
Fede G, D’Amico G, Arvaniti V, Tsochatzis E, Germani G, Georgiadis D et al (2012) Renal failure and cirrhosis. A systematic review of mortality and prognosis. J Hepatol 56:810–818
Kaplan EL, Meier P (1958) Nonparametric estimation from incomplete observations. J Am Stat Assoc 53:457–481
Kalbfleisch JD, Prentice RL (1980) The statistical analysis of failure time data. Wiley, New York
Rothman KJ (2002) Epidemiology: an introduction. Oxford University Press, New York
Cox DR (1972) Regression models and life-tables (with discussion). JR Stat Soc B 34:187–220
Grunkemeier GL, Jin R, Eijkemans MJC, Takkember JJM (2007) Actual and actuarial probabilities of competing risks: apples and lemons. Ann Thorac Surg 83:1586–1592
Andersen PK, Geskus RB, de Witte T, Putter H (2012) Competing risks in epidemiology: possibilities and pitfalls. Int J Epidemiol 41:861–870
Andersen PK, Keiding N (2012) Interpretability and importance of functionals in competing risks and multistate models. Stat Med 31:1074–1088
Jepsen P, Wilstrup H, Andersen PK (2014) The clinical course of cirrhosis. The importance of multistate models and competing risks analysis. Hepatology. doi:10.1002/hep.27598
Jepsen P, Ott P, Andersen PK et al (2010) The clinical course of alcoholic liver cirrhosis: a Danish population-based cohort study. Hepatology 51:1675–1682
Moons KGM, Kengne AP, Woodward M, Royston P, Vergouwe Y, Altman GD, Grobbee DE (2012) Risk prediction models: I. Development, internal validation, and assessing the incremental value of a new (bio)marker. Heart 98:683–690
Moons KGM, Kengne AP, Grobbee DE, Royston P, Vergouwe Y, Altman GD, Woodward M (2012) Risk prediction models: II. Esternal validation, model updating and impact assessment. Heart 98:691–698
Pintile M (2006) Competing risks: a practical perspective. Wiley, Chichester
Fine JP, Gray RJ (1999) A proportional hazards model for the subdistribution of competing risk. J Am Stat Assoc 94:496–509
Wolbers M, Koller MT, Wittenam JCM, Steyerberg EW (2009) Prognostic models with competing risks. Methods and application to coronary risk prediction. Epidemiology 20:555–561
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2016 Springer International Publishing Switzerland
About this paper
Cite this paper
D’Amico, G. (2016). Competing Risks and Prognostic Stages in Cirrhosis. In: de Franchis, R. (eds) Portal Hypertension VI. Springer, Cham. https://doi.org/10.1007/978-3-319-23018-4_3
Download citation
DOI: https://doi.org/10.1007/978-3-319-23018-4_3
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-23017-7
Online ISBN: 978-3-319-23018-4
eBook Packages: MedicineMedicine (R0)