Abstract
This chapter aims to discuss the evidence available to date from various laboratory and clinical studies about the use of glass-ionomer cements (GICs) in the management of deep caries. The contemporary minimally invasive approach to the operative management of cavitated deep lesions approaching the pulp relies on the selective removal of infected and/or affected dentine close to the pulp, followed by the use of a suitable adhesive restorative material to seal and bond to the underlying peripheral cavity margins/walls. In order to optimize the clinical outcome, an appreciation is required as to how this physico-chemical interaction occurs between GIC and sound as well as caries-affected substrates. The ionic transfer between GIC and tooth structure is described and discussed, with a particular emphasis on its anti-caries and remineralizing potential and also any effects, deleterious or otherwise, on the dental pulp when placed in close proximity to it. The clinical techniques available to restore teeth using high-viscosity GICs are outlined, including Atraumatic Restorative Treatment (ART) and the layered/laminate/sandwich restoration with resin composite. The findings of studies assessing the clinical longevity of such restorations in comparison to other direct plastic restorative materials are analyzed, both in the primary and secondary dentition. From the evidence presented, it is clear that GIC and its derivatives, whilst not perfect, have a major role to play in the minimally invasive restorative management of deep caries lesions.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Minimum Intervention care
- Minimally invasive dentistry
- Caries
- Enamel
- Dentine
- Pulp
- Adhesion
- Glass-ionomer cement
- Resin-modified glass-ionomer
- ART
4.1 Introduction: What is Minimum Intervention Dentistry?
Minimum intervention dentistry (MID) is a term that is used in contemporary dental practice. Its meaning, however, is often misconstrued and misunderstood, therefore misused and often maligned.
‘MID’ has two distinct but inter-related definitions:
4.1.1 Minimum Intervention Care (Fig. 4.1)
This describes the overall patient-centred oral and dental healthcare delivery framework where the oral healthcare team (dentist, dental therapist, hygienist, oral health educator, nurse, practice manager and technician) collectively advises patients and offers treatment that promotes health and prevents disease. Included in this framework are disease detection and diagnosis, its non-operative (non-invasive) prevention and control, operative management where necessary, all packaged together with suitably frequent recall consultations. At these vital recall appointments (often undersold to patients by the profession using the valueless term ‘check-ups’), the patient’s motivation, behaviour and adherence to the preventive lifestyle change advised are reassessed and reinforced. This is within the context of an outcome review of the treatment provided in the previous care episode(s) (restoration status, evidence of disease progression, changes in bacterial/ionic oral balance, etc). The periodicity of these consultations must be tailored to the individual patient’s need and ongoing disease risk/susceptibility assessment.
4.1.2 Minimally Invasive (MI) Dentistry
Traditional operative caries management relies upon complete excavation of carious tissue followed by modification of the resulting cavity in terms of its surface finish and overall internal shape to aid restoration retention. The extent of this modification is dictated by the nature of the direct, plastic restorative material used to fill the cavity. Classically, for many years, non-adhesive dental amalgam has been the material of choice for most clinicians, so much so that predetermined cavity shapes are associated erroneously with GV Black’s caries classification, classes 1–5, by many dental professionals.
With improved knowledge and understanding of the patho-physiology of the caries process and the subsequent defence reactions of the dentine-pulp complex, scientific and clinical evidence now shows clearly that not all carious tissue requires excavation during interventive operative surgical procedures (Banerjee and Watson 2015). The peripheral seal of restorations with healthy enamel and dentine close to the enamel-dentine junction (EDJ) is of paramount importance to ensure the tooth-restoration complex survives (Banerjee and Watson 2015). This is in conjunction with primary preventive methods instigated by the patient to disturb and/or remove the plaque biofilm, so preventing it from stagnating and becoming cariogenic in nature. Maximum tissue preservation along with maintaining pulp vitality (sensibility) is the tenet of minimally invasive dentistry, and the development of adhesive dentistry has promoted the MI philosophy into mainstream operative care.
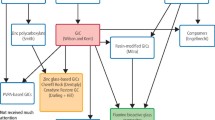
Glass-ionomer cements (GICs) are an important part of the contemporary restorative armamentarium for MI operative dentistry. Since their introduction by Wilson and Kent (1971), glass-ionomer cements have had a wide range of clinical uses due to their naturally adhesive, tooth-coloured nature and fluoride-leaching properties, their low coefficient of thermal expansion and ultimate biocompatibility with mineralised tissues (Burke and Lynch 1994; Olivia et al. 2000). Clinically, they have proven to be useful in restorative, lining, luting and sealing applications.
4.2 GIC Adhesion to Tooth Structure
4.2.1 Enamel
Glass-ionomer cements (GICs) bind chemically to calcium ions in hydroxyapatite (HAP), the main constituent of dental enamel. Enamel surface pre-treatment with conditioners including polyacrylic acid (PAA) improves the bond strength between enamel and GIC, based on the exchange of calcium and phosphate ions versus carboxyl ions at the enamel surface. Es-Souni et al. (1999) indicated that the improved adhesion of the GIC on polished and conditioned surfaces resulted from the combined beneficial effects of superficial surface cleanliness, better wettability and surface chemistry. They concluded that PAA conditioning of the enamel prior to GIC bonding led to the formation of a fine polymeric film on the surface. This film may act as a primer and be involved directly in the cement-building reactions, so creating a ‘stronger’ interfacial layer on the GIC aspect of the bond.
4.2.2 Fluoride and Mineralized Tissues
Bezerra et al. (2012) examined the levels of the fluoride, calcium and phosphate in the enamel and dentine alongside glass-ionomer-based restorations in vivo over time using a high-viscosity GIC (Fuji IX GP, GC Corp, Tokyo, Japan) and a resin-modified GIC (Vitremer, 3M ESPE, St Paul, MN, USA). They described a substantial increase in the fluoride ion concentration adjacent to the glass-ionomer-based restorations attributable to large differences in the ionic gradients and subsequent diffusion patterns (Ngo et al. 2006). This finding raises an important question: does the increase in fluoride ion concentrations in enamel and dentine contribute substantially to an increase in the acid resistance of these two substrates? In-vitro studies had shown an increase in the resistance to demineralization of enamel (Hatibovic-Kofman et al. 1997; Attar and Önen 2002) and dentine (Jang et al. 2001), which was attributed to the release of fluoride ions from the GIC-based restorations. Qvist et al. (2004) reported a reduction in carious lesion progression in enamel surfaces adjacent to glass-ionomer restorations in primary teeth as compared with amalgam restorations over a period of 8 years. This evidence certainly seems to indicate that the fluoride release from glass-ionomer-based materials can play a role in disease arrest in enamel and dentine that is in contact with these materials. When high-viscosity and resin-modified glass-ionomer restorations are used to restore carious lesions in primary molars using the atraumatic restorative treatment (ART) technique, fluoride ions are released into the adjacent enamel and, in particular, into the demineralized, caries-affected dentine.
4.2.3 Sound Dentine
The development of a minimally invasive adhesive approach to conservative dentistry has brought many advantages, such as preservation of tooth tissue, reinforcement/infiltration of weakened remaining tooth structure, reduced marginal leakage and the reduced potential for pulp sensitivity and maintenance of pulp vitality. Adhesive restorative materials should have a close affinity mechanically, physically and chemically to tooth tissue in a way that minimizes the risk of further ingress of bacteria and arrests disease activity. They should also have the ability to bond to a variety of overlying protective restorative materials including resin composite, metals and ceramics. One of the most attractive features of GICs is their ability to bond directly to dentine. Polyacrylate ions either react with apatite by displacing calcium and phosphate ions or bond directly to the calcium within the apatite via hydrogen bonds with the collagen and ionic bonds to the apatite within the dentine (Van Noort 2013).
There have been many studies published reporting varying bond strengths between dentine and GIC. Yip et al. (2001) measured the micro-tensile bond strength (μTBS) of three highly viscous glass-ionomer cements to sound coronal dentine; they found bond strengths in the range of 12–15 MPa, with interfacial (adhesive) and mixed modes of failure. However, previous studies (Cattani-Lorente et al. 1993; Burke and Lynch 1994; Berry and Powers 1994) suggested that bond strengths >5 MPa were seldom achieved using tensile or shear tests in vitro, with more cohesive failures occurring within the GIC (Nakajima et al. 1995). It was clear that much of the difference could be explained by variations in the experimental testing technique used, the inconsistencies in sample preparation and the varying specimen sizes as well as their geometry and configuration.
Both scanning and transmission electron microscopy (SEM/TEM) analysis has shown the presence of an intermediate layer between 0.5 and 1.5 mm thick (Ngo et al. 1997a, b; Yip et al. 2001). Depending on the type of GIC, the TEM observations ranged from surface interaction zones consisting of nanometer-sized plate-like structures of calcium and phosphate salt precipitates dispersed among denatured smear layer remnants to plate-like structures being present within the inter-fibrillar spaces of intact, banded collagen fibrils. The inclusion of either smear layer remnants or banded collagen fibrils within the surface intermediate layer may be explained by the aggressiveness of different conditioning protocols used to remove the smear layer and demineralizing the underlying intact dentine. This is associated with the concentration of the polyacrylic acid employed as well as the application time that is recommended by each manufacturer. When the dentine was conditioned with 10 % polyacrylic acid for 10 s (a conventional, clinically recommended protocol), the presence of smear layer remnants within the surface intermediate layer indicated the smear layer was not completely removed. Chemical bonding of polyacrylic acid or polyacrylic acid/maleic acid to the residual hydroxyapatite from the smear layer may result in the retention of these polyelectrolytes on the dentine surface instead of being rinsed off (Yoshida et al. 2000). This could help produce the gel-like, glass-free layer that facilitates subsequent chemical exchange between the leached ions from the setting glass-ionomer matrix and the calcium and phosphate ions from the partially demineralized smear layer. Such a surface intermediate layer that incorporates smear layer remnants was often retained on the dentine surface in specimens that exhibited interfacial or mixed interfacial failures (Yoshida et al. 2000). When a more aggressive conditioning protocol of treating the smear layer-covered dentine with 25 % polyacrylic acid for 25 s was employed, the dentine tubule orifices were rendered patent, and this encouraged the formation of micro-mechanical dentine tubule tags. Moreover, the smear layer was removed completely and the underlying dentine demineralized to a depth of about 0.5 mm.
Hosoya and Garcia-Godoy (1998) reported an absence of cement tags or a hybrid layer when using a highly viscous GIC (Ketac-Molar, 3MESPE, St Paul, MN, USA). Rinsing off the conditioner probably resulted in a collagen-rich zone that contained retained polyelectrolytes. Subsequent ion exchange between the setting GIC and the partially demineralized collagen fibrils could have resulted in the formation of a surface intermediate layer where the inter-fibrillar spaces were not infiltrated completely by the polyelectrolytes. This could have accounted for the lower bond strength observed when 25 % polyacrylic acid was used as the conditioner in that study. It is further speculated that the clinical situation may be worsened when conditioned and rinsed dentine is then desiccated by the operator before the application of the GIC, as collapse of the collagen network during air-drying will further limit polyelectrolyte diffusion (Gwinnett 1994). It could be concluded that complete removal of the smear layer with more aggressive conditioning protocols that effectively ‘etch’ into sound dentine does not enhance the dentine-GIC bond strength. The observation of short cement tags that pulled out of the dentine tubules further suggests that they have a limited micro-mechanical contribution to the ultimate retention of GICs.
4.2.4 Caries-Affected Dentine
The MI operative approach to cavitated carious lesion management aims to minimize the excavation of carious dental tissues and instead encourages their preservation, recovery and repair. Dentine caries results from a bacteriogenic demineralizing acid attack from the cariogenic, stagnating biofilm at the tooth surface followed by further enzymatic destruction of the organic, primarily collagenous, matrix in dentine, if the process is unopposed and uninterrupted for a period of time. This ongoing process causes a histo-pathological wave of tissue destruction, divided descriptively into caries-infected and caries-affected dentine zones based on the bacterial load, extent and reparability of the tissue damage sustained (Banerjee and Watson 2015). In the necrotic, caries-infected dentine zone in the heart of the dentine lesion just subjacent to the enamel-dentine junction (EDJ), the mineral and collagenous organic matrices are irreversibly damaged and the bacterial load high. The deeper caries-affected dentine is hypomineralized but with a partially sound collagenous fibrillar structure, which could be repaired and remineralized by the ongoing reparative biological activity of the dentine-pulp complex. The relatively slow progression of the caries process often allows a reparative biochemical reaction which can help restore the mineralized architecture of this zone, especially after having removed the soft, wet, highly infected layer using a minimally invasive operative approach. The interaction between GIC and wet dentine is in the form of an ion exchange where aluminium, fluoride and calcium/strontium leach out of the cement as the glass is dissolved by the polyacid; at the same time, calcium and phosphate ions also move from the underlying dentine as a result of the initial self-etching effect of the acid-base chemical reaction of the setting cement (Watson 1999; Yiu et al. 2004). The release of fluoride and calcium/strontium ions provides GICs with the potential for remineralization of carious tissues (Ngo et al. 2006), where ion exchange could replenish the demineralized tissues’ lost ions, thus tipping the balance in favour of mineral deposition/precipitation.
There is little evidence published about the immediate bond/sealing effectiveness or the long-term durability of the bonded interfaces produced by GIC to caries-affected dentine (Czarnecka et al. 2007; Alves et al. 2013). It is still unknown if the type of GIC has an effect on its clinical performance, as there is little published evidence to date regarding the bond strength of high-viscosity or resin-modified GICs (RMGICs) to caries-affected dentine. Some studies have evaluated bond strength degradation of GIC when bonded to sound dentine (De Munck et al. 2004; Fagundes et al. 2009). Bissoto Calvo et al. (2014) examined the in vitro bond strength of different GICs (a high-viscosity GIC and RM GICs with and without nano-particle fillers) to sound and caries-affected primary dentine immediately and after 2 years storage in vitro. No statistically significant differences in the immediate bond strength values between the tested materials to either sound or caries-affected dentine were reported. After 2 years, only the RMGIC without nano-particles showed stable bond strength values to both primary sound and caries-affected dentine. Previous to this, Marquezan et al. (2010) reported that a resin-modified GIC showed more resistance to degradation at the bonded interface with caries-affected primary dentine after pH- and load-cycling in vitro, compared to an adhesively bonded resin composite. Conventional GIC adheres primarily chemically to dentine, through the interaction of hydroxyapatite and polycarboxylate functional groups. On the other hand, in RMGICs both chemical and micro-mechanical adhesion are involved, which may contribute to the higher immediate and prolonged bond strength values measured.
Although the presence of nano-particles in the formulation of RMGIC potentially reinforces the material’s strength, the interfacial area between the nano-particles and the organic matrix is hydrolytically unstable and may favour water sorption and degradation over time. Additionally, the chemical bond of the nano-particulate RMGIC to dentine may be weaker than that produced by a conventional GIC or a conventional RMGIC. This may be due to the reduction in the polycarboxylate content, as a result of nano-particle inclusion, reducing the available functional groups to interact with hydroxyapatite.
The type of the substrate (sound or caries-affected dentine) did not appear to affect the bond strength of GICs, regardless of type or storage time in several studies (Way et al. 1996; Czarnecka et al. 2007; Marquezan et al. 2010). This can be attributed to the hydrophilic properties of GICs. Also, the type of adhesion is chemical and not purely micro-mechanical. In contrast, Cehreli et al. (2013) observed differences between the bond strength values of GICs to sound and simulated caries-affected dentine after 18 months; unfortunately, the immediate bond strength was not recorded, making it impossible to conclude if any bond strength degradation had occurred. Moreover, the authors used caries-affected dentine created artificially using acetic acid as a demineralizing solution. This method results in complete demineralization (Marquezan et al. 2009), which is different to that of the pH-cycling process of de-and remineralization employed in many other studies. This final point is a critical one: ‘artificial’ data can be produced when using an artificial substrate, and thus clinical extrapolation of such results must be made with considerable caution. The ideal substrate to use is natural caries-affected tissue which needs to be exposed carefully from naturally carious teeth either in situ or in vitro.
4.3 GIC and Remineralization
Remineralization of demineralized carious dentine using various types of GICs has been demonstrated in several laboratory and clinical studies (Creanor et al. 1998; Ngo 2002a, b). Remineralization can be defined as the deposition of mineral in demineralized defects at a molecular level (Arends and ten Bosch 1986). It has been suggested that the mineral deposited should be apatitic in nature and should not be different from the mineral structure of natural, sound enamel and dentine. Ngo et al. (2006) studied the chemical interaction between a highly viscous GIC and demineralized dentine in vivo to determine the level of ion exchange between them. The material they used was Fuji IX GP (GC Corp, Tokyo, Japan), which includes a strontium-containing glass as opposed to the more conventional calcium-based glass in other GICs. They found that a substantial amount of both strontium and fluoride ions crossed the interface into the partially demineralized caries-affected dentine subjacent to the GIC. As the freshly mixed material is placed against the cavity wall, there is a release of ions from the enamel and dentine also leading to the exchange of ions, termed ‘ion exchange adhesion’. It is suggested that the same ion exchange can occur in the presence of the partially demineralized carious dentine (Ngo et al. 2006). The ions released from both the GIC and the tooth structure will combine to buffer the low initial pH until such time that it rises to a level where ion activity ceases. During this period of activity there will be both fluoride and strontium ions available to promote mineral deposition in areas of demineralized dentine where the calcium ion levels are low, with strontium ions substituting them. It was suggested that this occurs through a diffusion process driven partly by the concentration gradient which exists between the GIC and the dentine with respect to these two elements. As both strontium and fluoride are apatite-forming elements, they react with the demineralized dentine. If the process is controlled purely by diffusion then one would expect to see the level of ionic strontium and fluoride to be highest at the GIC-dentine interface and lowest deeper towards the sound dentine. The above clinical findings support the laboratory evidence that glass-ionomer can contribute directly to the remineralization of carious dentine. However, there are two important requirements for this to happen: firstly, the restoration has to provide a total seal against the external environment, and secondly, there must be intimate contact between the glass-ionomer and the partly demineralized dentine.
4.4 Clinical Studies of GIC Use in the MI Management of Deep Caries
The treatment of deep carious lesions approaching a vital pulp presents a significant challenge to the practitioner. The traditional management of carious lesions dictates the removal of all infected and affected dentine to prevent further caries progress and to provide a sound base of dentine to support the overlying definitive restoration. In order to prevent, or at least minimize, the serious complications of complete excavation of carious dentine close to the pulp (the dreaded pulp exposure), the minimally invasive, tooth-preserving operative ‘stepwise’ excavation approach was developed. This involves initially excavating the more superficial soft, wet, necrotic infected dentine, followed by sealing the lesion with calcium hydroxide and a GIC provisional restoration. Some months later the clinician would revisit the lesion and finally remove all or most of the underlying arrested, dry and often darkly stained dentine. The rationale for this is that by this point, any residual bacteria will not have survived, the residual affected dentine will have remineralized and tertiary, reparative dentine will have been deposited. This will make it easier for the dentist to remove any remaining carious tissue without the risk of exposing the vital pulp (Thompson et al. 2008; Banerjee and Watson 2015). As it became increasingly clear that it is the effective peripheral seal of the restoration that is important in preventing the caries process from continuing within an existing cavitated lesion, a fully minimally invasive or ultraconservative approach was developed; this is also referred to as ‘partial/selective caries removal’. In this method, all of the infected dentine is removed, the peripheral enamel and dentine are prepared to optimize adhesion and the cavity is sealed (with or without indirect pulp protection) with the definitive adhesive restoration. The ‘trade-off’ for avoiding pulp exposure, which more often clinically leads to pulp death (Bjørndal et al. 2010), is retaining a layer of potentially radiolucent, affected dentine beneath the definitive restoration (see Fig. 4.2e). This can be defended by citing the substantial evidence that exists in the literature showing that cariogenic bacteria isolated from their source of nutrition by a restoration of sufficient integrity either die or remain quiescent and thus, given a vital pulp, pose no risk to the health of the dentition (Ricketts et al. 2013). Foley et al. (2004) compared the cariostatic effectiveness of alternative restorative materials in both selective and complete removal of carious tissue. They used a split-mouth design in 44 patients (aged 3.7–9.5 years) who had at least one pair of previously unrestored primary molars that had no pulp involvement. One tooth of each pair underwent complete caries removal, and the other had incomplete, selective caries removal followed by restoration using copper phosphate cement, GIC or a material ‘of the operator”s choice’ (such as amalgam). At 24 months post treatment, teeth that had undergone selective caries removal followed by restoration with copper phosphate cement exhibited greater abscess or sinus formation than did teeth that had undergone other treatments. Teeth treated with GIC alone after selective caries removal exhibited a durability and effectiveness comparable with those placed in teeth that had undergone complete caries removal.
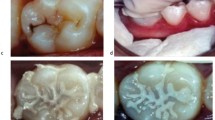
(a) Pre-operative periapical radiograph of LL6 and LL7 showing early caries on the distal aspect of LL6 and a large mesio-occlusal carious radiolucency associated with LL7. The LL7 exhibited symptoms of acute pulpitis and positive pulp sensibility tests and showed no loss of lamina dura/widening of the periodontal ligament space around the root apices in the pre-operative radiograph. (b) An occlusal clinical image of LL7 showing the underlying shadowing of the lesion mesially and cavitation in the midline fissure. (c) After minimally invasive selective caries removal, a GIC was placed as a provisional restoration. (d) At the 1-month review, the GIC was cut back and a resin composite restoration veneered onto its surface as a closed sandwich technique. (N.B: The red marks show the occlusal articulation at the 1-year review stage). (e) The 1-year post-op periapical radiograph: the differences in radiopacities of the GIC and overlying resin composite can be observed. The tooth-restoration complex was sound and the pulp remained vital. The slight radiolucency (arrowed) at the dentine border adjacent to the GIC is the retained caries-affected dentine, and is inactive (Hashem et al. 2015)
Marchi et al. (2006) studied the effectiveness of two materials as indirect protective pulp liners, a setting calcium hydroxide and a RMGIC, in the treatment of 27 primary molars with deep caries. Four years post treatment, the success rate using the former was 88.8 % and using the GIC was 93 %. The investigators defined ‘success’ essentially as the absence of any ‘clinical or radiographic signs or symptoms of irreversible pulp pathologies or necrosis’. The authors concluded that ‘indirect pulp capping in primary teeth arrests the progression of the underlying caries, regardless of the material used as a liner’. In order to provide evidence that the caries process was arrested in the sealed lesions, they sampled teeth for bacterial culture at periods ranging from 1 week to 2 years; at the latter stage, they found a substantial decrease in the number of cultivable micro-organisms in sealed lesions when compared with the unsealed control teeth. Interestingly, they found the greatest amount of bacterial reduction within 2 weeks after treatment. In another microbiological study of dentine samples taken from 40 carious lesions before and after undergoing atraumatic restorative treatment (ART), Bonecker et al. (2003) found significant reductions in the frequency and proportions of the total viable mutans streptococci (but not lactobacilli) in restorations sealed with GIC.
A more recent randomized clinical trial has compared the use of GIC and a calcium silicate cement to restore deep carious cavities in patients (Hashem et al. 2015). The affected teeth were symptomatic with acute pulpitis. Baseline periapical radiographs, CBCT (cone beam CT) and a full clinical examination were carried out before minimally invasive selective caries removal was performed using burs and hand instruments, assisted with Carisolv™ gel. No pulp exposures occurring at this stage of treatment were included in the study. These deep cavities were restored either with a high-viscosity GIC, Fuji IX (GC Corp, Tokyo, Japan), or a setting calcium silicate cement, Biodentine™ (Septodont, Saint-Maur-des-Fossés, France). They were reviewed after 1 month, and assuming the clinical signs and symptoms indicated healing, these provisional restorations were veneered with a resin composite (a layered definitive restoration – see Fig. 4.2). At the 1 year review, it was clear that both materials had a similar 83 % success rate in maintaining tooth structure as well as pulp vitality and the layered restorations were faring well. Thus, on the basis of the evidence cited, it can be reasonably concluded that the removal of all infected dentine in deep carious lesions is not required for successful caries treatment, provided that the restoration can seal the lesion from the oral environment effectively.
4.5 GIC and Atraumatic Restorative Treatment (ART)
A number of countries have already banned or are considering banning the use of dental amalgam, partly in response to the Minamata Treaty agreed by the United Nations Environment Programme (UNEP 2013). Since then, both the International Dental Federation (FDI) and the World Health Organization (WHO) have called for alternatives to amalgam to be developed for use to operatively manage carious lesions. One alternative is to use currently available glass-ionomer cements. Its high-viscosity variant has become the material of choice for atraumatic restorative treatment (ART). This minimally invasive caries management approach, involving the use of hand instruments only and the placement of a high-viscosity glass-ionomer cement (HVGIC), is considered an alternative to the more traditional maximally invasive restorative treatments (Raggio et al. 2013; Holmgren et al. 2013). In terms of restoration survival, a systematic review concluded that ART/HVGIC and amalgam restorations of the same size, type of dentition and follow-up period are equally successful clinically. However, because of the limited number of suitable data sets for the analysis, the authors of the review suggested that further studies should be carried out to confirm these findings (Mickenautsch et al. 2010).
A weak inherent feature of conventional GIC is its low fracture toughness. By increasing the powder-to-liquid ratio, the fracture toughness can be increased (Peez and Frank 2006), and it was suggested that by using this improved GIC with ART, the survival of ART restorations may be increased, especially in multiple-surface restorations. Hilgert et al. (2014) assessed and compared the cumulative survival rate of amalgam and ART using HVGIC restorations in primary molars over 3 years for single- and multiple-surface restorations. The survival rates over 3 years for all, single- and multiple-surface, amalgam restorations were not significantly different from those of comparable ART/HVGIC restorations. Single-surface restorations had higher survival rates than multiple-surface restorations for both procedures. A higher proportion of restorations failed due to mechanical reasons (94.8 %) than due to caries associated with restorations/sealants (CARS), i.e. secondary caries (5.2 %). The HVGIC used in conjunction with ART is a viable option for restoring carious dentine lesions in single surfaces in vital primary molars. However, the performance of proximal ART restorations using HVGIC is still far from ideal (Ersin et al. 2006; De Amorim et al. 2012). This may be attributed to the highly viscous consistency of the GIC which increases the difficulties with its handling and placement (Frencken and Holmgren 1999). These characteristics can lead to poor adaptation of the material to the cavity base resulting in gaps and leakage, lack of retention and ultimate loss of the restoration (Roeleveld et al. 2006; Bonifácio et al. 2009). More recent laboratory studies showed the insertion of a thin flowable GIC layer at the base of deep proximal cavities prior to the insertion of the regular HVGIC layer (two-layer technique) can improve the material’s adaptation to the cavity, so increasing the bond strength to sound tissues (Bonifácio et al. 2010; Lenzi et al. 2013). The success of ART restorations can be influenced by many factors, the most often reported being the operator-induced effect (Frencken et al. 2004; Kemoli et al. 2009). This includes the proper use of hand instruments, cavity pre-conditioning, correct mixing/manipulation of the HVGIC and, in cases of multi-surface restorations, factors such as correct matrix band application, moisture control and sufficient material obturation/adaptation (Kemoli et al. 2009). These differences in individual operative skills are always to be expected (Van Gemert-Schriks et al. 2007; Frencken and Leal 2010).
Bonifácio et al. (2013) investigated the use of a two-layer flowable technique for the insertion of GIC in proximal cavities and assessed the influence of the operator in the survival rate of proximal ART restorations in primary molars. Despite the small sample size, short evaluation period (12 months) and the lack of a control group for comparison, the results showed an acceptable survival rate and no detrimental operator effect over the time period investigated. The retention rate was similar to those previously reported in the literature (Carvalho et al. 2010; da Franca et al. 2011). A 1-year survival rate of 74 % is in agreement with that presented in the literature. In general, the 12-month survival rate of proximal ART restorations in primary posterior teeth ranges between 12 and 88 %, for studies conducted in schools (Ersin et al. 2006; van Gemert-Schriks et al. 2007; Deepa and Shobha 2010). High-viscosity GICs are difficult to handle and can lead to inadequate adaptation to the cavity walls and cervical gaps (Lenzi et al. 2013), both of which contribute to restoration failure (Roeleveld et al. 2006; Mhaville et al. 2006). Bonifácio et al. (2013) concluded that using a flowable layer of the GIC prior to the insertion of a conventional layer leads to an improvement in GIC adaptation and a reduction in the occurrence of the secondary caries (CARS, see earlier). The main reason for failure was bulk fracture or total loss of the restoration, which is in accordance with the published literature (Eden et al. 2006; Van Gemert-Schriks et al. 2007; Topaloglu-Ak et al. 2009; da Franca et al. 2011).
Bulk fractures are related generally to the mechanical properties of the GIC; the use of a flowable layer as a liner may contribute towards reducing the mechanical strength as the final material created has fewer glass particles/unit volume. However, Fonseca et al. (2010) reported that there is no difference in the diametral tensile strength of conventional GIC when the powder/liquid ratio was reduced by 50 %. A disadvantage of the two-layer technique may be that the second layer does not adhere properly to the first, so contributing to bulk fractures. To confirm the potential improvements delivered by the two-layer technique of applying GIC in ART proximal cavities, further studies in the form of controlled clinical trials as well as investigations of the mechanical and adhesive properties of this two-layered GIC should be conducted.
4.6 The Longevity of GIC Restorations
The ultimate success or failure of a restorative material is measured by its longevity in the oral environment whilst maintaining tooth tissue integrity and pulp vitality. As initial laboratory tests of new materials do not always reveal their full limitations or assets, clinical data is essential to provide empirical evidence. Unfortunately, at the present time, there is no consensus on the desired or ideal length of time of a clinical study to accurately predict the performance or clinical life expectancy of restorative dental materials. Differences between study variables, including the sample size, material and restoration type, method of assessment, operator and patient factors, often make data comparisons difficult. Nevertheless, information gleaned from longitudinal assessments is important in the hope that cumulatively, this information adds to the body of evidence to help make informed clinical decisions regarding treatment options. As a ‘major undertaking for general dental practitioners is the provision and assessment of dental restorations’, observations in clinical practice offer valuable evidence if interpreted appropriately (Sidhu 2010).
Clinical trials investigating the longevity of GICs in primary molars are mostly short-term studies of less than 3 years. The longest measured survival rates for GICs are in low stress-bearing areas including Class III and Class V restorations (Mount 1993). Vlietstra et al. (1978) reported that 75 % of the conventional glass-ionomer restorations in primary molars were intact after 1 year, and the marginal adaptation, contour and surface finish were all satisfactory. Others (Cho and Cheng 1999) reported that GICs in primary molars showed no significant differences with amalgam restorations in overall failure rates after 2 years. However, a previous 5-year follow-up of restorations showed that GICs had a significantly inferior survival time to amalgam (Welbury et al. 1991). Ostlund et al. (1992) compared Class II restorations of amalgam, resin composite and glass-ionomer cement in primary molars and reported a high failure rate for the glass-ionomer cement of 60 % after 2 years. In contrast, the failure rates for amalgam and resin composite restorations were 8 and 16 % respectively. It is worthwhile noting that these studies were carried out over two decades ago, and much has changed in the chemistry and application of GIC materials since then.
More recently, Fuks et al. (2000) compared the clinical performance of a GIC with amalgam in Class II restorations in primary molars. Only 9 of 101 glass-ionomer restorations met all the quality criteria after 1 year, whereas 90 % of the amalgam restorations met all the evaluation criteria after 3 years. Hickel et al. (2005) investigated the mean survival time of different types of restorations in primary molars and found that the mean survival time for glass-ionomer restorations was only 12 months compared to more than 5 years for stainless-steel crowns and amalgam restorations. The results of these and other studies indicate that conventional GIC is not an appropriate alternative to amalgam in the restoration of primary molars unless the teeth are expected to exfoliate in 1 or 2 years. Indeed, RMGICs may prove to have the highest success rates in terms of longevity in the primary dentition (Toh and Messer 2007).
With regard to the adult dentition, conclusive clinical evidence remains elusive to date. A systematic review of the literature up to 2010 indicated that the longevity of HVGIC restorations is site dependent but in buccal cervical restorations can last successfully over 6 years in clinical service (Frencken et al. 2006; Mickenautsch et al. 2010; see Fig. 4.3).
In the permanent dentition, longevity of ART restorations is ≥equivalent amalgam restorations up to 6.3 years, site-dependent. No difference is observed in primary teeth (Frencken et al 2006)
4.7 GIC and the Pulp Response
The use of GICs directly on the pulp or in deep cavities approaching the pulp has been a subject of controversy. Since the introduction of this material approaching five decades ago, the biocompatibility of this material has been studied intensively. Early studies showed that GICs are associated with an increased inflammatory cell infiltrate in the odontoblast layer compared to controls when placed in non-exposed deep cavities in human teeth. However, no symptoms were recorded during the observation periods, and the changes had mostly resolved towards the end of the experiments. Others demonstrated pulp inflammation and necrosis when glass-ionomer cements were placed directly on exposed molar rat teeth. This finding was corroborated by another in-vitro study assessing the cytotoxicity of eight different GICs by means of pulp cell culture. The authors found that some GICs are more cytotoxic than others and concluded that they should not be placed directly on or near pulps (Müller et al. 1990). However, great caution should be employed when extrapolating results from in-vitro studies to the clinical situation, as the natural protective effect of dentine is ignored and the individual defence and repair mechanisms, which increase tolerance to such materials, are not present.
Indeed, contrary to the above, animal studies have reported no adverse pulp reactions to GIC when placed in non-exposed deep cavities and observed over different time periods (Felton et al. 1991). These results are corroborated by more recent studies using improved GICs, which have shown minimal cytotoxic effects on the pulp. In a study by Six et al. (2000), a HVGIC (Fuji IX, GC Corp, Tokyo, Japan) was placed in deep non-exposed cavities of rat teeth and compared to unfilled cavities as a control. Observation after 8 days revealed few inflammatory cells in both groups, disruption of the odontoblast layer and dilatation of the blood vessels. The inflammatory reaction in the glass-ionomer group was slightly higher than in the control group. After 30 days, complete recovery of the pulp tissue was observed with no disruption of the odontoblast layer. A thick layer of reparative dentine had formed in both groups. The authors concluded that the GIC used (Fuji IX) is biocompatible with the pulp and does not induce any harmful effect on pulp cells.
Hume and Mount (1988) studied the effect of GICs when placed directly on a sterile tissue culture medium or indirectly through a layer of human dentine. It was found that glass-ionomer cement when placed directly had a higher cytotoxic effect while GIC through dentine had limited or no cytotoxicity. Freshly mixed GIC is acidic with a pH ranging between 0.9 and 1.6. However, dentine acts as a buffer, and even thin layers of dentine remaining between the restoration and the pulp are sufficient to prevent a reduction of pH affecting the pulp tissue. A mild inflammatory response has been noted by several authors, but as the pH rises within the first hour, the inflammatory cellular response is transient, resolving within 10–20 days. To a certain extent, the pulp irritation may be accounted for by the high buffering capacity of the hydroxyapatite itself. Also, the low mobility and chelating capacity of the large polyalkenoic acid molecules may be significant in this regard.
It can be concluded from these studies that GICs are not suitable when placed directly on the pulp. However, using them as indirect pulp protection/capping agents or as a dentine replacement material in deep cavities is widely accepted (Sidhu 2011).
4.8 Adhesion Between GIC and Resin Composite
Glass-ionomer cements have many different clinical applications including indirect pulp protection/capping. They consist of a calcium fluoro-alumino-silicate glass powder and an aqueous solution of a poly (acrylic acid—itaconic acid) copolymer containing tartaric acid (Smith 1998). The setting reaction involves the acid-base reaction of the polyacrylic acid and the glass particles and ions (Al3+, Ca2+) located in the glass network (Mount and Hume 1998). Modifications in both components have been made in various commercial brands for both patent and practical reasons (Smith 1998). GICs have been reported to demonstrate excellent sealing properties and good biocompatibility when placed in close proximity with, but not directly on, the pulp (Hilton 2009). In addition, they have the ability to adhere chemically to moist dentine through ionic exchange at the interface leading to the formation of a new intermediate dentine-GIC layer approximately 300 μm thick (Zoergiebel and Ilie 2013). This ionic exchange is triggered at the interface through a diffusion process. This process is partly driven by the concentration gradient which exists between the glass-ionomer and the dentine, with strontium, calcium and fluoride ions undertaking apatitic activity in relation to areas in dentine where the calcium ion levels are low (Ngo et al. 2006).
Drawbacks of GIC include its physical properties as it is susceptible to acid erosion and wear, therefore its successful use lies mainly in the field of dentine replacement in laminate/layered/‘sandwich’ restorations (Davidson 2006). GICs have been used in both open and closed sandwich restorations with a higher success rate reported for closed sandwich restorations (van Dijken 1994). This is because in open sandwich restorations, GIC is associated with an increased risk of dissolution due to its susceptibility to early moisture contamination. In addition, the proximal area is exposed to longer acid clearance times, which increases the erosion rate at the surface. If a GIC lining cement is used, the cement will be stressed continuously by masticatory forces transferred via the overlying restoration, resulting potentially in crack formation at the cement-restorative interface followed by fracture of the cement. This is due to the lack of strength of the thin cement layer (van Dijken 1994). This problem can be overcome by using a restorative version of the cement which is cut back rather than using a liner variety, resulting in a more robust restoration with better mechanical properties (Cattani-Lorente et al. 1993). This also has an advantage if an overlying resin composite restoration is used as the increased thickness of the GIC reduces the thickness of the resin composite leading to a reduction in the polymerization shrinkage (Woolford 1993).
An interaction between GIC and the overlying resin composite restoration was suggested where GIC reduced the hardness of the surface of resin composite adjacent to it up to a distance of 1 mm into the thickness of the resin composite. This detrimental interaction was observed when resin composite was placed on fresh GIC; therefore, it is recommended to leave the cement to mature as much as possible before the application of the resin composite – a two-visit clinical procedure (Woolford 1993).
4.9 Conclusions
This chapter has aimed to discuss the potential attributes of glass-ionomer cements and their use in the contemporary minimally invasive operative management of deep caries lesions. Indeed, low-viscosity GICs have been advocated for use as fissure sealant restorations in clinical cases of primary and tertiary minimally (non)invasive caries prevention. Even though resin composite sealants exhibit better durability and retention clinically, in scenarios with compromised moisture control, GIC sealants still have a part to play, especially in the primary dentition.
Glass-ionomer cements are not necessarily the strongest or toughest direct plastic restorative material available clinically to withstand the occlusal forces and the changing environment generated in the oral cavity. However, their ability to chemically bond to and seal enamel and dentine substrate without the need for a separate chemical adhesive, to leach ions that can aid mineralization, including fluoride, all help to encourage resistance and repair of caries-affected tissues and also the dentine-pulp complex. Clinically, modern GIC derivatives exhibit improved handling characteristics, aesthetics and strength/durability. With the potential global reduction in use of dental amalgam to restore teeth with carious cavities, GICs form an invaluable member of the remaining restorative armamentarium to the clinician treating dental caries in their patients.
References
Alves FBT, Hesse D, Lenzi TL, et al. The bonding of glass ionomer cements to caries-affected primary dentin. Pediatr Dent. 2013;35(4):320–4.
Arends J, ten Bosch JJ. In vivo de- and remineralization of dental enamel. In: Leach SA, editor. Factors relating to demineralization and remineralization of the teeth. Oxford: IRL Press; 1986. p. 1–11.
Attar N, Önen A. Artificial formed caries-like lesions around esthetic restorative materials. J Clin Pediatr Dent. 2002;26:289–96.
Banerjee A, Watson TF. Pickard’s guide to minimally invasive operative dentistry. 10th ed. London: Oxford University Press; 2015.
Berry EA, Powers JM. Bond strength of glass ionomers to coronal and radicular dentin. Oper Dent. 1994;19:122–6.
Bezerra AC, Novaes RC, Faber J, et al. Ion concentration adjacent to glass-ionomer restorations in primary molars. Dent Mater. 2012;28:e259–63.
Bissoto Calvo AF, Alves FBT, Lenzi TL, et al. Glass ionomer cements bond stability in caries-affected primary dentin. Int J Adhes Adhes. 2014;48:183–7.
Bjørndal L, Reit C, Bruun G, et al. Treatment of deep caries lesions in adults: randomised clinical trials comparing stepwise vs. direct complete excavation, and direct pulp capping vs. partial pulpotomy. Eur J Oral Sci. 2010;118(3):290–7.
Bonecker M, Toi C, Cleaton-Jones P. Mutans streptococci and lactobacilli in carious dentine before and after atraumatic restorative treatment. J Dent. 2003;31(6):423–8.
Bonifácio CC, Kleverlaan CJ, Raggio DP, et al. Physical-mechanical properties of glass ionomer cements indicated for atraumatic restorative treatment. Aust Dent J. 2009;54:233–7.
Bonifácio CC, van Amerongen WE, Meschini TG, et al. Flowable glass ionomer cement as a liner: improving marginal adaptation of atraumatic restorative treatment restorations. J Dent Child. 2010;77:12–6.
Bonifácio CC, Hesse D, Bönecker M, et al. A preliminary clinical trial using flowable glass-ionomer cement as a liner in proximal-ART restorations: the operator effect. Med Oral Patol Oral Cir Bucal. 2013;18(3):e529–32.
Burke F, Lynch E. Glass polyalkenoate bond strength to dentine after chemo-mechanical caries removal. J Dent. 1994;22:283–91.
Carvalho TS, Sampaio FC, Diniz A, et al. Two years survival rate of Class II ART restorations in primary molars using two ways to avoid saliva contamination. Int J Paediatr Dent. 2010;20:419–25.
Cattani-Lorente MA, Godin C, Meyer JM. Early strength of glass ionomer cements. Dent Mater. 1993;9:57–62.
Cehreli ZC, Akca T, Altay N. Comparison of bonding ability of single-step self- etching adhesives with different etching aggressiveness to root dentin. Am J Dent. 2013;16:47A–50.
Cho S, Cheng AC. A review of glass ionomer restorations in the primary dentition. J Can Dent Assoc. 1999;65:491–5.
Creanor SL, Awawdeh LA, Saunders WP, et al. The effect of a resin-modified glass ionomer restorative material on artificially demineralized dentine caries in vitro. J Dent. 1998;26(5–6):527–31.
Czarnecka B, Deregowska-Nosowicz P, Limanowska-Shaw H, et al. Shear bond strengths of glass-ionomer cements to sound and to prepared carious dentine. J Mater Sci. 2007;18(5):845–9.
Da Franca C, Colares V, Van Amerongen E. Two-year evaluation of the atraumatic restorative treatment approach in primary molars class I and II restorations. Int J Paediatr Dent. 2011;21:249–53.
Davidson CL. Advances in glass-ionomer cements. J Applied Oral Sci. 2006;14:3–9.
De Amorim RG, Leal SC, Frencken JE. Survival of atraumatic restorative treatment (ART) sealants and restorations: a meta-analysis. Clin Oral Investig. 2012;16:429–41.
De Munck J, Inoue S, Suzuki K, et al. Four-year water degradation of a resin-modified glass-ionomer adhesive bonded to dentin. Eur J Oral Sci. 2004;112(1):73–83.
Deepa G, Shobha T. A clinical evaluation of two glass ionomer cements in primary molars using a traumatic restorative treatment technique in India: 1 year follow up. Int J Paediatr Dent. 2010;20:410–8.
Eden E, Topaloglu-Ak A, Frencken JE, et al. Survival of self-etch adhesive Class II composite restorations using ART and conventional cavity preparations in primary molars. Am J Dent. 2006;19:359–63.
Ersin NK, Candan U, Aykut A, et al. A clinical evaluation of resin-based composite and glass ionomer cement restorations placed in primary teeth using the ART approach: results at 24 months. J Am Dent Assoc. 2006;137:1529–36.
Es-Souni M, Zimehl R, Fischer-Brandies H. Microscopic and electron spectroscopic characterization of dental enamel surfaces. Colloid Polym Sci. 1999;277:382–7.
Fagundes TC, Toledano M, Navarro MF, et al. Resistance to degradation of resin-modified glass-ionomer cements dentine bonds. J Dent. 2009;37(5):342–7.
Felton DA, Cox CF, Odom M, et al. Pulpal response to chemically cured and experimental light-cured glass ionomer cavity liners. J Prosthet Dent. 1991;65:704–12.
Foley J, Evans D, Blackwell A. Partial caries removal and cariostatic materials in carious primary molar teeth: a randomised controlled clinical trial. Br Dent J. 2004;197(11):697–701.
Fonseca RB, Branco CA, Quagliatto PS, et al. Influence of powder/liquid ratio on the radiodensity and diametral tensile strength of glass ionomer cements. J Appl Oral Sci. 2010;18:577–84.
Frencken JE, Holmgren CJ. How effective is ART in the management of dental caries? Community Dent Oral Epidemiol. 1999;27:423–30.
Frencken JE, Leal SC. The correct use of the ART approach. J Appl Oral Sci. 2010;18:1–4.
Frencken JE, Van’t Hof MA, Van Amerongen WE, et al. Effectiveness of single-surface ART restorations in the permanent dentition: a meta-analysis. J Dent Res. 2004;83:120–3.
Frencken JE, Taifour D, Van’t Hof MA. Survival of ART and amalgam restorations in permanent teeth of children after 6.3 years. J Dent Res. 2006;85:622–6.
Fuks AB, Araujo FB, Osorio LB, et al. Clinical and radiographic assessment of Class II aesthetic restorations in primary molars. Pediatr Dent. 2000;22(6):479–85.
Gwinnett AJ. Chemically conditioned dentin: a comparison of conventional and environmental scanning electron microscopy findings. Dent Mater. 1994;10:150–5.
Hashem D, Mannocci F, Patel S, et al. Efficacy of calcium silicate indirect pulp capping; a randomized controlled clinical trial. J Dent Res. 2015;94(4):562–8.
Hatibovic-Kofman S, Suljak JP, Koch G. Remineralization of natural carious lesions with a glass ionomer cement. Swed Dent J. 1997;21:11–7.
Hickel R, Kaaden CH, Paschos E, et al. Longevity of occlusally-stressed restorations in posterior primary teeth. Am J Dent. 2005;18(3):198–211.
Hilgert LA, de Amorim RG, Leal SC, et al. Is high-viscosity glass-ionomer-cement a successor to amalgam for treating primary molars? Dent Mater. 2014;30:1172–8.
Hilton TJ. Keys to clinical success with pulp capping: a review of the literature. Oper Dent. 2009;34:615–25.
Holmgren CJ, Roux D, Doméjean S. Minimal intervention dentistry: part 5. Atraumatic restorative treatment (ART) – a minimum intervention and minimally invasive approach for the management of dental caries. Br Dent J. 2013;214:11–8.
Hosoya Y, Garcia-Godoy F. Bonding mechanism of Ketac-Molar Applicap and Fuji IX GP to enamel and dentin. Am J Dent. 1998;11:235–9.
Hume WR, Mount GJ. In vitro studies on the potential for pulpal cytotoxicity of glass-ionomer cements. J Dent Res. 1988;67:915–8.
Jang KT, Garcia-Godoy F, Donly KJ, et al. Remineralizing effects of the glass ionomer restorations on adjacent interproximal caries. ASDC J Dent Child. 2001;68:125–8.
Kemoli AM, van Amerongen WE, Opinya G. Influence of the experience of operator and assistant on the survival rate of proximal ART restorations: two-year results. Eur Arch Paediatr Dent. 2009;10:227–32.
Lenzi TL, Bonifácio CC, Bönecker M, et al. Flowable glass ionomer cement layer bonding to sound and caries affected primary dentin. J Dent Child (Chic). 2013;80:20–4.
Marchi JJ, de Araujo FB, Froner AM, et al. Indirect pulp capping in the primary dentition: a 4 year follow-up study. J Clin Pediatr Dent. 2006;31(2):68–71.
Marquezan M, Corrêa FN, Sanabe ME, et al. Artificial methods of dentine caries induction: a hardness and morphological comparative study. Arch Oral Biol. 2009;54(12):1111–7.
Marquezan M, Osório Ciamponi AL, Toledano M. Resistance to degradation of bonded restorations to simulated caries-affected primary dentin. Am J Dent. 2010;23(1):47–52.
Mhaville RJ, van Amerongen WE, Mandari GJ. Residual caries and marginal integrity in relation to Class II glass ionomer restorations in primary molars. Eur Arch Paediatr Dent. 2006;7:81–4.
Mickenautsch S, Yengopal V, Banerjee A. Atraumatic restorative treatment versus amalgam restoration longevity: a systematic review. Clin Oral Invest. 2010;14:233–40.
Mount GJ. Clinical placement of modern glass ionomer cements. Quintessence Int. 1993;24:99–107.
Mount GJ, Hume WR. Preservation and restoration of tooth structure, chapter 8. St Louis: Mosby; 1998. p. 80–2.
Müller J, Bruckner G, Kraft E, et al. Reaction of cultured pulp cells to eight different cements based on glass ionomers. Dent Mater. 1990;6:172–7.
Nakajima M, San H, Burrow MF, et al. Tensile bond strength and SEM evaluation of caries-affected dentin using dentin adhesive. J Dent Res. 1995;74:1679–88.
Ngo H. Remineralization of artificial carious dentine exposed to two glass ionomers. J Dent Res. 2002a;81(Special Issue):386.
Ngo HC. Biological potential of glass-ionomer. In: Mount GJ, editor. An atlas of glass-ionomer cements. London: Martin Dunitz; 2002b. p. 43–55.
Ngo HC, Mount GJ, Peters MC. A study of glass-ionomer cement and its interface with enamel and dentin using a low-temperature, high resolution scanning electron microscopic technique. Quintessence Int. 1997a;28:63–9.
Ngo HC, Ruben J, Arends J, et al. Electron probe microanalysis and transverse microradiography studies of artificial lesions in enamel and dentin: a comparative study. Adv Dent Res. 1997b;11:426–32.
Ngo HC, Mount G, McIntyre J, et al. Chemical exchange between glass-ionomer restorations and residual carious dentine in permanent molars: an in vivo study. J Dent. 2006;34(8):608–13.
Olivia A, Della Ragione F, Salerno A. Biocompatibility studies on glass ionomer cements by primary cultures of human osteoblasts. Biomaterials. 2000;17:1351–6.
Ostlund J, Möller K, Koch G. Amalgam, composite resin and glass ionomer cement in Class II restorations in primary molars – a three year clinical evaluation. Swed Dent J. 1992;16(3):81–6.
Peez R, Frank S. The physical–mechanical performance of the new Ketac Molar Easymix compared to commercially available glass ionomer restoratives. J Dent. 2006;34:582–7.
Qvist V, Laurberg L, Poulsen A, et al. Eight-year study on conventional glass ionomer and amalgam restorations in primary teeth. Acta Odontol Scand. 2004;62:37–45.
Raggio DP, Hesse D, Lenzi TL, et al. Is atraumatic restorative treatment an option for restoring occluso-proximal caries lesions in primary teeth? A systematic review and meta-analysis. Int J Paediatr Dent. 2013;23:435–43.
Ricketts DNJ, Lamont T, Innes P et al (2013) Operative caries management in adults and children. Cochrane Database Syst Rev (3):CD003808
Roeleveld AC, van Amerongen WE, Mandari GJ. Influence of residual caries and cervical gaps on the survival rate of Class II glass ionomer restorations. Eur Arch Paediatr Dent. 2006;7:85–91.
Sidhu SK. Clinical evaluations of resin-modified glass-ionomer restorations. Dent Mater. 2010;2:7–12.
Sidhu SK. Glass-ionomer cement restorative materials: a sticky subject? Aust Dent J. 2011;56 Suppl 1:23–30.
Six N, Lasfargues JJ, Goldberg M. In vivo study of the pulp reaction to Fuji IX, a glass ionomer cement. J Dent. 2000;28:413–22.
Smith DC. Development of glass-ionomer cement systems. Biomaterials. 1998;19:467–78.
Thompson V, Craig RG, Curro FA, et al. Treatment of deep carious lesions by complete excavation or partial removal: a critical review. J Am Dent Assoc. 2008;139(6):705–12.
Toh SL, Messer LB. Evidence-based assessment of tooth-colored restorations in proximal lesions of primary molars. Pediatr Dent. 2007;29(1):8–15.
Topaloglu-Ak A, Eden E, Frencken JE, et al. Two year survival rate of class II composite resin restorations prepared by ART with and without a chemomechanical caries removal gel in primary molars. Clin Oral Investig. 2009;13:325–32.
United Nations Environment Programme (2013) Minamata Convention on Mercury UNEP(DTIE)/Hg/CONF/3
Van Dijken JW. A 6-year evaluation of a direct composite resin inlay/onlay system and glass ionomer cement-composite resin sandwich restorations. Acta Odontol. 1994;52:368–76.
Van Gemert-Schriks MC, van Amerongen WE, ten Cate JM, et al. Three-year survival of single- and two-surface ART restorations in a high-caries child population. Clin Oral Investig. 2007;11:337–43.
Van Noort R. Introduction to dental materials. 4th ed. Edinburgh/New York: Mosby Elsevier; 2013. p. 113.
Vlietstra JR, Plant CG, Shovelton DS, et al. The use of glass ionomer cement in deciduous teeth. Br Dent J. 1978;145:164–6.
Watson TF. Bonding glass-ionomer cements to tooth structure. In: Davidson CL, Mjör IA, editors. Advances in glass-ionomer cements. Germany: Quintessence Publishing Co Inc.; 1999.
Way JL, Caputo AA, Jedrychowski JR. Bond strength of light-cured glass ionomers to carious primary dentin. ASDC J Dent Child. 1996;63(4):261–4.
Welbury RR, Walls AW, Murray JJ, McCabe JF. The 5-year results of a clinical trial comparing a glass polyalkenoate (ionomer) cement restoration with an amalgam restoration. Br Dent J. 1991;170:177–81.
Wilson AD, Kent BE. The glass ionomer cement. A new translucent dental filling material. J Appl Chem Biotechnol. 1971;21:313.
Woolford M. Composite resin attached to glass polyalkenoate (ionomer) cement—the laminate technique. J Dent. 1993;21:31–8.
Yip HK, Tay FR, Ngo HC, et al. Bonding of contemporary glass ionomer cements to dentin. Dent Mater. 2001;17(5):456–70.
Yiu CK, Tay FR, King NM, et al. Interaction of glass-ionomer cements with moist dentin. J Dent Res. 2004;83(4):283–9.
Yoshida Y, Van Meerbeek B, Nakayama Y, et al. Evidence of chemical bonding at biomaterial-hard tissue interfaces. J Dent Res. 2000;79(2):709–14.
Zoergiebel J, Ilie N. An in vitro study on the maturation of conventional glass ionomer cements and their interface to dentin. Acta Biomater. 2013;9:9529–37.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2016 Springer International Publishing Switzerland
About this chapter
Cite this chapter
Banerjee, A. (2016). The Role of Glass-Ionomer Cements in Minimum Intervention (MI) Caries Management. In: Sidhu, S. (eds) Glass-Ionomers in Dentistry. Springer, Cham. https://doi.org/10.1007/978-3-319-22626-2_4
Download citation
DOI: https://doi.org/10.1007/978-3-319-22626-2_4
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-22625-5
Online ISBN: 978-3-319-22626-2
eBook Packages: MedicineMedicine (R0)