Abstract
Intracranial pressure (ICP) is routinely measured in patients with severe traumatic brain injury (TBI). We describe a novel technique that allowed us to monitor intraspinal pressure (ISP) at the injury site in 14 patients who had severe acute traumatic spinal cord injury (TSCI), analogous to monitoring ICP after brain injury. A Codman probe was inserted subdurally to measure the pressure of the injured spinal cord compressed against the surrounding dura. Our key finding is that it is feasible and safe to monitor ISP for up to a week in patients after TSCI, starting within 72 h of the injury. With practice, probe insertion and calibration take less than 10 min. The ISP signal characteristics after TSCI were similar to the ICP signal characteristics recorded after TBI. Importantly, there were no associated complications. Future studies are required to determine whether reducing ISP improves neurological outcome after severe TSCI.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
Introduction
After severe traumatic brain injury (TBI), the brain swells. This causes an increase in intracranial pressure (ICP) and a decrease in cerebral perfusion pressure (CPP; CPP = mean arterial pressure (MAP) − ICP), which may lead to secondary ischaemic brain damage [10]. The early management of severe TBI is aimed at reducing the elevated intracranial ICP and increasing CPP to reduce secondary brain damage [10]. To achieve this, patients with TBI are urgently transferred to a neurointensive care unit for ICP monitoring. Low CPP (<60 mmHg) and high ICP (>20 mmHg) [3, 6] are associated with a worse outcome after TBI.
In contrast to the management of severe TBI, that of severe acute traumatic spinal cord injury (TSCI) in the neurointensive care unit is variable. Many anaesthetists do not measure arterial blood pressure invasively, and the optimal levels of MAP and arterial pCO2 (paCO2) are arbitrary [11]. The American Association of Neurological Surgeons guidelines recommend MAP of 85–90 mmHg for 5–7 days after TSCI [5]. The UK National Spinal Cord Injury Strategy Board guidelines recommend systolic ABP of 90–100 mmHg [8]. However, there is insufficient evidence to support these guidelines.
A key reason why TSCI management is so different from TBI management is because there is no method in clinical use for measuring intraspinal pressure (ISP) after TSCI. Measuring ISP after TSCI would be analogous to measuring ICP after TBI. The ability to measure ISP would be a major advance in that it would allow the various principles that are used clinically for managing severe TBI (reducing ICP, increasing CPP, optimising cerebrovascular pressure reactivity) to be adapted for use in TSCI (reducing ISP, increasing spinal cord perfusion pressure [SCPP], optimising spinal cord vascular pressure reactivity). Here, we describe our technique for measuring ISP at the injury site after TSCI.
Materials and Methods
Inclusion and Exclusion Criteria
The Injured Spinal Cord Pressure Evaluation (ISCoPE) study was set up in 2009. The initial findings from the ISCoPE study are reported in Critical Care Medicine [12]. Approvals were obtained from the St George’s Joint Research Office and the South London, Maudsley and the Institute of Psychiatry Local Research Ethics Committee (No. 10/H0807/23). We recruited 18- to 70-year-old patients with severe TSCI (ASIA grades A–C). Exclusion criteria were the inability to consent and other major injuries or significant co-morbidities. ISP monitoring was started within 72 h of the TSCI and continued for up to a week.
Surgical Technique
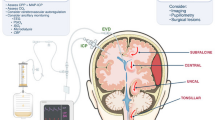
A Codman pressure probe was chosen because it is already licensed for use in patients and is widely used to measure ICP. The Codman wire is 1 m long (and therefore the patient does not lie on the connector), it has a small diameter of 0.7 mm (thus reducing the risk of spinal injury), it has a low 10-day zero drift (<0.2 mmHg/day) and a high response frequency (100 Hz). The ISP probe was placed subdurally following laminectomy or a small laminotomy. The insertion technique is summarised diagrammatically in Fig. 1a. After reducing and fixing the spinal fracture, and inserting metalwork to stabilise the spine, a 14-gauge introducer was used to tunnel a Codman pressure probe through the skin into the wound. We used a 21-gauge needle bent at 90° to perforate the dura one level below the injury. The Codman probe was calibrated and advanced through the dural hole until the probe tip was at the site of maximal spinal cord swelling according to the MR scan. The probe was secured to the skin using silk sutures. We found it important to insert a tightening stitch around the exit site to prevent CSF leakage. The probe was connected to a Codman ICP box linked via a ML 221 amplifier to a PowerLab running LabChart v.7 · 3 · 3 (AD Instruments, Oxford, UK). Data were captured at 100 Hz. Satisfactory probe position was confirmed by CT before data collection.
Insertion of pressure probe. (a) 1: A hole is made in the dura, using a 90° bent needle, one level below the injury site. 2: The needle is removed. Cerebrospinal fluid (CSF) flows through the dural puncture. 3: Using forceps, the Codman probe is inserted in the subdural space and advanced to lie between the swollen spinal cord and dura. 4: A tightening silk stitch is placed at the exit site and multiple stitches secure the probe cable to the skin. (b) Learning curve showing time taken to insert the probe vs patient number
Results
Patient Recruitment
Fourteen consecutive patients with TSCI were recruited between October 2010 and September 2012. All except patients who were approached consented to participation in the study, except one. Fifty seven percent of TSCI patients were male. Seventy-one percent had cervical injuries and 29 % had thoracic injuries. Fifty seven percent were ASIA A, 14 % ASIA B and 29 % ASIA C. Twenty-one percent of TSCI patients were recruited within 24 h, 36 % at 24–48 h, and 43 % at 48–72 h.
Surgery and Complications
Thirty-six percent of TSCI patients had anterior and posterior cervical fusion, 29 % posterior cervical fusion only, and 29 % thoracic pedicle screws. Sixty-four percent of TSCI patients had a laminectomy. There was a learning curve for the “probe insertion and calibration time”, such that initially it took 31–43 min and by the end it only took 6–8 min (Fig. 1b). There were no complications related to ISP monitoring such as wound infection, meningitis, cerebrospinal fluid (CSF) leak, pseudomeningocele, spinal cord or subdural haematoma (as assessed by MRI), or deterioration in ASIA score (before probe insertion vs after probe removal). Figure 2 shows preoperative and postoperative scans for a patient with cervical spinal cord injury. Follow-up of 10 patients at 5–13 months after the surgery showed that there were no wound-related complications.
ICP Signal Recording
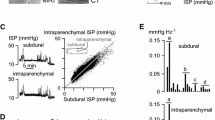
The ISP signal was recorded for up to a week. Figure 3a shows that the ISP waveform is similar to that of ICP, with three peaks corresponding to arterial pulsation, intracranial compliance and aortic valve closure [1–3]. Representative ISP recordings are shown in Fig. 3b. In some patients, ISP was high (>20 mmHg) during the recording period, with ISP reaching very high values (>40 mmHg). To put this in context, if these were ICP recordings after TBI (rather than ISP recordings after TSCI) then ICP > 20 mmHg would typically be treated and ICP > 40 mmHg would be characteristic of a patient at risk of imminent death.
Discussion
We described a novel technique for measuring subdural ISP. Our recordings indicate that after TSCI, ISP is elevated at the injury site in some patients. After TBI, high ICP is potentially lethal as it causes brain ischaemia and herniation [3, 6, 10]. Future studies are required to determine whether high ISP is harmful in TSCI.
Our ISP monitoring method is technically simple and analogous to the one used to measure ICP. The ISP signal was stable for at least a week without probe-related complications. With experience, the procedure took <10 min. Previous attempts to measure ISP after TSCI have had little success. Lumbar drains were inserted to measure CSF pressure below the injury [7], which (as shown here) differs from the ISP at the injury site. Pressure in a spinal radicular artery has been recorded [4], but is technically difficult, risks vascular damage to the spinal cord and does not measure SCPP at the injury site.
The normal spinal cord is surrounded by CSF, which is contained within a non-distensible dural sac. After TSCI, the injured section of the spinal cord swells so that there is no CSF between the spinal cord and the dura. At the injury site, the lack of CSF around the spinal cord decreases the local reserve capacity (which can be quantified using the parameter sRAP, as discussed by Czosnyka et al. in “Waveform Analysis of Intraspinal Pressure After Traumatic Spinal Cord Injury: An Observational Study (O-64)”). Further spinal cord swelling causes a rapid local rise in ISP (as the spinal cord becomes compressed against the dura), which in turn causes loss of autoregulation (quantified using the parameter sRAP, as discussed by Czosnyka et al. in “Waveform Analysis of Intraspinal Pressure After Traumatic Spinal Cord Injury: An Observational Study (O-64)”). Together, these findings suggest that the basic concepts developed for managing severe TBI, such as tissue pressure, perfusion pressure, reserve capacity and autoregulation might also be applicable when managing severe TSCI.
In the future, we envisage that after TSCI, patients will be admitted in neurointensive care units for ISP and arterial pressure monitoring to optimise ISP. For incomplete TSCI, the aim is to improve function below the level of injury and for complete TSCI, to limit cranial extension of the spinal cord damage. Perhaps the technique of ISP monitoring could also be applied to limit spinal cord damage in other conditions that cause high ISP, such as longitudinally extensive transverse myelitis [9].
References
Cardoso ER, Rowan JO, Galbraith S (1983) Analysis of the cerebrospinal fluid pulse wave in intracranial pressure. J Neurosurg 59:817–821
Czosnyka M, Guazzo E, Whitehouse M, Smielewski P, Czosnyka Z, Kirkpatrick P, Piechnik S, Pickard JD (1996) Significance of intracranial pressure waveform analysis after head injury. Acta Neurochir 138:531–541
Czosnyka M, Hutchinson PJ, Balestreri M, Hiler M, Smielewski P, Pickard JD (2006) Monitoring and interpretation of intracranial pressure after head injury. Acta Neurochir Suppl 96:114–118
Etz CD, Di Luozzo G, Zoli S, Lazala R, Plestis KA, Bodian CA, Griepp RB (2009) Direct spinal cord perfusion pressure monitoring in extensive distal aortic aneurysm repair. Ann Thor Surg 87:1764–1773
Hadley MN (2002) Blood pressure management after acute spinal cord injury. Neurosurgery 50:S58–S62
Juul N, Morris GF, Marshall SB, Marshall LF (2000) Intracranial hypertension and cerebral perfusion pressure: influence on neurological deterioration and outcome in severe head injury. The Executive Committee of the International Selfotel Trial. J Neurosurg 92:1–6
Kwon BK, Curt A, Belanger LM, Bernardo A, Chan D, Markez JA, Gorelik S, Slobogean GP, Umedaly H, Giffin M, Nikolakis MA, Street J, Boyd MC, Paquette S, Fisher CG, Dvorak MF (2009) Intrathecal pressure monitoring and cerebrospinal fluid drainage in acute spinal cord injury: a prospective randomized trial. J Neurosurg Spine 10:181–193
U.K. National Spinal Cord Injury Strategy Board website: The initial management of adults with spinal cord injuries: advice for major trauma networks and SCI centres on the development of joint protocols. With advice for clinicians in acute hospitals. http://www.excellence.eastmidlands.nhs.uk/welcome/improving-care/emergency-urgent-care/major-trauma/major-trauma-related-documents. Accessed 1 Sept 2012
Papadopoulos MC, Verkman AS (2012) Aquaporin 4 and neuromyelitis optica. Lancet Neurol 11:535–544
Rosenfeld JV, Maas AI, Bragge P, Morganti-Kossmann MC, Manley GT, Gruen RL (2012) Early management of severe traumatic brain injury. Lancet 380:1088–1098
Werndle MC, Zoumprouli A, Sedgwick P, Papadopoulos MC (2012) Variability in the treatment of acute spinal cord injury in the United Kingdom: results of a national survey. J Neurotrauma 29:880–888
Werndle MC, Saadoun S, Phang I, Czosnyka M, Varsos GV, Czosnyka ZH, Smielewski P, Jamous A, Bell BA, Zoumprouli Z, Papadopoulos MC (2014) Monitoring of spinal cord perfusion pressure in acute spinal cord injury: initial findings of the injured spinal cord pressure evaluation study. Crit Care Med 42(3):646–655. doi:10.1097/CCM.0000000000000028
Acknowledgements
Funded by the UK Spinal Cord Injury Research Network (UKSCIRN; grant to MCP) and the Neurosciences Research Foundation (NRF), Royal College of Surgeons of England and the London Deanery (fellowships to MCW). MCP is funded by the Guthy Jackson Charitable Foundation. MC, ZF, PS and GV are funded by the National Institute of Health Research (Neuroscience Theme), Cambridge. We thank P. Rich, T. Jones, M. Crocker, T. Bishop, J. Barnard (St George’s), M. Belci (Stoke Mandeville), D. Choi (National Hospital for Neurology and Neurosurgery), K. Rezajooi (Royal National Orthopaedic), D. Bell, N. Thomas (King’s College) and N. Burr (lay person).
Conflict of Interest
The authors report no conflicts of interest.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2016 Springer International Publishing Switzerland
About this chapter
Cite this chapter
Werndle, M.C. et al. (2016). Measurement of Intraspinal Pressure After Spinal Cord Injury: Technical Note from the Injured Spinal Cord Pressure Evaluation Study. In: Ang, BT. (eds) Intracranial Pressure and Brain Monitoring XV. Acta Neurochirurgica Supplement, vol 122. Springer, Cham. https://doi.org/10.1007/978-3-319-22533-3_64
Download citation
DOI: https://doi.org/10.1007/978-3-319-22533-3_64
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-22532-6
Online ISBN: 978-3-319-22533-3
eBook Packages: MedicineMedicine (R0)