Abstract
The aim of this study was to describe multimodal brain monitoring characteristics during plateau waves of intracranial pressure (ICP) in patients with head injury, using ICM+ software for continuous recording. Plateau waves consist of an abrupt elevation of ICP above 40 mmHg for 5–20 min. This is a prospective observational study of patients with head injury who were admitted to a neurocritical care unit and who developed plateau waves. We analyzed 59 plateau waves that occurred in 8 of 18 patients (44 %). At the top of plateau waves arterial blood pressure remained almost constant, but cerebral perfusion pressure, cerebral blood flow, brain tissue oxygenation, and cerebral oximetry decreased. After plateau waves, patients with a previously better autoregulation status developed hyperemia, demonstrated by an increase in cerebral blood flow and brain oxygenation. Pressure and oxygen cerebrovascular reactivity indexes (pressure reactivity index and ORxshort) increased significantly during the plateau wave as a sign of disruption of autoregulation. Bedside multimodal brain monitoring is important to characterize increases in ICP and give differential diagnoses of plateau waves, as management of this phenomenon differs from that of regular ICP.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Head injury
- Multimodal brain monitoring
- Intracranial pressure
- Plateau waves
- Pressure reactivity index
- Short oxygen reactivity index
- Cerebral blood flow index
Introduction
Plateau waves or A waves, as described by Lundberg and colleagues [12], “follow a specific pattern, characterized by a steep rise to a high level (60–100 mmHg) and, following some minutes, an often equally steep fall.” The increase in intracranial pressure (ICP) during plateau waves reflects vasodilation, with a subsequent increase in cerebral blood volume (CBV) [13] and a decrease in cerebral perfusion pressure (CPP) and cerebral blood flow (CBF). The end of the vasodilatory cascade of a plateau wave should be pursued [13, 14] because long duration of more than 30–40 min is associated with an unfavorable outcome [8]. Multimodal brain monitoring at the bedside helps to identify different patterns of high ICP and the consequences for cerebral hemodynamics combined with cerebral oxygenation. The aim of this study was to describe the characteristics of plateau waves with multimodal brain monitoring in patients with head injury admitted to neurocritical care.
Materials and Methods
This was a prospective observational study completed in 18 patients with severe head injury admitted to the neurocritical care unit (NCCU) at Centro Hospitalar Sao Joao, Porto. Patients were sedated and ventilated and CPP-oriented therapy was managed according to the Brain Trauma Foundation (BTF) guidelines [5–7] whenever we could not calculate optimal CPP using PRx [2]. Multimodal systemic and brain monitoring was applied to the patients for the first 10 days, and variables were continuously recorded using the dedicated software program ICM+. We defined primary variables for heart rate (HR), arterial blood pressure (ABP), ICP, CPP, pulse amplitude (AMP), end tidal CO2 (ETCO2), brain temperature (temp), brain tissue oxygenation pressure (PtO2), cerebral oximetry with transcutaneous near-infrared spectroscopy (CO), and CBF, and secondary variables related to the cerebral compensatory reserve (RAP) [3] and cerebrovascular reactivity indexes were calculated as a moving correlation coefficient using 10-s averages of primary variables over a moving window of 5 min in duration (PRx [9], PAx, [1], ORxshort [10], and CBFx [10]). Intraparenchymal probes were located in areas at risk of developing secondary lesions, mainly in the penumbra area. The compiled data were analyzed offline in patients who developed plateau waves, after local research ethics committee approval and with written consent from the patients’ next of kin.
To study the plateau waves we applied time averages of the primary and secondary variables for 30 min at baseline, during the plateau phase, and two consecutive 30-min intervals after the wave (first 30 min – Δ1 and second 30 min – Δ2).
Statistical analysis was performed using IBM software, SPSS 20. Repeated measures ANOVA were applied to test the significance of the results between baseline values and values found during and after plateau waves, between and within subjects. When applicable, the nonparametric Kruskal–Wallis test was used to test statistical relationships between variables. Values are presented as mean ± SD and statistical significance was considered for p values <0.05.
Results
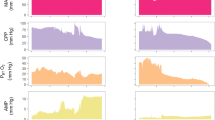
In this study, we identified 59 plateau waves with a mean duration of 8:47 min ± 7:12 that occurred in 44 % of the patients (8 of 18). Sixteen were male with a mean age of 42 years ± 15 and the mean Glasgow Coma Scale score at admission was 6 ± 3. Mortality rate at 3 months after hospital discharge was 17 % and median Glasgow Outcome Scale (GOS) score was 3. An example of a plateau wave using a multimodal monitoring setup is presented in Fig. 1.
Example of a plateau wave characterized by multimodal brain monitoring. The increase in intracranial pressure (ICP) is preceded by a sudden 2-mmHg rise in endtidal CO2 (ETCO 2 ), which may be the trigger of the vasodilatory cascade. During the tidal wave, arterial blood pressure (ABP) remained stable, but cerebral perfusion pressure (CPP), tissue oxygenation (ptO 2 ), and cerebral blood flow (CBF) decreased. After the end of the plateau wave, there was a hyperemic response, with a significant increase in CBF followed by a recovery of brain oxygenation
At baseline, mean ICP was 17.4 ± 5.3 mmHg, with a mean ICP amplitude of 2.7 ± 1.3, a mean CPP of 90.7 ± 11.4 mmHg, and brain tissue oxygenation of 21.4 ± 7.9 mmHg.
At the top of the plateau wave, mean ICP increased at a level of 47.3 ± 6.5 mmHg (p = 0), mean ICP pulse amplitude rose to 6.9 ± 2.7 (p = 0), and mean CPP decreased to 61.1 ± 14 mmHg (p < 0.0001). Cerebral blood flow and cerebrovascular resistance decreased, although not significantly, and brain oxygenation decreased significantly (p = 0.0004) to values below 20 mmHg. Simultaneously, brain hypoxia was detected by cerebral oximetry (CO) although with a less significant value (p = 0.021). We also saw a small increase in endtidal CO2 between baseline and plateau wave, although it was highly significant (p = 0.00042), which may be responsible for triggering the vasodilatory cascade. Detailed data and statistical analysis are presented in Table 1. After the end of plateau wave, a hyperemic response was recorded in 64 % of cases with a significant increase in cerebral blood flow (p = 0.03) and brain oxygenation (p = 0.009) above baseline. The higher magnitude of ICP (Δ ICP) during plateau waves was associated with better pressure and oxygen autoregulation status (PRx and ORx) and low brain oxygenation (CO) described by the model defined with multiple regression analysis (Δ ICP = 8.30*ORx + 0.37*CO − 8*PRx + 49).
Discussion
Head injury patients may develop episodes of high ICP and some of these events are plateau waves. Poor outcome after TBI is associated with sustained high ICP [15] or low oxygenation [4]. However, only long plateau waves of more than 30 min in duration seem to be related to unfavorable outcome [8].
In our NCCU, plateau waves are actively treated, which explains the short duration (mean duration less than 10 min) found in our data. Nevertheless, we were able to demonstrate the statistically significant negative impact of plateau waves on cerebral hemodynamics (CPP, CBF, and CVR) and cerebral oxygenation (CO, PtO2). Also, the transient reactive post-plateau hyperemia may well be a response following the brief period of brain tissue hypoperfusion and hypoxia.
When autoregulation evaluated using PRx was working at baseline, disruption of that phenomenon occurred during the wave phase. The same pattern was observed by the other cerebrovascular reactivity indexes PAx, ORxshort, and CBFx. Moreover, the magnitude of the plateau wave was greater in patients with intact cerebrovascular reactivity. The results presented in our study are consistent with the vasodilatory cascade theory [11] for patients with good autoregulation, but a worse compensatory reserve, as indicated by the shifts of PRx and RAP.
Conclusion
Multimodal brain monitoring permits the early identification of plateau waves and allows better understanding of these intrinsic brain vascular phenomena, which may have a positive influence on the management of head injury patients.
References
Aries MJ, Czosnyka M, Budohoski KP, Kolias AG, Radolovich DK, Lavinio A, Pickard JD, Smielewski P (2012) Continuous monitoring of cerebrovascular reactivity using pulse waveform of intracranial pressure. Neurocrit Care 17:67–76
Aries MJ, Czosnyka M, Budohoski KP, Steiner LA, Lavinio A, Kolias AG, Hutchinson PJ, Brady KM, Menon DK, Pickard JD, Smielewski P (2012) Continuous determination of optimal cerebral perfusion pressure in traumatic brain injury. Crit Care Med 40:2456–2463
Avezaat CJ, van Eijndhoven JH, Wyper DJ (1979) Cerebrospinal fluid pulse pressure and intracranial volume pressure relationships. J Neurol Neurosurg Psychiatry 42:687–700
Balestreri M, Czosnyka M, Hutchinson P, Steiner LA, Hiler M, Smielewski P, Pickard JD (2006) Impact of intracranial pressure and cerebral perfusion pressure on severe disability and mortality after head injury. Neurocrit Care 4:8–13
Brain Trauma Foundation, American Association of Neurological Surgeons, Congress of Neurological Surgeons, Joint Section on Neurotrauma and Critical Care, AANS/CNS, Bratton SL, Chestnut RM, Ghajar J, McConnell Hammond FF, Harris OA, Hartl R, Manley GT, Nemecek A, Newell DW, Rosenthal G, Schouten J, Shutter L, Timmons SD, Ullman JS, Videtta W, Wilberger JE, Wright DW (2007) Guidelines for the management of severe traumatic brain injury. I. Blood pressure and oxygenation. J Neurotrauma 24(Suppl 1):S7–S13
Brain Trauma Foundation, American Association of Neurological Surgeons, Congress of Neurological Surgeons, Joint Section on Neurotrauma and Critical Care, AANS/CNS, Bratton SL, Chestnut RM, Ghajar J, McConnell Hammond FF, Harris OA, Hartl R, Manley GT, Nemecek A, Newell DW, Rosenthal G, Schouten J, Shutter L, Timmons SD, Ullman JS, Videtta W, Wilberger JE, Wright DW (2007) Guidelines for the management of severe traumatic brain injury. XI. Anesthetics, analgesics, and sedatives. J Neurotrauma 24(Suppl 1):S71–S76
Bratton SL, Chestnut RM, Ghajar J (2007) Guidelines for the management of severe traumatic brain injury. IX. Cerebral perfusion thresholds. J Neurotrauma 24(suppl 1):S59–S64
Castellani G, Zweifel C, Kim DJ, Carrera E, Radolovich DK, Smielewski P, Hutchinson PJ, Pickard JD, Czosnyka M (2009) Plateau waves in head injured patients requiring neurocritical care. Neurocrit Care 11:143–150
Czosnyka M, Smielewski P, Kirkpatrick P, Laing RJ, Menon D, Pickard JD (1997) Continuous assessment of the cerebral vasomotor reactivity in head injury. Neurosurgery 41:11–17, discussion 17–19
Dias C, Maia I, Cerejo A, Varsos G, Smielewski P, Paiva JA, Czosnyka M (2013) Pressures, flow, and brain oxygenation during plateau waves of intracranial pressure. Neurocrit Care 21(1):124–132
Hayashi M, Kobayashi H, Handa Y, Kawano H, Hirose S, Ishii H (1991) Plateau-wave phenomenon (II). Occurrence of brain herniation in patients with and without plateau waves. Brain 114(Pt 6):2693–2699
Risberg J, Lundberg N, Ingvar DH (1969) Regional cerebral blood volume during acute transient rises of the intracranial pressure (plateau waves). J Neurosurg 31:303–310
Rosner MJ, Becker DP (1984) Origin and evolution of plateau waves. Experimental observations and a theoretical model. J Neurosurg 60:312–324
Ursino M, Di Giammarco P (1991) A mathematical model of the relationship between cerebral blood volume and intracranial pressure changes: the generation of plateau waves. Ann Biomed Eng 19:15–42
Vik A, Nag T, Fredriksli OA, Skandsen T, Moen KG, Schirmer-Mikalsen K, Manley GT (2008) Relationship of “dose” of intracranial hypertension to outcome in severe traumatic brain injury. J Neurosurg 109:678–684
Disclosure
The ICM+ software for brain monitoring (www.neurosurg.cam.ac.uk/imcplus) is licensed by the University of Cambridge (Cambridge Enterprise). Peter Smielewski and Marek Czosnyka have financial interests in part of the licensing fee. All other authors declare that they have no conflicts of interest.
Conflict of Interest Statement
We declare that we have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2016 Springer International Publishing Switzerland
About this chapter
Cite this chapter
Dias, C., Maia, I., Cerejo, A., Smielewski, P., Paiva, JA., Czosnyka, M. (2016). Plateau Waves of Intracranial Pressure and Multimodal Brain Monitoring. In: Ang, BT. (eds) Intracranial Pressure and Brain Monitoring XV. Acta Neurochirurgica Supplement, vol 122. Springer, Cham. https://doi.org/10.1007/978-3-319-22533-3_29
Download citation
DOI: https://doi.org/10.1007/978-3-319-22533-3_29
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-22532-6
Online ISBN: 978-3-319-22533-3
eBook Packages: MedicineMedicine (R0)