Abstract
Hepatitis B virus (HBV) replicates without killing infected hepatocytes or causing a prolonged activation of innate immunity in vivo. Transient infections can be cleared by the adaptive immune response even after infection has spread to the entire hepatocyte population. Resolution of transient infections requires weeks and is associated with killing of a major fraction of infected hepatocytes by cytotoxic T lymphocytes (CTLs). Cytokines also play a major role, clearing viral DNA replication intermediates from the cytoplasm of infected hepatocytes. A major issue concerns the clearance of covalently closed circular (ccc) DNA, the nuclear transcriptional template of the virus. If clearance is not achieved, infections become chronic, resulting in accelerated turnover of hepatocytes due to an ongoing CTL response. Because hepatocytes appear to form a closed self-renewing population, natural and, especially, CTL-stimulated turnover should lead to genetic narrowing of the hepatocyte population as the patient ages. This process will be further accelerated if any hepatocyte mutations facilitate escape from antiviral CTLs, or provide a growth advantage. Finally, the association of genetic narrowing and expansion of cell clones in the context of chronic liver disease may contribute to the development of hepatocellular carcinoma (HCC), the most serious sequela of chronic hepatitis B.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Hepatitis B virus
- Viral persistence
- HBV persistence
- Viral clearance
- HBV clearance
- Liver cancer
- Hepatocellular carcinoma
Introduction
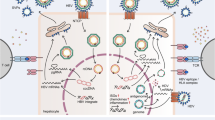
Hepatitis B virus (HBV) has the ability to persistently infect hepatocytes , which constitute about 70 % of the cells in the normal liver. An adult liver contains about 5 × 1011 hepatocytes, of which the vast majority are HBV susceptible. Persistence is probably due to the fact that productive infection (Fig. 6.1), per se, is not cytopathic. That is, infection in the absence of an adaptive immune response does not perceptibly accelerate hepatocyte death. In addition, the hepatocyte population has a low rate of spontaneous turnover and covalently closed circular viral DNA (cccDNA), the viral transcriptional template, appears to be highly stable in nondividing hepatocytes [1], in addition to being present at up to 50 copies per cell. There is evidence that cccDNA may be lost during cell division , as well as evidence that it survives [2–4]; thus, the findings remain controversial.
Cellular life cycle of HBV. The figure shows a model for the life cycle of hepadnaviruses (adapted from ref. [72]; for a detailed description see ref. [72]). Briefly, RNA and DNA containing capsids are shown in magenta and blue, respectively. Envelope proteins are shown in yellow. Upon infection mediated by the entry receptor, NTCP, viral capsids are transported to the nucleus. About 90 % of virus has relaxed circular (rc) DNA, which is converted to covalently closed circular DNA (cccDNA). cccDNA does not undergo semiconservative DNA synthesis. Early in infection, when envelope protein concentrations are low, newly made nucleocapsids, with their enclosed viral DNA, are transported to the nucleus to amplify cccDNA copy. When sufficient envelope is present, nucleocapsids are directed to the secretory pathway and cccDNA amplification ceases. About 10 % of virus has a double stranded linear (dsl) DNA genome as a result of an error in viral DNA replication. These viruses can also infect hepatocytes. cccDNA is formed from dsl DNA by nonhomologous recombination, resulting in a loss of sequences and, generally, rendering this cccDNA unable to support virus replication. Dsl DNA may also integrate into host DNA via nonhomologous recombination; this pathway does not appear to have a role in the virus life cycle, but occurs nonetheless. Even during transient infections, 0.01–0.1 % of hepatocytes may acquire integrated viral DNA
In the first weeks after infection, HBV can spread through the entire hepatocyte population , often without an apparent induction of innate immunity or an inflammatory response (hepatitis). Despite a long delay, often of six or more weeks, the immune system may eventually activate and clear the virus, without destroying the liver in the process. About 95 % of adults recover following HBV infection. In infants and young children the anti-HBV immune response appears to be less vigorous; >90 % of infants under a year of age become chronically infected [5].
HBV clearance is generally associated with an acute inflammatory immune response that destroys large numbers of hepatocytes over a period of a few weeks, and then abates. Residual virus may remain, but its replication is inhibited by the immune system. Thus, subsequent immune suppression can lead to virus reactivation [6, 7]. Antibodies to the hepatitis B surface antigens (HBsAg) present in the viral envelope proteins appear to be an important component of long-term protection against virus rebound, which is also associated with antiviral CTLs that persist in the circulation [8]. For those who do not resolve a productive infection, chronic liver disease often develops, driven to a major degree by the antiviral CTLs.
A hallmark of HBV infection of hepatocytes is that the target population may be entirely self-renewing. In addition, studies with animal models of HBV infection suggest that virus spreads into daughter cells as hepatocytes divide to compensate for death of other hepatocytes [3, 4]. Indeed, it is still unclear if there is a significant role for extracellular spread of virus to maintain infection once the liver is fully infected, or if persistence is maintained primarily by distribution of virus DNA to daughter cells.
In normal human liver, about 0.05 % of hepatocytes are in S phase, which provides a minimum estimate of liver turnover [9]. This rate may be much higher in chronic infection . For instance, in woodchucks chronically infected with woodchuck hepatitis virus (WHV), the hepatocyte turnover rate appears to be increased by ~tenfold, again by scoring S phase hepatocytes [10]. This enhanced turnover is thought to be immune mediated; for instance, it is not found during transient infections of woodchucks or chimpanzees , prior to the infiltration of inflammatory cells into the liver [11, 12]. The turnover rate during transient infections is elevated during the inflammatory response [12, 13].
Elevated turnover during decades of chronic infection has led to the idea that hepatocytes become increasingly senescent and, as a result, less able to contribute to liver maintenance as the disease progresses. Thus, it was thought that while hepatocyte replacement by division of mature hepatocytes declined due to senescence, it was compensated by emergence of oval cells, putative hepatocyte progenitor cells that differentiated into new hepatocytes [14]. This concept had been supported by studies of chemical carcinogenesis in the rat. These studies had suggested that under conditions of hepatocyte injury with agents that also inhibited hepatocyte proliferation, progenitor/stem cells present near the portal triad (e.g., Canals of Hering and bile ductules) gave rise to a proliferating population of oval cells that eventually differentiated into mature hepatocytes to maintain liver size and function [15, 16]. An important implication was that hepatocyte replacement during chronic HBV infection might become increasingly dependent upon a specialized progenitor/stem cell compartment.
However, recent studies suggest that the concepts of hepatocyte senescence and replacement from specialized hepatocyte stem/progenitor cells may be more complicated. First, in serial transplantation studies in mice, using either mouse or human hepatocytes, both normal hepatocytes, and hepatocytes with markers of senescence, were found to enter the cell cycle and contribute to maintenance of liver mass [17]. Second, studies using hepatocyte transplantation to distinguish resident cells from transplanted cells suggested that hepatocyte regeneration, during times of stress leading to oval cell proliferation, still depended, as in the healthy liver, upon the mature hepatocyte population [18, 19]. A recent tweak on this observation suggested that oval cells can contribute to hepatocyte replacement in times of stress, but that these oval cells are formed from the mature hepatocytes, not in a specialized stem/progenitor cell compartment [20]. Dedifferentiation of mature hepatocytes to an oval cell phase may allow their temporary escape from an environment that inhibits proliferation of mature hepatocytes, providing an efficient pathway for hepatocyte replacement. Finally, subsequent findings failed to support the evidence that progenitor cells in the bone marrow contribute to hepatocyte replacement [21, 22]. This notion came from the observation that genetically tagged bone marrow cells appeared to migrate to the liver to form hepatocytes, but this now seems due to marker transfer as a result of cell fusion with hepatocytes [23].
In brief, it appears that virus persistence vs. clearance needs to be understood in the context of a target cell population that may be entirely self-renewing. This raises major questions about the mechanisms for immune clearance of HBV from the fully infected liver and the consequences of persistent immune pressure upon the hepatocyte population in the chronically infected liver. However, it may also rationalize a large body of data that suggest that HCC originates from hepatocytes and not a specialized progenitor cell compartment.
Transient Infection
As noted above, the persistence of HBV is believed to be aided by the stability of cccDNA in the nucleus of infected hepatocytes. Experiments with primary hepatocyte cultures prepared from woodchucks and infected with WHV indicated that cccDNA is stable in nondividing hepatocytes [1] as well as in hepatocytes that have been induced to divide [24]. In contrast, there is a lack of consensus on whether cccDNA survives mitosis in vivo [2–4], and how cccDNA responds to antiviral cytokines [25, 26].
During resolution of transient hepadnavirus infection , killing of infected hepatocytes is due to the cell-mediated adaptive immune response. This response is accompanied by the expression of cytokines including IFNγ and TNFα, that can change the physiological status of infected hepatocytes and lead to loss of virus DNA replication intermediates from the cytoplasm (Fig. 6.1), which appears to occur before loss of cccDNA [12]. The key question is what contributes to the subsequent loss of cccDNA? Is cccDNA loss entirely due to cell death? If so, how are uninfected hepatocytes generated? Is it that cccDNA does not survive mitosis, so that infected hepatocytes that divide, to compensate for the death of other infected hepatocytes, are cured? Or is elimination of cccDNA the result of dilution, requiring that hepatocytes pass though multiple rounds of mitosis to eliminate all the cccDNA copies that were present in the parent cell. Finally, does the inflammatory response activate cellular mechanisms that cause cccDNA destruction or contribute to the observed loss of cccDNA, perhaps during cell division or even in the absence of cell division?
The past 30 years have witnessed efforts to gain insights into the mechanisms that control clearance of HBV from infected livers, as it is observed during transient transfection in human patients, as well as in experimentally infected chimpanzees, woodchucks and ducks. The goal has been to establish a time line for the dynamics of viral and immunological markers during the course of transient infections. While there is large variation between individual patients and experimental animals, the following pattern is common to most transient infections, as shown in mammalian hosts. The clearance phase can last several weeks and is accompanied by the activation of an adaptive immune response manifested by an influx of CD4+ and CD8+ T lymphocytes into the liver parenchyma, expression of IFNγ, TNFα and other cytokines, and by an increased rate of hepatocyte death. During the same time period, cytoplasmic rcDNA and nuclear cccDNA levels decline and become undetectable. Clearance of the virus from the infected liver will occur even when all hepatocy tes have been infected, as determined by immunohistochemistry and/or in situ hybridization for viral antigens and nucleic acids, respectively [12, 27, 28]. Protective antibodies against the surface antigen become detectable during or following the clearance phase.
Observations in human patients are generally limited to analyses of blood samples for the presence of reactive T-cells, serological markers and enzymatic assays to determine hepatocyte cell death. These studies revealed the presence of IFNγ-producing CD4+ and CD8+ T cells that proliferate in vitro upon stimulation with HBV-specific peptides. Consistent with a role of CD8+ CTLs and the presence of an inflammatory response against infected hepatocytes, serum ALT levels are elevated during the clearance phase. These observations provided the basis for a model where a combination of hepatocyte killing and regeneration and inflammatory cytokines are responsible for the natural cure of an HBV infection. However, due to the lack of available human liver biopsy tissue, these studies did not provide any direct information about the fraction of infected hepatocytes, the extent of liver damage or the exact sequence of events that leads to clearance .
The duck and woodchuck model for HBV infections permitted a more detailed study of transient infection, and collection of liver tissue biopsies at regular intervals during the recovery phase, to establish that recovery could occur even after all hepatocytes were infected [27–29]; that is, that full infection of the hepatocyte population did not of necessity lead to chronicity (for technical reasons, full infection means >95 %, beyond which uninfected cells would be virtually impossible to detect even if present). However, a major limitation of these models was the lack of commercially available reagents to follow the course of the immune response. This handicap was partially overcome with the cloning of woodchuck genes coding for the major T cell markers as well as IFNγ and TNFα.
The observation that recovery could occur after apparent infection of the entire hepatocyte population raised the question about the origin of hepatocytes in the recovered liver. Do they really arise from infected hepatocytes, as suggested above and consistent with the notion of a self-contained hepatocyte population, or do they arise from a progenitor/stem cell compartment? Studies of more than 20 woodchucks revealed that the time for recovery as well as the extent of liver damage varies among animals [11, 28–30], and can begin as early as 2–3 weeks post infection, though generally later. The fraction of apoptotic hepatocytes observed in liver biopsies ranged from less than 1 % up to 10 % of the hepatocyte population. While the results from these studies were consistent with a model where killing of hepatocytes played a major role during clearance, they did not reveal the cumulative killing of hepatocyte during the resolution of an infection or identify the origin of virus-free hepatocytes.
An experimental strategy, taking advantage of the occasional integration of viral DNA into chromosomal DNA, provided a solution to both of these problems, the amount of hepatocyte destruction and the origin of uninfected hepatocytes [13]. Integration is a byproduct of hepadnavirus infection (Fig. 6.1) and, as such, not required for the viral life cycle. However, an analysis of the complexity and abundance of the viral-cell DNA junctions can be used to determine the fate of hepatocytes during recovery. The first observation was that the fraction of hepatocytes with integrated WHV DNA remains roughly constant during infection and in the recovered liver. This indicated that a large fraction of recovered hepatocytes were derived from infected hepatocytes, consistent with the idea that progenitor/stem cells do not play a major role in maintenance of the hepatocyte population.
These studies also provided an estimate of the amount of hepatocyte death during virus clearance . Since integration occurs at random sites in host DNA, the complexity of the collection of integration sites will remain at 1.0 unless these hepatocytes divide (complexity = (unique integration sites)/(total integration sites)). In fact, the complexity was much lower in recovered woodchucks, ~0.5 rather than 1.0, indicating that initially infected hepatocytes divided to fill in the space created by hepatocyte killing. Thus, the second major result of this experimental approach was an estimate that cumulative hepatocyte turnover equaled or exceeded 0.7 complete livers [11, 13].
These data, indicating that cured hepatocytes can be derived from infected ones, are again consistent with the more recent evidence that hepatocyte renewal occurs primarily from the hepatocyte population, not from a specialized stem/progenitor cell compartment [20, 31]. Thus, to explain recovery, there must be a mechanism for curing hepatocytes of HBV infection. A major gap in knowledge concerns, obviously, the mechanisms responsible for the loss of cccDNA. In particular, does a cure depend on the action of cytokines to destroy cccDNA or to increase loss of cccDNA during cell division? The notion that cytokines could play an important role in the clearance of HBV was derived from experiments with transgenic mice expressing HBV. In this system, IFNγ and TNFα, for instance, caused the rapid clearance of cytoplasmic DNA replication intermediates [32]. However, a major limitation of the HBV transgenic mouse model is that cccDNA is not formed.
Similar to those described in woodchucks, experiments were also conducted with a small number of HBV infected chimpanzees. The observations made with the primate model expanded upon results with the woodchuck model, especially as many more reagents were available to assess the host response to the infection [12]. Mathematical modeling of the declines in viral markers in the chimpanzee experiments suggested that non-cytolytic clearance of cccDNA due to action of antiviral cytokines (i.e., a mechanism in which cccDNA is eliminated even from nondividing hepatocytes) played a significant role in virus clearance [33].
In contrast, mathematical modeling of data from the woodchuck, this time focused on the amount of hepatocyte turnover during recovery, suggested that there is enough hepatocyte turnover to explain cccDNA clearance, provided (a) new cccDNA formation from DNA replication intermediates is blocked (Fig. 6.1) and (b) existing cccDNA is lost during cell division [11]. This modeling did not invoke non-cytolytic clearance of cccDNA from nondividing hepatocytes.
While the concept of cccDNA loss during cell division seemed at odds with apparent cccDNA survival through cell division (e.g., during treatment of chronically WHV-infected woodchucks with an antiviral nucleoside analog (NA) [3], or in primary woodchuck hepatocyte cultures induced to divide [24]), a recent study with the duck model calls this conclusion into question. This study raised the possibility that NA inhibition of viral DNA synthesis may be incomplete when hepatocytes are dividing [4], allowing new cccDNA formation in dividing cells, a process presumably prevented during immune clearance.
However, interpretations of results from in vivo experiments to immune recovery have to be considered with caution, particularly because analysis of the results depended, ultimately, upon assumptions about the fate of cccDNA in nondividing hepatocytes in the presence of antiviral cytokines. Is it completely stable, or can it really be destabilized by antiviral cytokines? This is a particularly difficult question to approach in vivo because of the large amount of cell death during recovery from transient infections, as well as the inherent problem in obtaining adequate numbers of liver biopsy samples for precise analyses.
How can the information currently available from experimentally infected animals be interpreted and incorporated into a model for the mechanisms responsible for the cure of a transient HBV infection? In consideration of all the available data from woodchucks and chimpanzee, we envision the following model for the resolution of transient infections, which reflects our own bias that cccDNA is refractory to degradation in nondividing cells. Significant revision of this view will of course be necessary if support is obtained for recent evidence that cytokines destabilize cccDNA by APOBEC deamination of cytosine, followed by depurination and degradation by cellular nucleases [25]. To date, we have not found evidence that APOBEC editing of cccDNA is frequent enough to explain its non-cytolytic loss during resolution of transient infections (C. Seeger, unpublished observations).
In brief, infected hepatocytes are recognized by the immune system resulting in an influx of CD4+ and CD8+ T cells, activation of various other inflammatory cells, and production of IFNγ and TNFα. Second, the presence of cytokines in the liver parenchyma leads to an inhibition of HBV replication and elimination of viral DNA replication intermediates from infected cells [12], preventing new cccDNA synthesis. Third, CD8+ CTLs kill infected hepatocytes. Fourth, infected hepatocytes that have lost replicative intermediates, due to cytokines exposure, divide to replace killed hepatocytes and in the process loose cccDNA, resulting in a new population of “cured” hepatocytes. How cured hepatocytes are protected from immediate reinfection cannot be predicted based on the experimental data. It is conceivable that a combination of events is required. They include, besides a lack of HBV production due to inhibition of viral replication, reduced susceptibility of hepatocytes to de novo infection. This might occur via induction of an antiviral state by cytokines expressed by monocytes and T cells. Whether antibodies against viral envelope proteins play a role during these early steps of recovery is doubtful, because anti-HBsAg antibodies often appear weeks after viral DNA becomes undetectable [28, 29].
HBV Persistence During Chronic Infection
Chronic infection , typically acquired in early childhood, may proceed through a number of phases, including a prolonged immune tolerant phase, a clearance phase, a quiescent immune control phase, and sometimes, a virus rebound phase [34, 35].
The immune tolerant phase is so named because patients in this phase, which may last several decades, will, by definition, have high serum virus titers of 109 per ml or more, and normal ALTs. Where liver biopsies are available, these patients should also have little or no evidence of fibrosis or hepatic inflammation. It should be noted, however, that normal ATLs do not necessary indicate immune tolerance and, at least for some patients, this stage may be misdiagnosed in the absence of a liver biopsy [35–38].
The immune tolerant phase may progress to an immune clearance phase, which has many of the hallmarks of recovery from transient infections, except that it may be more prolonged and involve multiple cycles of partial virus clearance and rebound [39]. Optimally this leads to immune control of the infection, with virus titers often <104 per ml, and often at lower levels (e.g., ≤102 per ml). HBsAg titers in the circulation may also be lowered, but not nearly as much as virus titers [40]. The reason HBsAg is more refractory to immune control mechanisms than virus production is unclear. One source of HBsAg production during the immune control phase may be integrated HBV DNA, which is probably present in 1–10 % or more of hepatocytes by this time. Importantly, liver disease may progress during the immune control phase, especially if virus titers exceed 105 per ml [35], probably because immune control of virus replication is incomplete, at least in this situation, so that elevated hepatocyte destruction persists. A more complete loss of control can lead to a severe exacerbation of liver disease [6, 34].
Unfortunately, progression through the immune clearance phase and into the immune control phase is often accompanied by a high incidence of cirrhosis. The risk of HCC in these patients is high, up to 20–30 % or more, especially in those that do not achieve full control of HBV replication [35]. Antiviral therapy with NAs during the immune clearance and immune control phase will reduce the short-term (e.g., 5-year) incidence of HCC ~fivefold, especially in patients with advanced liver disease, less so in patients with less advanced disease and, therefore, a lower short term HCC risk [41]. NA therapy can also lead to some reversal of fibrosis and cirrhosis [42, 43], probably by facilitating progression to and/or replacement of infected by uninfected cells, thereby causing a quantitative reduction in hepatocyte killing. It is not yet known if beginning NA therapy even earlier, during the immune tolerant phase, would have a greater impact on HCC incidence. The effect on HCC risk beyond that achieved by starting NA therapy during later stages of infection remains to be determined [41]. It seems likely, based on existing data [43], that initiating therapy during the imm une tolerance phase would prevent cirrhosis. Cirrhosis may increase the risk of HCC.
In the following sections, we focus on virus persistence, with emphasis on its effects on the hepatocyte population that may influence the progression of chronic hepatitis B (CHB), and contribute to the cancer risk and might, at least based on present knowledge, be mitigated by earlier initiation of antiviral therapy. The emphasis is on the effects of turnover in a closed population, in which dying hepatocytes are ultimately replaced by division of existing hepatocytes. Cirrhosis is not discussed explicitly, though it presumably exacerbates effects that occur in its absence, by providing strong pressure for clonal expansion of rare hepatocytes that are able to survive in the toxic environment of cirrhotic nodules, in which normal blood flow, lobular structure, etc. are impaired. More detailed information on immune control of virus replication, including the possible role of methylation of cccDNA, is discussed in Chap. 4.
Hepatocyte Evolution and Chronic Infection
If the hepatocyte population is closed, as indicated by recent data, even random death of some hepatocytes will lead to clonal expansion of others (including those with initiating mutations that may contribute, ultimately, to neoplasia). That is, in a closed population, some hepatocyte lineages will be lost over time due to random cell death and division, as others divide to maintain liver mass. The larger a lineages becomes (clone size), the lower its risk of being eliminated by random destruction. In comparison, lineages with only one or a few cells would be at greater risk of elimination. In effect, if a hepatocyte does not die early, there is a possibility that it will be able to clonally expand nonselectively, simply due to natural processes. Clonal expansion would be aided if the hepatocyte had a survival or growth advantage as a result of mutational or stable epigenetic changes. Subsequent changes could enhance its rate of clonal expansion even more, facilitating a progression towards neoplasia.
Cirrhotic nodules may show extensive clonality, as suggested above [44–50]. But what is the evidence that clonally expanded hepatocytes exist in the “non-cirrhotic liver”? And is clone size in the non-cirrhotic liver compatible with an origin via random death and division?
First, during chronic infection the emergence of foci of virus negative hepatocytes occurs in the non-cirrhotic liver, even in the presence of high titer viremia, as illustrated for example in a study of chronically infected chimpanzees [51] and, to a lesser extent, woodchucks [52]. Second, clonal expansion of hepatocytes has been detected by using randomly integrated viral DNA as a hepatocyte lineage marker. These assays have detected clones of up to 60,000 hepatocytes in older non-cirrhotic human carriers. This is much larger than expected from random death and regeneration (see below), implying that these cells had either a survival advantage, growth advantage, or both [53, 54]. Even in immune tolerant carriers under the age of 30, unexpectedly large hepatocyte clones have been detected (Kennedy, Bertoletti, Mason, unpublished observations). In addition, early studies of HBV infection provide clear evidence that in long-term carriers many hepatocytes are not productively infected (e.g., [55, 56]). Second, Su and colleagues have reported on focal expansion of ground glass hepatocytes expressing variant HBsAg that has lost immune-dominant epitopes due to deletion mutations [57]. Thus, one likely basis for clonal expansion is failure to support HBV replication, so that cells are no longer targeted by antiviral CTLs. Another is loss of virus epitopes by deletion or mutation. Another might be failure to present viral antigens to antiviral CTLs.
It should be noted, however, that while large numbers of virus negative hepatocytes that may also be HBV resistant arise during the course of chronic infections, this progression does not appear to lead to the immune control stage of chronic hepatitis. The data are more consistent with the current notion that the low level of infection during the immune control stage reflects, predominately, immune control of HBV replication, since immune suppression can lead to reemergence of the virus and acute liver disease [6]. Possible effectors of the immune control of HBV replication are discussed in Chap. 4.
In any case, an obvious concern is that extensive clonal expansion of hepatocytes, especially in an environment predisposing to DNA damage , may be an HCC risk factor. It is noteworthy that clonal hepatocyte expansion leading to liver repopulation and survival is seen in genetic diseases in response to host gene mutations that are toxic to hepatocytes (e.g., alpha-1-antitrypsin deficiency [58]). Repopulation involves hepatocytes with secondary mutations that bypass the toxicity. These patients have an elevated risk of HCC [59]. As in HBV patients, the mechanistic basis for the elevated HCC risk is unknown. It is interesting, though perhaps coincidental, that preneoplastic lesions and HCCs often appear to be resistant to HBV (or, in the woodchuck, WHV) infection [52, 60–64].
Liver cancer is thought to progress through stages of initiation, promotion, and progression [65]. Initiation would be a mutation or epigenetic change that is stably inherited by daughter hepatocytes and gives them a growth or survival advantage . Promotion involves the proliferation of the altered cell population (s). In the context of chemical carcinogenesis , for example, this is typically due to the ability of rare initiated hepatocytes to escape deleterious, growth inhibitory effects an initiating agent has on growth of the bulk of the hepatocyte population. Progression involves the subsequent changes that convert cells in initiated lineages to cancer cells. Possible initiation and progression events during chronic HBV infection would include viral DNA integration, excess mutations due to inhibition of DNA nucleotide excision repair by HBx [66, 67] and oxidative DNA damage, which may be due in part to the action of viral proteins including HBsAg and HBx [68]. Promotion in the context of HBV infection could, in theory, be either selective or nonselective, and likely coexists with progression. The analyses below suggest that promotion in HBV carriers is more likely selective than nonselective. Possible selective advantages could be due to hepatocyte mutations/epigenetic changes that either prevent HBV infection/replication or inhibit presentation of viral antigens to antiviral CTLs, in either case facilitating immune evasion.
Mathematical Modeling of Hepatocyte Dynamics
To illustrate the possible dynamics of clonal hepatocyte repopulation in an HBV patient, we have made some calculations based on estimated rates of hepatocyte turnover in the normal and chronically infected liver. We have based these simulations on a liver specimen size of one million hepatocytes, which reflected a practical computational limit using PCs. (Because the computer program (comp10, Ref. 51) employs a stochastic model , every run gives similar but not the exact same results. In what follows, we show results of single runs). Specifically, we first considered a scenario for expansion of hepatocytes with no survival or growth advantage. We then considered hepatocytes with survival advantages over the remainder of the hepatocyte population due to evasion from antiviral CTLs. Finally, we considered the effect on clonal hepatocyte repopulation if hepatocytes that can evade antiviral CTLs also have a slightly enhanced growth response; that is, they are more likely to divide to replace dying hepatocytes. For instance, studies with heterologous systems have suggested that HBV infection may retard progression of hepatocytes through the cell cycle (e.g., [69]). Initiated hepatocytes that did not support HBV expression would not be subject to this theoretical effect.
It has been reported that 0.05 % of hepatocytes are in S phase at any given time in the normal adult liver [9]. For the purpose of modeling, we assume that the rate of hepatocyte turnover is about three times higher (assuming S phase is about 8 h). This would give a daily turnover rate constant of 0.0015 (0.15 %) in normal liver. We also assume for the purpose of discussion that elevated hepatocyte turnover due to CTL killing usually begins in teenagers [36–38] and that, prior to this, the genetic complexity of the liver is 1; that is, all hepatocytes are uniquely tagged (for the purpose of modeling) but do not contain any deleterious mutations resulting, for instance, from oxidative DNA damage to hepatocytes due to antiviral immune responses. Thus, we only consider events that occur from this time on to expand individual hepatocyte lineages to reduce the complexity of the liver and, occasionally, lead to the emergence of clones with mutations that may predispose to subsequent neoplastic progression.
Effect of Random Death and Regeneration on Complexity and Clonal Expansion in the Hepatocyte Population
We first illustrate a scenario for immune-tolerant patients with neither immune pressure on the hepatocyte population, nor initiating events in hepatocytes that provide a survival or growth advantage. In this model, the daily rate constant for turnover of the hepatocyte population was 0.15 % (0.15 % of hepatocytes die every day), characteristic of healthy liver [9]. Under these conditions, about 0.55 livers worth of hepatocytes would die and be replaced by surviving hepatocytes every year. This rate of turnover, if due to random hepatocyte death and compensating division, would reduce the genetic complexity of the liver about ~12-fold after 20 years and ~30-fold after 50 years (Fig. 6.2). Thus, for immune tolerant HBV carriers as well as healthy individuals in their 30s, about the time the HCC incidence begins to rise, maximum hepatocyte clone sizes should still be relatively small, mostly less than 100 hepatocytes (Fig. 6.3a). If the daily turnover rate was tenfold higher in immune tolerant patients (i.e., if they were not truly immune tolerant), the complexity would be reduced ~100-fold fold after 20 years and ~300-fold after 50 years (Fig. 6.2), and the maximum clone size after 20 years would be larger, but <1500 (Fig. 6.3b). These clone sizes, predicted by random death and regeneration, do not explain the larger clone sizes observed in chronically infected woodchucks, chimpanzees and humans [51, 53, 70]. While it would be possible to achieve larger clones sizes by presuming extraordinarily high rates of random death and regeneration (e.g., see Ref. 51), in what follows, we point out that this is much more readily achieved even for low rates of turnover by assuming clones are composed of hepatocytes with a selective advantage.
Effects of death and division on the complexity of the hepatocyte population. Changes in complexity were calculated using a computer program, comp10 [51] (available upon request). This program tracks the individual fate of an array of one million hepatocytes during daily cycles of killing and division to maintain liver mass. At the start, hepatocytes are designated as either infected or uninfected. Uninfected and infected hepatocytes can have different daily death rates, which are considered here to be first order (i.e., of the form dN/dt = kN(t), where N is the number of infected or uninfected hepatocytes at time t). Output includes the hepatocyte clone size distribution and complexity ((distinct hepatocyte lineages at time t)/(lineages at time zero (one million)) of the hepatocyte population (assuming a complexity of 1 at time 0). In the first three scenarios shown here, all hepatocytes are assumed to be infected and dying with a rate constant ki = 0.0005, 0.0015, or 0.015 (i.e., 0.05, 0.15, and 1.5 % per day). In the next two scenarios, the liver is assumed to contain 0.01 % hepatocytes at t = 0 that are resistant to CTL killing and die at with a background rate constant kr = 0.0015. Typical infected hepatocytes are assumed to die with ki = 0.015 or ki = 0.003
Effects of random death and regeneration on clone size distribution of the hepatocyte population. All hepatocytes were assumed to be infected and die with a rate constant of ki = 0.0015 (panel a) or ki = 0.015 (panel b) (0.15 or 1.5 % per day). Clone size distribution is the fraction of the liver of one million cells that is comprised of clones of the sizes indicated on the abscissa (log scale). The effects on complexity are shown in Fig. 6.2
Clonal Expansion of Hepatocytes that are Resistant to CTL Killing
To illustrate the effect of even a small survival advantage on complexity and clonal hepatocyte expansion, we assumed that typical infected hepatocytes were killed at twice background (0.3 % per day), and that, initially, 1/10,000 hepatocytes had a survival advantage (i.e., due to evasion of CTL killing), dying at the background rate (0.15 % per day). (Note that we are referring to first order rate constants, so the number of cells killed every day will be the indicated percentage of cells in each population.) As illustrated in Fig. 6.2, between 20 and 30 years, a precipitous increase in the rate of complexity loss takes place; this occurs because starting at about 20 years, infected hepatocytes with the higher turnover rate constant are primarily replaced by hepatocytes which evade CTL killing. After either 20 or 30 years, the effect on clone size is dramatic. By 20 years, ~80–90 % of hepatocytes will have a resistant phenotype, dying at the background rate of 0.015 % per day, and these resistant hepatocytes will be in clones up to ~75,000 cells in size (Fig. 6.4b). The loss of complexity is mainly in the infected cell population; after 20 years, half of the original clones of resistant cells remain (clone size ~2,500–50,000), vs. only a few percent of the original infected clones (clone size ~1–50). This trend does not appreciably change after 30 years (Fig. 6.4c), with <1 % of the original infected cell clones remaining, again vs. half of the resistant clones. For contrast, very little happens in a short time of say, 2 years (Fig. 6.4a).
Effects on clone size distribution when a minor population of hepatocytes is resistant to CTL killing and dies at a background rate. 99.99 % of infected hepatocytes at time zero were assumed to die with a rate constant of ki = 0.003 (0.3 % per day). 0.01 % of the hepatocytes at time zero were assumed to be resistant to CTL killing and to die with a background rate constant of kr = 0.0015. This lower turnover rate could be because the cells were resistant to infection or virus gene expression, and/or failed to present virus antigens to antiviral CTLs. Panels (a), (b), and (c) show the effects on clone size distribution after 2, 20, and 30 years, respectively. Effects on complexity are shown in Fig. 6.2
The effect on complexity and clonal expansion is even faster when typical infected hepatocytes die at ten times the background death rate (1.5 % per day) (Figs. 6.2 and 6.5). Again, a small fraction (1/10,000) of hepatocytes present, at the start, are able to evade CTL killing, dying instead at a background rate of 0.15 % per day. By 2 years, resistant cells will constitute 40 % of the hepatocyte population with clones of 10,000–100,000 hepatocytes, suggesting that even a short period of persistent hepatitis might have a dramatic impact on the hepatocyte population if there are cells present in the liver that can evade antiviral CTLs. In this situation, ~90 % of the resistant clones remain (clone size ~40–40,000).
Effects of a higher rate of killing of infected hepatocytes on the expansion of a minor population of resistant hepatocytes. 99.99 % of hepatocytes at time zero were assumed to die with a rate constant of ki = 0.015, five times higher than in Fig. 6.4. 0.01 % of hepatocytes at time zero were assumed to be resistant to CTL killing and to die at with a background rate constant kr = 0.0015. Panels (a), (b) and (c) show the hepatocyte clone size distribution after 1, 2 and 20 years, respectively. The effects on com plexity are shown in Fig. 6.2
While we assume that hepatocytes that can evade antiviral CTLs are present from time zero, they may, of course, arise later with the same result. We also realize that there may be anatomic restrictions on clonal expansion of hepatocytes with a normal growth response. These considerations point out that any stable heterogeneity in the hepatocyte population effecting death rates (e.g., resistance to antiviral CTLs) could lead to significant hepatocyte repopulation, especially within the hepatic lobule. This highlights the major implications of the reports that the hepatocyte population is self-renewing, even in hepatic disease [31], for selective expansion of rare hepatocytes with a survival advantage. Essentially the same picture is seen if the initial fraction of such cells is 1/100,000. These scenarios could explain the larger clone sizes observed in chronically infected woodchucks, chimpanzees and humans [51, 53, 70].
Effects of Enhanced Growth Rate on the Hepatocyte Population
We also considered the effect of an enhanced growth rate for clonal expansion of rare hepatocytes that are able to evade antiviral CTLs. As in Fig. 6.4, we assumed that typical infected cells were killed twice as fast as resistant cells. However, in contrast to that model, we added a proviso that resistant cells were twice as likely to respond to a signal to divide, to maintain liver mass. In the absence of this growth advantage, clone sizes remain <2000 hepatocytes even after 10 years (Fig. 6.6a). In contrast, if the resistant hepatocytes are also twice as likely to divide to maintain liver mass, clones of 10,000–100,000 hepatocytes appear (Fig. 6.6b), resistant cells constituting >90 % of the hepatocyte population. A selective growth advantage alone, without a survival advantage, had similar effects on clonal expansion (Fig. 6.6c) as a selective survival advantage with a growth advantage (Fig. 6.6b). Hepatocytes with a growth advantage (Fig. 6.6c) constitute ~70 % of the hepatocyte population after 10 years. (The effect on clone size with a growth advantage alone is similar to the scenario including a survival advantage because of the higher rate of hepatocyte turnover throughout (0.3 % per day)).
Effects on clonal expansion of hepatocytes with growth and/or survival advantages. 99.99 % hepatocytes at time zero were assumed to die with a rate constant ki = 0.03, twice background. Panel (a): 0.01 % of hepatocytes at time zero were assumed to be resistant to CTL killing and to die with a rate constant of kr = 0.0015. All hepatocytes were assumed to have the same probability of dividing to maintain liver mass. Panel (b): As in panel (a), but resistant hepatocytes were assume to divide with twice the rate constant as the remaining hepatocytes. In this scenario, cells are selected individually and at random to divide, but on each call, a resistant hepatocyte will divide, whereas the remainder has only a 50 % chance of dividing. If not selected, another hepatocyte (either resistant or not) is selected at random and the process repeated until a cell divides. Panel (c): As in panel (b), except all hepatocytes die with the same rate constant, ki = kr = 0.003, twice the background rate of normal liver (see text)
In summary, the biology of the liver as currently understood is conducive to a scenario in which HBV infection promotes clonal expansion of a subset of hepatocytes which have resistance to immune killing, and therefore a greater risk of progressing to HCC, especially if additional changes are acquired that also facilitate neoplastic progression. Based on appearance of foci of virus negative hepatocytes during the course of infection, we assume many of these hepatocytes achieve CTL escape via resistance to HBV. It is important to note that damages that promote survival or growth will be cumulative in a closed population. It is also important to note, however, that the clonal hepatocyte expansion mentioned here largely involves normal appearing hepatocytes, not preneoplastic lesions [53, 54]. Thus, the idea that selective growth of hepatocytes that are resistant to antiviral immunity also reflects promotion of carcinogenesis remains an inference, though in our opinion a plausible one. As noted, studies of genetic diseases of the liver hav e led to similar conclusions about selective growth of disease resistant hepatocytes [59].
Persistence of HBV, Clonal Expansion , and Virus Evolution
The biological properties of large he patocyte clones is not yet understood and it is conceivable that differences exist among clones in terms of their susceptibility to HBV infections as well as NA therapy. Indeed, differences among hepatocyte clones might also influence the dynamics of virus evolution because of continued selection of the fittest members within an existing viral population. Virus evolution during chronic infection is well documented, and has been attributed to immune selection against viral epitopes (Chap. XXX), i.e., immune evasion. This is thought to explain, for example, the HBeAg positive to HBeAg negative transition that sometimes occurs during the immune clearance phase, in which HBeAg negative variants of HBV emerge to become the predominant genotype and virus titers remain detectable and often, but not always, at levels many logs less than before their emergence (e.g., [71, 72]).
Evolution of the virus population to escape the antiviral immune response might occur at two levels, spread of resistant virus and selective clonal expansion, via immune escape, of hepatocytes that become infected by these variants. In the absence of selective pressure, as during the immune clearance phase, HBV variants probably exist in proportion to the estimated HBV mutation frequency of 10−4 to 10−5 per nt. However, hepatocytes infected by an immune escape mutant might be able to expand clonally at the expense of cells infected by wt HBV, if a low level immune selection occurs during the prolonged immune tolerant phase. It might also be that HBV variants with a higher replication rate expand in the liver even without a need for immune evasion. As noted, super-infection resistance is high [73]. However, there is currently no way to know if some leakiness could allow expansion of a mutant with a higher replication rate over the many decades that can characterize the immune tolerant phase of infection. Unfortunately, while there is evidence that some HBeAg negative mutants replicate at a higher rate than wt [74] in cell culture, it is not known if this is true in vivo, especially during chronic infections.
In addition, during the immune clearance phase, at least two events might coexist, differential killing of hepatocytes infected with wt HBV and, if there are one or more rebound phases, spread of mutant and to a lesser extent, wt HBV, to hepatocytes which had been cured of their infection. Thus, elevated killing of hepatocytes infected with wt HBV, as compared to an HBeAg negative variant, might facilitate emergence of the mutant. Whether either of these scenarios actually occurs, in vivo, is unknown.
In summary, virus persistence in patients with active hepatitis seems facilitated by the expansion of immune escape variants of HBV, while wt HBV probably remains predominant in patients with low disease activity, as in the immune tolerance phase. Emergence of immune escape variants of HBV is probably associated with virus spread during the immune clearance phase, as well as clonal expansion of hepatocytes infected by these variants. Spread by super-infection does not, a priori, appear to be a helpful scenario for supplanting the wt unless the mutant has a higher replication rate; whether such variants occur naturally is unknown. To the extent that clonal expansion of hepatocytes leads to promotion and/or progression to HCC, immune escape variants of HBV might be intrinsically procarcinogenic, irrespective of any more direct role they may have in carcinogenesis.
Implications for Future Antiviral Therapies and Prevention of HCC
Investigations on the hepatocyte lineage in mouse models indicate that differentiated hepatocytes are the source for regenerated cells. Consistent with this concept, studies on WHV infections in woodchucks demonstrated that previously infected hepatocytes repopulate the liver after the resolution of transient infections. Moreover, the concept that the hepatocyte population is closed has potentially important implications for the progression to HCC during chronic hepatitis B. In essence, if the population is closed, hepatocyte turnover will inevitably lead to clonal expansion of some lineages at the expense of others. Clonal expansion can influence disease outcome in many ways, depending on properties of individual hepatocytes clones, in terms of their permissiveness for virus replication, their sensitivity to CTL killing and their growth rate. For example, HCC development might be influenced by early genetic lesions, or epigenetic events that are key to HCC development, but require additional changes to eventually lead to cancer.
Because non-cytocidal genetic damage to the hepatocyte population will be cumulative, it would seem useful to consider earlier NA treatment to restrict the accumulation of mutations and expansion of mutant cell populations. Similarly, expansion of clones could have an effect on the outcome of NA-based antiviral therapies, especially if the NAs have any negative impact on cell viability, even a small one. This might explain “leakiness” observed with antiviral therapies. If so, antiviral therapies would benefit from new, non-NA drugs, such as the previously described capsid formation inhibitors .
References
Moraleda G, Saputelli J, Aldrich CE, Averett D, Condreay L, Mason WS. Lack of effect of antiviral therapy in nondividing hepatocyte cultures on the closed circular DNA of woodchuck hepatitis virus. J Virol. 1997;71:9392–9.
Lutgehetmann M, Volz T, Kopke A, Broja T, Tigges E, Lohse AW, et al. In vivo proliferation of hepadnavirus-infected hepatocytes induces loss of covalently closed circular DNA in mice. Hepatology. 2010;52:16–24.
Zhu Y, Yamamoto T, Cullen J, Saputelli J, Aldrich CE, Miller DS, et al. Kinetics of hepadnavirus loss from the liver during inhibition of viral DNA synthesis. J Virol. 2001;75:311–22.
Reaiche-Miller GY, Thorpe M, Low HC, Qiao Q, Scougall CA, Mason WS, et al. Duck hepatitis B virus covalently closed circular DNA appears to survive hepatocyte mitosis in the growing liver. Virology. 2013;446:357–64.
Stevens CE, Neurath RA, Beasley RP, Szmuness W. HBeAg and anti-HBe detection by radioimmunoassay: correlation with vertical transmission of hepatitis B virus in Taiwan. J Med Virol. 1979;3:237–41.
Hoofnagle JH. Reactivation of hepatitis B. Hepatology. 2009;49(5 Suppl):S156–65.
Menne S, Cote PJ, Butler SD, Toshkov IA, Gerin JL, Tennant BC. Immunosuppression reactivates viral replication long after resolution of woodchuck hepatitis virus infection. Hepatology. 2007;45:614–22.
Rehermann B, Ferrari C, Pasquinelli C, Chisari FV. The hepatitis B virus persists for decades after patients recovery from acute viral hepatitis despite active maintenance of a cytotoxic T-lymphocyte response. Nat Med. 1996;2:1104–8.
Mancini R, Marucci L, Benedetti A, Jezequel AM, Orlandi F. Immunohistochemical analysis of S-phase cells in normal human and rat liver by PC10 monoclonal antibody. Liver. 1994;14:57–64.
Mason WS, Cullen J, Moraleda G, Saputelli J, Aldrich CE, Miller DS, et al. Lamivudine therapy of WHV-infected woodchucks. Virology. 1998;245:18–32.
Mason WS, Xu C, Low HC, Saputelli J, Aldrich CE, Scougall C, et al. The amount of hepatocyte turnover that occurred during resolution of transient hepadnavirus infections was lower when virus replication was inhibited with entecavir. J Virol. 2009;83(4):1778–89.
Wieland SF, Spangenberg HC, Thimme R, PUrcell RH, Chisari FV. Expansion and contraction of the hepatitis B virus transcriptional template in infected chimpanzees. Proc Natl Acad Sci U S A. 2004;101:2129–34.
Summers J, Jilbert AR, Yang W, Aldrich CE, Saputelli J, Litwin S, et al. Hepatocyte turnover during resolution of a transient hepadnaviral infection. Proc Natl Acad Sci U S A. 2003;100:11652–9.
Roskams T. Liver stem cells and their implications in hepatocellular and cholangiocarcinoma. Oncogene. 2006;25:3818–22.
Hsia CC, Evarts RP, Nakatsukasa H, Marsden ER, Thorgeirsson SS. Occurrence of oval-type cells in hepatitis B virus-associated human hepatocarcinogenesis. Hepatology. 1992;16:1327–33.
Evarts RP, Nagy P, Marsden E, Thorgeirsson SS. A precursor-product relationship exists between oval cells and hepatocytes in rat liver. Carcinogenesis. 1987;8:1737–40.
Wang MJ, Chen F, Li JX, Liu CC, Zhang HB, Xia Y, et al. Reversal of hepatocyte senescence after continuous in vivo cell proliferation. Hepatology. 2014;60:349–61.
Yanger K, Knigin D, Zong Y, Maggs L, Gu G, Akiyama H, et al. Adult hepatocytes are generated by self-duplication rather than stem cell differentiation. Cell Stem Cell. 2014;15:340–9.
Schaub JR, Malato Y, Gormond C, Willenbring H. Evidence against a stem cell origin of new hepatocytes in a common mouse model of chronic liver injury. Cell Rep. 2014;8:933–9.
Tarlow BD, Pelz C, Naugler WE, Wakefield L, Wilson EM, Finegold MJ, et al. Bipotential adult liver progenitors are derived from chronically injured mature hepatocytes. Cell Stem Cell. 2014;15:605–18.
Theise ND, Krause DS. Bone marrow to liver: the blood of Prometheus. Semin Cell Dev Biol. 2002;13:411–7.
Petersen BE, Bowen WC, Patrene KD, Mars WM, Sullivan AK, Murase N, et al. Bone marrow as a potential source of hepatic oval cells. Science. 1999;284:1168–70.
Wang X, Willenbring H, Akkari Y, Torimaru Y, Foster M, Al-Dhalimy M, et al. Cell fusion is the principal source of bone-marrow-derived hepatocytes. Nature. 2003;422:897–901.
Dandri M, Burda MR, Will H, Petersen J. Increased hepatocyte turnover and inhibition of woodchuck hepatitis B virus replication by adefovir in vitro do not lead to reduction of the closed circular DNA. Hepatology. 2000;32:139–46.
Lucifora J, Xia Y, Reisinger F, Zhang K, Stadler D, Cheng X, et al. Specific and nonhepatotoxic degradation of nuclear hepatitis B virus cccDNA. Science. 2014;343:1221–8.
Chisari FV, Mason WS, Seeger C. Virology. Comment on “Specific and nonhepatotoxic degradation of nuclear hepatitis B virus cccDNA”. Science. 2014;344:1237.
Jilbert AR, Wu T-T, England JM, Hall PM, Carp NZ, O’Connell AP, et al. Rapid resolution of duck hepatitis B virus infections occurs after massive hepatocellular involvement. J Virol. 1992;66:1377–88.
Kajino K, Jilbert AR, Saputelli J, Aldrich CE, Cullen J, Mason WS. Woodchuck hepatitis virus infections: very rapid recovery after a prolonged viremia and infection of virtually every hepatocyte. J Virol. 1994;68:5792–803.
Ponzetto A, Cote PJ, Ford EC, Purcell RH, Gerin JL. Core antigen and antibody in woodchucks after infection with woodchuck hepatitis virus. J Virol. 1984;52:70–6.
Guo J-T, Zhou H, Liu C, Aldrich C, Saputelli J, Whitaker T, et al. Apoptosis and regeneration of hepatocytes during recovery from transient hepadnavirus infection. J Virol. 2000;74:1495–505.
Grompe M. Liver stem cells, where art thou? Cell Stem Cell. 2014;15:257–8.
Guidotti LG, Ishikawa T, Hobbs MV, Matzke B, Schreiber R, Chisari FV. Intracellular inactivation of the hepatitis B virus by cytotoxic T lymphocytes. Immunity. 1996;4:25–36.
Murray JM, Wieland SF, Purcell RH, Chisari FV. Dynamics of hepatitis B virus clearance in chimpanzees. Proc Natl Acad Sci U S A. 2005;102:17780–5.
Yim HJ, Lok AS. Natural history of chronic hepatitis B virus infection: what we knew in 1981 and what we know in 2005. Hepatology. 2006;43(2 Suppl 1):S173–81.
Chen CJ, Yang HI. Natural history of chronic hepatitis B REVEALed. J Gastroenterol Hepatol. 2011;26:628–38.
Wang HY, Chien MH, Huang HP, Chang HC, Wu CC, Chen PJ, et al. Distinct hepatitis B virus dynamics in the immunotolerant and early immunoclearance phases. J Virol. 2010;84:3454–63.
Kennedy PT, Sandalova E, Jo J, Gill U, Ushiro-Lumb I, Tan AT, et al. Preserved T-cell function in children and young adults with immune-tolerant chronic hepatitis B. Gastroenterology. 2012;143:637–45.
Bertoletti A, Kennedy PT. The immune tolerant phase of chronic HBV infection: new perspectives on an old concept. Cell Mol Immunol. 2014;12(3):258–63.
Davis GL, Hoofnagle JH, Waggoner JG. Spontaneous reactivation of chronic hepatitis B virus infection. Gastroenterology. 1984;86(2):230–5.
Evans AA, O’Connell AP, Pugh JC, Mason WS, Shen FM, Chen GC, et al. Geographic variation in viral load among hepatitis B carriers with differing risks of hepatocellular carcinoma. Cancer Epidemiol Biomark Prev. 1998;7:559–65.
Hosaka T, Suzuki F, Kumada H. Long-term entecavir treatment reduces hepatocellular carcinoma incidence in patients with hepatitis B virus infection. Hepatology. 2013;58:96–107.
Marcellin P, Gane E, Buti M, Afdhal N, Sievert W, Jacobson IM, et al. Regression of cirrhosis during treatment with tenofovir disoproxil fumarate for chronic hepatitis B: a 5-year open-label follow-up study. Lancet. 2013;381:468–75.
Tana MM, Hoofnagle JH. Scar undone: long-term therapy of hepatitis B. Lancet. 2013;381:433–4.
Furuya K, Nakamura M, Yamamoto T, Togel K, Otsuka H. Macroregenerative nodule of the liver. A clinicopathologic study of 345 autopsy cases of chronic liver disease. Cancer. 1988;61:99–105.
Mashal RD, Lester SC, Sklar J. Clonal analysis by study of X chromosome inactivation in formalin-fixed paraffin-embedded tissue. Cancer Res. 1993;53:4676–9.
Paradis V, Laurendeau I, Vidaud M, Bedossa P. Clonal analysis of macronodules in cirrhosis. Hepatology. 1998;28:953–8.
Piao Z, Park YN, Kim H, Park C. Clonality of large regenerative nodules in liver cirrhosis. Liver. 1997;17:251–6.
Robinson WS, Klote L, Aoki N. Hepadnaviruses in cirrhotic liver and hepatocellular carcinoma. J Med Virol. 1990;31:18–32.
Yeh SH, Chen PJ, Shau WY, Chen YW, Lee PH, Chen JT, et al. Chromosomal allelic imbalance evolving from liver cirrhosis to hepatocellular carcinoma. Gastroenterology. 2001;121:699–709.
Aoki N, Robinson WS. State of hepatitis B viral genomes in cirrhotic and hepatocellular carcinoma nodules. Mol Biol Med. 1989;6:395–408.
Mason WS, Low HC, Xu C, Aldrich CE, Scougall CA, Grosse A, et al. Detection of clonally expanded hepatocytes in chimpanzees with chronic hepatitis B virus infection. J Virol. 2009;83:8396–408.
Xu C, Yamamoto T, Zhou T, Aldrich CE, Frank K, Cullen JM, et al. The liver of woodchucks chronically infected with the woodchuck hepatitis virus contains foci of virus core antigen-negative hepatocytes with both altered and normal morphology. Virology. 2007;359:283–94.
Tu T, Mason WS, Clouston AD, Shackel NA, McCaughan GW, Yeh MM, et al. Clonal expansion of hepatocytes with a selective advantage occurs during all stages of chronic hepatitis B virus infection. J Viral Hepat. 2015;22(9):737–53.
Mason WS, Liu C, Aldrich CE, Litwin S, Yeh MM. Clonal expansion of normal-appearing human hepatocytes during chronic hepatitis B virus infection. J Virol. 2010;84:8308–15.
Burrell CJ, Gowans EJ, Jilbert AR, Lake JR, Marmion BP. Hepatitis B virus DNA detection by in situ cytohybridization: Implications for viral replication strategy and pathogenesis of chronic hepatitis. Hepatology. 1982;2:85S–91.
Gowans EJ, Burrell CJ, Jilbert AR, Marmion BP. Detection of hepatitis B virus DNA sequences in infected hepatocytes by in situ cytohybridisation. J Med Virol. 1981;8:67–78.
Su IJ, Wang HC, Wu HC, Huang WY. Ground glass hepatocytes contain pre-S mutants and represent preneoplastic lesions in chronic hepatitis B virus infection. J Gastroenterol Hepatol. 2008;23:1169–74.
Rudnick DA, Perlmutter DH. Alpha-1-antitrypsin deficiency: A new paradigm for hepatocellular carcinoma in generic liver disease. Hepatology. 2005;42:514–21.
Marongiu F, Doratiotto S, Montisci S, Pani P, Laconi E. Liver repopulation and carcinogenesis: two sides of the same coin? Am J Pathol. 2008;172:857–64.
Hsu HC, Wu TT, Sheu JC, Wu CY, Chiou TJ, Lee CS, et al. Biologic significance of the detection of HBsAg and HBcAg in liver and tumor from 204 HBsAg-positive patients with primary hepatocellular carcinoma. Hepatology. 1989;9:747–50.
Govindarajan S, Conrad A, Lim B, Valinluck B, Kim AM. P. S. Study of preneoplastic changes in liver cells by immunohistochemical and molecular hybridization techniques. Arch Pathol Lab Med. 1990;114:1042–5.
Yang D, Alt E, Rogler CE. Coordinate expression of N-myc 2 and insulin-like growth factor II in pre-cancerous altered hepatic foci in woodchuck hepatitis virus carriers. Cancer Res. 1993;53:2020–7.
Li Y, Hacker H, Kopp-Schneider A, Protzer U, Bannasch P. Woodchuck hepatitis virus replication and antigen expression gradually decrease in preneoplastic hepatocellular lineages. J Hepatol. 2002;37:478–85.
Radaeva S, Li Y, Hacker HJ, Burger V, Kopp-Schneider A, Bannasch P. Hepadnaviral hepatocarcinogenesis: in situ visualization of viral antigens, cytoplasmic compartmentation, enzymic patterns, and cellular proliferation in preneoplastic hepatocellular lineages in woodchucks. J Hepatol. 2000;33:580–600.
Farber E, Sarma DS. Hepatocarcinogenesis: a dynamic cellular perspective. Lab Invest. 1987;56(1):4–22.
Becker SA, Lee TH, Butel JS, Slagle BL. Hepatitis B virus X protein interferes with cellular DNA repair. J Virol. 1998;72:266–72.
Minor MM, Slagle BL. Hepatitis B virus HBx protein interactions with the ubiquitin proteasome system. Viruses. 2014;6:4683–702.
Higgs MR, Chouteau P, Lerat H. ‘Liver let die’: oxidative DNA damage and hepatotropic viruses. J Gen Virol. 2014;95:991–1004.
Wu BK, Li CC, Chen HJ, Chang JL, Jeng KS, Chou CK, et al. Blocking of G1/S transition and cell death in the regenerating liver of Hepatitis B virus X protein transgenic mice. Biochem Biophys Res Commun. 2006;340:916–28.
Mason WS, Jilbert AR, Summers J. Clonal expansion of hepatocytes during chronic woodchuck hepatitis virus infection. Proc Natl Acad Sci U S A. 2005;102:1139–44.
Wursthorn K, Lutgehetmann M, Dandri M, Volz T, Buggisch P, Zollner B, et al. Peginterferon alpha-2b plus adefovir induce strong cccDNA decline and HBsAg\ reduction in patients with chronic hepatitis B. Hepatology. 2006;44:675–84.
Seeger C, Zoulim F, Mason WS. Hepadnaviruses. 6 ed. Knipe DM, Howley P, editors. Philadelphia: Lippincott, Williams and Wilkins; 2013.
Rodrigues L, Freitas N, Kallakury BV, Menne S, Gudima SO. Super-infection with woodchuck hepatitis virus (WHV) strain WHVNY of the livers chronically infected with the strain WHV7. J Virol. 2014;89:384–405.
Parekh S, Zoulim F, Ahn SH, Tsai A, Li J, Kawai S, et al. Genome replication, virion secretion, and e antigen expression of naturally occurring hepatitis B virus core promoter mutants. J Virol. 2003;77:6601–12.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2016 Springer International Publishing Switzerland
About this chapter
Cite this chapter
Seeger, C., Litwin, S., Mason, W.S. (2016). Hepatitis B Virus: Persistence and Clearance. In: Liaw, YF., Zoulim, F. (eds) Hepatitis B Virus in Human Diseases. Molecular and Translational Medicine. Humana Press, Cham. https://doi.org/10.1007/978-3-319-22330-8_6
Download citation
DOI: https://doi.org/10.1007/978-3-319-22330-8_6
Publisher Name: Humana Press, Cham
Print ISBN: 978-3-319-22329-2
Online ISBN: 978-3-319-22330-8
eBook Packages: MedicineMedicine (R0)