Abstract
This chapter provides a review of the history of home mechanical ventilation (HMV) models in Europe. It also provides an analysis of patient prevalence in HMV programs, patient inclusion, and follow-up in HMV programs, and discussed the role that home telemonitoring can play in HMV programs.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
In the history of mechanical ventilation over the last 100 years, from 1928, when an iron lung was first used at the Children’s Hospital of Boston (Massachusetts), through its highest level of use in the 1940s and 1950s during the poliomyelitis epidemics, until our current times, we realize it is a two-speed journey. The fast track was taken in the 1950s, when iron lungs started to be replaced by positive airway pressure through intubation, and the second track started when the facial mask started to be used as a noninvasive ventilation method. Thus, the possibility of avoiding long hospital stays became a reality with the creation of the first home mechanical ventilation (HMV) programs.
The prevalence rate of HMV has considerably increased in Europe in recent years, both in countries that traditionally had low prevalence rates like Switzerland [1] and the Netherlands [2], in those that had the highest rates, such as France (data source is ANTADIR) [3], and also in countries such as Australia and New Zealand (9.9–12/100,000) [4].
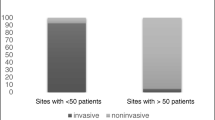
In the beginning, most patients (70 %) were invasively ventilated with positive pressure mechanical ventilators through a tracheostomy tube, and the rate of patients whose ventilation was noninvasive was low. The interfaces used for these were either mouth or nasal pieces (16 %), or negative pressure ventilators (14 %). In the 1980s, nasal masks started to be used in patients with Duchenne’s disease, and negative pressure ventilators were limited to exceptional use [5].
After that time, the use of noninvasively implemented mechanical ventilators spread quickly as the technique of choice among patients with restrictive respiratory failure. Patients adapted to the ventilator in a hospital setting and there were later followed-up in their homes; this is when the first positive pressure noninvasive ventilation (NIV) home programs appeared. In 1994, Leger et al. [6] published the first series of patients treated with positive pressure NIV at home, with a follow-up of 276 patients during 5 years. In this study, the benefit for patients with kyphoscoliosis or sequelae from tuberculosis or Duchenne’s disease was clear. Moreover, the study showed that patients with chronic obstructive pulmonary disease (COPD) and bronchiectasis also benefited from this method. In 1992, the first pressure support ventilator was created and, since then, the technology has improved and different ventilation methods have appeared.
HMV programs have evolved in recent years and, at the same time, technological progress has allowed for an increase in home monitoring of patients on NIV. Moreover, HMV program implementation aims to bring NIV as close to patients’ homes as possible.
2 HMV Prevalence in Europe
HMV’s introduction into Europe has been uneven and has differed according to country. In 2001 and 2002, to assess the pattern of HMV use in Europe, questionnaires were sent to 483 centers in 16 European countries. A total of 329 replied, which amounts to between 62 and 79 % of HMV users in Europe. This data was published in the EUROVENT [7] study. The average prevalence in Europe was estimated at 6.6 patients/100,000 citizens, although there was a large variation between countries: France was the country with the highest prevalence (17/100,000) whereas Poland had the lowest (0.1/100,000) (Table 92.1). Prevalence variation could be related partly to the average years since NIV started to be implemented. Differences in the relative proportion of patients with obstructive disease, rib cage pathology, and neuromuscular disease were also made clear.
In fact, it is highly likely that data obtained from EUROVENT is not up to date. This is the case with Poland, which went from a 0.1/100,000 prevalence in 2002, to 2.5/100,000 in 2010, with a reduction from over 80 % of neuromuscular patients in 2002 to 51 % in 2010 as a result of the increase in the number of patients with respiratory disease [8].
A similar case is that of the Spanish region of Valencia. Spain showed a prevalence of HMV in 1999 of 4.59/100,000 according to a study by De Lucas et al. [9], and of 6.3/100,000 in 2002 according to the EUROVENT study (close to the European average) [7]. In 1999, the Valencia region showed a prevalence of 4.83 [5]. In a study carried out in 2007 by Chiner et al. [10] in the Valencia region, HVM prevalence was proven to have risen to 29/100,000. Although this is data from just one region in Spain, it can probably be extrapolated to the rest of the country.
3 HMV Models
NIV use in patients with chronic respiratory failure is covered by national health systems, however, only a few countries have clear guidelines about how NIV should be started and in which patient groups. Public national health systems and private insurance companies usually hire private home therapy companies to provide and maintain NIV equipment prescribed by patients’ doctors to use when patients are at home. These companies have paramedical staff that can train patients and their families to correctly use NIV. The frequency of visits depends on the type of ventilator prescribed for each patient; the interface type can be adjusted and humidifiers can be provided. In some cases, these companies can also offer other home services, for instance SatO2 night monitoring can be provided. If problems arise at patients’ homes, they are communicated to the prescribing doctor; good coordination with the reference hospital is key [11].
Sometimes, the relationship between these companies and the national health systems is poor and often there is no formal infrastructure. Thus, a European study that was carried out in 16 countries including more than 20,000 ventilated patients showed that, in 62 % of centers, an external company carried out services provided to patients. It also showed that the maintenance frequency ranged between 3 and 12 months; that interaction between the service-providing companies and the hospitals was scarce; that the participation of hospitals in the quality control of equipment was poor; and that there were important differences not only between countries but also within the same country [10, 12].
An outstanding exception is France’s HMV program. The French model’s efficacy is partly attributed to local and regional services, which get support from a specialized center. The services network is effectively maintained as a result of the national capacity to gather data to advance and support the service assessment and research [13].
4 NIV Adaptation in HMV Programs
NIV adaptation can take place during a programmed hospital stay, although it can also be effectively implemented in day hospitals, outpatient settings, and even at the patient’s home. Pallero et al. [14] carried out a multicenter, randomized, prospective study to compare efficacy and costs according to whether the HMV program was started in a hospital setting or an outpatient setting, with patients who had stable chronic respiratory failure with NIV indication. The main study variable was the PaCO2 drop 6 months after NIV initiation. They found a significant decrease in both groups, although they did not find significant differences between them. Direct costs of both interventions were estimated. The hospital setting intervention was estimated to have a cost of 2692 euros, whereas the outpatient setting intervention had a cost of 1500 euros. Therefore, the conclusion was that, because adapting NIV in the outpatient setting is equivalent to doing it in the hospital setting from a therapeutic perspective, adaptation in the outpatient setting could lead to cost-savings for the health system.
5 HMV Programs Follow-Up
There are no data on how frequently patients should see their specialized center doctor, and it depends on different factors such as the patient’s pathology, how they adapt to NIV, and how easy it is for them to travel to the hospital. If a private home services company is involved, some of the follow-up can be done in patients’ homes. The information can be transmitted to the prescribing doctor who, every 3 months, can systematically check the patients’ approximate symptoms, quality of life, ventilation-related side effects, and compliance. After receiving this information, the doctor will be able to determine whatever adjustments are needed.
Some of the tests can only be carried out in the hospital; therefore, it seems that some kind of hospital follow-up is necessary. Generally, the average number of outpatient visits per year is three. Follow-up complementary tests during these visits include arterial blood gas, chest X-rays, and respiratory function tests. The nocturnal evaluation is carried out at home, if possible, or in the hospital to control ventilation quality during the night, and, if possible, in the 3 months following NIV initiation. Patient nocturnal follow-up during the HMV program’s first year includes monitoring O2 saturation, if possible with capnography, respiratory polygraphy, polysomnography, and arterial blood gas first thing in the morning. For many of the more restrictive patients, once they are stable, the supervision required is minimal. Unstable patients or patients who are insufficiently stabilized with NIV need closer follow-up (e.g., those with rapidly progressing neuromuscular diseases such as amyotrophic lateral sclerosis (ALS), and to a lesser extent, patients with muscular dystrophy caused by Duchenne’s disease or COPD) [11].
6 Telemonitoring
Telemonitoring can be used to check a ventilator’s compliance and performance. Although not widely available at the moment, the situation is likely to improve in the future. In 2010, Pinto et al. [15] published a prospective study on 40 patients with ALS who were divided into two groups. In the intervention group, the ventilator data was received by modem, whereas in the control group, compliance and ventilator parameters were checked on official visits. The study did not find differences between groups regarding compliance, although the number of visits to the doctor and to the ER was significantly lower in the group in which telemonitoring was carried out (p < 0.0001). Moreover, although there were no significant differences, survival showed a positive trend in the group with telemonitoring (p = 0.13), the conclusion being that telemonitoring reduces the need to use health services and probably has a favorable impact on costs, survival, and performance status.
Telemonitoring might also be useful for patients other than those with ALS. In 2015, Borel et al. [16] published a study that observed that an increase in the breathing rate and the percentage of respiratory cycles caused by the patient were predictive of a COPD exacerbation. Both parameters can be registered by NIV software.
7 Conclusions
A progressive increase of the NIV prevalence rate by all European Union member states demands cost-effective schemes to manage patients on HMV. Moreover, telemonitoring these patients should be the first option to effectively solve this public health issue, although prospective, multicenter studies that ensure its feasibility are still needed.
Key Major Recommendations
-
The exponential and global growth, in Europe, of patients on HMV programs generates a key area for development.
-
There is a need for European guidelines on home assistance management models.
-
Unique quality indicators for the HMV model are needed.
-
The cost-efficiency of telemonitoring programs requires assessment.
Abbreviations
- ALS:
-
Amyotrophic lateral sclerosis
- COPD:
-
Chronic obstructive pulmonary disease
- HMV:
-
Home mechanical ventilation
- NIV:
-
Noninvasive ventilation
References
Janssens JP, Derivaz S, Breitenstein E, De Muralt B, Fitting JW, Chevrolet JC, Rochat T. Changing patterns in long-term noninvasive ventilation: a 7-year prospective study in the Geneva Lake area. Chest. 2003;123:67–79.
Hazenberg A, Cobben NA, Kampelmacher MJ, Rischen J, Wijkstra PJ. Home mechanical ventilation in the Netherlands. Ned Tijdschr Geneeskd. 2012;156:A3609.
Antadir. www.antadir.com. 2014.
Garner DJ, Berlowitz DJ, Douglas J, Harkness N, Howard M, McArdle N, Naughton MT, Neill A, Piper A, Yeo A, Young A. Home mechanical ventilation in Australia and New Zealand. Eur Respir J. 2013;41:39–45.
Diaz Lobato S, Mayorales AS. Modern non-invasive mechanical ventilation turns 25. Arch Bronconeumol. 2013;49:475–9.
Eger P, Bedicam JM, Cornette A, Reybet-Degat O, et al. Nasal intermittent positive pressure. Long-term follow-up in patients with severe chronic respiratory insufficiency. Chest. 1994;105:100–5.
Lloyd-Owen SJ, Donaldson GC, Ambrosino N, et al. Patterns of home mechanical ventilation use in Europe: results from the Eurovent survey. Eur Respir J. 2005;25:1025–31.
Nasiłowski J, Wachulski M, Trznadel W, et al. The evolution of home mechanical ventilation in Poland between 2000 and 2010. Respir Care. 2015;60:577–85.
De Lucas Ramos P, Rodríguez González-Moro JM, Paz González L, et al. Estado actual de la ventilación domiciliaria en España: resultados de una encuesta de ámbito nacional. Arch Bronconeumol. 2000;36:545–50.
Chiner E, Llombart M, Martínez-García MA, Fernández-Fabrellas E, et al. Noninvasive mechanical ventilation in Valencia, Spain: from theory to practice. Arch Bronconeumol. 2009;45:118–22.
Leger P, Laier-Groeneveld G. Infrastructure, funding and follow-up in a programme of noninvasive ventilation. Eur Respir J. 2002;20:1573–8.
Farré R, Lloyd-Owen SJ, Ambrosino N, et al. Quality control of equipment in home mechanical ventilation: a European survey. Eur Respir J. 2005;26:86–94.
Stuart M, Weinrich M. Integrated health system for chronic disease management – lessons learned from France. Chest. 2004;125:695–703.
Pallero M, Puy C, Güell R, et al. Ambulatory adaptation to noninvasive ventilation in restrictive pulmonary disease: a randomized trial with cost assessment. Respir Med. 2014;108:1014–22.
Pinto A, Almeida JP, Pinto S, Pereira J, Oliveira AG, de Carvalho M. Home telemonitoring of non-invasive ventilation decreases healthcare utilisation in a prospective controlled trial of patients with amyotrophic lateral sclerosis. J Neurol Neurosurg Psychiatr. 2010;81:1238–42.
Borel JC, Pelletier J, Taleux N, et al. Parameters recorded by software of non-invasive ventilators predict COPD exacerbation: a proof-of-concept study. Thorax. 2015;70:284–5.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2016 Springer International Publishing Switzerland
About this chapter
Cite this chapter
Ribas-Solís, F.J., Garcia-Fuertes, J.A., Egea-Santaolalla, C.J. (2016). European Models of Home Noninvasive Mechanical Ventilation: What Have We Learned? Evidence and Key Determinants. In: Esquinas, A. (eds) Noninvasive Mechanical Ventilation. Springer, Cham. https://doi.org/10.1007/978-3-319-21653-9_92
Download citation
DOI: https://doi.org/10.1007/978-3-319-21653-9_92
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-21652-2
Online ISBN: 978-3-319-21653-9
eBook Packages: MedicineMedicine (R0)