Abstract
Rathke’s cleft cysts (RCCs) are benign, nonadenomatous lesions of the sellar and supra-parasellar areas, which are included in the differential diagnosis with other cystic lesions in such regions, such as craniopharyngiomas, arachnoid cysts, epidermoid cysts, cystic pituitary adenomas, etc.RCCs may remain located within the sella or even extending into the suprasellar space or, conversely, arising as purely suprasellar lesions.Indeed, symptomatic RCCs have historically been felt to be uncommon, determining mass effect on the surrounding structures causing endocrinological and/or neurological dysfunction.Symptomatic patients may present with headaches, visual disturbance, hyperprolactinemia, and/or varying degrees of hypopituitarism, thus requiring surgical removal. The optimal surgical strategy varies according to both clinical status and cyst volume and location (intrasellar/intra-suprasellar cysts Vs. purely suprasellar cysts). With the advent, refinement, and widespreading of the endoscopic endonasal technique for removing pituitary lesion, this technique has been advocated for the treatment of different sellar and suprasellar lesions, including the Rathke’scleft cysts.Thus, lesions that are purely intrasellar or intra-/suprasellar can be removed via a “standard” endoscopic endonasal approach, whereas patients with supraglandular cysts may be candidate to a transtuberculum transplanum “extended” approach. A key point in the surgical management of RCCs is that the simple cyst emptying with a limited removal of any nonadherent cyst wall as specimens for the histopathological diagnosis is usually sufficient to improve or even resolve the preoperative symptoms, mainly related with the mass effect due to the cyst enlargement over time.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
Cysts of the Rathke’s cleft or pouch are benign, nonadenomatous lesions of the sellar and supra-parasellar areas, which are included in the differential diagnosis with other cystic lesions in such regions, such as craniopharyngiomas, arachnoid cysts, epidermoid cysts, cystic pituitary adenomas, etc. The pathogenesis of Rathke’s cleft cysts (RCCs) remains controversial: cuboidal or ciliated columnar epithelial cells line the majority of RCCs, and the leading theory is that they represent remnants from the incomplete obliteration of Rathke’s pouch during embryological development [1]. As a matter of fact, the Rathke’s pouch represents a superiorly directed evagination from the stomodeum of the 4-week-old human embryo, which becomes entirely obliterated with the exception of its cranial portion by the seventh week of gestation. The anterior wall of the remaining small cavity forms the anterior lobe of the pituitary gland (adenohypophysis), and its posterior wall proliferates much less to become the pars intermedia of the gland. The posterior lobe and the pituitary stalk form from an inferiorly directed evagination from the diencephalon, which meets the Rathke’s pouch at the level of the sella turcica.
Indeed, residual clefts of Rathke’s pouch may persist, and a residual cavity between the anterior and posterior lobes may be commonly found even in the adult life as small fluid cysts. Such facts explain the reason why growing RCCs, when intrasellar or intra-suprasellar, typically split the gland, pushing and compressing the adenohypophysis anteriorly and the neurohypophysis posteriorly in sagittal MRI images. There are several routine autopsy studies that describe the finding of asymptomatic cysts of the Rathke’s pouch in up to 13–33 % of normal pituitary glands and, actually, account for the 2–9 % of all the intracranial tumors removed via a transsphenoidal approach [2–16].
Typically, RCCs are lined with cuboidal or columnar epithelial cells, often ciliated, and include mucin-secreting goblet cells, which stain positively by the periodic acid-Schiff method. Stratified or pseudostratified squamous epithelium may also be present and may rest on a collagenous connective tissue stroma. However, since stratified squamous epithelial cells (like those typically seen in craniopharyngiomas) are sometimes noted to line a portion of RCCs, some authors have speculated that RCCs and craniopharyngiomas have continuum of cystic sellar lesions [1].
In most instances RCCs remain asymptomatic, remaining located within the sella or even extending into the suprasellar space or, conversely, arising as purely suprasellar lesions. Indeed, symptomatic RCCs have historically been felt to be uncommon, determining mass effect on the surrounding structures causing endocrinological and/or neurological dysfunction. Symptomatic patients may present with headaches, visual disturbance, hyperprolactinemia, and/or varying degrees of hypopituitarism. In such cases, RCCs require surgical removal [1, 6–8, 10, 11, 13, 17–19].
2 Endoscopic Endonasal Transsphenoidal Approach to RCCs
RCCs are characterized based on preoperative and postoperative magnetic resonance (MR) findings and categorized as (a) purely intrasellar, (b) intrasellar/suprasellar, or (c) purely suprasellar [1]. Therefore, the optimal surgical strategy varies according to both clinical status and cyst volume and location. Furthermore, during the last 20 years, with the advent, refinement, and wide-spreading of the endoscopic endonasal technique for removing pituitary lesion, this technique has been advocated for the treatment of different sellar and suprasellar lesions, including the Rathke’s cleft cyst [5, 9, 15, 16, 20–22]. More recently, the introduction of the endoscopic endonasal “extended” approaches permitted to access the suprasellar area thanks to the additional removal of the tuberculum sellae and posterior portion of the sphenoidal planum, rendering amenable the excision of purely suprasellar lesions, traditionally removed via a transcranial route only [23].
As a matter of fact, the surgical treatment of the RCCs can be performed via a “standard” endoscopic endonasal approach for those lesions that are purely intrasellar or intra-/suprasellar, whereas patients with supraglandular cysts may be candidate to transtuberculum transplanum “extended” approach.
The procedure is typically performed using a rigid 0° endoscope, 18 cm in length and 4 mm in diameter (Karl Storz Endoscopy, Tuttlingen, Germany), as the sole visualizing tool. The 30–45° angled endoscopes are usually employed to explore large intra-suprasellar tumor residual cavities. The details of the surgical procedures have been already described in previous publications [20, 23–25]. However, there are significant differences in the surgical management of the RCC with an intra- or intra-suprasellar location and those with a purely suprasellar location, so that we will analyze relative peculiar features separately.
2.1 Intra-suprasellar RCCs
The nasal and sphenoidal steps of the procedure are performed following the same principles of the standard pituitary approach for pituitary adenomas: a binarial 3–4 hands technique is usually adopted; as for standard pituitary surgery, no middle turbinate is routinely removed in both nostrils; they are simply lateralized with an elevator and are repositioned back at the end of the procedure. In purely intrasellar RCC, the sellar floor is extensively removed down to the clival recess to grant proper maneuverability of the surgical instruments inside the sella. Anyway, it can be useful to preserve a reliable extradural plane undermining the bony edges, in order to allow an effective extradural closure of the sellar floor in case of intraoperative CSF leak. After the opening of the dura mater, one may directly access the cyst or may first see the normal pituitary gland, since, usually, the adenohypophysis is pushed anteriorly by the cyst. Though, the cyst is entered and emptied and any floating part of the cyst wall is taken out by sharp maneuvers, in order to have the histopathological confirm of the diagnosis. As the residual cavity is wide enough, the endoscope is inserted inside the residual cavity: by continuous irrigation through the irrigation sheath, the so-called diving technique is performed, which is a similar technique used by laparoscopic surgeons in creating the pneumoperitoneum [26]. This permits the removal of any cystic content remnant eventually adherent to the cyst wall. Should the cyst wall be tightly adherent to the pituitary tissue, the dissection maneuvers should be limited or even avoided in order to lower the chance of causing any postoperative further impairment of the pituitary function. At the end of the procedure, no sellar closure is performed, unless an intraoperative CSF leak occurred [27].
2.2 Purely Suprasellar RCCs
In purely suprasellar RCCs, the sellar cavity is usually not enlarged and an endoscopic endonasal transtuberculum/transplanum approach is needed to access the suprasellar area. As already described elsewhere [20–23, 25, 28], the approach is realized through both nostrils with a middle turbinectomy on one side, resection of the posterior portion of the nasal septum and a wider anterior sphenoidotomy. Owing that RCCs content is in most cases fluid, large bone opening over the planum sphenoidale is usually not required and extensive drilling at the level of the medial opto-carotid recess or over the planum sphenoidale is not mandatory [29]. The cyst is usually clearly identified after the dura opening. Such maneuver usually causes the creation of a large CSF leakage, since the anterior part of the cyst wall is surrounded by or even intimately adherent to the arachnoid of the suprasellar cistern; the remaining part of the cyst wall can be attached to the pituitary stalk – which is usually pushed contralaterally – the superior hypophyseal arteries and/or the optic chiasm. In such cases it is of utmost importance to avoid tractions in order to prevent injuries to these neurovascular structures. Bimanual microsurgical dissection is performed while dealing with the anatomical structures in the suprasellar area: one surgeon works bimanually, with either sharp and blunt instruments, to dissect, if easily possible and remove the cyst wall, while a second surgeon drives dynamically the endoscope.
Concerning the reconstruction of the skull base and dural defects, the techniques vary according to the surgical procedure adopted and the grade of the intraoperative CSF leak [27]. In case of a standard approach without any evidence of intraoperative CSF leakage, no reconstruction is usually performed; anyway, a single layer of dural substitute can be placed extradurally in order to close the dural opening. Should a CSF leak occur, especially in cases of extended approaches with the creation of a large CSF leak, one of the reliable methods for the reconstruction is the intradural filling of the dead space with a gasket-seal extradural closure of the osteodural defect eventually supported by a pedicled nasoseptal flap [27, 30–32].
2.3 Outcome
2.3.1 Extent of Resection, Clinical Symptoms, and Recurrence Rate
In terms of the extent of resection, a gross total removal can be defined as a condition of complete cyst content evacuation with cyst wall removal, while subtotal removal is usually intended as the cyst drainage with eventual partial removal or even a simple biopsy of the cyst wall. One should always balance the opportunity to seek a total removal of the cyst wall with the possibility of creating new postoperative hormonal deficits of either the anterior or the posterior gland and also injury of the neurovascular structures, thus risking the creation of a new visual deficit or the worsening of any preoperative visual impairment. On the other side, a subtotal removal poses higher risks of cyst recurrence. It should be noted that, usually, a standard approach is reserved for the treatment of a purely intrasellar or intra- and suprasellar cysts, while the extended endoscopic surgical techniques are adopted for the removal of those cysts with a purely suprasellar location or to remove the suprasellar component of intra-suprasellar cysts. Having such principles in mind, the most common occurrence in case of RCCs is the subtotal removal. As a matter of fact, the simple cyst emptying with a limited removal of any nonadherent cyst wall as specimens for the histopathological diagnosis is usually sufficient to improve or even resolve the preoperative symptoms, mainly related with the mass effect due to the cyst enlargement. Indeed, in many clinical series on RCCs, the most common presenting symptom – i.e., headache – resolves in the majority of cases [1, 6–9, 11, 12, 15, 16, 19, 21, 22]. Besides, the visual symptoms may improve with simple cyst content evacuation, while the creation of new visual deficits with the endoscopic endonasal approach is rare [6, 33].
Concerning the endocrine outcome, it should be noted that symptomatic RCCs predominantly occur in women; however, this may simply reflect a detection bias as irregular menses often trigger an endocrine evaluation [1]. Anyway, preoperative endocrine deficits are those that more inconstantly improve postoperatively. In some series an anterior pituitary insufficiency has been reported to improve in roughly half of the patients, while in others such symptoms are recorded to not improve at all [1, 6–9, 11, 12, 15, 16, 19, 21, 22]. Conversely, the creation of new hormonal deficits has been reported in a more consistent way; the occurrence of new anterior pituitary insufficiency and also diabetes insipidus (either transient or permanent) is a fairly common evidence, especially when the surgeon attempts to extensively remove the cyst wall. It is worth of note that patients with the higher chances of new hormonal deficiencies usually have suprasellar RCCs that are intimately attached to the pituitary stalk, making their removal considerably more challenging [1]. Also the rate of delayed hyponatremia has been described to be higher (17 %) in RCCs than the 2–17 % rate reported after transsphenoidal surgery for pituitary adenomas [1]. Delayed hyponatremia after pituitary surgery is generally attributed to the syndrome of inappropriate secretion of antidiuretic hormone (SIADH). It is possible that a higher rate of hyponatremia after RCC surgery may result from the closer anatomical association of RCC with the pituitary stalk and neurohypophysis [1].
Concerning the possibility of cyst recurrence, several authors have concluded that a relatively high rate of recurrence may indicate a link between RCCs and craniopharyngiomas, while the extent of resection of the cyst wall was not associated with an increased rate of recurrence, founding no differences in recurrence rates between radical and subtotal resections [34].
It can be concluded that the removal of a different Rathke’s cleft cyst can benefit from the adaptability of the endoscopic endonasal approach, having the possibility of extending the surgical route to the tuberculum sellae and the posterior sphenoid in those cases where the cyst cannot be effectively drained via a standard transsphenoidal corridor or when the cyst is purely located in the suprasellar area. As well, the cyst wall total removal does not represent a key step to gain the resolution of the pathology; it can be thought reasonable to leave a residual behind when it is tightly attached to the surrounding neurovascular structures [20]. Particularly, there is no conclusive evidence that a more aggressive resection of the cyst wall can result in a lower risk of recurrence (Figs. 10.1, 10.2, 10.3 and 10.4).
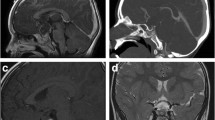
Contrast-enhanced sellar MRI, sagittal image: typical appearance of a Rathke’s cleft cyst. The cyst has split the pituitary gland: the adenohypophysis has been moved and compressed anteriorly, and the neurohypophysis has been moved and compressed posteriorly. The cystic content does not show contrast enhancement
References
Cohan P, Foulad A, Esposito F, Martin NA, Kelly DF (2004) Symptomatic Rathke’s cleft cysts: a report of 24 cases. J Endocrinol Invest 27(10):943–948
Arai T, Horiguchi K, Saeki N, Oka H, Saito T, Takahashi-Fujigasaki J, Sakamoto H, Kato N, Dobashi H, Tanaka T, Hasegawa Y, Abe T (2011) Surgical treatment of a calcified Rathke’s cleft cyst with endoscopic extended transsphenoidal surgery–case report. Neurol Med Chir (Tokyo) 51(7):535–538
Brassier G, Morandi X, Tayiar E, Riffaud L, Chabert E, Heresbach N, Poirier JY, Carsin-Nicol B (1999) Rathke’s cleft cysts: surgical-MRI correlation in 16 symptomatic cases. J Neuroradiol 26(3):162–171
Ceylan S, Koc K, Anik I (2009) Extended endoscopic approaches for midline skull-base lesions. Neurosurg Rev 32(3):309–319; discussion 318–309. doi:10.1007/s10143-009-0201-9
Dusick JR, Esposito F, Kelly DF, Cohan P, DeSalles A, Becker DP, Martin NA (2005) The extended direct endonasal transsphenoidal approach for nonadenomatous suprasellar tumors. J Neurosurg 102(5):832–841
el-Mahdy W, Powell M (1998) Transsphenoidal management of 28 symptomatic Rathke’s cleft cysts, with special reference to visual and hormonal recovery. Neurosurgery 42(1):7–16; discussion 16–17
Fan J, Peng Y, Qi S, Zhang XA, Qiu B, Pan J (2013) Individualized surgical strategies for Rathke cleft cyst based on cyst location. J Neurosurg 119(6):1437–1446. doi:10.3171/2013.8.JNS13777
Fan MC, Wang QL, Wang JF, Deng WS, Li LD, Wang ZH, Sun P (2012) Surgical treatment of symptomatic Rathke’s cleft cysts: clinical features, therapy considerations and outcomes. Chin Med J (Engl) 125(16):2919–2924
Frank G, Sciarretta V, Mazzatenta D, Farneti G, Modugno GC, Pasquini E (2005) Transsphenoidal endoscopic approach in the treatment of Rathke’s cleft cyst. Neurosurgery 56(1):124–128; discussion 129
Jahangiri A, Molinaro AM, Tarapore PE, Blevins L Jr, Auguste KI, Gupta N, Kunwar S, Aghi MK (2011) Rathke cleft cysts in pediatric patients: presentation, surgical management, and postoperative outcomes. Neurosurg Focus 31(1):E3. doi:10.3171/2011.5.FOCUS1178
Kim E (2012) Symptomatic Rathke cleft cyst: clinical features and surgical outcomes. World Neurosurg 78(5):527–534. doi:10.1016/j.wneu.2011.12.091
Laws ER, Kanter AS (2004) Rathke cleft cysts. J Neurosurg 101(4):571–572; discussion 572. doi:10.3171/jns.2004.101.4.0571
Potts MB, Jahangiri A, Lamborn KR, Blevins LS, Kunwar S, Aghi MK (2011) Suprasellar Rathke cleft cysts: clinical presentation and treatment outcomes. Neurosurgery 69(5):1058–1068; discussion 1068–1057. doi:10.1227/NEU.0b013e318228bcea
Trifanescu R, Ansorge O, Wass JA, Grossman AB, Karavitaki N (2012) Rathke’s cleft cysts. Clin Endocrinol (Oxf) 76(2):151–160. doi:10.1111/j.1365-2265.2011.04235.x
Xie T, Hu F, Yu Y, Gu Y, Wang X, Zhang X (2011) Endoscopic endonasal resection of symptomatic Rathke cleft cysts. J Clin Neurosci 18(6):760–762. doi:10.1016/j.jocn.2010.10.014
Zada G (2011) Rathke cleft cysts: a review of clinical and surgical management. Neurosurg Focus 31(1):E1. doi:10.3171/2011.5.FOCUS1183
Benveniste RJ, King WA, Walsh J, Lee JS, Naidich TP, Post KD (2004) Surgery for Rathke cleft cysts: technical considerations and outcomes. J Neurosurg 101(4):577–584. doi:10.3171/jns.2004.101.4.0577
Han SJ, Rolston JD, Jahangiri A, Aghi MK (2014) Rathke’s cleft cysts: review of natural history and surgical outcomes. J Neurooncol 117(2):197–203. doi:10.1007/s11060-013-1272-6
Koutourousiou M, Grotenhuis A, Kontogeorgos G, Seretis A (2009) Treatment of Rathke’s cleft cysts: experience at a single centre. J Clin Neurosci 16(7):900–903. doi:10.1016/j.jocn.2008.10.007
Cavallo LM, Prevedello D, Esposito F, Laws ER, Dusick JR, Messina A, Jane JA, Kelly DF, Cappabianca P (2008) The role of the endoscope in the transsphenoidal management of cystic lesions of the sellar region. Neurosurg Rev 31(1):55–64. doi:10.1007/S10143-007-0098-0
Jahangiri A, Potts M, Kunwar S, Blevins L, El-Sayed IH, Aghi MK (2014) Extended endoscopic endonasal approach for suprasellar Rathke’s cleft cysts. J Clin Neurosci 21(5):779–785. doi:10.1016/j.jocn.2013.07.023
Madhok R, Prevedello DM, Gardner P, Carrau RL, Snyderman CH, Kassam AB (2010) Endoscopic endonasal resection of Rathke cleft cysts: clinical outcomes and surgical nuances. J Neurosurg 112(6):1333–1339. doi:10.3171/2009.10.JNS09348
Cappabianca P, Cavallo LM, Esposito F, de Divitiis O, Messina A, de Divitiis E (2008) Extended endoscopic endonasal approach to the midline skull base: the evolving role of transsphenoidal surgery. In: Pickard JD, Akalan N, Di Rocco C et al (eds) Advances and technical standards in neurosurgery. Springer, Wien New York, pp 152–199
Cappabianca P, Cavallo LM, de Divitiis E (2004) Endoscopic endonasal transsphenoidal surgery. Neurosurgery 55(4):933–940; discussion 940–941
de Divitiis E, Cavallo LM, Cappabianca P, Esposito F (2007) Extended endoscopic endonasal transsphenoidal approach for the removal of suprasellar tumors: part 2. Neurosurgery 60(1):46–58; discussion 58–59
Locatelli D, Canevari FR, Acchiardi I, Castelnuovo P (2010) The endoscopic diving technique in pituitary and cranial base surgery: technical note. Neurosurgery 66(2):E400–E401; discussion E401. doi:10.1227/01.NEU.0000363746.84763.A5
Esposito F, Dusick JR, Fatemi N, Kelly DF (2007) Graded repair of cranial base defects and cerebrospinal fluid leaks in transsphenoidal surgery. Neurosurgery 60(2):ONS1–ONS9
Kassam A, Snyderman CH, Mintz A, Gardner P, Carrau RL (2005) Expanded endonasal approach: the rostrocaudal axis. Part I. Crista galli to the sella turcica. Neurosurg Focus 19(1):E3:1–E3:12
de Notaris M, Solari D, Cavallo LM, D’Enza AI, Ensenat J, Berenguer J, Ferrer E, Prats-Galino A, Cappabianca P (2012) The “suprasellar notch,” or the tuberculum sellae as seen from below: definition, features, and clinical implications from an endoscopic endonasal perspective. J Neurosurg 116(3):622–629. doi:10.3171/2011.11.JNS111162
Cavallo LM, Messina A, Esposito F, de Diviths O, Dal Fabbro M, de Diviths E, Cappabianca P (2007) Skull base reconstruction in the extended endoscopic transsphenoidal approach for suprasellar lesions. J Neurosurg 107(4):713–720. doi:10.3171/Jns-07/10/0713
Leng LZ, Brown S, Anand VK, Schwartz TH (2008) “Gasket-seal” watertight closure in minimal-access endoscopic cranial base surgery. Neurosurgery 62(5 Suppl 2):ONSE342–ONSE343; discussion ONSE343. doi:10.1227/01.neu.0000326017.84315.1f 00006123-200805002-00010 [pii]
Cappabianca P, Esposito F, Magro F, Cavallo LM, Solari D, Stella L, de Divitiis O (2010) Natura Abhorret a Vacuo-use of fibrin glue as a filler and sealant in neurosurgical “dead spaces”. Technical note. Acta Neurochir (Wien) 152(5):897–904. doi:10.1007/S00701-009-0580-2
Cappabianca P, Cavallo LM, Esposito F, De Divitiis O, Messina A, De Divitiis E (2008) Extended endoscopic endonasal approach to the midline skull base: the evolving role of transsphenoidal surgery. Adv Tech Stand Neurosurg 33:151–199
Aho CJ, Liu C, Zelman V, Couldwell WT, Weiss MH (2005) Surgical outcomes in 118 patients with Rathke cleft cysts. J Neurosurg 102(2):189–193. doi:10.3171/jns.2005.102.2.0189
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
1 Electronic Supplementary Material
Below is the link to the electronic supplementary material.
Video 10.1
(MP4 39735 kb)
Rights and permissions
Copyright information
© 2016 Springer International Publishing Switzerland
About this chapter
Cite this chapter
Esposito, F. (2016). Rathke’s Cleft Cyst: Endoscopic Endonasal Transsphenoidal Approach. In: Cappabianca, P., Cavallo, L., de Divitiis, O., Esposito, F. (eds) Midline Skull Base Surgery. Springer, Cham. https://doi.org/10.1007/978-3-319-21533-4_10
Download citation
DOI: https://doi.org/10.1007/978-3-319-21533-4_10
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-21532-7
Online ISBN: 978-3-319-21533-4
eBook Packages: MedicineMedicine (R0)