Abstract
The utricular otolith and the mechanosensory lateral line of the toadfish, Opsanus tau, were investigated for sensitivity to multimodal sensory input by recording neural activity from free swimming fish. The utricle was sensitive to horizontal body movement, and displayed broad sensitivity to low frequency (80–200 Hz) sound. The lateral line was sensitive to water currents, swimming, prey movements, and sound with maximal sensitivity at 100 Hz. Both systems showed directional sensitivity to pure tones and toadfish vocalizations, indicating potential for sound localization. Thus, toadfish possess two hair cell based sensory systems that integrate information from disparate sources. However, swimming movements or predation strikes can saturate each system and it is unclear the effect that self-generated movement has on sensitivity. It is hypothesized that the toadfish’s strategy of short distance swim movements allows it to sample the acoustical environment while static. Further study is needed to determine the integration of the two systems and if they are able to segregate and/or integrate multimodal sensory input.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
The inner ear of teleosts developed over 400 million years ago and consists of three otolith organs, the saccule, utricle, and lagena and three semicircular canals, all of which contain sensory hair cells. The colonization of the terrestrial environment by vertebrates led to the development of the outer ear to detect airborne sound and segregation of the inner ear components into vestibular (otoliths and semicircular canals) and auditory (cochlea or cochlea duct) organs (Fritzsch 1999). However, the dual auditory/vestibular function of the otoliths persists in extant teleosts, and it remains unclear how these organs simultaneously integrate bimodal sensory input such as sound and self-generated movement.
Fish also possess a hair cell based mechanosensory lateral line which functions in schooling behavior (Partridge and Pitcher 1980), rheotaxis (Montgomery et al. 1997), localization of underwater objects (Weissert and von Campenhausen 1981), and predator/prey interactions (Montgomery et al. 1995). Unlike the inner ear, the lateral line was not retained throughout vertebrate evolution and is limited to fish and aquatic amphibians. Although the role of the lateral line in sound detection has long been debated (see Braun et al. 2002 for review), several studies have suggested that the fish’s mechanosensory lateral line may play a role in sound localization (Higgs and Radford 2013; Mirjany and Faber 2011; Mirjany et al. 2011; Radford and Mensinger 2014; Weeg and Bass 2002). Thus, similar to the otoliths, the mechanosensory lateral line receives multimodal (i.e., vibration and sound) input.
A long-standing question in neuroethology is how fish localize sound underwater. Although the saccule is considered the primary auditory endorgan in fish (Popper and Fay 1993, 2011), both the saccule and utricle are sensitive to linear acceleration and acoustic particle motion, and display directional sensitivity, functioning predominantly as low frequency (60–1000 Hz) detectors (Boyle et al. 2001; Fay 1984; Fay and Edds-Walton 2000; Lu et al. 2004; Mensinger 2006). However, the mechanism by which otoliths contribute to sound localization remains unclear. While terrestrial vertebrates use interaural time delays to localize sound in the azimuth (Schnupp and Carr 2009), the small distances between otolith pairs, the low density of the cerebral spinal fluid and/or brain tissue in the intervening space and the relatively rapid underwater speed of sound, makes using time disparities challenging for teleosts. Further complicating matters is the otoliths’ vestibular role as any self-generated movement may impact auditory sensitivity.
The traditional neurophysiological method of recording from restrained, anesthetized fish complicates investigating bimodal sensory input, especially associated with self-movement (i.e., respiration, swimming). Semi-submerged preparations make it difficult to deliver and/or quantify the sound impacting the otoliths, while submerged preparations often are complicated by echoes produced during sound presentation in small tanks (Mensinger and Deffenbaugh 2000). Furthermore, animal care regulations mandate the use of anesthesia with restrained and/or paralyzed fish, which may depress neural sensitivity (Palmer and Mensinger 2004). The development of the shaker table by the Fay laboratory (Fay 1984) allowed for very accurate measurement of acoustic sensitivity that partially offset previous testing problems, however it remained limited to restrained fish which makes it difficult to test bimodal stimuli.
It has long been the goal of neuroethologists to record from freely moving animals in their natural state. While significant advances have been made with terrestrial animals using radio telemetry for monitoring physiological processes (Kramer and Kinter 2003), the use of these techniques in the aquatic medium has been tempered by its opacity to radio waves and viscosity that produces drag on external devices. Tethered preparations using swivels have been successful with animals exhibiting two dimensional movement (i.e., mice in the horizontal plane) (Young and Davisson 2011), but less amenable for actively swimming fish that can quickly entangle themselves in the wire. The development of chronically implanted microwire electrodes and telemetry tag (Mensinger and Deffenbaugh 1998, 2000) or tether provided the ability for stable, long-term recording (up to a week) in freely moving fish. This chapter summarizes the use of this system for exploring the sensory physiology of the inner ear and lateral line. The eventual goal is to determine the relative contribution and the possible integration of each system during multimodal stimulation. For example, fish swimming will stimulate both otoliths and the lateral line, and it is unclear how these organs will process auditory input during movement.
1.1 The Toadfish
Batrachoid fish (Opsanus sp. and Porichthys sp.) have been developed into important biological models for investigating muscle physiology (Elemans et al. 2014; Harwood et al. 2011), excretory function (Walsh et al. 2008), and vestibular physiology (Rabbitt et al. 1995). However, as sound generation and reception is an integral part of their natural history, they also have become subjects for neuroethology and bioacoustic studies. The Fay laboratory has detailed the neuroanatomy and the auditory physiology of the saccular endorgan of the toadfish, Opsanus tau (Edds-Walton et al. 1999, 2013; Edds-Walton and Fay 2003, 2005a, b, 2008, 2009) demonstrating its ability to encode pure tones in the range of fish vocalizations. Both male and female fish produce broadband grunts by means of rapid contraction of sonic muscles surrounding the swim bladder. However, only sexually mature male toadfish produce a bimodal vocalization, termed a boatwhistle, which is used to acoustically attract females to nesting sites (Fine et al. 1977; Gray and Winn 1961). The boatwhistle consists of a brief, irregular initial grunt (broadband) followed by an extended period of regular pulsing (fundamental frequency < 200 Hz) (Edds-Walton et al. 2002). Although the production and reception of the sound has resulted in many investigations on sonic muscle and auditory physiology (Harwood et al. 2011; Mensinger 2014; Walsh et al. 2008), the mechanism by which female fish locate the males, and which characteristics (i.e., amplitude, frequency, duration) of the call influence mate choice remain largely unknown.
2 Materials and Methods
2.1 Telemetry Tag
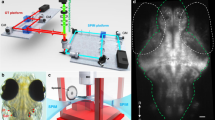
An inductive telemetry system was developed for recording neural activity from free swimming fish. The system consisted of three channel microwire electrodes, a cylindrical (38 × 15 mm dia) transmitting telemetry tag and receiver coils. The tritrodes (impedance 0.5–1.5 MΩ) were fabricated with three strands of insulated 20 μm-diameter 10 % platinum/iridium wire that terminated into a multipin underwater connector which joined to the telemetry tag. Two miniature capacitors, a differential amplifier, low (400 Hz) and high (4 kHz) filters, and a circular inductive coil were contained in the tag (Fig. 1a). The neural signals were transmitted as a frequency-modulated magnetic field (90 kHz carrier, 20 kHz bandwidth), to the receiver coils embedded in a recharging habitat and stage (RECHABS) (Fig. 1b). The RECHABS consisted of a cylindrical habitat (12 cm internal diameter × 30 cm) that opened onto an octagonal stage (16 cm per side), and served to receive the telemetry signal and recharge the tag. Telemetry and recharging was possible whenever the fish was within the footprint of the RECHABS up to an elevation of approximately 15 cm above the stage. Magnetic induction fully powered the tag in less than 30 s and provided telemetry for up to 20 min between charging. Alternatively, the microwire electrodes were connected via a transdermal lead to a long, thin tether (~2.0 m) that terminated into the head stage of the amplifier outside of the tank. Sufficient slack remained in the cable to allow the toadfish to freely move around the aquarium.
(a) Photograph of the neural telemetry tag. (A) Electrode lead; (B) waterproof connector; (C) tag body with amplifier, capacitors, and filters: (D) Inductive coil. (b) Dorsal view of the experimental arena. The recharging habitat and stage (RECHABS) consists of the cylindrical habitat (H) and the octagonal stage (S). Neural telemetry and tag recharging could transpire when the fish was in the habitat or over the stage. Fish movements were recorded with an overhead video camera (C). Drawing is not to scale. Modified from Palmer et al. (2005)
The electrodes were implanted chronically into the utricular or anterior lateral line nerves. All implants were performed on anesthetized (MS-222) and paralyzed (pancuronium bromide) toadfish. Extracellular potentials were differentially amplified and monitored on a portable computer. The two recording channels that provided the highest fidelity signal were chosen for the experiments. Cyanoacrylate gel was used to affix the electrode to the skull and seal the craniotomy with the overlying tissue sutured to provide a watertight seal over the implant and around the transdermal electrode lead.
Immediately after surgery, the toadfish was placed in an opaque round fiberglass tank (~1 m dia) with a water depth of 30 cm and left undisturbed for a minimum of 90 min, a time previously shown to eliminate any effects of anesthesia on neural recordings (Palmer and Mensinger 2004). A University Sound UW-30 speaker (frequency response 80 Hz–10 kHz) was suspended vertically in the water column approximately 80 cm from the fish, and a hydrophone was placed directly above the toadfish head.
Pure tones and previously recorded male toadfish vocalizations were used as auditory stimuli. The front of the RECHABS cylinder habitat was maintained 80 cm from the speaker, and fish were only presented with sounds while in the habitat with their head facing out near the opening. As the fish were free to move, small displacements inside the RECHABS of ±5 cm from the opening and/or ±5° left or right were possible and allowed. However, if fish exited the habitat or retreated further than 5 cm into the habitat, the experiment was suspended and the fish repositioned in the cylinder. The habitats were rotated in 45° increments relative to the speaker to test for directional sensitivity with the distance from the front of the habitat to the speaker kept constant (i.e., the endorgans remained the same distance from the speaker). For sound presentation to the lateral line, the habitat was removed to streamline sound presentation and only the tether was used for recording.
Thresholds were determined for each test frequency along the axis of best directional sensitivity by starting with a supra-threshold intensity followed by decreasing intensities until the afferent no longer responded to the stimulus. For the utricle experiments, a calibrated hydrophone (Brüel and Kjær 8103 or High Tech HTI-94) recorded the sound stimulus reaching the toadfish. Relative sound pressure levels (SPL) were calculated for each frequency and intensity by measuring the root mean square (rms) voltage at the position of the fish head and converted to SPL in dBrms re: 1 μPa. For the lateral line experiments, the frequency response of the underwater loudspeaker was measured using a calibrated HTI-96-MIN hydrophone (High Tech Inc.) and a B&K 4524 triaxial accelerometer (Brüel and Kjær) positioned at the location of the fish’s head during the experiments. Relative sound pressure and particle motion were calculated using an oscilloscope and adjusted with the attenuator to ensure that the sound pressure and particle motion at all frequencies were of equal amplitude (±2 dB) (Radford and Mensinger 2014).
Single and multiunit recordings were amplified (x1000; Dagan Ex-1), filtered (300 Hz–3 kHz), recorded for up to 7 days after implant, stored on a portable computer using Chart5 software and analyzed offline with CED Spike2 software. Although microwires often yielded multiunit activity, neuron discrimination was usually limited to one or two units that yielded the greatest amplitude and had clearly distinguishable waveforms above the noise level. To verify that the same afferent(s) was consistently recorded during an experiment, individual fibers were distinguished using waveform analysis in addition to spike amplitude. All statistical analysis was performed using GraphPad Software (San Diego, CA, USA) or SigmaStat for Windows version 3.10 (Systat Software, Inc., Richmond, CA, USA). All data represent mean values ± 1 S.E.M. unless otherwise indicated.
2.2 Data Analysis
Neural responses to tones were quantified for vector strength (VS or synchronization coefficient, R) and evoked spikes rates across the entire stimulus cycle. Spike rates for directional responses were expressed as the maximum evoked spike rate minus the mean resting rate for each neuron (e.g., peak-DC) (Goldberg and Brown 1969). VS varies from zero (random distribution; no phase locking) to one (all spikes in the same bin; strong phase locking) and has been determined to be a better predictor for auditory frequency encoding among vertebrates than maximum evoked spike rates for frequencies ≤1 kHz (Fay 1978, 1982, 1994; Javel and Mott 1988; Sisneros and Bass 2003). The significance of phase locking was determined by the calculation of the Rayleigh statistic, Z, which is defined as R 2 × N, where R is the coefficient of synchronization (or vector strength) and N is the total number of spikes sampled. Responses with Z ≥ 4.5 (P = 0.01, utricle) or Z ≥ 6.9 (P = 0.001, lateral line) were considered significantly phase locked (Batschelet 1981). Threshold was defined at the lowest intensity to evoke an increase in spike rate above spontaneous activity, or a significant Z value as described in other studies (Lu and Fay 1993; Maruska and Tricas 2009) and determined from 80 to 400 Hz. Directional responses for each individual neuron were calculated at the same supra-threshold stimulus strength (~5–10 dB above threshold) at each of the eight different stimulus orientations and examined as both spike rate (spikes/sec) and vector strength.
3 Results
3.1 Initial Experiments with the Telemetry Tag
The effectiveness of the telemetry tag was first demonstrated in the toadfish mechanosensory lateral line. Since ethical constraints make it difficult to justify removing anesthesia from restrained or paralyzed animals, it has been problematic to assess the effect of anesthesia on the fish nervous system, as it would entail removing the drug from immobilized animals. The telemetry tag proved effective in assessing the common fish anesthetic MS-222 on neural sensitivity. The electrodes were implanted under anesthesia, and the fish allowed to recover and resume normal activity. Subsequent challenges with anesthesia showed depression of neural activity correlated with increasing concentrations of MS-222 (Fig. 2). The results suggested that care should be taken when using the anesthetic and that once the surgical plane of anesthesia is achieved, the minimal dose that maintains the animal in this state should be used throughout the experiments (Palmer and Mensinger 2004).
MS-222 Dose response curve. Normalized firing rate of lateral line fibers (n = 17: 11 spontaneous and 6 silent) is plotted vs. increasing MS-222 concentrations. Firing rate is normalized as a percent of the preanesthetized firing activity. Spontaneous activity (black circle) represents the resting discharge rate from spontaneously active lateral line fibers. Evoked activity (white circle) is the firing rate from both spontaneous and silent fibers in response to water current. Error bars = 1 SE. Modified from Palmer and Mensinger (2004)
The tag was next used to monitor neural activity from the anterior lateral line nerve in response to water movements generated by natural prey. Previous studies using vibrating dipoles had determined that the lateral line can detect water displacements, and that is a relatively short range sensory system (one to two body lengths) (Coombs and Janssen 1990). Nocturnal studies determined that juvenile toadfish only attacked prey within approximately one half of toadfish body length (Price and Mensinger 1999), however it was uncertain if this was the range at which the prey were detected or w hen the attack commenced. Subsequent studies, using the telemetry tag, with large (30 cm sl) adult toadfish, indicated that small baitfish were only detectable by the lateral line at approximately 10 cm or a 1/3 of a body length suggesting that, at least in toadfish, the lateral line mediates predator prey interactions at relatively short distances (Palmer et al. 2005) (Fig. 3).
Lateral line detection of prey. The diagram depicts the head of the toadfish projecting out of its habitat and the sequential positions of the approaching prey: (A) 10 cm; (B) 3.5 cm; (C) 1.0 cm. Images were reconstructed from single video frames. The letter next to the prey fish corresponds to neural activity from a superficial neuromast on the suborbital portion of the infraorbital lateral line. Although multiunit activity is visible in the trace, data analysis was restricted to the fiber with the greatest amplitude. Modified from Palmer et al (2005)
3.2 The Utricle
The otolithic endorgans in teleost fishes (saccule, utricle, and lagena) have dual vestibular and auditory roles and function to encode linear particle motion. The saccule is the largest otolith and considered the primary auditory endorgan in most fish species (Popper and Fay 1993). The response characteristics of saccular afferents have been studied across a wide variety of fishes including goldfish (Fay 1978), midshipman (Sisneros and Bass 2005), sleeper goby (Lu et al. 1998), and toadfish (Fay and EddsWalton 1997), and are sensitive to linear acceleration and directionally sensitive to acoustic particle motion functioning predominantly as a low frequency detector (60–1000 Hz). The toadfish saccule is well adapted to detect the fundamental frequency of the male boatwhistle sound (~150 to 200 Hz) and grunt vocalizations (~50 to 250 Hz) (Edds-Walton et al. 1999, 2002; Maruska and Mensinger 2009).
Unlike its congener Poricththys, the saccular nerve in Opsanus sp. was difficult to access, thin and variably branched and not amenable to implants. In contrast, the utricular nerve was easily accessible and of sufficient size for microwire insertion. The smaller utricular otolith has received less attention than the saccule and there is limited information on its physiology, having been examined in only a few species of fishes. Its vestibular role as a linear accelerometer had been established in normal (Rabbitt et al. 1995) and post space flight toadfish (Boyle et al. 2001). Utricular afferents also were determined to be sound sensitive and showed directional responses to 140 Hz in the goldfish (Fay 1984) and 50–400 Hz in the sleeper goby Dormitater latifrons (Lu et al. 2004). Therefore, the utricle provided a good candidate to investigate multimodal sensory input.
Wild and captive toadfish normally spend long periods of time motionless inside sheltered habitats with occasional brief forays limited mainly to foraging. Pre- and post-operative fish displayed similar behavior, and the tag or tether did not restrict movement, inhibit respiration or precipitate behavior to dislodge the devices. Recording fidelity was similar between direct recording nerve recording and using the telemetry tag or tether. The toadfish showed full recovery from the anesthesia within 2 h and resumed feeding within 24 h indicating that the fish quickly recovered and was displaying normal behavior during the testing.
Utricular neurons were quite sensitive to horizontal but not vertical movements of the toadfish. Sustained body movements of several seconds during either natural or evoked swimming led to continuous, elevated, and often maximal discharge rates in utricular afferents. Small (1–3 cm) lateral movements also evoked robust responses. The units also were modulated by ventilation and in large fish (>25 cm sl), breathing movements rarely displaced the quiescent toadfish more than ±2 mm and in many cases, there was no discernable body movement demonstrating the high sensitivity of these fibers to small displacements (Boyle et al. 2001). Therefore, the ability to integrate of environmental stimuli must also take into account the input from breathing movements.
The underwater speaker precluded testing frequencies less than 80 Hz, however the toadfish utricular neurons were most sensitive from 80 to 200 Hz with decreasing sensitivity at higher frequencies (Fig. 4). Most afferents consistently fired during sound presentation, and increased stimulus intensity resulted in greater firing rates. The sensitivity corresponded with the fundamental frequency of toadfish grunts (80–120 Hz) and male boatwhistles (100–200 Hz), and the utricle was responsive to playbacks of toadfish boatwhistles (Fig. 5). Thus not only was the utricle sensitive to low frequency sound, it is also well designed for detecting the frequencies of toadfish vocalizations used for intraspecific communication and therefore has the potential to assist in sound localization (Maruska and Mensinger 2009).
Utricular afferent tuning curve. The sound threshold (relative amplitude) needed to invoke the criterion response is plotted versus sound frequency (Hz) in toadfish (N = 15). Error bars = 1 SE. Modified from Maruska and Mensinger (2009)
Response of a single utricular afferent to playback of toadfish boatwhistle vocalizations. Top trace is the playback of three toadfish boatwhistles presented via the underwater speaker and recorded by the hydrophone at the toadfish head, while the bottom trace is the waveform of the utricular neural activity recorded from a tethered toadfish. Modified from Maruska and Mensinger (2009)
3.3 Sound Localization
One requirement for sound localization is that the endorgan exhibits directional sensitivity to sound. The majority (75 %) of utricular neurons (n = 12) displayed directional sensitivity, suggesting the utricle may be involved in sound localization, particularly in the azimuth (Fig. 6). Non-directional (or omnidirectional) neurons still responded robustly to acoustic stimuli, however, did not display clear directionality. Several additional neurons were acceleration sensitive and responded to fish movement, but were relatively insensitive to sound frequencies tested suggesting dichotomy in utricular hair cells with some hair cells functioning primarily as low frequency vestibular and not auditory sensors. Whether the converse is true is unknown, as only candidate fibers that responded to horizontal movement of the vibration isolation table during the implant were selected for sound tests. Alternatively, as these cells were not tested for sound sensitivity between 5 and 80 Hz, these may be representative of the lower frequency fibers found in the sleeper goby (Lu et al. 2004).
Diversity of directional responses of utricular (a) and lateral line primary afferents (b) in the toadfish. Polar plots of neural responses using vector strength analysis from three representative primary afferents are shown to demonstrate directional responses (a: green 0–180°; red 90–270°; blue 45–225°; b: green 45–225°; red 90–270°; blue 0–180°). Plots were constructed from recordings at the best frequency of each afferent at 5 dB above threshold. The distance from the central origin to each data point represents the vector strength, or coefficient of synchronization (ranges from 1.0 representing strong phase locking, to 0.0 representing no phase-locking or random firing), at each angle 0–180° represents the rostro-caudal fish axis with fish’s head point towards 0°. Modified from (a) Maruska and Mensinger (2015) and (b) Radford and Mensinger (2014)
The ability of fish to localize sound sources is complicated by small interaural distances and the high speed of sound underwater. The saccule has been implicated as the main endorgan of hearing and is certainly the largest otolith in toadfish. However, the caudal ends of the bilaterally positioned saccules are in close proximity, and even in adult fish, sound arrives at the posterior of each endorgan virtually simultaneously. The smaller utricles, on the other hand, are rostral to the saccules and in large, adult toadfish, are separated by distances of 1–3 cm. Whether this spacing provides a sufficient delay to localize sounds based on interaural time differences remains to be determined.
What is clear, however, is that body movements and normal ventilation can also stimulate the utricle, and while these latter cyclic movements may be filtered in higher order processing centers (Montgomery and Bodznick 1994), the ability to hear and/or find the sound source may be compromised by self-generated movement. While male toadfish remain relatively stationary during advertisement calling, female fish must swim to find suitable males. Swimming movements can cause maximal excitation of utricle afferents and the ability to pin point sound sources during these forays may be compromised. Observations of female fish movement in the field are complicated by poor water visibility, cryptic coloration, and/or nocturnal movements. However, if the utricle is important in localizing sound, the female may need to alternate swimming with stationary pauses. Spontaneous toadfish movements in outdoor ponds and large tanks suggests that a typical toadfish “swim” consist of short legs, typically less than 1 m interspersed with pauses rather than long distance sustained bursts. While this behavior is more likely to have evolved to minimize alerting prey or predators outside of their protective habitats, it may also allow the fish to sample its acoustic environment without the added complications of self-generated movement.
The sensitivity of the utricle in the horizontal plane suggests it may function in detecting particle motion in azimuth, while the more vertically oriented saccule and lagena better detect particle motion in elevation. For the benthic-dwelling toadfish, sound detection in the horizontal plane is likely extremely important for detecting sounds generated by conspecifics, predators, and prey. Further studies are needed, however, to determine the relative role of each of the different otolithic endorgans and how they contribute to sound localization in fishes.
3.4 The Lateral Line
The toadfish anterior lateral line responds to water flow, opercular displacement, prey movements, and swimming (Palmer et al. 2005; Palmer and Mensinger 2004). Although otoliths organs may be too close to use interaural time differences, the mechanosensory lateral has widely spaced neuromasts that may encode sufficient time delays for sound localization. The location, innervation, and morphological type of the neuromasts in the toadfish anterior lateral line has been established (Clapp 1891; Pankratz 1930). In comparison with other teleosts, the anterior lateral canals are reduced, with only 20 external canal pores on each side of the head. The superficial neuromasts (N = ~ 40 per side) are surrounded by paired finger like projections with the hair cells aligned perpendicular to the appendages, consequently the directional sensitivity of the neuromasts can be predicted by external morphology (Marranzino et al. 2013).
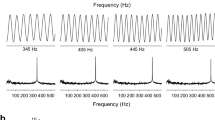
Neural activity was monitored from the anterior lateral line during the presentation of pure tones (80–400 Hz) and toadfish vocalizations. All units showed greatest sensitivity to 100 Hz (Fig. 7). While overall sensitivity was independent of stimulus source location, the nerves’ phase-locking ability was directly related to fish orientation (Fig. 7). Two types of units were classified, Type 1 (tonic), Type 2 (phasic) with Type 1 fibers further divided into sub-types based on their frequency response, which was hypothesized to be related to canal (Type 1–1) and superficial (Type 1–2) neuromast innervation. Lateral line units also exhibited phase locking to boatwhistle vocalizations, with greatest spike rates exhibited at the onset of the call. These results provide the first direct evidence that oyster toadfish can use their lateral line to detect behaviorally relevant sound stimuli, and potentially function in sound localization (Radford and Mensinger 2014).
Lateral line turning curves. Toadfish anterior lateral line thresholds (dB re 1 ms−1) are plotted versus frequency. Each point represents the mean threshold for each fiber. The Type 1 response is split into 2 responses Type 1–1 (black circle) responded to the entire frequency range (n = 25) and Type 1–2 (triangle) responded to a maximum frequency of 250 Hz (n = 8). Phasic (inverted triangle Type 2, n = 4), inhibitory (square, Type 3, n = 1). Modified from Radford and Mensinger (2014)
The lateral line responds to the near field component of sound (particle motion) and has the potential to contribute to hearing sensitivity and sound localization (Mirjany et al. 2011). While delay lines such as found in the owl (Carr and Konishi 1988) have not been discovered, interneuromast distance, combined with afferent nerve length and conduction velocities may be sufficient to use sound delays to locate the source. Anterior lateral line neuromasts can be separated by over 10 cm with distances between anterior and posterior lateral line neuromasts capable of exceeding 25 cm. For example, underwater sound (35 ppt, 20 °C) directly in front of a 25 cm sl fish will impact the foremost anterior lateral neuromasts 16 μs prior to arriving at neuromasts located at the base of the caudal fin. However, factoring in toadfish cranial nerve diameters [1–12 μm (Mensinger and Highstein 1999)], conduction speeds associated with myelinated nerves of these diameters (10 or 50 m/s), and afferent lengths to second order neurons (up to 5 cm length for anterior and 20 cm for posterior; Mensinger unpublished), delays to the central nervous system would range from approximately 400 μs to 2 ms, which is within the time frame used for interaural delays found in other vertebrates.
4 Discussion
4.1 Detection Distance
The experiments demonstrate that the utricle and lateral line are well designed to detect toadfish vocalizations and they may play a role in sound localization. However, it remains unclear what is the functional range of each system, how acoustic input to both systems is integrated, and the effect of self-generated movement on hearing sensitivity. Male toadfish often nest in high densities (up to 10–12 m2) in estuaries near Woods Hole, MA, and produce loud [~140 dB re 1 μPa (Tavolga 1971)] boatwhistles with fundamental frequencies ranging between 90 and 250 Hz depending on season and geographical location (Fine 1978), which is within the sensitivity and range for the utricle and lateral line. Calls can propagate several meters underwater with distance influenced by toadfish size, water depth, and substrate composition (Fine and Lenhardt 1983) with hydrophones able to detect calls at least 5 m from toadfish nests (Mensinger 2014), although it remains to be determined at what range the females can detect the signal or what aspect of the boatwhistle influences mate choice.
Underwater acoustic stimulus consists of two components, the “nearfield,” which is dominated by hydrodynamic flow and the “farfield,” which is modulated by the propagating pressure wave (Popper and Fay 1993). The lateral line is sensitive to hydrodynamic flow within short distances from the source (nearfield), but is relatively insensitive to pressure (farfield) (Montgomery et al. 1995; Webb et al. 2008). Therefore, it is likely that the acoustic stimulation of the lateral line of teleosts will transpire in close proximity to the source. The near field dominates the acoustic field up to a distance of λ/2π from the source (Bass and Clarke 2002), and should extend at least 1–3 m from the nests based on the fundamental frequencies of the boatwhistle, which would place the female fish well within the range of the acoustic field that would provide lateral line stimulation. The utricle may be able to detect vocalizations outside of the near field, providing the toadfish with both a long and short range acoustic detection system.
4.2 Self-generated Movement
Body movements, including normal ventilation, stimulate both the utricle and the anterior lateral line, and it is unclear the effects this has on sound sensitivity. While rhythmic movements may be filtered in higher order processing centers (Montgomery and Bodznick 1994), the ability to hear and/or find the sound source may be compromised by nonrhythmic or spontaneous movement. During predation strikes, the anterior lateral line was saturated in toadfish and unlikely to be able to integrate additional prey information (Palmer et al. 2005). However, the toadfish is an ambush predator that launches ballistic strikes, and its large mouth provides sufficient margin for error that sensory feedback during the strike is probably unnecessary.
Male toadfish also remain relatively stationary during advertisement calling and alternate calls to avoid overlap. However, they remain sensitive to conspecific signals which allows them to generate disruptive grunts during competitors’ boatwhistles (Mensinger 2014). However, there is no evidence that they can detect these grunts, as the caller’s auditory system is either saturated by boatwhistle generation or efferently modulated to avoid potentially damaging sound production. Thus, their quiescent nature during acoustical advertisement generates little movement and allows toadfish to maintain hearing sensitivity during inter call intervals.
The situation is more complicated for mobile females as they need to localize the males and swim to the nest. Swimming can cause maximal excitation of utricle and lateral line afferents and degrade the ability to pin point sound sources during these forays. Although in situ observations of female fish approaching males from a distance are complicated by the poor environmental visibility, one would predict that if the utricle is important in localizing sound, that the females may need to alternate swimming with stationary pauses to assist in locating the sound. This intermittent swimming strategy which has been observed in captivity may allow the fish to sample the acoustic field during stops using both the saccule and utricle and help localize the sound source.
However, not all movement may degrade acoustic sensitivity. The superficial neuromasts in the toadfish are surrounded by paired finger like projections and arrayed in different orientations. As the projections may act to restrict water flow along the neuromast, swimming fish would have a proportional of their neuromasts not impacted by movement/water flow and remain sensitive to acoustic stimulus. Additionally, the utricular organs act as linear accelerometers, and once the fish achieve constant velocity they could regain full sensitivity to acoustic input. Although in toadfish, the short, intermittent swimming motions make achieving constant velocity problematic, other species that display constant, steady swimming could maintain auditory sensitivity. Additionally, as both the lateral line and utricle are innervated by first order neurons, central nervous system filtering may also factor in modulating sensitivity.
4.3 Future Directions
It is equally important to investigate the effects of simultaneous bimodal sensory input into two systems. Preliminary experiments have proven the efficacy of implanting bilateral electrodes in the lateral line (Radford and Mensinger, unpublished). Future experiments aim to implant electrodes in both the lateral line and utricle to determine how these systems encode and integrate similar stimuli, and determine how the utricle and lateral line function during free swimming and sound localization behavior.
5 Summary
The neural telemetry tag has allowed exploration of multiple sensory input such as self-generated movement and sound in both the utricle and lateral line. It has allowed neural sensitivity to be explored in freely moving fish without the complications of anesthesia. Both systems were sensitive to sounds consistent with toadfish vocalizations and showed directional sensitivity, indicating a role in sound localization.
References
Bass AH, Clarke CW (2002) The physical acoustics of underwater sound communication. Acoust Commun 16:15–64
Batschelet E (1981) The Rayleigh test. In: Batschelet E (ed) Circular statistics in biology. Academic, New York, pp 54–58
Boyle R, Mensinger AF, Yoshida K, Usui S, Intravaia A, Tricas T, Highstein SM (2001) Neural readaptation to earth's gravity following return from space. J Neurophysiol 86:2118–2122
Braun CB, Coombs S, Fay RR (2002) What is the nature of multisensory interaction between octavolateralis sub-systems? Brain Behav Evol 59:162–176
Carr CE, Konishi M (1988) Axonal delay-lines for time measurement in the owls brain-stem. Proc Natl Acad Sci U S A 85:8311–8315
Clapp CM (1891) Some points in the development of the Toad-fish (Batachus tau). J Morphol 5:494–501
Coombs S, Janssen J (1990) Behavioral and neurophysiological assessment of lateral line sensitivity in the mottled sculpin Cottus bairdi. J Comp Physiol A 167:557–567
Edds-Walton PL, Fay RR (2003) Directional selectivity and frequency tuning of midbrain cells in the oyster toadfish, Opsanus tau. J Comp Physiol A 189:527–543
Edds-Walton PL, Fay RR (2005a) Projections to bimodal sites in the torus semicircularis of the toadfish, Opsanus tau. Brain Behav Evol 66:73–87
Edds-Walton PL, Fay RR (2005b) Sharpening of directional responses along the auditory pathway of the oyster toadfish, Opsanus tau. J Comp Physiol A 191:1079–1086
Edds-Walton PL, Fay RR (2008) Directional and frequency response characteristics in the descending octaval nucleus of the toadfish (Opsanus tau). J Comp Physiol A 194:1013–1029
Edds-Walton PL, Fay RR (2009) Physiological evidence for binaural directional computations in the brainstem of the oyster toadfish, Opsanus tau (L.). J Exp Biol 212:1483–1493
Edds-Walton PL, Fay RR, Highstein SM (1999) Dendritic arbors and central projections of physiologically characterized auditory fibers from the saccule of the toadfish, Opsanus tau. J Comp Neurol 411:212–238
Edds-Walton PL, Mangiamele LA, Rome LC (2002) Variations of pulse repetition rate in boatwhistle sounds. Bioacoustics 13:153–173
Edds-Walton P, Matos S, Fay R (2013) Does the magnocellular octaval nucleus process auditory information in the toadfish, Opsanus tau? J Comp Physiol A 199:353–363
Elemans CPH, Mensinger AF, Rome LC (2014) Vocal production complexity correlates with neural instructions in the oyster toadfish (Opsanus tau). J Exp Biol 217:1887–1893
Fay RR (1978) Phase-locking in goldfish saccular nerve fibers accounts for frequency discrimination capacities. Nature 275:320–322
Fay RR (1982) Neural mechanisms of an auditory temporal discrimination by goldfish. J Comp Physiol 147:201–216
Fay RR (1984) The goldfish ear codes the axis of acoustic particle motion in 3 dimensions. Science 225:951–954
Fay RR (1994) Perception of temporal acoustic patterns by the goldfish (Carassius auratus). Hear Res 76:158–172
Fay RR, EddsWalton PL (1997) Directional response properties of saccular afferents of the toadfish, Opsanus tau. Hear Res 111:1–21
Fay RR, Edds-Walton PL (2000) Directional encoding by fish auditory systems. Philos Trans R Soc Lond B Biol Sci 355:1281–1284
Fine ML (1978) Seasonal and geographic variation of mating call of oyster toadfish, Opsanus tau L. Oecologia 36:45–57
Fine ML, Winn HE, Joest L, Perkins PJ (1977) Temporal aspects of calling behavior in the oyster toadfish, Opsanus tau. Fish Bull 75:871–874
Fine M, Lenhardt M (1983) Shallow-water propagation of the toadfish mating call. Comp Biochem Physiol a-Physiol 76:225–231
Fritzsch B (1999) Hearing in two worlds: theoretical and actual adaptive changes for the aquatic and terrestrial ear for sound reception. In: Fay R, Popper A (eds) Comparative hearing: fish and amphibians. Springer, New York, pp 15–42
Goldberg J, Brown P (1969) Response of binaural neurons of dog superior olivary complex to dichotic tonal stimuli – some physiological mechanisms of sound localization. J Neurophysiol 32:613–636
Gray GA, Winn HE (1961) Reproductive ecology and sound production of the toadfish Opsanus tau. Ecology 28:9
Harwood C, Young I, Tikunov B, Hollingworth S, Baylor S, Rome L (2011) Paying the piper: the cost of Ca2+ pumping during the mating call of toadfish. J Physiol 589:5467–5484
Higgs D, Radford C (2013) The contribution of the lateral line to ‘hearing’ in fish. J Exp Biol 216:1484–1490
Javel E, Mott J (1988) Physiological and psychophysical correlates of temporal processes in hearing. Hear Res 34:275–294
Kramer K, Kinter LB (2003) Evaluation and applications of radiotelemetry in small laboratory animals. Physiol Genomics 13:197–205
Lu Z, Fay RR (1993) Acoustic response properties of single units in the torus semicircularis of the goldfish, Carassius auratus. J Comp Physiol A 173:33–48
Lu Z, Song J, Popper A (1998) Encoding of acoustic directional information by saccular afferents of the sleeper goby, Dormitator latifrons. J Comp Physiol A 182:805–815
Lu Z, Xu Z, Buchser W (2004) Coding of acoustic particle motion by utricular fibers in the sleeper goby, Dormitator latifrons. J Comp Physiol A 190:923–938
Marranzino A, Frank M, Lindemann S, Guiffrida B, Sipper K, Webb J, Mensinger A (2013) Functional morphology of cephalic protuberances in the oyster toadfish, Opsanus tau. Integr Comp Biol 53:E325
Maruska KP, Mensinger AF (2009) Acoustic characteristics and variations in grunt vocalizations in the oyster toadfish Opsanus tau. Environ Biol Fishes 84:325–337
Maruska K, Tricas T (2009) Encoding properties of auditory neurons in the brain of a soniferous damselfish: response to simple tones and complex conspecific signals. J Comp Physiol A 195:1071–1088
Maruska KP, Mensinger AF (2015) Directional sound sensitivity in utricular afferents in the toadfish Opsanus tau. J Exp Biol 218:1759–1766
Mensinger AF (2006) Sensitivity of utricular afferent fibers to intraspecific calling via inductive neural telemetry in free ranging oyster toadfish, Opsanus tau. Integr Comp Biol 46:E97
Mensinger AF (2014) Disruptive communication: stealth signaling in the toadfish. J Exp Biol 217:344–350
Mensinger AF, Deffenbaugh M (1998) Prototype rechargeable tag for acoustical neural telemetry. Biol Bull 195:194–195
Mensinger AF, Deffenbaugh M (2000) Anechoic aquarium for ultrasonic neural telemetry. Philos Trans R Soc Lond B Biol Sci 355:1305–1308
Mensinger AF, Highstein SM (1999) Characteristics of regenerating horizontal semicircular canal afferent and efferent fibers in the toadfish, Opsanus tau. J Comp Neurol 410:653–676
Mirjany M, Faber D (2011) Characteristics of the anterior lateral line nerve input to the Mauthner cell. J Exp Biol 214:3368–3377
Mirjany M, Preuss T, Faber D (2011) Role of the lateral line mechanosensory system in directionality of goldfish auditory evoked escape response. J Exp Biol 214:3358–3367
Montgomery JC, Bodznick D (1994) An adaptive filter that cancels self-induced noise in the electrosensory and lateral-line mechanosensory systems of fish. Neurosci Lett 174:145–148
Montgomery J, Coombs S, Halstead M (1995) Biology of the mechanosensory lateral-line in fishes. Rev Fish Biol Fish 5:399–416
Montgomery J, Baker C, Carton A (1997) The lateral line can mediate rheotaxis in fish. Nature 389:960–963
Palmer LM, Mensinger AF (2004) Effect of the anesthetic tricaine (MS-222) on nerve activity in the anterior lateral line of the oyster toadfish, Opsanus tau. J Neurophysiol 92:1034–1041
Palmer LM, Deffenbaugh M, Mensinger AF (2005) Sensitivity of the anterior lateral line to natural stimuli in the oyster toadfish, Opsanus tau (Linnaeus). J Exp Biol 208:3441–3450
Pankratz DS (1930) The cranial-nerve components in the toadfish (Opsanus tau). J Comp Neurol 50:247–286
Partridge B, Pitcher T (1980) The sensory basis of fish schools: relative roles of lateral line and vision. J Comp Physiol A 130:315–325
Popper AN, Fay RR (1993) Sound detection and processing by fish – critical-review and major research questions. Brain Behav Evol 41:14–38
Popper AN, Fay RR (2011) Rethinking sound detection by fishes. Hear Res 273:25–36
Price NN, Mensinger AF (1999) Predator–prey interactions of juvenile toadfish, Opsanus tau. Biol Bull 197:246–247
Rabbitt RD, Boyle R, Highstein SM (1995) Mechanical indentation of the vestibular labyrinth and its relationship to head rotation in the toadfish, Opsanus tau. J Neurophysiol 73:2237–2260
Radford CA, Mensinger AF (2014) Anterior lateral line nerve encoding to tones and play-back vocalisations in free-swimming oyster toadfish, Opsanus tau. J Exp Biol 217:1570–1579
Schnupp JWH, Carr CE (2009) On hearing with more than one ear: lessons from evolution. Nat Neurosci 12:692–697
Sisneros JA, Bass AH (2003) Seasonal plasticity of peripheral auditory frequency sensitivity. J Neurosci 23:1049–1058
Sisneros JA, Bass AH (2005) Ontogenetic changes in the response properties of individual, primary auditory afferents in the vocal plainfin midshipman fish Porichthys notatus Girard. J Exp Biol 208:3121–3131
Tavolga WN (1971) Sound production and detection. In: Hoar WS, Randall DJ (eds) Fish physiology, vol 5. Academic, New York, pp 135–205
Walsh P, Mensinger A, Highstein S (2008) Toadfish as biomedical models. In: Walsh P, Smith S, Fleming L, Solo-Gabriele H, Gerwick WH (eds) Oceans and human health: risks and remedies from the seas. Academic, Burlington, VT, pp 547–558
Webb J, Montgomery J, Mogdans J (2008) Bioacoustics and the lateral line system of fishes. In: Webb J, Popper A, Fay R (eds) Fish bioacoustics. Springer, New York
Weeg M, Bass A (2002) Frequency response properties of lateral line superficial neuromasts in a vocal fish, with evidence for acoustic sensitivity. J Neurophysiol 88:1252–1262
Weissert R, von Campenhausen C (1981) Discrimination between stationary objects by the blind cavefish Anoptichthys jordani (Characidae). J Comp Physiol A 143:375–381
Young CN, Davisson RL (2011) In vivo assessment of neurocardiovascular regulation in the mouse: principles, progress, and prospects. Am J Physiol 301:H654–H662
Acknowledgements
I am grateful to Karen Maruska (utricle) and Craig Radford (lateral line) for performing the bulk of the experiments and data analysis reported in this chapter and to the Grass foundation for providing their support. Thanks to Lucy Palmer and Max Deffenbaugh for initial help in developing the tag. Funding was provided by NSF grants IOS 0316130, 0843735, and 1354745.
I would also thank Dick Fay and Art Popper for their contributions to fish bioacoustics. I first met Dick Fay while I was a post-doc in the Highstein lab during our summer toadfish research at the Marine Biological Laboratory in Woods Hole, Massachusetts. While our study sites in the toadfish brain were just mm apart, our interests at the time were quite divergent as Dick was investigating the saccule and I was concentrating on nerve regeneration and developing the telemetry tag. Dick was always quite supportive and encouraging of my research, and I appreciated his input and guidance. Although his shaker table and experiments were cutting edge, his patience for neurophysiology was certainly old school. There was never any need to ask Dick how the experiments were going, because the frequency of his outdoor “breaks” were inversely correlated with experimental success. Although I interacted with Art Popper less frequently, I always looked forward to our interactions at the MBL or scientific meetings. Art was also quite supportive of my career and always took the time to ask about my current research.
I cannot think of another scientific pair that so defined a field and yet were so generous with their time and support for students and colleagues. Thank you Dick and Art for your support, generosity and “sound” advice
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2016 Springer International Publishing Switzerland
About this chapter
Cite this chapter
Mensinger, A.F. (2016). Multimodal Sensory Input in the Utricle and Lateral Line of the Toadfish, Opsanus tau . In: Sisneros, J. (eds) Fish Hearing and Bioacoustics. Advances in Experimental Medicine and Biology, vol 877. Springer, Cham. https://doi.org/10.1007/978-3-319-21059-9_13
Download citation
DOI: https://doi.org/10.1007/978-3-319-21059-9_13
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-21058-2
Online ISBN: 978-3-319-21059-9
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)