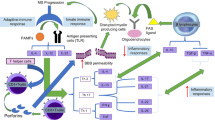
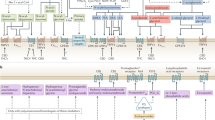
Abstract
There are numerous reports that people with multiple sclerosis (MS) have for many years been self-medicating with illegal street cannabis or more recently medicinal cannabis to alleviate the symptoms associated with MS and also amyotrophic lateral sclerosis (ALS). These anecdotal reports have been confirmed by data from animal models and more recently clinical trials on the ability of cannabinoids to alleviate limb spasticity, a common feature of progressive MS (and also ALS) and neurodegeneration. Experimental studies into the biology of the endocannabinoid system have revealed that cannabinoids have efficacy, not only in symptom relief but also as neuroprotective agents which may slow disease progression and thus delay the onset of symptoms. This review discusses what we now know about the endocannabinoid system as it relates to MS and ALS and also the therapeutic potential of cannabinoid therapeutics as disease-modifying or symptom control agents, as well as future therapeutic strategies including the potential for slowing disease progression in MS and ALS.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Amyotrophic lateral sclerosis
- Endocannabinoid
- Experimental autoimmune encephalomyelitis
- Multiple sclerosis
- Neurodegeneration
- Neuroinflammation
- Neuroprotection
- Symptom management
1 Introduction
Multiple sclerosis (MS) and amyotrophic lateral sclerosis (ALS) are two relatively common neurological conditions which are major causes of disability in adults (and also some children in the case of paediatric MS). The course of disease in MS is typically slow, with eventual increasing disability leading to death whereas the course of disease in ALS is typically rapid from diagnosis, with rapidly evolving disability and typically death within 2 years. This review will discuss what is known about the influence of the endocannabinoid system in these diseases, what is the potential influence of endocannabinoids in disease progression and what these findings may hold for the potential exploitation of the endocannabinoid system as a therapeutic strategy in MS and ALS.
2 Multiple Sclerosis
2.1 Natural History
Multiple sclerosis (MS) is an inflammatory, autoimmune, demyelinating disease of the central nervous system (CNS) and is the most common cause of non-traumatic neurological disability in young adults of northern European descent (Compston and Coles 2002, 2008). This disease affects approximately 100,000 people within the United Kingdom. The absolute number of cases of MS around the world has gradually increased, which may be as a result of improved diagnosis, as well as other factors, and currently affects 2–3 million people worldwide (Kurtzke 1993). The incidence of MS is geographically restricted and particularly occurs with a high incidence in Northern Europe and in regions colonized by white Northern Europeans, such as Canada and Northern USA, Australia and New Zealand, with a gradient of higher incidence further from the equator (Compston and Coles 2002). MS is more common in females compared to males, with an increasing ratio of historically 2:1–3:1 today, with a more pronounced female incidence in younger MS patients with relapsing-remitting disease (RRMS) (Runmarker and Andersen 1993). The highest incidence of MS reported is in the Orkney Isles with an incidence of 1 in 170 females (Visser et al. 2012). The susceptibility to the development of MS is influenced by genetics, as shown by an increased concordance of MS in monozygotic twins (~30 %) compared to dizygotic twins (~5 % concordance rate), and this susceptibility is polygenically controlled (Compston and Coles 2002, 2008). MS is associated with the expression of certain MHC haplotypes such as HLADRB1*1501 and is also influenced by over 150 other immune-related, susceptibility genes (Prat et al. 2005; Sawcer et al. 2011). However, the discrepancy in concordance of disease incidence in identical twins demonstrates that other, environmental, factors may influence susceptibility. Migration studies from low to high incidence areas suggest that the environmental trigger is acquired before the age of 15 (Compston and Coles 2002). Some have suggested that it may relate to age of infection and there is a long-standing hypothesis that this could relate to Epstein Barr Virus (EBV) infection (Ascherio and Munger 2010). The vast majority of people with MS, if not all, have been shown to have been infected with EBV compared to 90 % of the general population, and there is increased frequency of MS in people who developed glandular fever (Handel et al. 2010). This is indirectly supported by the geographic distribution of people with MS (Ebers and Sadovnick 1993). Vitamin D levels can influence the immune response and may even be important in utero (Willer et al. 2005), and a “month of birth” effect has been reported in a number of studies where in the northern hemisphere, there was a 5 % excess of cases among patients born in April and 5–8 % reductions in MS risk associated with birth in October or November. This suggests that ambient UV radiation and hence maternal vitamin D levels are prenatal environmental modulators of MS risk. Importantly, a number of genes associated with MS, such as certain human leucocyte antigen (HLA) haplotypes, contain vitamin D responsive elements in their promoter regions that can influence expression and may link environmental and genetic susceptibility elements (Ramagopalan et al. 2009, 2010). MS most commonly (approximately 80 %) presents as a series of relapsing-remitting episodes of loss of neurological function due to conduction block in axons that eventually develops into a chronic, secondary progressive MS (SPMS) phase with no remission and with increasing disability over time, which correlates with CNS atrophy and axonal loss, particularly in the spinal cord (Bjartmar et al. 2000). In addition, a subtype of MS, primary progressive MS (PPMS) presents as a progressive degenerative phenotype in 10–15 % of patients after an initial bout of CNS inflammation (particularly in those with a disease onset later in life), which along with secondary progressive MS is largely refractory to currently available MS therapies such as immunomodulation (Miller and Leary 2007) and where neuroprotective strategies are urgently indicated. Clinically, PPMS develops at a later age than RRMS, with onset in the fourth decade rather than the third decade as seen in RRMS (Andersson et al. 1999) and with a lower female preponderance. As such, about 80 % of people with MS will be severely disabled within 25 years from disease onset.
2.2 Pathology, Symptoms and Disability
MS is associated with blood:brain barrier dysfunction due to high levels of mononuclear cell infiltration that arises around post-capillary venules in the CNS in a tissue where the normal level of leucocyte traffic is extremely limited. Leucocytes then invade the brain parenchyma leading to an expanding ring of macrophage-mediated myelin destruction. This leads to the pathological hallmark of MS, which is demyelination of the white and grey matter, due to loss of oligodendrocytes and myelin. Although initially there is remyelination (shadow plaques), the innate capacity to repair eventually becomes exhausted and astrogliotic scars are formed within demyelinated plaques and there is significant neuronal loss. Whilst lesion load is decreased following successful immunosuppressive treatment (Jones and Coles 2010; Polman et al. 2006), suggesting that leucocyte infiltration to the CNS is part of the primary insult in MS, it has also been suggested that damage to the astrocyte or oligodendrocyte may be the primary event followed by infiltration of mononuclear cells (Barnett and Prineas 2004; Parratt and Prineas 2010).
As the disease evolves, inflammatory attacks in the CNS increase the burden of demyelination and a dystrophic environment that leads to eventual neuronal and axonal loss, which impairs normal levels of neurotransmission. As a consequence of neuronal/axonal loss, there is the increasing development of additional distressing symptoms such as incontinence, limb tremor, pain, spasms, fatigue and spasticity, which have a major negative impact on quality of life indices (Compston and Coles 2002; Confavreux and Vukusic 2006). The time taken to convert to a secondary progressive neurodegenerative phenotype can vary widely between individuals and may reflect differences in an individual’s ability to cope with episodes of neuronal insult, perhaps consistent with genetic control and heterogeneity of disease (Compston and Coles 2002). In approximately a quarter of cases, neurological disability does not reach a level where it impinges on daily living, but conversely, in around 15 % of cases the progression to disability is rapid. The prognosis for patients is better in cases where sensory symptoms dominate the course of disease, and there is a complete recovery from these symptoms at remission whereas the prognosis is poorer when there is motor involvement such as deficits of pyramidal, visual, sphyncteric and cerebellar systems (Amato and Ponziani 2000). Frequent relapses and incomplete recovery plus a short time period between the initial neurological event and the subsequent relapse also have a poorer prognosis. There is also a poorer prognosis for the disease in older men who develop MS (Compston and Coles 2002). However, once a threshold of disability has been reached, disability progression is remarkably uniform (Confavreux et al. 2000), and approximately 90 % of RRMS patients will develop progressive disease after 25 years of clinical follow-up (Weinshenker et al. 1989). It may be that given enough time, all RRMS patients will eventually convert to the progressive phase of the disease. A recent study demonstrated that disability progression seems to follow a two stage course. The first stage, corresponding to clinical disease onset to irreversible Kurztke expanded disability status scale (EDSS) level 3, is dependent on ongoing focal neuroinflammation. There is a second stage, from irreversible disability scale 3 to irreversible disability scale 6, which is independent of ongoing focal neuroinflammation where neuroprotective strategies are indicated, rather than immunomodulatory therapies which are indicated for the phase one stage of MS (Leray et al. 2010).
Whilst immune-mediated conduction block and destruction of CNS myelin, followed by lesion resolution and limited myelin repair, may account for the relapsing-remitting nature of the disease, what is less clear are the mechanisms that account for the conversion to the chronic neurodegenerative secondary phase, which appears to be independent of, though worsened by, the accumulated neuronal dysfunction accompanying relapses (Bjartmar et al. 2003). A gradual degeneration of predominantly the pyramidal and cerebellar systems evolves which is often accompanied by sphincter and sexual dysfunction (Amato and Ponziani 2000). Axonal pathology during MS has been re-examined in recent years, a shift away from the predominant focus on demyelination, and it has been established that CNS atrophy and axonal loss occurs, coincidentally with inflammatory lesion formation, early in the relapsing-remitting phase. This may be accommodated initially by utilisation of spare neuronal capacity in the CNS, remodelling of neuronal circuits (neural plasticity) or an increase in the number of neural precursors in some lesioned areas contiguous with subventricular zones (Chang et al. 2008). However, as the disease continues, a threshold is reached, beyond which permanent impairment and increasing disability are established (Bjartmar et al. 2000, 2003; Confavreux et al. 2000; Confavreux and Vukusic 2006). This suggests that axonal loss rather than myelin damage is the key determinant of progressive disability in MS. In addition, a doubling in the levels of glutamate, an excitatory amino acid that has been shown to be neurotoxic in excess, is seen in the CSF of MS patients undergoing an inflammatory episode (Stover et al. 1997).
In experimental allergic encephalomyelitis (EAE), an animal model of MS induced by the development of autoimmunity against myelin antigens, 15–30 % of spinal cord axons can be lost before permanent locomotor impairment is noted (Bjartmar et al. 2000). After a number of relapse events, permanent disability develops with significant axonal loss in the spinal cord (40–80 %, as also occurs in MS), and the development of hind limb spasticity and tremor (Baker et al. 2000), which may reflect preferential loss of inhibitory circuits in certain locations of the spinal cord and their influence on signalling to skeletal muscles. Whilst inflammatory events are associated with axonal transections, chronic demyelination may contribute to a slow degenerative process.
As increasing numbers of axons are lost, this creates an extra burden on the remaining neurons and potential neurototoxicity due to increased metabolic demand or excitotoxic mechanisms on these neurons within the neural circuitry. Thus, a slow amplifying cascade of neuronal death may be triggered, which could occur independently of significant inflammation. This would be compatible with the slow progression in secondary progressive MS and the inability of potent immunosuppressive agents, which successfully suppress disease activity during the relapsing-remitting phase, to inhibit this aspect of disease despite their efficacy in reducing blood:brain barrier dysfunction and the relapse rate (Confavreux and Vukusic 2006). During all neurodegenerative diseases, symptoms occur because homeostatic control of neurotransmission is lost and may result from increased neurotransmission by excessive signalling of excitatory circuits or loss of inhibitory circuits or vice versa. As it appears that an important function of the endogenous cannabinoid system is the modulation of neurotransmitter release via CB1 receptor expression at pre-synaptic nerve terminals (Wilson and Nicoll 2002), this raises the possibility of therapeutic intervention in CNS events for symptom control or disease modification by the manipulation of this system.
2.3 Endocannabinoids in Multiple Sclerosis
2.3.1 Experimental Evidence
The first evidence for the involvement of the endocannabinoid system in multiple sclerosis was obtained from a mouse model of multiple sclerosis (EAE). Here it was shown that mice that had entered the chronic phase of disease, accompanied by profound neurodegeneration leading to the development of hind-limb spasticity, had elevated levels of the endocannabinoids anandamide [arachidonoylethanolamide (AEA)], the anandamide congener palmitoylethanolamide (PEA) and 2-arachidonoyl glycerol (2-AG) in the brain and spinal cord, compared to non-spastic EAE mice and normal controls. Spasticity was ameliorated by CB1 receptor agonists but also by elevation of the endogenous levels of endocannabinoids via inhibition of their uptake or degradation (Baker et al. 2000, 2001). Importantly, spasticity was transiently increased after the administration of Rimonabant®, a CB1 receptor inverse agonist/antagonist and strongly suggested the presence of an endogenous endocannabinoid tone which was elevated in response to the development of limb spasticity. This amelioration of hind-limb spasticity is CB1-dependent (Pryce and Baker 2007) and can be achieved by the elevation of the endogenous levels of endocannabinoids via inhibition of the putative AEA transporter (Baker et al. 2001; de Lago et al. 2004, 2006), inhibition of the degradation of AEA via fatty acid amide hydrolase (FAAH) inhibition (Pryce et al. 2013) or inhibition of the degradation of 2-AG via the inhibition of monoacylglycerol lipase (MAG lipase) (Pryce et al. 2013).
Endocannabinoids also have an important role in neurodegenerative processes arising from neuroinflammation, which was confirmed by the observation that CB1-deficient mice show an increased rate of neurodegeneration compared to wild-type mice undergoing EAE or experimental autoimmune uveitis (Jackson et al. 2005; Pryce et al. 2003).
In another study, EAE-induced alterations of glutamate-mediated cortico-striatal excitatory postsynaptic current (EPSC) frequencies were exacerbated in mice lacking CB1 receptors on glutamatergic neurons (Glu-CB1R-KO), indicating that this subset of receptors controls the effects of inflammation on glutamate release and mediates the potential excitotoxic effects of enhanced glutamate levels (Musella et al. 2014).
The rate of neurodegeneration in EAE can be decreased by the exogenous administration of either CB1 agonists (Croxford et al. 2008), by exogenous 2-AG administration (Lourbopoulos et al. 2011), via pharmacological inhibition of endocannabinoid (AEA) degradation/uptake (Cabranes et al. 2005) or by the genetic ablation of FAAH, the enzyme that degrades AEA (Webb et al. 2008; Rossi et al. 2011; unpublished observation from this laboratory). Also, oligodendrocyte (the cell responsible for the production of myelin) excitotoxicity and white matter damage have been reported to be ameliorated by the administration of a MAG lipase inhibitor via enhancing endogenous levels of 2-AG, in contrast to the inhibition of FAAH, which had no effect in this animal model of MS (Bernal-Chico et al. 2015). In another study, EAE was also ameliorated via the selective inhibition of MAG lipase (Hernández-Torres et al. 2014).
Endocannabinoid (AEA and 2-AG) levels have been reported to be decreased in EAE in response to neuroinflammation (Cabranes et al. 2005) and in another study 2-AG was also found to be decreased (Witting et al. 2006). However, in contrast, an increase in AEA but not 2-AG has also been reported in EAE (Centonze et al. 2007). Excessive glutamate-mediated synaptic transmission and secondary excitotoxicity have been proposed as key determinants of the neurodegenerative damage in MS arising from neuroinflammation (Smith et al. 2000), and the primary action of neural CB1 receptors is to regulate synaptic transmission via regulation of glutamate release from synaptic vesicles (Marsicano et al. 2003). Further evidence that supports this hypothesis is the observation that mice with genetically deleted CB1 receptors show enhanced neurodegeneration as a result of neuroinflammation (Pryce et al. 2003), via enhanced neuronal apoptosis mediated by caspase-3 (Jackson et al. 2005) and loss of the neuroprotective effect of pharmacological potentiation of CB1 signalling during neuroinflammation (Croxford et al. 2008). This protective action of CB1 receptors may be via the suppression of tumour necrosis factor α (TNFα)-mediated potentiation of striatal spontaneous glutamate-mediated EPSCs, which may contribute to the inflammation-induced neurodegenerative damage observed in EAE mice (Rossi et al. 2011). Decreases in CB1 receptor levels in certain brain regions (striatum and cortex) in rat EAE have been reported, but this is accompanied by an increased coupling to GTP-binding protein-mediated signalling pathways, indicating a potential compensatory mechanism is in play here (Berrendero et al. 2001).
The role of the endocannabinoids in CB2 receptor-mediated modulation of cells of the immune system in neuroinflammation is more contentious. It has been reported that selective stimulation of CB2 receptors in C57Bl/6 mouse EAE can ameliorate disease (Kong et al. 2014). However, in a study with CB2-deficient mice using a genetic strain (Biozzi ABH) that shows far more robust disease than C57Bl/6, it was shown that immunosuppression obtained by administration of high-dose Δ9-tetrahydrocannabinol (THC) is maintained in CB2-deficient mice. This indicates that THC-induced suppression of neuroinflammation does not result from CB2 receptor activation but probably, rather from CB1 receptor-mediated stimulation of glucocorticoid release via the hypothalamic/pituitary/adrenal axis (Sisay et al. 2013), with no immunomodulatory effect induced by a CB2 selective receptor agonist in wild-type mice. That this immunosuppressive activity of THC is CB1 receptor mediated is confirmed by the observation that THC-induced immunosuppression is lost in either global CB1 knockout animals or where CB1 is conditionally deleted in nerve cells (Maresz et al. 2007).
In summary, experimental studies have revealed that the endocannabinoid system is actively involved in protection against neurodegeneration arising from neuroinflammation and also positive benefits from CB1 agonists such as THC and Sativex® in symptom control in MS point to the potential benefits of endocannabinoid modulation in these conditions.
2.3.2 Clinical Evidence
Endocannabinoids have been reported to be implicated in the pathophysiology of MS. AEA has been reported to be elevated in the cerebrospinal fluid (CSF) of MS patients compared to controls. Elevated levels of AEA were also seen in peripheral blood lymphocytes in this patient cohort (Centonze et al. 2007). In another study, increased plasma levels of AEA, oleoylethanolamide (OEA) and PEA were detected in MS patients, and increased AEA was seen in relapsing-remitting (RRMS), secondary progressive (SPMS) and primary progressive (PPMS) MS (Jean-Gilles et al. 2009). An increase in PEA was seen in RRMS and SPMS plasma but not PPMS. OEA was increased in the plasma of SPMS patients only. The levels of 2-AG was unchanged compared to controls across all three MS variants. A caveat here is that blood levels of endocannabinoids may not correlate to the levels of endocannabinoids that pertain to the more clinically relevant setting of the CNS. However, it has also been reported that both AEA and 2-AG levels are significantly reduced in the CSF of MS patients versus control subjects with lower values detected in the SPMS group. Higher levels of AEA and PEA, although below those of controls, were found in the CSF of relapsing-remitting MS patients during a relapse and increased levels of AEA, 2-AG and OEA were found in patients with active neuroinflammatory lesions (Di Filippo et al. 2008). Further evidence for the potential reduction of endocannabinoids in MS was provided by the observation that enhanced FAAH expression was detected in active lesions in MS brain tissue (Benito et al. 2007).
To date there are no reports of clinical trials on the efficacy of the modulation of endocannabinoid levels in multiple sclerosis for either symptom relief or neuroprotection. However, the efficacy of cannabinoid agonists (THC, Sativex®) in the amelioration of spasticity has now been proven in a number of clinical studies (Fernández et al. 2014; Flachenecker et al. 2014; Lorente Serpell et al. 2013; Zajicek et al. 2005, 2012). Sativex® has been licensed for the treatment of spasticity in MS. There has also been one study investigating the neuroprotective potential of THC to slow the development of disability in MS (Zajicek et al. 2013). This stemmed from follow-up studies in symptomatic trials that suggested a neuroprotective effect of THC (Zajicek et al. 2005). Participants in the neuroprotective trial were randomly assigned to receive THC capsules or placebo capsules, to be taken by mouth over a period of 3 years. 329 people were allocated to receive the THC capsules and 164 were allocated to the placebo group. For each participant, the first 4 weeks of the trial were devoted to establishing the best tolerated dose of study treatment. For the remainder of the study period, participants remained on a stable dose of trial treatment, as far as possible, before the dose was gradually reduced to zero at the end of the treatment period. The study was “double-blind”, meaning that neither the participants nor the doctors and nurses involved at the study sites knew which treatment group they were in. Despite the abundant experimental evidence that cannabinoid therapy has a neuroprotective role in a spectrum of neurological diseases, overall the study found no evidence that THC had an effect on MS progression in either of the main outcomes [the EDSS neurological assessments conducted by doctors at the study clinics or the 29-item multiple sclerosis impact scale (MSIS-29) questionnaire responses provided by the participants]. The EDSS and MSIS-29 scores showed little change over the course of the study, and no difference was found between the active and placebo groups. A confounding finding was that the placebo group had not progressed as expected, which complicates assessing the value of the trial. However, and potentially importantly, there was some evidence from subgroup analysis that THC might have a significant (p < 0.01) beneficial effect in participants at the lower end of the EDSS disability scale (<5.5 EDSS) where people tend to progress more rapidly (Leray et al. 2010). There was also some evidence from the two main study assessments (EDSS and MSIS-29) that participants with less disability had some slowing of MS progression, but the number of people in this category was too small (in statistical terms) to conclude with any certainty that THC is effective in slowing MS progression. More research will be needed to investigate these findings, and patients will need to be selected at the lower end of the disability spectrum before meaningful conclusions on the neuroprotective ability of cannabinoids or endocannabinoids to slow the rate of disease progression in MS can be drawn.
3 Amyotrophic Lateral Sclerosis
3.1 Natural History
ALS is a fatal progressive neurodegenerative condition predominantly of later life that primarily affects motor neurons in the spinal cord, brainstem and motor cortex, leading to complete paralysis and death usually within 3–5 years from diagnosis. The majority of cases of ALS are sporadic, but there are also familial cases which are now increasingly identified. One is the inheritance of autosomal dominant mutations in the superoxide dismutase (SOD-1) gene encoding for the antioxidant enzyme Cu/Zn-superoxide dismutase (Rosen et al. 1993). Another more recently discovered cause of hereditary ALS is the inheritance of an autosomal dominant hexanucleotide repeat expansion in the non-coding region of the gene C9ORF72 (De Jesus-Hernandez et al. 2011; Renton et al. 2011), which is also associated with fronto-temporal dementia (FTD).
The annual incidence rate of ALS in Europe is 2.16 per 100,000 person years, with similar incidence rates across all countries studied. The incidence was higher among men (3.0 per 100,000 person years) than among women (2.4 per 100,000 person years), and incidence decreases dramatically after 80 years of age (Logroscino et al. 2010). Both upper motor neurons and lower motor neurons degenerate in ALS and the communication between the neuron and muscle is lost, prompting progressive muscle weakening and the appearance of fasciculations (persistent muscle twitches). In the later disease stages, the patients become progressively paralyzed and up to 50 % of people with ALS can show cognitive impairment, particularly involving more severe executive dysfunction and mild memory decline.
To date the anti-glutamatergic agent Riluzole (Rilutek®) is the only licensed medication for ALS whose mechanism of action is by blockade of voltage-dependent sodium channels on motor neurons (Cheah et al. 2010). However, only a modest increase in survival time is seen with Riluzole, indicating the urgent need for better therapeutics for ALS. The major problem with ALS currently is that at the time of diagnosis, the disease progression is already well advanced, with significant motor neuron loss, and so the administration of therapeutics is unlikely to significantly affect disease course. However, particularly in familial ALS, those at risk of developing ALS could be identified well before significant pathology has developed, and so neuroprotective agents may be administered prophylactically to hopefully delay or prevent the development of ALS.
3.2 Pathology, Symptoms and Disability
The pathogenesis of ALS is still incompletely understood, but a number of mechanisms have been implicated including neurofilament accumulation leading to cellular inclusions via defective protein processing, disruption of axonal transport, neurotransmitter-mediated excitotoxicity, oxidative stress, mitochondrial dysfunction and also neuroinflammation with extensive microglial activation (Bilsland et al. 2008; Malik et al. 2013; Rao and Weiss 2004; Ström et al. 2008; Zhao et al. 2008). An established hallmark of ALS is the presence of various inclusion bodies in degenerating neurones and surrounding reactive astrocytes. Ubiquitinated inclusions are the most common and specific type of inclusion in ALS and are found in lower motor neurons of the spinal cord and brainstem (Matsumoto et al. 1993). ALS typically presents as muscle weakness and/or fasciculations, which gradually worsen, bulbar symptoms (speech problems and difficulty swallowing) and eventually respiratory problems leading to failure. Spasticity can develop in weakened atrophic limbs affecting dexterity and gait, and at the later stages of disease flexor spasms may develop due to excessive activation of the flexor arc in a spastic limb (Wijesekera and Leigh 2009).
Many of these mechanisms and symptoms may be amenable to manipulation via the pharmacological action of cannabinoid receptor agonists or by the manipulation of the levels of endogenous endocannabinoids.
3.3 Endocannabinoids in ALS
3.3.1 Experimental Evidence
Compared to MS, there is a relative paucity of studies on endocannabinoids and ALS. The main source of evidence for the potential role of endocannabinoids and cannabinoids in the amelioration of ALS comes from studies conducted in the G93A-SOD1 mouse, which is a transgenic mouse strain expressing the human mutated SOD-1 autosomal dominant gene expressed in familial cases of ALS (Tu et al. 1996). This mouse model displays many of the clinical and pathological hallmarks of ALS, and evidence for the involvement of the endocannabinoid system has been obtained by several groups using this model system.
Increases in the levels of both AEA and 2-AG have been reported in the lumbar regions of the spinal cords of G93A-SOD 1 mice, which is the first portion of the spinal cord to show neurodegeneration before overt motor impairment (Witting et al. 2004). It is postulated that the increase in endocannabinoid levels is an endogenous neuroprotective mechanism in response to neurodegenerative processes. Supportive evidence for this was provided by the observation that genetic ablation of FAAH in G93A-SOD 1 mice, thereby increasing AEA levels, significantly delayed disease progression, which was also demonstrated by treating G93A-SOD 1 mice with the CB1/CB2 agonist WIN 55,212 (Bilsland et al. 2006). However, WIN 55,212-2, or elevation of endocannabinoid levels by FAAH ablation, had no effect on life span. Genetic depletion of the CB1 receptor, in contrast, had no effect on disease onset in G93A-SOD 1 mice but significantly extended life span. These results showed that cannabinoids have significant neuroprotective effects in this model of ALS but suggested that these beneficial effects may be mediated by non-CB1 receptor mechanisms such as the activation of CB2 receptors (also activated by WIN 55-212) that putatively suppress neuroinflammatory processes. Further evidence for the involvement of the CB2 receptor was demonstrated by the observation that CB2 receptors are dramatically upregulated in the spinal cords of G93A-SOD 1 mice. This is presumably in activated cells of the immune system such as microglia, and administration of the selective CB2 agonist AM-1241 at onset of neurological signs increased the survival interval after disease onset by 56 % (Shoemaker et al. 2007).
The sensitivity of cannabinoid CB1 receptors controlling both glutamate and gamma aminobutyric acid (GABA) transmission was potentiated in ALS mice, indicating that adaptations of the endocannabinoid system might be involved in the pathophysiology of ALS (Rossi et al. 2010). Excitatory and inhibitory synaptic transmission was investigated in the striatum of G93A-SOD1 ALS mice, along with the sensitivity of these synapses to cannabinoid CB1 receptor stimulation. There was a reduced frequency of glutamate-mediated EPSCs and increased frequency of GABA-mediated spontaneous inhibitory postsynaptic currents recorded from striatal neurons of ALS mice, possibly due to presynaptic defects in neurotransmitter release via CB1 receptor overactivity (Rossi et al. 2010). In hippocampal neuron cultures, there was a blocking of the TNF-α (a proinflammatory cytokine), induced increase of the expression of AMPA glutamate receptors via CB1 receptor stimulation and a subsequent protection from excitotoxic death (Zhao et al. 2010).
Increases in N-acylphosphatidylethanolamine phospholipase D (one of the enzymes responsible for the generation of AEA) and CB2 receptors were detected in the spinal cords of male G93A-SOD 1 mice but in female mice only increases in CB2 receptors were reported, pointing to an increase in neuroinflammation in these animals (Moreno-Martet et al. 2014). Treatment of these mice with a Sativex®-like combination of phytocannabinoids only produced weak improvements in the progression of neurological deficits and survival, particularly in females (Moreno-Martet et al. 2014). These experimental data point to the potential role of the endocannabinoid system as a promising therapeutic avenue for the treatment of ALS although much further work needs to be done.
3.3.2 Clinical Evidence
To date, there is little clinical evidence on endocannabinoids or cannabinoid therapy in ALS, but the preclinical experimental data indicate that there may be a clinical state of endocannabinoid deficiency involved. People with ALS have reported that cannabis can alleviate some of the symptoms associated with ALS such as pain and muscle spasms, appetite improvement and alleviation of depression and excessive drooling (sialorrhea) due to the reduction in saliva production (Amtmann et al. 2004). Patients who were able to get access to cannabis found it preferable to prescribe medication for their symptoms (Carter et al. 2010). In addition to pain, spasticity is also a major problem for patients with ALS and patients report that cannabis can subjectively improve spasticity (Amtmann et al. 2004). However, a small scale study on cramps in ALS did not demonstrate a subjective improvement in cramp intensity in 27 ALS patients in a randomised double-blind crossover trial with 5 mg THC twice daily (Weber et al. 2010). The situation for cannabis and ALS is analogous to that of MS at the beginning of this century, with anecdotal patient reports of potential efficacy that needs to be followed up by properly designed randomised clinical trials that can properly examine the therapeutic benefit of cannabis or the endocannabinoids in symptom management or their potential for disease modification.
4 Summary
The beneficial therapeutic effects of cannabinoids and potentially the endocannabinoids in symptom management in MS can now be said to be proven, particularly for symptoms such as spasticity. The potential for cannabis in slowing disease progression in MS is less clear, but experimental evidence clearly suggests that cannabis and the endocannabinoids are definitely neuroprotective, and the findings of a single clinical trial performed with THC capsules, though certainly not definitive, do suggest that there was a neuroprotective benefit in a sub-group of patients with a lower initial level of disability (although the numbers in this group were too low for a definitive conclusion). Such a clinical trial needs to be repeated in a larger group of MS patients with lower levels of disability on entering the study. In ALS, the field is some years behind that of MS. Experimental studies do point to a potential role of cannabis and the endocannabinoids in the management of this disease, particularly with regard to symptoms such as pain and spasticity, but also potentially in the modification of disease progression, and the need for clinical trials in this area to investigate this is indicated.
Abbreviations
- 2-AG:
-
2-Arachidonoyl glycerol
- AEA:
-
Anandamide
- ALS:
-
Amyotrophic lateral sclerosis
- EDSS:
-
Expanded disability status scale
- EPSC:
-
Excitatory post-synaptic current
- FAAH:
-
Fatty acid amide hydrolase
- FTD:
-
Fronto-temporal dementia
- GABA:
-
Gamma aminobutyric acid
- MAG lipase:
-
Monoacylglycerol lipase
- MS:
-
Multiple sclerosis
- OEA:
-
Oleoylethanolamide
- PEA:
-
Palmitoylethanolamide
- SOD-1:
-
Superoxide dismutase 1
- THC:
-
Δ9-tetrahydrocannabinol
References
Amato MP, Ponziani G (2000) A prospective study on the prognosis of multiple sclerosis. Neurol Sci 21:S831–S838. doi:10.1007/s100720070021
Amtmann D, Weydt P, Johnson KL, Jensen MP, Carter GT (2004) Survey of cannabis use in patients with amyotrophic lateral sclerosis. Am J Hosp Palliat Care 21:95–104. doi:10.1177/104990910402100206
Andersson PB, Waubant E, Gee L, Goodkin DE (1999) Multiple sclerosis that is progressive from the time of onset: clinical characteristics and progression of disability. Arch Neurol 56:1138–1142. doi:10.1001/archneur.56.9.1138
Ascherio A, Munger KL (2010) Epstein-Barr virus infection and multiple sclerosis: a review. J Neuroimmune Pharmacol 5:271–277. doi:10.1007/s11481-010-9201-3
Baker D, Pryce G, Croxford JL, Brown P, Pertwee RG, Huffman JW, Layward L (2000) Cannabinoids control spasticity and tremor in a multiple sclerosis model. Nature 404:84–87. doi:10.1038/35003583
Baker D, Pryce G, Croxford JL, Brown P, Pertwee RG, Makriyannis A, Khanolkar A, Layward L, Fezza F, Bisogno T, Di Marzo V (2001) Endocannabinoids control spasticity in a multiple sclerosis model. FASEB J 15:300–302. doi:10.1096/fj.00-0399fje
Barnett MH, Prineas JW (2004) Relapsing and remitting multiple sclerosis: pathology of the newly forming lesion. Ann Neurol 55:458–468. doi:10.1002/ana.20016
Benito C, Romero JP, Tolón RM, Clemente D, Docagne F, Hillard CJ, Guaza C, Romero J (2007) Cannabinoid CB1 and CB2 receptors and fatty acid amide hydrolase are specific markers of plaque cell subtypes in human multiple sclerosis. J Neurosci 27:2396–2402. doi:10.1523/JNEUROSCI.4814-06.2007
Bernal-Chico A, Canedo M, Manterola A, Victoria Sánchez-Gómez M, Pérez-Samartín A, Rodríguez-Puertas R, Matute C, Mato S (2015) Blockade of monoacylglycerol lipase inhibits oligodendrocyte excitotoxicity and prevents demyelination in vivo. Glia 63:163–176. doi:10.1002/glia.22742
Berrendero F, Sánchez A, Cabranes A, Puerta C, Ramos JA, García-Merino A, Fernández-Ruiz J (2001) Changes in cannabinoid CB(1) receptors in striatal and cortical regions of rats with experimental allergic encephalomyelitis, an animal model of multiple sclerosis. Synapse 41:195–202. doi:10.1002/syn.1075
Bilsland LG, Dick JR, Pryce G, Petrosino S, Di Marzo V, Baker D, Greensmith L (2006) Increasing cannabinoid levels by pharmacological and genetic manipulation delay disease progression in SOD1 mice. FASEB J 20:1003–1005. doi:10.1096/fj.05-4743fje
Bilsland LG, Nirmalananthan N, Yip J, Greensmith L, Duchen MR (2008) Expression of mutant SOD1 in astrocytes induces functional deficits in motoneuron mitochondria. J Neurochem 107:1271–1283. doi:10.1111/j.1471-4159.2008.05699.x
Bjartmar C, Kidd G, Mork S, Rudick R, Trapp BD (2000) Neurological disability correlates with spinal cord axonal loss and reduced N-acetyl aspartate in chronic multiple sclerosis patients. Ann Neurol 48:893–901. doi:10.1002/1531-8249(200012)48:6<893::AID-ANA10>3.0.CO;2-B
Bjartmar C, Wujek JR, Trapp BD (2003) Axonal loss in the pathology of MS: consequences for understanding the progressive phase of the disease. J Neurol Sci 206:165–171. doi:10.1016/S0022-510X(02)00069-2
Cabranes A, Venderova K, de Lago E, Fezza F, Sánchez A, Mestre L, Valenti M, García-Merino A, Ramos JA, Di Marzo V, Fernández-Ruiz J (2005) Decreased endocannabinoid levels in the brain and beneficial effects of agents activating cannabinoid and/or vanilloid receptors in a rat model of multiple sclerosis. Neurobiol Dis 20:207–217. doi:10.1016/j.nbd.2005.03.002
Carter GT, Abood ME, Aggarwal SK, Weiss MD (2010) Cannabis and amyotrophic lateral sclerosis: hypothetical and practical applications, and a call for clinical trials. Am J Hosp Palliat Care 27:347–356. doi:10.1177/104990910402100206
Centonze D, Bari M, Rossi S, Prosperetti C, Furlan R, Fezza F, De Chiara V, Battistini L, Bernardi G, Bernardini S, Martino G, Maccarrone M (2007) The endocannabinoid system is dysregulated in multiple sclerosis and in experimental autoimmune encephalomyelitis. Brain 130:2543–2553. doi:10.1093/brain/awm160
Chang A, Smith MC, Yin X, Fox RJ, Staugaitis SM, Trapp BD (2008) Neurogenesis in the chronic lesions of multiple sclerosis. Brain 131:2366–2375. doi:10.1093/brain/awn157
Cheah BC, Vucic S, Krishnan AV, Kiernan MC (2010) Riluzole, neuroprotection and amyotrophic lateral sclerosis. Curr Med Chem 17:1942–1949. doi:10.2174/ 092986710791163939
Compston A, Coles A (2002) Multiple sclerosis. Lancet 359:1221–1231. doi:10.1016/S0140-6736(02)08220-X
Compston A, Coles A (2008) Multiple sclerosis. Lancet 372:1502–1517. doi:10.1016/S0140-6736(08)61620-7
Confavreux C, Vukusic S (2006) Accumulation of irreversible disability in multiple sclerosis: from epidemiology to treatment. Clin Neurol Neurosurg 108:327–332. doi:10.1016/j.clineuro.2005.11.018
Confavreux C, Vukusic S, Moreau T, Adeleine P (2000) Relapses and progression of disability in multiple sclerosis. N Engl J Med 343:1430–1438. doi:10.1056/NEJM200011163432001
Croxford JL, Pryce G, Jackson SJ, Ledent C, Giovannoni G, Pertwee RG, Yamamura T, Baker D (2008) Cannabinoid-mediated neuroprotection, not immunosuppression, may be more relevant to multiple sclerosis. J Neuroimmunol 193:120–129. doi:10.1016/j.jneuroim.2007.10.024
De Jesus-Hernandez M, Mackenzie IR, Boeve BF, Boxer AL, Baker M, Rutherford NJ, Nicholson AM, Finch NA, Flynn H, Adamson J, Kouri N, Wojtas A, Sengdy P, Hsiung GY, Karydas A, Seeley WW, Josephs KA, Coppola G, Geschwind DH, Wszolek ZK, Feldman H, Knopman DS, Petersen RC, Miller BL, Dickson DW, Boylan KB, Graff-Radford NR, Rademakers R (2011) Expanded GGGGCC hexanucleotide repeat in noncoding region of C9ORF72 causes chromosome 9p-linked FTD and ALS. Neuron 72:245–256. doi:10.1016/j.neuron.2011.09.011
de Lago E, Ligresti A, Ortar G, Morera E, Cabranes A, Pryce G, Bifulco M, Baker D, Fernandez-Ruiz J, Di Marzo V (2004) In vivo pharmacological actions of two novel inhibitors of anandamide cellular uptake. Eur J Pharmacol 484:249–257. doi:10.1016/j.ejphar.2003.11.027
de Lago E, Fernández-Ruiz J, Ortega-Gutiérrez S, Cabranes A, Pryce G, Baker D, López-Rodríguez M, Ramos JA (2006) UCM707, an inhibitor of the anandamide uptake, behaves as a symptom control agent in models of Huntington’s disease and multiple sclerosis, but fails to delay/arrest the progression of different motor-related disorders. Eur Neuropsychopharmacol 16:7–18. doi:10.1016/j.euroneuro.2005.06.001
Di Filippo M, Pini LA, Pelliccioli GP, Calabresi P, Sarchielli P (2008) Abnormalities in the cerebrospinal fluid levels of endocannabinoids in multiple sclerosis. J Neurol Neurosurg Psychiatry 79:1224–1229. doi:10.1136/jnnp.2007.139071
Ebers GC, Sadovnick AD (1993) The geographic distribution of multiple sclerosis: a review. Neuroepidemiology 12:1–5. doi:10.1159/000110293
Flachenecker P, Henze T, Zettl UK (2014) Nabiximols (THC/CBD oromucosal spray, Sativex®) in clinical practice–results of a multicenter, non-interventional study (MOVE 2) in patients with multiple sclerosis spasticity. Eur Neurol 71:271–279. doi:10.1159/000357427
Handel AE, Williamson AJ, Disanto G, Handunnetthi L, Giovannoni G, Ramagopalan SV (2010) An updated meta-analysis of risk of multiple sclerosis following infectious mononucleosis. PLoS One 5, e12496. doi:10.1371/journal.pone.0012496
Hernández-Torres G, Cipriano M, Hedén E, Björklund E, Canales A, Zian D, Feliú A, Mecha M, Guaza C, Fowler CJ, Ortega-Gutiérrez S, López-Rodríguez ML (2014) A reversible and selective inhibitor of monoacylglycerol lipase ameliorates multiple sclerosis. Angew Chem Int Ed Engl 53(50):13765–13770. doi:10.1002/anie.201407807
Jackson SJ, Pryce G, Diemel LT, Cuzner ML, Baker D (2005) Cannabinoid-receptor 1 null mice are susceptible to neurofilament damage and caspase 3 activation. Neuroscience 134:261–268. doi:10.1016/j.neuroscience.2005.02.045
Jean-Gilles L, Feng S, Tench CR, Chapman V, Kendall DA, Barrett DA, Constantinescu CS (2009) Plasma endocannabinoid levels in multiple sclerosis. J Neurol Sci 287:212–215. doi:10.1016/j.jns.2009.07.021
Jones JL, Coles AJ (2010) New treatment strategies in multiple sclerosis. Exp Neurol 225:34–39. doi:10.1016/j.expneurol.2010.06.003
Kong W, Li H, Tuma RF, Ganea D (2014) Selective CB2 receptor activation ameliorates EAE by reducing Th17 differentiation and immune cell accumulation in the CNS. Cell Immunol 287:1–17. doi:10.1016/j.cellimm.2013.11.002
Kurtzke JF (1993) Epidemiologic evidence for multiple sclerosis as an infection. Clin Microbiol Rev 6:382–427
Leray E, Yaouanq J, Le Page E, Coustans M, Laplaud D, Oger J, Edan G (2010) Evidence for a two-stage disability progression in multiple sclerosis. Brain 133:1900–1913. doi:10.1093/brain/awq076
Logroscino G, Traynor BJ, Hardiman O, Chiò A, Mitchell D, Swingler RJ, Millul A, Benn E, Beghi E, EURALS (2010) Incidence of amyotrophic lateral sclerosis in Europe. J Neurol Neurosurg Psychiatry 81:385–390. doi:10.1136/jnnp.2009.183525
Lorente Fernández L, Monte Boquet E, Pérez-Miralles F, Gil Gómez I, Escutia Roig M, Boscá Blasco I, Poveda Andrés JL, Casanova-Estruch B (2014) Clinical experiences with cannabinoids in spasticity management in multiple sclerosis. Neurologia 29:257–260. doi:10.1016/j.nrl.2013.06.014
Lourbopoulos A, Grigoriadis N, Lagoudaki R, Touloumi O, Polyzoidou E, Mavromatis I, Tascos N, Breuer A, Ovadia H, Karussis D, Shohami E, Mechoulam R, Simeonidou C (2011) Administration of 2-arachidonoylglycerol ameliorates both acute and chronic experimental autoimmune encephalomyelitis. Brain Res 1390:126–141. doi:10.1016/j.brainres.2011.03.020
Malik B, Nirmalananthan N, Gray AL, La Spada AR, Hanna MG, Greensmith L (2013) Co-induction of the heat shock response ameliorates disease progression in a mouse model of human spinal and bulbar muscular atrophy: implications for therapy. Brain 136:926–943. doi:10.1093/brain/aws343
Maresz K, Pryce G, Ponomarev ED, Marsicano G, Croxford JL, Shriver LP, Ledent C, Cheng X, Carrier EJ, Mann MK, Giovannoni G, Pertwee RG, Yamamura T, Buckley NE, Hillard CJ, Lutz B, Baker D, Dittel BN (2007) Direct suppression of CNS autoimmune inflammation via the cannabinoid receptor CB1 on neurons and CB2 on autoreactive T cells. Nat Med 13:492–497. doi:10.1038/nm1561
Marsicano G, Goodenough S, Monory K, Hermann H, Eder M, Cannich A, Azad SC, Cascio MG, Gutiérrez SO, van der Stelt M, López-Rodriguez ML, Casanova E, Schütz G, Zieglgänsberger W, Di Marzo V, Behl C, Lutz B (2003) CB1 cannabinoid receptors and on-demand defense against excitotoxicity. Science 302:84–88. doi:10.1126/science.1088208
Matsumoto SI, Goto S, Kusaka H, Imai T, Hashizume Y, Okazaki H, Hirano A (1993) Ubiquitin-positive inclusion in anterior horn cells in subgroups of motor neuron diseases: a comparative study of adult-onset amyotrophic lateral sclerosis, juvenile amyotrophic lateral sclerosis and Werdnig-Hoffmann disease. J Neurol Sci 115:208–213. doi:10.1016/0022-510X(93)90226-O
Miller DH, Leary SM (2007) Primary-progressive multiple sclerosis. Lancet Neurol 6:903–912. doi:10.1016/S1474-4422(07)70243-0
Moreno-Martet M, Espejo-Porras F, Fernández-Ruiz J, de Lago E (2014) Changes in endocannabinoid receptors and enzymes in the spinal cord of SOD1(G93A) transgenic mice and evaluation of a Sativex(®)-like combination of phytocannabinoids: interest for future therapies in amyotrophic lateral sclerosis. CNS Neurosci Ther 20:809–815. doi:10.1111/cns.12262
Musella A, Sepman H, Mandolesi G, Gentile A, Fresegna D, Haji N, Conrad A, Lutz B, Maccarrone M, Centonze D (2014) Pre- and postsynaptic type-1 cannabinoid receptors control the alterations of glutamate transmission in experimental autoimmune encephalomyelitis. Neuropharmacology 79:567–572. doi:10.1016/j.neuropharm.2014.01.007
Parratt JD, Prineas JW (2010) Neuromyelitis optica: a demyelinating disease characterized by acute destruction and regeneration of perivascular astrocytes. Mult Scler 16:1156–1172. doi:10.1177/1352458510382324
Polman CH, O’Connor PW, Havrdova E, Hutchinson M, Kappos L, Miller DH, Phillips JT, Lublin FD, Giovannoni G, Wajgt A, Toal M, Lynn F, Panzara MA, Sandrock AW, Investigators AFFIRM (2006) A randomized, placebo-controlled trial of natalizumab for relapsing multiple sclerosis. N Engl J Med 354:899–910. doi:10.1056/NEJMoa044397
Prat E, Tomaru U, Sabater L, Park DM, Granger R, Kruse N, Ohayon JM, Bettinotti MP, Martin R (2005) HLA-DRB5*0101 and -DRB1*1501 expression in the multiple sclerosis-associated HLA-DR15 haplotype. J Neuroimmunol 167:108–119. doi:10.1016/j.jneuroim.2005.04.027
Pryce G, Baker D (2007) Control of spasticity in a multiple sclerosis model is mediated by CB1, not CB2, cannabinoid receptors. Br J Pharmacol 150:519–525. doi:10.1038/sj.bjp.0707003
Pryce G, Ahmed Z, Hankey DJ, Jackson SJ, Croxford JL, Pocock JM, Ledent C, Petzold A, Thompson AJ, Giovannoni G, Cuzner ML, Baker D (2003) Cannabinoids inhibit neurodegeneration in models of multiple sclerosis. Brain 126:2191–2202. doi:10.1093/brain/awg224
Pryce G, Cabranes A, Fernández-Ruiz J, Bisogno T, Di Marzo V, Long JZ, Cravatt BF, Giovannoni G, Baker D (2013) Control of experimental spasticity by targeting the degradation of endocannabinoids using selective fatty acid amide hydrolase inhibitors. Mult Scler 19:1896–1904. doi:10.1177/1352458513485982
Ramagopalan SV, Maugeri NJ, Handunnetthi L, Lincoln MR, Orton SM, Dyment DA, Deluca GC, Herrera BM, Chao MJ, Sadovnick AD, Ebers GC, Knight JC (2009) Expression of the multiple sclerosis-associated MHC class II Allele HLA-DRB1*1501 is regulated by vitamin D. PLoS Genet 5:e1000369. doi:10.1371/journal.pgen.1000369
Ramagopalan SV, Heger A, Berlanga AJ, Maugeri NJ, Lincoln MR, Burrell A, Handunnetthi L, Handel AE, Disanto G, Orton SM, Watson CT, Morahan JM, Giovannoni G, Ponting CP, Ebers GC, Knight JC (2010) A ChIP-seq defined genome-wide map of vitamin D receptor binding: associations with disease and evolution. Genome Res 2:1352–1360. doi:10.1101/gr.107920.110
Rao SD, Weiss JH (2004) Excitotoxic and oxidative cross-talk between motor neurons and glia in ALS pathogenesis. Trends Neurosci 27:17–23. doi:10.1016/j.tins.2003.11.001
Renton AE, Majounie E, Waite A, Simón-Sánchez J, Rollinson S, Gibbs JR, Schymick JC, Laaksovirta H, van Swieten JC, Myllykangas L, Kalimo H, Paetau A, Abramzon Y, Remes AM, Kaganovich A, Scholz SW, Duckworth J, Ding J, Harmer DW, Hernandez DG, Johnson JO, Mok K, Ryten M, Trabzuni D, Guerreiro RJ, Orrell RW, Neal J, Murray A, Pearson J, Jansen IE, Sondervan D, Seelaar H, Blake D, Young K, Halliwell N, Callister JB, Toulson G, Richardson A, Gerhard A, Snowden J, Mann D, Neary D, Nalls MA, Peuralinna T, Jansson L, Isoviita VM, Kaivorinne AL, Hölttä-Vuori M, Ikonen E, Sulkava R, Benatar M, Wuu J, Chiò A, Restagno G, Borghero G, Sabatelli M, ITALSGEN Consortium, Heckerman D, Rogaeva E, Zinman L, Rothstein JD, Sendtner M, Drepper C, Eichler EE, Alkan C, Abdullaev Z, Pack SD, Dutra A, Pak E, Hardy J, Singleton A, Williams NM, Heutink P, Pickering-Brown S, Morris HR, Tienari PJ, Traynor BJ (2011) A hexanucleotide repeat expansion in C9ORF72 is the cause of chromosome 9p21-linked ALS-FTD. Neuron 72:257–268. doi:10.1016/j.neuron.2011.09.010
Rosen DR, Siddique T, Patterson D, Figlewicz DA, Sapp P, Hentati A, Donaldson D, Goto J, O’Regan JP, Deng HX, Rahmani Z, Krizus A, McKenna-Yasek D, Cayabyab A, Gaston SM, Berger R, Tanzi RE, Halperin JJ, Herzfeldt B, Van den Bergh R, Hung W, Bird T, Deng G, Mulder DW, Smyth C, Laing NG, Soriano E, Pericak-Vance MA, Haines J, Rouleau GA, Gusella JS, Horvitz HR, Robert H, Brown RH Jr (1993) Mutations in Cu/Zn superoxide dismutase gene are associated with familial amyotrophic lateral sclerosis. Nature 362:59–62. doi:10.1038/362059a0
Rossi S, De Chiara V, Musella A, Cozzolino M, Bernardi G, Maccarrone M, Mercuri NB, Carrì MT, Centonze D (2010) Abnormal sensitivity of cannabinoid CB1 receptors in the striatum of mice with experimental amyotrophic lateral sclerosis. Amyotroph Lateral Scler 11:83–90. doi:10.3109/17482960902977954
Rossi S, Furlan R, De Chiara V, Muzio L, Musella A, Motta C, Studer V, Cavasinni F, Bernardi G, Martino G, Cravatt BF, Lutz B, Maccarrone M, Centonze D (2011) Cannabinoid CB1 receptors regulate neuronal TNF-α effects in experimental autoimmune encephalomyelitis. Brain Behav Immun 25:1242–1248. doi:10.1016/j.bbi.2011.03.017
Runmarker B, Andersen O (1993) Prognostic factors in a multiple sclerosis incidence cohort with twenty-five years of follow-up. Brain 116:117–134. doi:10.1093/brain/116.1.117
Sawcer S, Hellenthal G, Pirinen M, Spencer CC, Patsopoulos NA, Moutsianas L, Dilthey A, Su Z, Freeman C, Hunt SE, Edkins S, Gray E, Booth DR, Potter SC, Goris A, Band G, Oturai AB, Strange A, Saarela J, Bellenguez C, Fontaine B, Gillman M, Hemmer B, Gwilliam R, Zipp F, Jayakumar A, Martin R, Leslie S, Hawkins S, Giannoulatou E, D’alfonso S, Blackburn H, Martinelli BF, Liddle J, Harbo HF, Perez ML, Spurkland A, Waller MJ, Mycko MP, Ricketts M, Comabella M, Hammond N, Kockum I, McCann OT, Ban M, Whittaker P, Kemppinen A, Weston P, Hawkins C, Widaa S, Zajicek J, Dronov S, Robertson N, Bumpstead SJ, Barcellos LF, Ravindrarajah R, Abraham R, Alfredsson L, Ardlie K, Aubin C, Baker A, Baker K, Baranzini SE, Bergamaschi L, Bergamaschi R, Bernstein A, Berthele A, Boggild M, Bradfield JP, Brassat D, Broadley SA, Buck D, Butzkueven H, Capra R, Carroll WM, Cavalla P, Celius EG, Cepok S, Chiavacci R, Clerget-Darpoux F, Clysters K, Comi G, Cossburn M, Cournu-Rebeix I, Cox MB, Cozen W, Cree BA, Cross AH, Cusi D, Daly MJ, Davis E, de Bakker PI, Debouverie M, D’hooghe MB, Dixon K, Dobosi R, Dubois B, Ellinghaus D, Elovaara I, Esposito F, Fontenille C, Foote S, Franke A, Galimberti D, Ghezzi A, Glessner J, Gomez R, Gout O, Graham C, Grant SF, Guerini FR, Hakonarson H, Hall P, Hamsten A, Hartung HP, Heard RN, Heath S, Hobart J, Hoshi M, Infante-Duarte C, Ingram G, Ingram W, Islam T, Jagodic M, Kabesch M, Kermode AG, Kilpatrick TJ, Kim C, Klopp N, Koivisto K, Larsson M, Lathrop M, Lechner-Scott JS, Leone MA, Leppä V, Liljedahl U, Bomfim IL, Lincoln RR, Link J, Liu J, Lorentzen AR, Lupoli S, Macciardi F, Mack T, Marriott M, Martinelli V, Mason D, McCauley JL, Mentch F, Mero IL, Mihalova T, Montalban X, Mottershead J, Myhr KM, Naldi P, Ollier W, Page A, Palotie A, Pelletier J, Piccio L, Pickersgill T, Piehl F, Pobywajlo S, Quach HL, Ramsay PP, Reunanen M, Reynolds R, Rioux JD, Rodegher M, Roesner S, Rubio JP, Rückert IM, Salvetti M, Salvi E, Santaniello A, Schaefer CA, Schreiber S, Schulze C, Scott RJ, Sellebjerg F, Selmaj KW, Sexton D, Shen L, Simms-Acuna B, Skidmore S, Sleiman PM, Smestad C, Sørensen PS, Søndergaard HB, Stankovich J, Strange RC, Sulonen AM, Sundqvist E, Syvänen AC, Taddeo F, Taylor B, Blackwell JM, Tienari P, Bramon E, Tourbah A, Brown MA, Tronczynska E, Casas JP, Tubridy N, Corvin A, Vickery J, Jankowski J, Villoslada P, Markus HS, Wang K, Mathew CG, Wason J, Palmer CN, Wichmann HE, Plomin R, Willoughby E, Rautanen A, Winkelmann J, Wittig M, Trembath RC, Yaouanq J, Viswanathan AC, Zhang H, Wood NW, Zuvich R, Deloukas P, Langford C, Duncanson A, Oksenberg JR, Pericak-Vance MA, Haines JL, Olsson T, Hillert J, Ivinson AJ, De Jager PL, Peltonen L, Stewart GJ, Hafler DA, Hauser SL, McVean G, Donnelly P, Compston A (2011) Genetic risk and a primary role for cell-mediated immune mechanisms in multiple sclerosis. Nature 476:214–219. doi:10.1038/nature10251
Serpell MG, Notcutt W, Collin C (2013) Sativex long-term use: an open-label trial in patients with spasticity due to multiple sclerosis. J Neurol 260:285–295. doi:10.1007/s00415-012-6634-z
Shoemaker JL, Seely KA, Reed RL, Crow JP, Prather PL (2007) The CB2 cannabinoid agonist AM-1241 prolongs survival in a transgenic mouse model of amyotrophic lateral sclerosis when initiated at symptom onset. J Neurochem 101:87–98. doi:10.1111/j.1471-4159.2006.04346.x
Sisay S, Pryce G, Jackson SJ, Tanner C, Ross RA, Michael GJ, Selwood DL, Giovannoni G, Baker D (2013) Genetic background can result in a marked or minimal effect of gene knockout (GPR55 and CB2 receptor) in experimental autoimmune encephalomyelitis models of multiple sclerosis. PLoS One 8(10):e76907. doi:10.1371/journal.pone.0076907
Smith T, Groom A, Zhu B, Turski L (2000) Autoimmune encephalomyelitis ameliorated by AMPA antagonists. Nat Med 6:62–66. doi:10.1038/71548
Stover JF, Pleines UE, Morganti-Kossmann MC, Kossmann T, Lowitzsch K, Kempski OS (1997) Neurotransmitters in cerebrospinal fluid reflect pathological activity. Eur J Clin Invest 27:1038–1043. doi:10.1046/j.1365-2362.1997.2250774.x
Ström AL, Shi P, Zhang F, Gal J, Kilty R, Hayward LJ, Zhu H (2008) Interaction of amyotrophic lateral sclerosis (ALS)-related mutant copper-zinc superoxide dismutase with the dynein-dynactin complex contributes to inclusion formation. J Biol Chem 283:22795–22805. doi:10.1074/jbc.M800276200
Tu PH, Raju P, Robinson KA, Gurney ME, Trojanowski JQ, Lee VM (1996) Transgenic mice carrying a human mutant superoxide dismutase transgene develop neuronal cytoskeletal pathology resembling human amyotrophic lateral sclerosis lesions. Proc Natl Acad Sci U S A 93:3155–3160
Visser EM, Wilde K, Wilson JF, Yong KK, Counsell CE (2012) A new prevalence study of multiple sclerosis in Orkney, Shetland and Aberdeen city. J Neurol Neurosurg Psychiatry 83:719–724. doi:10.1136/jnnp-2011-301546
Weber M, Goldman B, Truniger S (2010) Tetrahydrocannabinol (THC) for cramps in amyotrophic lateral sclerosis: a randomised, double-blind crossover trial. J Neurol Neurosurg Psychiatry 81:1135–1140. doi:10.1136/jnnp.2009.200642
Webb M, Luo L, Ma JY, Tham CS (2008) Genetic deletion of fatty acid amide hydrolase results in improved long-term outcome in chronic autoimmune encephalitis. Neurosci Lett 439:106–110. doi:10.1016/j.neulet.2008.04.090
Weinshenker BG, Bass B, Rice GP, Noseworthy J, Carriere W, Baskerville J, Ebers GC (1989) The natural history of multiple sclerosis: a geographically based study. I. Clinical course and disability. Brain 112:133–146. doi:10.1093/brain/112.1.133
Wijesekera LC, Leigh PN (2009) Amyotrophic lateral sclerosis. Orphanet J Rare Dis 4:3. doi:10.1186/1750-1172-4-3
Willer CJ, Dyment DA, Sadovnick AD, Rothwell PM, Murray TJ, Ebers GC, Canadian Collaborative Study Group (2005) Timing of birth and risk of multiple sclerosis: population based study. BMJ 330:120–123. doi:10.1136/bmj.38301.686030.63
Wilson RI, Nicoll RA (2002) Endocannabinoid signalling in the brain. Science 296:678–682. doi:10.1126/science.1063545
Witting A, Weydt P, Hong S, Kliot M, Moller T, Stella N (2004) Endocannabinoids accumulate in spinal cord of SOD1 G93A transgenic mice. J Neurochem 89:1555–1557. doi:10.1111/j.1471-4159.2004.02544.x
Witting A, Chen L, Cudaback E, Straiker A, Walter L, Rickman B, Möller T, Brosnan C, Stella N (2006) Experimental autoimmune encephalomyelitis disrupts endocannabinoid-mediated neuroprotection. Proc Natl Acad Sci U S A 103:6362–6367. doi:10.1073/pnas.0510418103
Zajicek JP, Sanders HP, Wright DE, Vickery PJ, Ingram WM, Reilly SM, Nunn AJ, Teare LJ, Fox PJ, Thompson AJ (2005) Cannabinoids in multiple sclerosis (CAMS) study: safety and efficacy data for 12 months follow up. J Neurol Neurosurg Psychiatry 76:1664–1669. doi:10.1136/jnnp.2005.070136
Zajicek JP, Hobart JC, Slade A, Barnes D, Mattison PG, MUSEC Research Group (2012) Multiple sclerosis and extract of cannabis: results of the MUSEC trial. J Neurol Neurosurg Psychiatry 83:1125–1132. doi:10.1136/jnnp-2012-302468
Zajicek J, Ball S, Wright D, Vickery J, Nunn A, Miller D, Gomez Cano M, McManus D, Mallik S, Hobart J, CUPID investigator group (2013) Effect of dronabinol on progression in progressive multiple sclerosis (CUPID): a randomised, placebo-controlled trial. Lancet Neurol 12:857–865. doi:10.1016/S1474-4422(13)70159-5
Zhao P, Ignacio S, Beattie EC, Abood ME (2008) Altered presymptomatic AMPA and cannabinoid receptor trafficking in motor neurons of ALS model mice: implications for excitotoxicity. Eur J Neurosci 27:572–579. doi:10.1111/j.1460-9568.2008.06041.x
Zhao P, Leonoudakis D, Abood ME, Beattie EC (2010) Cannabinoid receptor activation reduces TNFalpha-induced surface localization of AMPAR-type glutamate receptors and excitotoxicity. Neuropharmacology 58:551–558. doi:10.1016/j.neuropharm.2009.07.035
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2015 Springer International Publishing Switzerland
About this chapter
Cite this chapter
Pryce, G., Baker, D. (2015). Endocannabinoids in Multiple Sclerosis and Amyotrophic Lateral Sclerosis. In: Pertwee, R. (eds) Endocannabinoids. Handbook of Experimental Pharmacology, vol 231. Springer, Cham. https://doi.org/10.1007/978-3-319-20825-1_7
Download citation
DOI: https://doi.org/10.1007/978-3-319-20825-1_7
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-20824-4
Online ISBN: 978-3-319-20825-1
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)