Abstract
Since the discovery of the two cannabinoid receptors, CB1 and CB2, several molecules, commonly defined as endocannabinoids, able to bind to and functionally activate these receptors, have been discovered and characterized. Although the general thought was that the endocannabinoids were mainly derivatives of the n-6 fatty acid arachidonic acid, recent data have shown that also derivatives (ethanolamides) of n-3 fatty acids may be classified as endocannabinoids. Whether the n-3 endocannabinoids follow the same biosynthetic and metabolic routes of the n-6 endocannabinoids is not yet clear and so warrants further investigation. In this review, we describe the primary biosynthetic and metabolic pathways for the two well-established endocannabinoids, anandamide and 2-arachidonoylglycerol.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
1 Endocannabinoids and Endocannabinoid-Like Compounds
The discovery, in 1988, of a high-affinity, stereoselective and pharmacologically distinct cannabinoid receptor in rat brain tissue (Devane et al. 1988), led to a continuous search for natural endogenous ligands. Since then, several molecules have been identified and collectively named as “endocannabinoids”. Endocannabinoids are defined as derivatives (amides, esters and ethers) of a long-chain polyunsaturated fatty acid (PUFA), mainly arachidonic acid (AA), capable of binding and functionally activating the cannabinoid receptors (Di Marzo et al. 2004). Although anandamide (AEA) and 2-arachidonoylglycerol (2-AG) are the best characterized endocannabinoids (an N-acylethanolamine and a monoacylglycerol, respectively), other endogenous compounds that may also bind to the cannabinoid receptors have been discovered and suggested to be endocannabinoids: N-dihomo-γ-linolenoyl ethanolamine and N-oleoyl dopamine (OLDA) (Pertwee 2005), 2-arachidonoylglycerol ether (noladin ether, 2-AGE) (Hanus et al. 2001), O-arachidonoylethanolamine (virodhamine) (Porter et al. 2002), and N-arachidonoyldopamine (NADA) (Huang et al. 2002). It is now well established that the two most studied endocannabinoids (AEA and 2-AG) do not interact only with cannabinoid CB1 and CB2 receptors, and exhibit instead a degree of promiscuity that applies also to the less-studied arachidonic acid-derived endocannabinoids (Zygmunt et al. 1999; Hanus et al. 2001; Huang et al. 2002; Porter et al. 2002; Rozenfeld and Devi 2008; den Boon et al. 2012). Anandamide (AEA), from the Sanskrit word ananda, which means bliss, was the first endogenous cannabinoid identified (Devane et al. 1992; Hanus 2007). This is the ethanolamide of arachidonic acid and behaves as a partial agonist at both cannabinoid CB1 and CB2 receptors (Pertwee et al. 2010). Interestingly, it is now well established that AEA possesses an ability to interact also with other receptors, such as the transient receptor potential vanilloid 1 (TRPV1) (Zygmunt et al. 1999) and the peroxisome proliferator-activated receptor (PPAR) family (O’Sullivan 2007). Indeed, some of the effects of AEA are non-CB1/non-CB2 receptor mediated (Breivogel et al. 2001; Monory et al. 2002). 2-Arachidonoylglycerol (2-AG) is the arachidonate ester of glycerol that was isolated from peripheral tissues. This molecule can activate both CB1 and CB2 receptors with similar potency and efficacy (Mechoulam et al. 1995; Sugiura et al. 1995) as well as γ-aminobutyric acid receptors (Sigel et al. 2011). 2-Arachidonoy-glyceryl ether (noladin ether) binds to CB1 receptors, and very weakly to CB2 receptors, and also affects AEA uptake (Fezza et al. 2002; Páldyová et al. 2008). Recently, its classification as an endocannabinoid has been questioned because of its very low concentration in the brain (Oka et al. 2003). Virodhamine (from the Sanskrit word virodha, which means opposite) is the ester of arachidonic acid, and it has been reported to behave as a full agonist at CB2 receptors and as both a partial agonist/antagonist at CB1 receptors and a weak inhibitor of AEA uptake (Porter et al. 2002). This molecule can also interact with PPAR-α receptors (Sun et al. 2006) and GPR55 receptors (Sharir et al. 2012). N-arachidonoyl-dopamine (NADA), like AEA, behaves both as an endovanilloid and an endocannabinoid (Bisogno et al. 2000; Huang et al. 2002). It also interacts with PPAR-ƴ receptors (O’Sullivan 2007) and can antagonize the melastatin type-8 (TRPM8) cation channel (De Petrocellis et al. 2007).
We recently discovered that in addition to n-6 long-chain PUFA endocannabinoids, the ethanolamides of two n-3 fatty acids derived mainly from fish oils in the human diet, DHA (C22:6) and EPA (C20:5), should also be classified as endocannabinoids (Brown et al. 2010; Cascio 2013). These n-3 fatty acid ethanolamides are docosahexaenoyl-ethanolamide (DHEA) and eicosapentaenoyl-ethanolamide (EPEA), both of which bind to and partially activate CB1 and CB2 receptors and are produced both in vivo and in vitro after administering fish oil or individual n-3 long-chain PUFA (Sugiura et al. 1996; Bisogno et al. 1999; Berger et al. 2001; Brown et al. 2010, 2011; Maccarrone et al. 2010; Cascio 2013). These n-3 endocannabinoids show anti-inflammatory properties in macrophages and adipocytes (Fezza et al. 2014) and can inhibit cell growth in breast cancer by triggering autophagy via PPAR-γ receptors (Fezza et al. 2014). Interestingly, we recently reported evidence that both DHEA and EPEA possess cannabinoid receptor-dependent and -independent anti-proliferative effects in androgen receptor-positive and -negative prostate cancer cell lines (Brown et al. 2010).
Finally, endocannabinoids are produced together with cannabinoid receptor-inactive saturated and mono- or di-unsaturated compounds that are defined as endocannabinoid-like compounds. These compounds have been reported to exert their cannabimimetic effects by acting as “entourage molecules” that prevent endocannabinoids being degraded by specific metabolic enzymes (Cascio 2013). Palmitoylethanolamide (PEA) possesses both anti-inflammatory and analgesic activity, likely mediated by the TRPV1 and PPAR-α receptors (Costa et al. 2008; Ho et al. 2008; Di Cesare et al. 2013; Esposito et al. 2014), and it also interacts with GPR55 receptors (Moriconi et al. 2010). Stearoylethanolamide (SEA) produces anti-inflammatory, immunomodulatory as well as anorexic effects (Maccarrone et al. 2002; Dalle Carbonare et al. 2008; Ghafouri et al. 2013). Oleoyethanolamide (OEA) can activate both GPR119 and GPR55 receptors (Overton et al. 2008; Moriconi et al. 2010), and it regulates food intake in rodents by a mechanism that involves the activation of PPAR-α receptors (Rodríguez de Fonseca 2004). Oleamide is an unsaturated fatty acid amide isolated from the cerebrospinal fluid of sleep-deprived cats (Cravatt et al. 1995) which behaves as a full cannabinoid CB1 receptor agonist (Leggett et al. 2004). Other compounds that have been recently classified as endocannabinoids-like compounds are N-arachidonoylglycine (NAGly) and N-arachidonoylserine (NArS). NAGly interacts with both GPR18 and GPR92 receptors (Kohno et al. 2006; Oh et al. 2008; Fezza et al. 2014; McHugh et al. 2014) and behaves as a FAAH inhibitor (Cascio et al. 2004). The chemical structures of the endocannabinoids can be found in this volume in Pertwee “Endocannabinoids and Their Pharmacological Actions”.
2 Biosynthesis of the Endocannabinoids
It is generally accepted that endocannabinoids are not stored in cells awaiting release, but are rather synthesized on demand in a Ca2+-dependent manner in response to physiological and pathological stimuli (Di Marzo and Deutsch 1998). However, recent data suggest that AEA can also be stored inside the cell (Oddi et al. 2008). AEA as well as other N-acyl-ethanolamines were initially considered as terminal products of post mortem tissue degradation , and their physiological role remained controversial until the identification of their biosynthetic and metabolic pathways (Piomelli 2014).
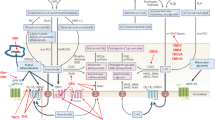
The principal pathway of AEA biosynthesis includes a first step, catalysed by a calcium-dependent N-acyltransferase (NAT), in which an acyl chain is transferred from the sn-1 position of a glycerophospholipid to the amino group of the hydroxyethyl moiety of phosphatidylethanolamine (PE), and a second step in which the generated N-acylphosphatidylethanolamine (NAPE) is hydrolysed to NAE and phosphatidic acid, through a reaction catalysed by a phosphodiesterase of the phospholipase D-type (NAPE-PLD) (Fig. 1). NAPE-PLD, that is chemically and enzymatically distinct from other known PLDs, is a member of the β-lactamase family of zinc-metal hydrolases, is highly conserved in mouse, rat and human, is stimulated by calcium, is highly expressed in the brain as well as in kidney, spleen, lung, heart and liver and is involved in the formation of other, cannabinoid-receptor inactive, N-acyl-ethanolamines (C16:0, C18:0 and C18:1) (Petersen and Hansen 1999; Ueda et al. 2001a; Liu et al. 2002; Okamoto et al. 2004). Interestingly, studies performed using NAPE-PLD knockout mice suggested that while an increase in endogenous levels of NAPEs with saturated and monounsaturated N-acyl chains was observed, few or no changes were observed in the levels of polyunsaturated NAPEs and NAEs, thus suggesting the existence of alternative AEA biosynthetic pathways (Brown et al. 2013; Cascio 2013; Fonseca et al. 2013). There is evidence too that: (1) AEA is formed from N-acyl-lysophosphatidylethanolamine by a lysophospholipase-D-enzyme (lyso-PLD) (Sun et al. 2004) (Fig. 1); (2) AEA is also formed in a pathway in which a crucial role is played by an additional enzyme, α/β-hydrolase 4 (Abh4), which can act on either NAPE or lyso-NAPE to generate glycerophospho-arachidonoylethanolamide (GpAEA), that is subsequently converted to AEA in the presence of a phosphodiesterase (Simon and Cravatt 2006) (Fig. 1) and finally (3) that NAPE can also be hydrolysed, by phospholipase C, to phosphoanandamide (pAEA) which, in turn, is dephosphorylated by phosphatases to AEA (Liu et al. 2006) (Fig. 1). Interestingly, an alternative biosynthetic pathway for AEA might also exist that involves direct condensation of free arachidonic acid and ethanolamine, catalysed by an AEA synthase. However, this pathway requires high “non-physiological” concentrations of both substrates (Sugiura et al. 1996; Ueda et al. 1996).
Schematic representation of anandamide biosynthesis and degradation . NArPE N-arachidonoylphosphatidyl-ethanolamine, PLC phospholipase C, PTPN22 protein tyrosine phosphatase, PLA 2 phospholipase A2, PE phosphatidyl-ethanolamine, PLD phospholipase D, Abh4 α/β-hydrolase 4, PG prostaglandin, HPETEA hydroxyperoxyeicosatetraenoylethanolamide, LOX lypoxygenase, COX cyclooxygenase, FAAH fatty acid amide hydrolase, NAAA N-acylethanolamine-hydrolysing acid amidase, R 1 ethanolamine
For 2-AG, the most accepted biosynthetic pathway is the hydrolysis of membrane phospholipids that is catalysed by phospholipase C (PLC) and that produces 1,2-diacylglycerol (DAG), which in turn is converted to 2-AG by diacylglycerol lipase (DGL) (Bisogno et al. 2003) (Fig. 2). DGL exists in two closely related forms designated α and β, that are both active at pH 7, both stimulated by calcium and glutathione (GSH) and both inhibited by inhibitors of Ser/Cis-hydrolases, such as p-hydroxy-benzoate-mercuric and HgCl2 but not by phenylmethylsulphonyl fluoride (Bisogno et al. 2003). Both enzymes are also inhibited by RHC80267, which is able to block the formation of 2-AG by intact cells (Bisogno et al. 2003). Pharmacological studies have revealed that during neuronal development, localization of DGLα and DGLβ changes from pre- to post-synaptic elements, i.e. from axonal tracts in the embryo to dendritic fields in the adult, suggesting a different need for 2-AG synthesis from the pre- to the post-synaptic compartment during brain development (Bisogno et al. 2003; Williams et al. 2003). Furthermore, there is evidence too that DGLα plays an essential role in the regulation of retrograde synaptic plasticity and neurogenesis (Gao et al. 2010; Tanimura et al. 2010; Savinainen et al. 2012). Like AEA, 2-AG can also be synthesized via other pathways. However, the physiological importance of these proposed pathways is not yet clear.
Main pathways for 2-arachidonoylglycerol biosynthesis and degradation . PLC phospholipase C, PLA 1 phospholipase A1, PI phospatidyl-inositol, DGL diacylglycerol lipase, HETE-G hydroxyeicosatetraenoyl-glycerol, HPETE-G hydroxyperoxyeicosatetraenoyl-glycerol, LOX lypoxygenase, COX cyclooxygenase, MGL monoacylglycerol lipase, ABHD α/β-hydrolase domain, R 1 glycerol
3 Uptake of the Endocannabinoids : Proposed Mechanisms
Once released into the extracellular space, endocannabinoids exert the majority of their effects by acting, as retrograde messengers, at CB1 cannabinoid receptors present on the surface of presynaptic nerve terminals (Piomelli 2014). So far, it is not clear how the endocannabinoids access their metabolic enzymes. Indeed, while monoacylglycerol lipase (MGL, the main metabolic enzyme of 2-AG) is localized pre-synaptically, fatty acid amide hydrolase (FAAH, the main metabolic enzyme of AEA) is localized post-synaptically, thus at a certain distance from the site of action of AEA (Piomelli 2014). To explain the mechanism(s) by which AEA would be taken up by cells, several interesting hypotheses have been proposed (Fowler 2012, 2013). Briefly, AEA is a lipophilic molecule and as such it could easily diffuse through the cell membrane. However, a simple diffusion through the membrane would cease once the equilibrium in the AEA gradient between the extracellular and intracellular environment is reached, unless this equilibrium is prevented by intracellular metabolism induced by FAAH (Fowler 2012, 2013). However, although FAAH may, of course, influence the uptake of AEA (at least the speed with which this process takes place), the uptake is clearly distinct from FAAH. Indeed, (1) compounds able to selectively inhibit the cellular uptake of AEA, but not FAAH, have been identified (De Petrocellis et al. 2000; Di Marzo et al. 2001, 2002; López-Rodríguez et al. 2001; Ortar et al. 2003); (2) inhibitors of FAAH increase, and inhibitors of AEA uptake decrease, the accumulation of AEA within cells (Kathuria et al. 2003); (3) cells that do not express FAAH rapidly internalize AEA (Di Marzo et al. 1999; Deutsch et al. 2001); (4) NADA and noladin, although they are not FAAH substrates, are rapidly internalized by cells (Fezza et al. 2002; Huang et al. 2002) and (5) a saturable AEA accumulation was observed in synaptosomes and cells prepared from genetically modified mice that do not express FAAH (Fegley et al. 2004; Ligresti et al. 2004).
In addition, since endocannabinoid uptake is rapid, temperature-dependent, selective for anandamide over other acylethanolamides and saturable, the hypothesis that AEA uptake may occur through a facilitated transport mechanism has also been proposed (Di Marzo et al. 1994; Hillard and Jarrahian 2000; Fezza et al. 2008). Unfortunately, the protein responsible for this transport, better known as “Endocannabinoid Membrane Transporter” or EMT, has not yet been cloned and its existence is supported only by indirect evidence. More recent studies have shown the existence of carrier proteins that would facilitate diffusion of AEA through the plasma membranes. Examples are the fatty acid binding proteins FABP5 and FABP7, but not FABP3 (Kaczocha et al. 2009), the heat shock protein 70 (Hsp70) and albumin (Oddi et al. 2009), and the most recently identified FAAH-like anandamide transporter (FLAT). FLAT is a dimeric protein lacking the membrane-anchoring domain of the FAAH dimer, and its overexpression in HEK-293 cells increases AEA uptake (Fu et al. 2012). Other hypotheses have also been proposed (Oddi et al. 2008; Di Pasquale et al. 2009; Fowler 2013).
Several synthetic compounds that are able to inhibit the cellular uptake of AEA have been developed so far, some examples being AM404, VDM11, UCM707, OMDM1, OMDM2 and LY21832110 (Pertwee 2014). These compounds have been reported to possess, at least in animals, promising pharmacological properties for the treatment of cancer, pain, multiple sclerosis, Parkinson’s disease, Huntington disease and anxiety (Pertwee 2014). Interestingly, AM404 has also been reported to be effective against nicotine-seeking behaviour and obsessive compulsive disorders (Pertwee 2014).
4 Degradation of the Endocannabinoids
Two main metabolic pathways have been identified so far: one hydrolytic and the other oxidative (Figs. 1 and 2). AEA is mainly hydrolyzed by FAAH (Cravatt et al. 1996; Giang and Cravatt 1997; Bracey et al. 2002), while 2-AG is mainly hydrolyzed by MGL (Dinh et al. 2002) and also by FAAH. In addition to these two enzymes, an N-acylethanolamine-selective acid amidase (NAAA) (Ueda et al. 1999) and, more recently, a second FAAH (FAAH-2) (Wei et al. 2006), as well as two other enzymes ABHD6 and ABHD12, (Blankman et al. 2007) have been reported to participate in the degradation of several endocannabinoids. Both AEA and 2-AG can also be degraded by enzymes of the arachidonate cascade, such as cyclooxygenase-2 (COX-2), lipoxygenases (LOXs) as well as cytochrome P450 enzymes, to produce the corresponding hydroxy- (in the case of lipoxygenases) and epoxy- (in the case of cytochrome P450 monooxidases) derivatives or to produce prostamides and prostaglandin glycerol esters (in the case of cyclooxygenases and prostaglandin synthases) (Piscitelli and Di Marzo 2012) (Figs. 1 and 2). While both hydroxy- and epoxy-endocannabinoids have been reported to act at both cannabinoid CB1 and CB2 receptors as well as at the vanilloid receptors, TRPV1 (hydroxy-endocannabinoids) and TRPV4 (epoxy-endocannabinoids), both prostamides and prostaglandin-glycerol esters are inactive at cannabinoid receptors. It has been suggested that they act at new, not yet identified, receptors (Piscitelli and Di Marzo 2012). Below, we report a brief description of the enzymes involved in the hydrolysis of endocannabinoids.
4.1 FAAH and NAAA
FAAH, which was first cloned by Cravatt et al. (1996), is an integral membrane protein widely distributed in various tissues of rat (Desarnaud et al. 1995; Cravatt et al. 1996; Katayama et al. 1997), mouse (Sun et al. 2005), and human (Giang and Cravatt 1997). This enzyme, active at pH 8–10, is mainly localized on microsomal membranes and contains 597 amino acids, with a short “amidase” sequence enriched in glycine and serine residues. The isolation of FAAH was made possible by the previous development of potent transition state inhibitors, one of which was used to carry out affinity chromatography purification (Cravatt et al. 1996; Petrosino and Di Marzo 2010). A covalent inhibitor, instead, was used to facilitate the formation of crystals of a slightly modified, soluble form of FAAH and to obtain its structure by X-ray crystallography (Bracey et al. 2002; Petrosino and Di Marzo 2010). FAAH catalytic triad is composed of Ser-Ser-Lys, in which Ser241 plays a critical role as both acid and base in the hydrolytic cycle, whereas Lys142 is the activator of Ser241, and Ser217 participates in the catalytic mechanism of FAAH by facilitating the nucleophile attack and the exit of the leaving group (Petrosino and Di Marzo 2010). Importantly, it has been reported that the promoter region of the FAAH gene is up-regulated by progesterone and leptin and down-regulated by estrogens and glucocorticoids (Puffenbarger et al. 2001; Waleh et al. 2002; Maccarrone et al. 2003a, b). Ergetova and co-workers (Egertová et al. 1998) analysed the distribution of FAAH in rat brain and compared its cellular localization with CB1-type cannabinoid receptors using immunocytochemistry. High concentrations of FAAH were detected in the cerebellum, hippocampus and neocortex, which are enriched with cannabinoid receptors. Immunocytochemical analysis of these brain regions revealed a complementary pattern of FAAH and CB1 expression with CB1 immunoreactivity occurring in fibres surrounding FAAH-immunoreactive cell bodies and/or dendrites (Egertová et al. 1998). In the cerebellum, FAAH was expressed in the cell bodies of Purkinje cells and CB1 was expressed in the axons of granule cells and basket cells, neurons which are presynaptic to Purkinje cells (Egertová et al. 1998).
FAAH is also able to metabolize other fatty acid amides such as N-arachidonoyl-dopamine and a large number of mono-unsaturated and saturated compounds (Ueda 2002; Fegley et al. 2005; Ho and Hillard 2005; Lo Verme et al. 2005). Examples are PEA (De Petrocellis et al. 2001; Ueda et al. 2001b; Ueda 2002; Lo Verme et al. 2005), oleoylethanolamide, N-arachidonoylserine and N-arachidonoylglycine, the latter two of which have also been reported to be FAAH inhibitors (Sheskin et al. 1997; Bradshaw and Walker 2005; Ho and Hillard 2005). Recently, a second isoform of FAAH, FAAH-2, has been identified. It shows ~20 % sequence similarity with FAAH at the amino acid level and is expressed in several species, including human, primates, frog, chicken, pufferfish and zebrafish, but not in rodents (Wei et al. 2006). FAAH-1 and FAAH-2 are located on the cytosolic and luminal sides of intracellular membranes, respectively. Both FAAH enzymes have distinct tissue distribution. Indeed, FAAH-2 was detected in the heart and ovary, but not in the brain, small intestine or testis, which are known to express FAAH-1. However, FAAH-1 and FAAH-2 were both detected in the prostate, lung, kidney and liver (Wei et al. 2006). FAAH is also involved in the hydrolysis of 2-AG (Di Marzo and Deutsch 1998), although it has been observed that levels of 2-AG, unlike those of AEA, are not increased in FAAH-knockout mice (Lichtman et al. 2002). Interestingly, recent reports have shown that FAAH is involved in the production of symptoms of a variety of disorders and that FAAH inhibitors may be effective at ameliorating acute, inflammatory, visceral and neuropathic pain as well as osteoarthritic pain and hyperalgesia induced by bladder inflammation (Pertwee 2014). Importantly, unlike direct CB1 agonists, FAAH inhibitors produce antinociception in mice at doses that do not induce hypomotility, hypothermia, catalepsy and hyperphagia or signs of physical or psychological dependence (Pertwee 2014). A few examples of FAAH inhibitors are URB597, OL135, O-1887, URB532, AM374 (palmitylsulphonyl fluoride), N-arachidonoylglycine and N-arachidonoyl serotonin, JNJ1661010 and CAY10401, AM3506 and AM5206, ST4070, PF3845 and PF04457845 (Pertwee 2014).
One other enzyme involved in AEA hydrolysis is NAAA. This enzyme is a cysteine hydrolase belonging to the N-terminal nucleophile hydrolase superfamily, is present in cellular lysosomes or in the Golgi apparatus of cells, is active only at acidic pH and shows higher selectivity for PEA than for AEA (Brown et al. 2013; Ueda et al. 2013). Millimolar concentrations of dithiothreitol (DTT) as well as non-ionic detergents such as Triton X-100 and Nonidet P-40 are required to promote its full activity (Brown et al. 2013; Ueda et al. 2013). NAAA is highly expressed in a number of blood cell lines, as well as in macrophages in various rodent tissues. In humans, NAAA mRNA is expressed most abundantly in prostate followed by leukocytes, liver, spleen, kidney and pancreas (Ueda et al. 2010). Prostate cancer cell lines like PC3, LNCaP and DU-145 also express high levels of NAAA (Ueda et al. 2010). Interestingly, due to its selectivity towards PEA, selective NAAA inhibitors that can increase local levels of endogenous PEA are expected to be anti-inflammatory and analgesic drugs (Petrosino et al. 2010; Ueda et al. 2013).
4.2 MGL, ABHD6 and ABHD12
MGL is a serine hydrolase responsible for about 85 % of the 2-AG hydrolyzing activity of mouse brain (Blankman et al. 2007). This enzyme of about 303 amino acids is present in both membrane and cytosolic subcellular fractions and can recognize other unsaturated monoacylglycerols also as substrates, which in some cases compete with 2-AG inactivation (Ben-Shabat et al. 1998; Di Marzo and Deutsch 1998). MGL is sensitive to sulphydryl-specific reagents, and comparison models strongly suggest that cysteine residues present near its binding site play a role in the catalytic mechanism (Saario et al. 2005), although the catalytic triad of this enzyme also involves Ser122, Asp239 and His269 (Karlsson et al. 1997). The distribution of MGL was studied in rat, and it was shown to be ubiquitous (Karlsson et al. 1997). Specifically, MGL mRNA was reported to be present in adrenal gland, heart, adipose tissue, kidney, ovary, testis, spleen, lung, liver, skeletal muscle and brain (particularly in hippocampus, cortex, thalamus and cerebellum, where CB1 receptors are highly expressed) (Dinh et al. 2002). Ultrastructural localization studies show that MGL is mainly pre-synaptic and often co-localizes with CB1 receptors in the axon terminals (Savinainen et al. 2012). The complimentary localization in the brain for MGL and FAAH, pre-synaptic and post-synaptic, respectively, has suggested different roles for the two main endocannabinoids in the central nervous system (Gulyas et al. 2004). Several MGL inhibitors have been developed so far. Methylarachidonoylfluorophosphonate (MAFP) inhibits MGL irreversibly but lacks selectivity, since it inhibits most metabolic serine hydrolases (Saario et al. 2004; Savinainen et al. 2010, 2012). N-arachidonoylmaleimide (NAM) selectively, but only partially (85 %), inhibits MGL (Saario et al. 2005; Blankman et al. 2007; Savinainen et al. 2012). Other MGL inhibitors include the non-competitive/irreversible inhibitors, URB602 and JZL184, and the reversible inhibitor, OMDM169 (Petrosino and Di Marzo 2010). Like FAAH inhibitors, MGL inhibitors have been found to have potential therapeutic applications, as indicated, for example, by results obtained from experiments using animal models of acute, visceral, inflammatory, neuropathic or bone cancer pain (Pertwee 2014). Interestingly, MGL inhibitors have also been reported to be efficacious against signs of breast, ovarian, skin and prostate cancer in animal models (Pertwee 2014). Recent data have shown that MGL inhibitors such as JZL184 and URB602 can protect neurons from β amyloid peptide-induced neurodegeneration and apoptosis, suggesting a therapeutic potential for the treatment of Alzheimer’s disease (Pertwee 2014). Unfortunately, it has been reported that JZL184 shares the ability of direct CB1 agonists to induce both physical and psychological dependence in mice as well as tolerance to their antinociceptive effects (Schlosburg et al. 2010; Ghosh et al. 2013; Pertwee 2014).
2-AG metabolism is also catalysed by two integral membrane proteins, α/β-hydrolase domain containing protein-6 (ABHD6) and -12 (ABHD12). Both enzymes belong to the α/β-hydrolase superfamily, with the postulated catalytic triad serine-aspartic acid-histidine (Savinainen et al. 2012). ABHD6, in neurones, is localized at sites of 2-AG generation, including post-synaptic dendrites of principal glutamatergic neurones as well as some GABAergic interneurons (Savinainen et al. 2012). ABHD12 is highly expressed in microglia, macrophages and osteoclasts (Fiskerstrand et al. 2010). Interestingly, it was observed that mutations in the ABHD12 gene are causally linked to a neurodegenerative disease called PHARC (polyneuropathy, hearing loss, ataxia, retinitis pigmentosa and cataract) (Fiskerstrand et al. 2010; Savinainen et al. 2012).
5 Conclusions
Over the past 20 years, substantial progress has been made in the understanding of the endocannabinoid system. In particular, new molecules have been classified as endocannabinoids (e.g., the ethanolamides of two omega-3 fatty acids), and new targets other than cannabinoid CB1 and CB2 receptors have been identified and held accountable for some of the effects of the endocannabinoids. Moreover, substantial progress has also been made in the identification as well as in the characterization of the enzymes responsible for both the biosynthesis and the metabolism of the main endocannabinoids, AEA and 2-AG. It still remains to be established whether these enzymes catalyse the formation or degradation of other, less studied, endocannabinoids. In addition, several molecules have been developed that are able to interact more or less selectively or more or less potently with enzymes or uptake processes of the endocannabinoid system. Many of these molecules, such as FAAH and MGL inhibitors as well as endocannabinoid uptake inhibitors, have been discovered, albeit only in animal models, to possess notable therapeutic potential for the treatment of diseases such as cancer, pain, neurodegenerative diseases and so on. Unfortunately, some of these molecules, such as MGL inhibitors, have also been shown to share the ability of direct CB1 cannabinoid receptor agonists to cause physical and psychological dependence. This problem still needs to be overcome. Finally, as recently and elegantly discussed by Piomelli (Piomelli 2014), one important question about the endocannabinoids that still remains unresolved is how such lipophilic molecules are able to cover the distance between their site(s) of action and the site(s) of their enzymatic degradation. This distance is quite short for 2-AG, whose main metabolic enzyme (MGL) is localized presynaptically, and thus close to the pre-synaptic CB1 receptors on which 2-AG acts, but longer for anandamide , which after acting on presynaptic CB1 receptors must travel trans-synaptically in order to be metabolized by FAAH, which is primarily postsynaptic. The research on the understanding of the endocannabinoid system never ends.
Abbreviations
- 2-AG:
-
2-Arachidonoylglycerol
- 2-AGE:
-
2-Arachidonoylglyceryl ether
- AA:
-
Arachidonic acid
- Abh4:
-
Alpha/beta hydrolase 4
- ABHD:
-
Alpha/beta hydrolase domain
- AEA:
-
Anandamide
- Asp:
-
Aspartic acid
- CB:
-
Cannabinoid
- Cis:
-
Cysteine
- COX-2:
-
Cyclooxygenase-2
- DAG:
-
Diacylglycerol
- DGL:
-
Diacylglycerol lipase
- DHA:
-
Docosahexaenoic acid
- DHEA:
-
Docosahexaenoyl-ethanolamide
- DTT:
-
Dithiothreitol
- EMT:
-
Endocannabinoid membrane transporter
- EPA:
-
Eicosapentaenoic acid
- EPEA:
-
Eicosapentaenoyl-ethanolamide
- FAAH:
-
Fatty acid amide hydrolase
- FABP:
-
Fatty acid binding protein
- FLAT:
-
FAAH-like anandamide transporter
- GpAEA:
-
Glycerophospho-arachidonoylethanolamide
- GPR:
-
G-protein coupled receptor
- GSH:
-
Glutathione
- HEK:
-
Human embryonic kidney
- His:
-
Histidine
- Hsp:
-
Heat shock protein
- LOX:
-
Lipoxygenase
- Lys:
-
Lysine
- MAFP:
-
Methylarachidonoylfluorophosphonate
- MGL:
-
Monoacylglycerol lipase
- NAAA:
-
N-acylethanolamine-selective acid amidase
- NADA:
-
N-arachidonoyldopamine
- NAE:
-
N-acyl-ethanolamine
- NAGly:
-
N-arachidonoylglycine
- NAM:
-
N-arachidonoylmaleimide
- NAPE:
-
N-acylphosphatidylethanolamine
- NArS:
-
N-arachidonoylserine
- NAT:
-
N-acyltransferase
- OEA:
-
Oleoyethanolamide
- OLDA:
-
N-oleoyl dopamine
- pAEA:
-
Phosphoanandamide
- PE:
-
Phosphatidylethanolamine
- PEA:
-
Palmitoylethanolamide
- PHARC:
-
Polyneuropathy, hearing loss, ataxia, retinitis pigmentosa, cataract
- PL:
-
Phospholipase
- PPAR:
-
Peroxisome proliferator-activated receptor
- PUFA:
-
Polyunsaturated fatty acid
- SEA:
-
Stearoylethanolamide
- Ser:
-
Serine
- TRPM:
-
Transient receptor potential melastatin
- TRPV:
-
Transient receptor potential vanilloid
References
Ben-Shabat S, Fride E, Sheskin T, Tamiri T, Rhee MH, Vogel Z, Bisogno T, De Petrocellis L, Di Marzo V, Mechoulam R (1998) An entourage effect: inactive endogenous fatty acid glycerol esters enhance 2-arachidonoyl-glycerol cannabinoid activity. Eur J Pharmacol 353:23–31
Berger A, Crozier G, Bisogno T, Cavaliere P, Innis S, Di Marzo V (2001) Anandamide and diet: inclusion of dietary arachidonate and docosahexaenoate leads to increased brain levels of the corresponding N-acylethanolamines in piglets. Proc Natl Acad Sci U S A 98:6402–6406
Bisogno T, Delton-Vandenbroucke I, Milone A, Lagarde M, Di Marzo V (1999) Biosynthesis and inactivation of N-arachidonoylethanolamine (anandamide) and N-docosahexaenoylethanolamine in bovine retina. Arch Biochem Biophys 370:300–307
Bisogno T, Melck D, Bobrov MY, Gretskaya NM, Bezuglov VV, De Petrocellis L, Di Marzo V (2000) N-acyl-dopamines: novel synthetic CB(1) cannabinoid-receptor ligands and inhibitors of anandamide inactivation with cannabimimetic activity in vitro and in vivo. Biochem J 351:817–824
Bisogno T, Howell F, Williams G, Minassi A, Cascio MG, Ligresti A, Matias I, Schiano-Moriello A, Paul P, Williams EJ, Gangadharan U, Hobbs C, Di Marzo V, Doherty P (2003) Cloning of the first sn1-DAG lipases points to the spatial and temporal regulation of endocannabinoid signaling in the brain. J Cell Biol 163:463–468
Blankman JL, Simon GM, Cravatt BF (2007) A comprehensive profile of brain enzymes that hydrolyze the endocannabinoid 2-arachidonoylglycerol. Chem Biol 14:1347–1356
Bracey MH, Hanson MA, Masuda KR, Stevens RC, Cravatt BF (2002) Structural adaptations in a membrane enzyme that terminates endocannabinoid signaling. Science 298:1793–1796
Bradshaw HB, Walker JM (2005) The expanding field of cannabimimetic and related lipid mediators. Br J Pharmacol 144:459–465, Review
Breivogel CS, Griffin G, Di Marzo V, Martin BR (2001) Evidence for a new G protein-coupled cannabinoid receptor in mouse brain. Mol Pharmacol 60:155–163
Brown I, Cascio MG, Wahle KW, Smoum R, Mechoulam R, Ross RA, Pertwee RG, Heys SD (2010) Cannabinoid receptor-dependent and -independent anti-proliferative effects of omega-3 ethanolamides in androgen receptor-positive and -negative prostate cancer cell lines. Carcinogenesis 31:1584–1591
Brown I, Wahle KW, Cascio MG, Smoum-Jaouni R, Mechoulam R, Pertwee RG, Heys SD (2011) Omega-3 N-acylethanolamines are endogenously synthesised from omega-3 fatty acids in different human prostate and breast cancer cell lines. Prostaglandins Leukot Essent Fatty Acids 85:305–310
Brown I, Cascio MG, Rotondo D, Pertwee RG, Heys SD, Wahle KW (2013) Cannabinoids and omega-3/6 endocannabinoids as cell death and anticancer modulators. Prog Lipid Res 52:80–109, Review
Cascio MG (2013) PUFA-derived endocannabinoids: an overview. Proc Nutr Soc 72:451–459
Cascio MG, Minassi A, Ligresti A, Appendino G, Burstein S, Di Marzo V (2004) A structure-activity relationship study on N-arachidonoyl-amino acids as possible endogenous inhibitors of fatty acid amide hydrolase. Biochem Biophys Res Commun 314:192–196
Costa B, Comelli F, Bettoni I, Colleoni M, Giagnoni G (2008) The endogenous fatty acid amide, palmitoylethanolamide, has anti-allodynic and anti-hyperalgesic effects in a murine model of neuropathic pain: involvement of CB(1), TRPV1 and PPARgamma receptors and neurotrophic factors. Pain 139:541–550
Cravatt BF, Prospero-Garcia O, Siuzdak G, Gilula NB, Henriksen SJ, Boger DL, Lerner RA (1995) Chemical characterization of a family of brain lipids that induce sleep. Science 268:1506–1509
Cravatt BF, Giang DK, Mayfield SP, Boger DL, Lerner RA, Gilula NB (1996) Molecular characterization of an enzyme that degrades neuromodulatory fatty-acid amides. Nature 384:83–87
Dalle Carbonare M, Del Giudice E, Stecca A, Colavito D, Fabris M, D’Arrigo A, Bernardini D, Dam M, Leon A (2008) A saturated N-acylethanolamine other than N-palmitoyl ethanolamine with anti-inflammatory properties: a neglected story. J Neuroendocrinol 20(Suppl 1):26–34
De Petrocellis L, Bisogno T, Davis JB, Pertwee RG, Di Marzo V (2000) Overlap between the ligand recognition properties of the anandamide transporter and the VR1 vanilloid receptor: inhibitors of anandamide uptake with negligible capsaicin-like activity. FEBS Lett 483:52–56
De Petrocellis L, Davis JB, Di Marzo V (2001) Palmitoylethanolamide enhances anandamide stimulation of human vanilloid VR1 receptors. FEBS Lett 506:253–256
De Petrocellis L, Starowicz K, Moriello AS, Vivese M, Orlando P, Di Marzo V (2007) Regulation of transient receptor potential channels of melastatin type 8 (TRPM8): effect of cAMP, cannabinoid CB(1) receptors and endovanilloids. Exp Cell Res 313:1911–1920
den Boon FS, Chameau P, Schaafsma-Zhao Q, van Aken W, Bari M, Oddi S, Kruse CG, Maccarrone M, Wadman WJ, Werkman TR (2012) Excitability of prefrontal cortical pyramidal neurons is modulated by activation of intracellular type-2 cannabinoid receptors. Proc Natl Acad Sci U S A 109:3534–3539
Desarnaud F, Cadas H, Piomelli D (1995) Anandamide amidohydrolase activity in rat brain microsomes. Identification and partial characterization. J Biol Chem 270:6030–6035
Deutsch DG, Glaser ST, Howell JM, Kunz JS, Puffenbarger RA, Hillard CJ, Abumrad N (2001) The cellular uptake of anandamide is coupled to its breakdown by fatty-acid amide hydrolase. J Biol Chem 276:6967–6973
Devane WA, Dysarz FA 3rd, Johnson MR, Melvin LS, Howlett AC (1988) Determination and characterization of a cannabinoid receptor in rat brain. Mol Pharmacol 34:605–613
Devane WA, Hanus L, Breuer A, Pertwee RG, Stevenson LA, Griffin G, Gibson D, Mandelbaum A, Etinger A, Mechoulam R (1992) Isolation and structure of a brain constituent that binds to the cannabinoid receptor. Science 258:1946–1949
Di Cesare ML, D’Agostino G, Pacini A, Russo R, Zanardelli M, Ghelardini C, Calignano A (2013) Palmitoylethanolamide is a disease-modifying agent in peripheral neuropathy: pain relief and neuroprotection share a PPAR-alpha-mediated mechanism. Mediators Inflamm 2013:328797
Di Marzo V, Deutsch DG (1998) Biochemistry of the endogenous ligands of cannabinoid receptors. Neurobiol Dis 5:386–404
Di Marzo V, Fontana A, Cadas H, Schinelli S, Cimino G, Schwartz JC, Piomelli D (1994) Formation and inactivation of endogenous cannabinoid anandamide in central neurons. Nature 372:686–691
Di Marzo V, De Petrocellis L, Bisogno T, Melck D (1999) Metabolism of anandamide and 2-arachidonoylglycerol: an historical overview and some recent developments. Lipids 34(Suppl):S319–S325, Review
Di Marzo V, De Petrocellis L, Bisogno T (2001) Endocannabinoids Part I: molecular basis of endocannabinoid formation, action and inactivation and development of selective inhibitors. Expert Opin Ther Targets 5:241–265
Di Marzo V, Griffin G, De Petrocellis L, Brandi I, Bisogno T, Williams W, Grier MC, Kulasegram S, Mahadevan A, Razdan RK, Martin BR (2002) A structure/activity relationship study on arvanil, an endocannabinoid and vanilloid hybrid. J Pharmacol Exp Ther 300:984–991
Di Marzo V, Bifulco M, De Petrocellis L (2004) The endocannabinoid system and its therapeutic exploitation. Nat Rev Drug Discov 3:771–784, Review
Di Pasquale E, Chahinian H, Sanchez P, Fantini J (2009) The insertion and transport of anandamide in synthetic lipid membranes are both cholesterol-dependent. PLoS One 4, e4989
Dinh TP, Carpenter D, Leslie FM, Freund TF, Katona I, Sensi SL, Kathuria S, Piomelli D (2002) Brain monoglyceride lipase participating in endocannabinoid inactivation. Proc Natl Acad Sci U S A 99:10819–10824
Egertová M, Giang DK, Cravatt BF, Elphick MR (1998) A new perspective on cannabinoid signalling: complementary localization of fatty acid amide hydrolase and the CB1 receptor in rat brain. Proc Biol Sci 265:2081–2085
Esposito G, Capoccia E, Turco F, Palumbo I, Lu J, Steardo A, Cuomo R, Sarnelli G, Steardo L (2014) Palmitoylethanolamide improves colon inflammation through an enteric glia/toll like receptor 4-dependent PPAR-α activation. Gut 63:1300–1312
Fegley D, Kathuria S, Mercier R, Li C, Goutopoulos A, Makriyannis A, Piomelli D (2004) Anandamide transport is independent of fatty-acid amide hydrolase activity and is blocked by the hydrolysis-resistant inhibitor AM1172. Proc Natl Acad Sci U S A 101:8756–8761
Fegley D, Gaetani S, Duranti A, Tontini A, Mor M, Tarzia G, Piomelli D (2005) Characterization of the fatty acid amide hydrolase inhibitor cyclohexyl carbamic acid 3’-carbamoyl-biphenyl-3-yl ester (URB597): effects on anandamide and oleoylethanolamide deactivation. J Pharmacol Exp Ther 313:352–358
Fezza F, Bisogno T, Minassi A, Appendino G, Mechoulam R, Di Marzo V (2002) Noladin ether, a putative novel endocannabinoid: inactivation mechanisms and a sensitive method for its quantification in rat tissues. FEBS Lett 513:294–298
Fezza F, Oddi S, Di Tommaso M, De Simone C, Rapino C, Pasquariello N, Dainese E, Finazzi-Agrò A, Maccarrone M (2008) Characterization of biotin-anandamide, a novel tool for the visualization of anandamide accumulation. J Lipid Res 49:1216–1223
Fezza F, Bari M, Florio R, Talamonti E, Feole M, Maccarrone M (2014) Endocannabinoids, related compounds and their metabolic routes. Molecules 19:17078–17106, Review
Fiskerstrand T, H’mida-Ben Brahim D, Johansson S, M’zahem A, Haukanes BI, Drouot N, Zimmermann J, Cole AJ, Vedeler C, Bredrup C, Assoum M, Tazir M, Klockgether T, Hamri A, Steen VM, Boman H, Bindoff LA, Koenig M, Knappskog PM (2010) Mutations in ABHD12 cause the neurodegenerative disease PHARC: an inborn error of endocannabinoid metabolism. Am J Hum Genet 7:410–417
Fonseca BM, Costa MA, Almada M, Correia-da-Silva G, Teixeira NA (2013) Endogenous cannabinoids revisited: a biochemistry perspective. Prostaglandins Other Lipid Mediat 102–103:13–30, Review
Fowler CJ (2012) Anandamide uptake explained? Trends Pharmacol Sci 33:181–185, Review
Fowler CJ (2013) Transport of endocannabinoids across the plasma membrane and within the cell. FEBS J 280:1895–1904, Review
Fu J, Bottegoni G, Sasso O, Bertorelli R, Rocchia W, Masetti M, Guijarro A, Lodola A, Armirotti A, Garau G, Bandiera T, Reggiani A, Mor M, Cavalli A, Piomelli D (2012) A catalytically silent FAAH-1 variant drives anandamide transport in neurons. Nat Neurosci 15:64–69
Gao Y, Vasilyev DV, Goncalves MB, Howell FV, Hobbs C, Reisenberg M, Shen R, Zhang MY, Strassle BW, Lu P, Mark L, Piesla MJ, Deng K, Kouranova EV, Ring RH, Whiteside GT, Bates B, Walsh FS, Williams G, Pangalos MN, Samad TA, Doherty P (2010) Loss of retrograde endocannabinoid signaling and reduced adult neurogenesis in diacylglycerol lipase knock-out mice. J Neurosci 30:2017–2024
Ghafouri N, Ghafouri B, Larsson B, Stensson N, Fowler CJ, Gerdle B (2013) Palmitoylethanolamide and stearoylethanolamide levels in the interstitium of the trapezius muscle of women with chronic widespread pain and chronic neck-shoulder pain correlate with pain intensity and sensitivity. Pain 154:1649–1658
Ghosh S, Wise LE, Chen Y, Gujjar R, Mahadevan A, Cravatt BF, Lichtman AH (2013) The monoacylglycerol lipase inhibitor JZL184 suppresses inflammatory pain in the mouse carrageenan model. Life Sci 92:498–505
Giang DK, Cravatt BF (1997) Molecular characterization of human and mouse fatty acid amide hydrolases. Proc Natl Acad Sci U S A 94:2238–2242
Gulyas AI, Cravatt BF, Bracey MH, Dinh TP, Piomelli D, Boscia F, Freund TF (2004) Segregation of two endocannabinoid-hydrolyzing enzymes into pre- and postsynaptic compartments in the rat hippocampus, cerebellum and amygdala. Eur J Neurosci 20:441–458
Hanus LO (2007) Discovery and isolation of anandamide and other endocannabinoids. Chem Biodivers 4:1828–1841
Hanus L, Abu-Lafi S, Fride E, Breuer A, Vogel Z, Shalev DE, Kustanovich I, Mechoulam R (2001) 2-arachidonyl glyceryl ether, an endogenous agonist of the cannabinoid CB1 receptor. Proc Natl Acad Sci U S A 98:3662–3665
Hillard CJ, Jarrahian A (2000) The movement of N-arachidonoylethanolamine (anandamide) across cellular membranes. Chem Phys Lipids 108:123–134, Review
Ho WS, Hillard CJ (2005) Modulators of endocannabinoid enzymic hydrolysis and membrane transport. Handb Exp Pharmacol 168:187–207, Review
Ho WS, Barrett DA, Randall MD (2008) ‘Entourage’ effects of N-palmitoylethanolamide and N-oleoylethanolamide on vasorelaxation to anandamide occur through TRPV1 receptors. Br J Pharmacol 155:837–846
Huang SM, Bisogno T, Trevisani M, Al-Hayani A, De Petrocellis L, Fezza F, Tognetto M, Petros TJ, Krey JF, Chu CJ, Miller JD, Davies SN, Geppetti P, Walker JM, Di Marzo V (2002) An endogenous capsaicin-like substance with high potency at recombinant and native vanilloid VR1 receptors. Proc Natl Acad Sci U S A 99:8400–8405
Kaczocha M, Glaser ST, Deutsch DG (2009) Identification of intracellular carriers for the endocannabinoid anandamide. Proc Natl Acad Sci U S A 106:6375–6380
Karlsson M, Contreras JA, Hellman U, Tornqvist H, Holm C (1997) cDNA cloning, tissue distribution, and identification of the catalytic triad of monoglyceride lipase. Evolutionary relationship to esterases, lysophospholipases, and haloperoxidases. J Biol Chem 272:27218–27223
Katayama K, Ueda N, Kurahashi Y, Suzuki H, Yamamoto S, Kato I (1997) Distribution of anandamide amidohydrolase in rat tissues with special reference to small intestine. Biochim Biophys Acta 1347:212–218
Kathuria S, Gaetani S, Fegley D, Valiño F, Duranti A, Tontini A, Mor M, Tarzia G, La Rana G, Calignano A, Giustino A, Tattoli M, Palmery M, Cuomo V, Piomelli D (2003) Modulation of anxiety through blockade of anandamide hydrolysis. Nat Med 9:76–81
Kohno M, Hasegawa H, Inoue A, Muraoka M, Miyazaki T, Oka K, Yasukawa M (2006) Identification of N-arachidonylglycine as the endogenous ligand for orphan G-protein-coupled receptor GPR18. Biochem Biophys Res Commun 347:827–832
Leggett JD, Aspley S, Beckett SR, D’Antona AM, Kendall DA, Kendall DA (2004) Oleamide is a selective endogenous agonist of rat and human CB1 cannabinoid receptors. Br J Pharmacol 141:253–262
Lichtman AH, Hawkins EG, Griffin G, Cravatt BF (2002) Pharmacological activity of fatty acid amides is regulated, but not mediated, by fatty acid amide hydrolase in vivo. J Pharmacol Exp Ther 302:73–79
Ligresti A, Morera E, Van Der Stelt M, Monory K, Lutz B, Ortar G, Di Marzo V (2004) Further evidence for the existence of a specific process for the membrane transport of anandamide. Biochem J 380:265–272
Liu Q, Tonai T, Ueda N (2002) Activation of N-acylethanolamine-releasing phospholipase D by polyamines. Chem Phys Lipids 115:77–84
Liu J, Wang L, Harvey-White J, Osei-Hyiaman D, Razdan R, Gong Q, Chan AC, Zhou Z, Huang BX, Kim HY, Kunos G (2006) A biosynthetic pathway for anandamide. Proc Natl Acad Sci U S A 103:13345–13350
Lo Verme J, Fu J, Astarita G, La Rana G, Russo R, Calignano A, Piomelli D (2005) The nuclear receptor peroxisome proliferator-activated receptor-alpha mediates the anti-inflammatory actions of palmitoylethanolamide. Mol Pharmacol 67:15–19
López-Rodríguez ML, Viso A, Ortega-Gutiérrez S, Lastres-Becker I, González S, Fernández-Ruiz J, Ramos JA (2001) Design, synthesis and biological evaluation of novel arachidonic acid derivatives as highly potent and selective endocannabinoid transporter inhibitors. J Med Chem 44:4505–4508
Maccarrone M, Cartoni A, Parolaro D, Margonelli A, Massi P, Bari M, Battista N, Finazzi-Agrò A (2002) Cannabimimetic activity, binding, and degradation of stearoylethanolamide within the mouse central nervous system. Mol Cell Neurosci 21:126–140
Maccarrone M, Di Rienzo M, Finazzi-Agrò A, Rossi A (2003a) Leptin activates the anandamide hydrolase promoter in human T lymphocytes through STAT3. J Biol Chem 278:13318–13324
Maccarrone M, Bari M, Di Rienzo M, Finazzi-Agrò A, Rossi A (2003b) Progesterone activates fatty acid amide hydrolase (FAAH) promoter in human T lymphocytes through the transcription factor Ikaros. Evidence for a synergistic effect of leptin. J Biol Chem 278:32726–32732
Maccarrone M, Dainese E, Oddi S (2010) Intracellular trafficking of anandamide: new concepts for signaling. Trends Biochem Sci 35:601–608
McHugh D, Roskowski D, Xie S, Bradshaw HB (2014) Δ(9)-THC and N-arachidonoyl glycine regulate BV-2 microglial morphology and cytokine release plasticity: implications for signaling at GPR18. Front Pharmacol 4:162
Mechoulam R, Ben-Shabat S, Hanus L, Ligumsky M, Kaminski NE, Schatz AR, Gopher A, Almog S, Martin BR, Compton DR et al (1995) Identification of an endogenous 2-monoglyceride, present in canine gut, that binds to cannabinoid receptors. Biochem Pharmacol 50:83–90
Monory K, Tzavara ET, Lexime J, Ledent C, Parmentier M, Borsodi A, Hanoune J (2002) Novel, not adenylyl cyclase-coupled cannabinoid binding site in cerebellum of mice. Biochem Biophys Res Commun 292:231–235
Moriconi A, Cerbara I, Maccarrone M, Topai A (2010) GPR55: current knowledge and future perspectives of a purported “Type-3” cannabinoid receptor. Curr Med Chem 17:1411–1429, Review
Oddi S, Fezza F, Pasquariello N, De Simone C, Rapino C, Dainese E, Finazzi-Agrò A, Maccarrone M (2008) Evidence for the intracellular accumulation of anandamide in adiposomes. Cell Mol Life Sci 65:840–850
Oddi S, Fezza F, Pasquariello N, D’Agostino A, Catanzaro G, De Simone C, Rapino C, Finazzi-Agrò A, Maccarrone M (2009) Molecular identification of albumin and Hsp70 as cytosolic anandamide-binding proteins. Chem Biol 16:624–632
Oh DY, Yoon JM, Moon MJ, Hwang JI, Choe H, Lee JY, Kim JI, Kim S, Rhim H, O’Dell DK, Walker JM, Na HS, Lee MG, Kwon HB, Kim K, Seong JY (2008) Identification of farnesyl pyrophosphate and N-arachidonylglycine as endogenous ligands for GPR92. J Biol Chem 283:21054–21064
Oka S, Tsuchie A, Tokumura A, Muramatsu M, Suhara Y, Takayama H, Waku K, Sugiura T (2003) Ether-linked analogue of 2-arachidonoylglycerol (noladin ether) was not detected in the brains of various mammalian species. J Neurochem 85:1374–1381
Okamoto Y, Morishita J, Tsuboi K, Tonai T, Ueda N (2004) Molecular characterization of a phospholipase D generating anandamide and its congeners. J Biol Chem 279:5298–5305
Ortar G, Ligresti A, De Petrocellis L, Morera E, Di Marzo V (2003) Novel selective and metabolically stable inhibitors of anandamide cellular uptake. Biochem Pharmacol 65:1473–1481
O’Sullivan SE (2007) Cannabinoids go nuclear: evidence for activation of peroxisome proliferator-activated receptors. Br J Pharmacol 152:576–582, Review
Overton HA, Fyfe MC, Reynet C (2008) GPR119, a novel G protein-coupled receptor target for the treatment of type 2 diabetes and obesity. Br J Pharmacol 153(Suppl 1):S76–S81, Review
Páldyová E, Bereczki E, Sántha M, Wenger T, Borsodi A, Benyhe S (2008) Noladin ether, a putative endocannabinoid, inhibits mu-opioid receptor activation via CB2 cannabinoid receptors. Neurochem Int 52:321–328
Pertwee RG (2005) The therapeutic potential of drugs that target cannabinoid receptors or modulate the tissue levels or actions of endocannabinoids. AAPS J 7:E625–E654, Review
Pertwee RG (2014) Elevating endocannabinoid levels: pharmacological strategies and potential therapeutic applications. Proc Nutr Soc 73:96–105
Pertwee RG, Howlett AC, Abood ME, Alexander SP, Di Marzo V, Elphick MR, Greasley PJ, Hansen HS, Kunos G, Mackie K, Mechoulam R, Ross RA (2010) International union of basic and clinical pharmacology. LXXIX. Cannabinoid receptors and their ligands: beyond CB1 and CB2. Pharmacol Rev 62:588–631, Review
Petersen G, Hansen HS (1999) N-acylphosphatidylethanolamine-hydrolysing phospholipase D lacks the ability to transphosphatidylate. FEBS Lett 455:41–44
Petrosino S, Di Marzo V (2010) FAAH and MAGL inhibitors: therapeutic opportunities from regulating endocannabinoid levels. Curr Opin Investig Drugs 11:51–62, Review
Petrosino S, Iuvone T, Di Marzo V (2010) N-palmitoyl-ethanolamine: biochemistry and new therapeutic opportunities. Biochimie 92:724–727, Review
Piomelli D (2014) More surprises lying ahead. The endocannabinoids keep us guessing. Neuropharmacology 76(Pt B):228–234, Review
Piscitelli F, Di Marzo V (2012) “Redundancy” of endocannabinoid inactivation: new challenges and opportunities for pain control. ACS Chem Neurosci 3:356–363, Review
Porter AC, Sauer JM, Knierman MD, Becker GW, Berna MJ, Bao J, Nomikos GG, Carter P, Bymaster FP, Leese AB, Felder CC (2002) Characterization of a novel endocannabinoid, virodhamine, with antagonist activity at the CB1 receptor. J Pharmacol Exp Ther 301:1020–1024
Puffenbarger RA, Kapulina O, Howell JM, Deutsch DG (2001) Characterization of the 5′-sequence of the mouse fatty acid amide hydrolase. Neurosci Lett 314:21–24
Rodríguez de Fonseca F (2004) The endocannabinoid system and food intake control. Rev Med Univ Navarra 48(2):18–23
Rozenfeld R, Devi LA (2008) Regulation of CB1 cannabinoid receptor trafficking by the adaptor protein AP-3. FASEB J 22:2311–2322
Saario SM, Savinainen JR, Laitinen JT, Järvinen T, Niemi R (2004) Monoglyceride lipase-like enzymatic activity is responsible for hydrolysis of 2-arachidonoylglycerol in rat cerebellar membranes. Biochem Pharmacol 67:1381–1387
Saario SM, Salo OM, Nevalainen T, Poso A, Laitinen JT, Järvinen T, Niemi R (2005) Characterization of the sulfhydryl-sensitive site in the enzyme responsible for hydrolysis of 2-arachidonoyl-glycerol in rat cerebellar membranes. Chem Biol 12:649–656
Savinainen JR, Yoshino M, Minkkilä A, Nevalainen T, Laitinen JT (2010) Characterization of binding properties of monoglyceride lipase inhibitors by a versatile fluorescence-based technique. Anal Biochem 399:132–134
Savinainen JR, Saario SM, Laitinen JT (2012) The serine hydrolases MAGL, ABHD6 and ABHD12 as guardians of 2-arachidonoylglycerol signalling through cannabinoid receptors. Acta Physiol (Oxf) 204:267–276
Schlosburg JE, Blankman JL, Long JZ, Nomura DK, Pan B, Kinsey SG, Nguyen PT, Ramesh D, Booker L, Burston JJ, Thomas EA, Selley DE, Sim-Selley LJ, Liu QS, Lichtman AH, Cravatt BF (2010) Chronic monoacylglycerol lipase blockade causes functional antagonism of the endocannabinoid system. Nat Neurosci 13:1113–1119
Sharir H, Console-Bram L, Mundy C, Popoff SN, Kapur A, Abood ME (2012) The endocannabinoids anandamide and virodhamine modulate the activity of the candidate cannabinoid receptor GPR55. J Neuroimmune Pharmacol 7:856–865
Sheskin T, Hanus L, Slager J, Vogel Z, Mechoulam R (1997) Structural requirements for binding of anandamide-type compounds to the brain cannabinoid receptor. J Med Chem 40:659–667
Sigel E, Baur R, Rácz I, Marazzi J, Smart TG, Zimmer A, Gertsch J (2011) The major central endocannabinoid directly acts at GABA(A) receptors. Proc Natl Acad Sci U S A 108:18150–18155
Simon GM, Cravatt BF (2006) Endocannabinoid biosynthesis proceeding through glycerophospho-N-acyl ethanolamine and a role for alpha/beta-hydrolase 4 in this pathway. J Biol Chem 281:26465–26472
Sugiura T, Kondo S, Sukagawa A, Nakane S, Shinoda A, Itoh K, Yamashita A, Waku K (1995) 2-Arachidonoylglycerol: a possible endogenous cannabinoid receptor ligand in brain. Biochem Biophys Res Commun 215:89–97
Sugiura T, Kondo S, Sukagawa A, Tonegawa T, Nakane S, Yamashita A, Waku K (1996) N-arachidonoylethanolamine (anandamide), an endogenous cannabinoid receptor ligand, and related lipid molecules in the nervous tissues. J Lipid Mediat Cell Signal 14:51–56, Review
Sun YX, Tsuboi K, Okamoto Y, Tonai T, Murakami M, Kudo I, Ueda N (2004) Biosynthesis of anandamide and N-palmitoylethanolamine by sequential actions of phospholipase A2 and lysophospholipase D. Biochem J 380:749–756
Sun YX, Tsuboi K, Zhao LY, Okamoto Y, Lambert DM, Ueda N (2005) Involvement of N-acylethanolamine-hydrolyzing acid amidase in the degradation of anandamide and other N-acylethanolamines in macrophages. Biochim Biophys Acta 1736:211–220
Sun Y, Alexander SP, Kendall DA, Bennett AJ (2006) Cannabinoids and PPARalpha signalling. Biochem Soc Trans 34:1095–1097
Tanimura A, Yamazaki M, Hashimotodani Y, Uchigashima M, Kawata S, Abe M, Kita Y, Hashimoto K, Shimizu T, Watanabe M, Sakimura K, Kano M (2010) The endocannabinoid 2-arachidonoylglycerol produced by diacylglycerol lipase alpha mediates retrograde suppression of synaptic transmission. Neuron 65:320–327
Ueda N (2002) Endocannabinoid hydrolases. Prostaglandins Other Lipid Mediat 68–69:521–534, Review
Ueda N, Kurahashi Y, Yamamoto K, Yamamoto S, Tokunaga T (1996) Enzymes for anandamide biosynthesis and metabolism. J Lipid Mediat Cell Signal 14:57–61
Ueda N, Yamanaka K, Terasawa Y, Yamamoto S (1999) An acid amidase hydrolyzing anandamide as an endogenous ligand for cannabinoid receptors. FEBS Lett 454:267–270
Ueda N, Liu Q, Yamanaka K (2001a) Marked activation of the N-acylphosphatidylethanolamine-hydrolyzing phosphodiesterase by divalent cations. Biochim Biophys Acta 1532:121–127
Ueda N, Yamanaka K, Yamamoto S (2001b) Purification and characterization of an acid amidase selective for N-palmitoylethanolamine, a putative endogenous anti-inflammatory substance. J Biol Chem 276:35552–35557
Ueda N, Tsuboi K, Uyama T (2010) N-acylethanolamine metabolism with special reference to N-acylethanolamine-hydrolyzing acid amidase (NAAA). Prog Lipid Res 49:299–315, Review
Ueda N, Tsuboi K, Uyama T (2013) Metabolism of endocannabinoids and related N-acylethanolamines: canonical and alternative pathways. FEBS J 280:1874–1894
Waleh NS, Cravatt BF, Apte-Deshpande A, Terao A, Kilduff TS (2002) Transcriptional regulation of the mouse fatty acid amide hydrolase gene. Gene 291:203–210
Wei BQ, Mikkelsen TS, McKinney MK, Lander ES, Cravatt BF (2006) A second fatty acid amide hydrolase with variable distribution among placental mammals. J Biol Chem 281:36569–36578
Williams EJ, Walsh FS, Doherty P (2003) The FGF receptor uses the endocannabinoid signaling system to couple to an axonal growth response. J Cell Biol 160:481–486
Zygmunt PM, Petersson J, Andersson DA, Chuang H, Sørgård M, Di Marzo V, Julius D, Högestätt ED (1999) Vanilloid receptors on sensory nerves mediate the vasodilator action of anandamide. Nature 400:452–457
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2015 Springer International Publishing Switzerland
About this chapter
Cite this chapter
Cascio, M.G., Marini, P. (2015). Biosynthesis and Fate of Endocannabinoids. In: Pertwee, R. (eds) Endocannabinoids. Handbook of Experimental Pharmacology, vol 231. Springer, Cham. https://doi.org/10.1007/978-3-319-20825-1_2
Download citation
DOI: https://doi.org/10.1007/978-3-319-20825-1_2
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-20824-4
Online ISBN: 978-3-319-20825-1
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)